Abstract
Neuroimaging and transcranial magnetic stimulation provide insights into the neuronal mechanisms underlying speech disfluencies in chronic persistent stuttering. In the present paper, the goal is not to provide an exhaustive review of existing literature, but rather to highlight robust findings. We, therefore, conducted a meta-analysis of diffusion tensor imaging studies which have recently implicated disrupted white matter connectivity in stuttering. A reduction of fractional anisotropy in persistent stuttering has been reported at several different loci. Our meta-analysis revealed consistent deficits in the left dorsal stream and in the interhemispheric connections between the sensorimotor cortices. In addition, recent fMRI meta-analyses link stuttering to reduced left fronto-parieto-temporal activation while greater fluency is associated with boosted co-activations of right fronto-parieto-temporal areas. However, the physiological foundation of these irregularities is not accessible with MRI. Complementary, transcranial magnetic stimulation (TMS) reveals local excitatory and inhibitory regulation of cortical dynamics. Applied to a speech motor area, TMS revealed reduced speech-planning-related neuronal dynamics at the level of the primary motor cortex in stuttering. Together, this review provides a focused view of the neurobiology of stuttering to date and may guide the rational design of future research. This future needs to account for the perpetual dynamic interactions between auditory, somatosensory, and speech motor circuits that shape fluent speech.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stuttering is a speech disorder which most often occurs between the age of 3 and 6 years [1]. Lifespan incidence is higher than 5 %, with high rates of recovery (52–87 %) [2, 3]. Lifespan prevalence is 0.72 % with a sex ratio of 2.3 [4]. Neither etiology nor pathogenesis is known [5]; thus, stuttering is characterized by its symptoms. The hallmark signs of stuttering are involuntary sound and syllable repetitions, sound prolongations, and speech blocks [6]. In some cases, additional facial and limb movements such as grimacing, hand tapping, or stamping with one’s foot accompany these speech motor signs. Strategies to avoid stuttering include word substitutions, sentence reordering, but also to fall silent in certain situations. Failure in communication provokes negative emotions such as fear and embarrassment. The course of stuttering varies across individuals and distinct phenotypes emerge. Depending on severity, stuttering critically compromises quality of life [7].
Similar to other behaviourally defined disorders, the cause of stuttering is multifactorial and is associated with various genetic and environmental risk factors. The large presence of familial stuttering and the high concordance rate in twins support a genetic role in stuttering [8]. To date, few linkage studies have nominated contributing genes [9, 10]. Genome-wide significance [10] still awaits replication [11••] and more genome-wide association studies are required [12]. It remains to be seen whether future efforts will demonstrate the polygenetic basis of stuttering and thus shed light on the questions of involved transmission models, chromosomes, genes, or sex factors.
The phenomenon of stuttering has given rise to manifold theories, each shaped by the perspective of a certain field such as for example analytic psychology [13], speech and language pathology [1, 5, 14], psychology [15, 16], linguistics [17–19], biomechanics [20–22], and neuroscience [23–27]. Neuroscience-based hypotheses have included an aberrant dominant hemisphere structure [28–30], basal ganglia dysfunction [23], a disconnection syndrome [31], altered brain timing networks [25, 26, 32, 33], or an altered sensorimotor integration [20, 34, 35], mostly interrelating with each other. This multiplicity of causes is plausible due to the fact that a broad assortment of linguistic, cognitive, and sensorimotor processes are involved in speech production. Speech is a very complex sensorimotor action, and its intimate connection to language, a defining feature of human cognition, makes speech and stuttering a very complicated field of study for neuroscientists and neurologists. In the last 30 years, studies on the neurobiology of stuttering have improved our understanding of potential mechanisms, but there are still fundamental questions open. Here, we will summarize the main neuroscientific findings on chronic persistent stuttering.
The Continuous Speech Stream
The ultimate readout of language planning and speech motor control is articulation that results in an audible, smooth, continuous stream of speech. Articulation is a demanding coordinative challenge because it requires the orchestration of respiratory, laryngeal, and supralaryngeal structures by using approximately 100 muscles [36]. The respiratory system regulates the outflow of air during speech and thus provides energy for the acoustic targets of speech. The laryngeal system generates the quasiperiodic and tone-like sound fundamental for pitch modulation, vowels, and voiced consonants (e.g., [b], [z], and [m]). Voiceless and aspirated consonants (e.g., [p], [s], and [h]) require timely voice offsets transmitted by short transient glottal abductions. The supralaryngeal system comprises the pharyngeal, oral, and nasal cavities whose architecture and configuration shape the timbre and sound of the generated acoustic signal. The supralaryngeal system, also called the vocal tract, can be constricted at different places, for example via lip closure; lip protrusion; tongue tip or body elevation, or retraction; and velum elevation. Characteristic sound features of speech vowels are generated by articulatory gestures such as jaw lowering, tongue body elevation, and lip protrusion. In contrast, distinct acoustic features of consonants are generated by the magnitude of obstruction, resulting in bursts due to closure and friction-like noise due to fine-tuned constriction.
During speaking, our articulators are continuously in motion [37]. Our thoughts are transformed into coupled articulatory patterns that carry specific melodies and rhythms. Prosody and articulation are built upon motor units that act on multiple timescales. Their execution happens simultaneously, in an overlapping or subsequent manner continuously adapting to ever-changing contexts due to changes in speaking rate, co-articulation, or emotional load. Imagine a machine buildup of all necessary effectors and degrees of freedom enabling the spatio-temporal dynamics of sound production. Why would such a machine only produce scattered sounds but not smooth, fluid speech? One aspect is the unsolved problem of prosodic modeling in speech synthesis [38]. The other problem is a missing feedback system in current speech synthesis programs. Human speech production is closely coupled to its perception. The key to fluent speech is a production-perception interaction. The timely sequencing and context-dependent binding of speech units are constantly monitored and adjusted by an effective sensorimotor integration [39]. Feedback-related control couples not only perception and production processes but also internal models that closely relate to the sound envelop of a corresponding utterance [40] possibly translating auditory targets into motor commands. For this reason, it is necessary to consider the output and input systems as well as internal models, interfaces, and monitors to comprehensively elucidate the neurobiology of stuttering.
Neural Underpinnings of Persistent Stuttering: from Structure to Function
Chronic persistent stuttering is highly heterogeneous with regard to symptoms, avoidance behavior, applied strategies to overcome disfluencies, and severity. Therefore, it is not surprising that imaging studies have produced diverse, puzzling, and sometimes contradictory results [41]. It has been suggested that the “core” of the stuttered response may have nothing to do with changes in functional imaging observed at rest, during speech, or following therapy [42]. This review will not outline the diverging neuroimaging findings of the last 30 years. In fact, we rather have concentrated on published findings from diffusion tensor imaging (DTI) in an informative meta-analysis to obtain the most robust white matter changes in persistent stuttering currently reported. Subsequently, we relate these structural findings to irregular brain function as described in two recent activation likelihood estimation (ALE) meta-analyses [43••, 44••]. To account for the fact that the neural organization of speaking employs recurrent networks working at a high temporal resolution, we complement the view by reviewing results of those few transcranial magnetic stimulation (TMS) studies available.
DTI—the Left Dorsal Stream and Interhemispheric Somatosensory Connections Are Affected in Stuttering
Fractional anisotropy (FA) is the most frequently reported parameter of DTI. It measures the directionality of water molecule mobility on a submillimeter scale. This directedness is especially high along the myelinated axons of the white matter, though orientation distribution of axons and the degree of myelination are not the only influencing factors. Axon diameter distribution and the axonal tissue fraction or density affect the magnitude of the FA as well. Moreover, the macroscopic geometrical arrangement of white matter bundles such as crossing or fanning fibers comes into play especially at the low resolutions of 2–3 mm3 usually employed in human diffusion-weighted MRI. However, a reduced FA is commonly interpreted as less coherent white matter structure [45]. Group comparisons of neuroimaging parameters are not trivial, as individually shaped brains need to be aligned to a common space. To render DTI group statistics possible, this normalization is most often achieved by the projection of voxels with the highest FA in the center of each gyrus or white matter tract to a skeleton that represents a common tract-based template for the studied group (tract-based spatial statistics, TBSS [46]).
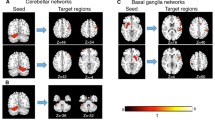
To date, nine DTI studies have reported whole-brain FA reductions from white matter regions in cases with persistent stuttering (Table 1). Sixty widespread loci result from the seven studies that examined subjects older than 14. Loci number and variability increase when adding studies in children (aged 3 to 12) as well (Fig. 1a). To reduce dimensionality, we calculated an informative meta-analysis of the coordinates of decreased FA using the ALE method. This method was introduced for the meta-analysis of functional MRI activation maps and detects three-dimensional conjunctions of coordinates, weighted by sample size [47]. The 60 loci that were included were from seven studies which interrogated 121 persons who stutter and 124 fluent speakers aged 14 to 52 years. Higher FA values in persons who stutter were not considered because increases are infrequently reported. The current analysis yielded three clusters of lower FA values in persons who stutter (p < 0.001; FDR q < 0.05; Fig. 1b), located in the left hemisphere and in the corpus callosum.
DTI (a–c) and fMRI (d, e) imaging changes associated with persistent chronic stuttering. a Loci of reduced FA as reported in multiple studies were shown on a transparent isosurface of the MNI brain. Red spheres indicate foci from studies of persons aged 14 to 52 who stutter, and orange spheres indicate loci from children aged 3 to 10 who stutter [63]. b Blue illustrates clusters of reduced FA in persistent stuttering as derived from a meta-analysis using the activation likelihood estimation (ALE) method (p < 0.001; FDR q < 0.05). c Diffusion tractography derived from full brain deterministic fiber tracking in [125] an ultra-high-resolution DTI data set of a single subject [49]. Tracts are shown that cross a sphere with a diameter of 5 mm surrounding the MNI coordinates of the meta-analysis after a linear registration to the subject’s native space. d Trait stuttering is captured by contrasts between persons who stutter and fluent speakers. Therefore, it reveals brain areas that are either more active (red and orange dots) or less active (blue and light blue dots) in persons who stutter compared to fluent speakers. Right hemisphere over-activations reside in the precentral gyrus, lip motor cortex, rolandic operculum, insula, IFG pars opercularis, IFG pars orbitalis, pre-SMA, middle frontal gyrus, IPL, and SPL. Left hemisphere over-activations reside in the SMA and in the SPL. Left hemisphere under-activations are located in the left larynx motor cortex, left MTG, left superior temporal sulcus, cerebellar vermis, and the red nucleus [43••, 44••]. Trait stuttering contrasts enlighten brain abnormalities that cause stuttering or that compensate for it. e Supplementary, state stuttering analyses capture within-group contrasts which enlighten areas in the brain that are more active when fluency is enhanced (green and light green dots) compared to areas that are more active when fluency is worse (purple and violet dots). Disfluency-related activations reside in Broca’s area in the right IFG pars orbitalis, the left IFG pars opercularis and pars triangularis, bilaterally in the SMA, the somatosensory cortex, and the cerebellum, and in the left precuneus and the left globus pallidus. Fluency-related activations reside mostly in the right hemisphere, namely the Heschl gyrus, the planum temporale, the posterior STG, MTG, SMG, IPL, IFG pars opercularis, and the MFG. Left hemisphere correlates are in the IPL [43••, 44••]. State stuttering contrasts might reveal causes of stuttering events, attempts to compensate for stuttering, or the correlates of stuttering as a motor act [44••]
Subsequent deterministic DTI tractography served to estimate the course of the white matter connections passing through the significant clusters of the current meta-analysis. The chosen high-quality diffusion tensor image of a representative single young healthy subject has an isotropic resolution of 1 mm acquired on an ultra-high-field MRI scanner using 60 diffusion directions and 4 averages [48, 49]. The first cluster was located in the left superior longitudinal fasciculus (SLF III, 344 mm3 centered at [41, 53, 42]; Fig. 1c left) of the inferior parietal lobe (IPL) adjacent to the angular gyrus and the posterior division of the supramarginal gyrus (SMG). Reconstructed connections terminated in the postcentral gyrus, in the ventral premotor cortex, and in the posterior-ventral area of the inferior frontal gyrus (IFG) pars opercularis as part of Broca’s area. The second cluster was located below the fundus of the left central sulcus in the left SLF but this time also including fibers of the arcuate fasciculus (AF, 280 mm3 centered at [38, 22, 30]; Fig. 1c middle). Connections terminated frontally in the ventral motor cortex, in the ventral premotor cortex, and in the posterior part of Broca’s area, the IFG pars opercularis; parietal terminations reached the SMG and the angular gyrus; and temporal terminations reached the posterior superior temporal gyrus (STG) and in the middle temporal gyrus (MTG). The third cluster was placed in the posterior midbody of the corpus callosum (240 mm3 centered at [3, 22, 25]; Fig. 1c right) where interhemispheric fibers pass through and terminate at the postcentral and precentral gyri close to the vertex.
Our current meta-analysis related the most robust white matter changes in stuttering to the left dorsal language stream. This is in line with diffusion tractography studies reporting a reduced FA in these streams [50], the absence of streamlines in a large portion of the left AF [51], as well as a reduced tractography density of the left SLF III [52] in persons who stutter compared to fluent speakers. The four branches of the SLF and AF are the prominent fiber bundles mediating the interaction between frontal, parietal, and temporal regions [53–55]—also evident in the current tractography results.
Another robust outcome of the current meta-analysis was the reduced FA in the interhemispheric fibers of the posterior midbody of the corpus callosum. Our deterministic fiber tracking showed that the disrupted callosal connections most likely connect sensorimotor regions (Fig. 1c right). The reconstructed pathways link medial regions of the post- and precentral gyri, but the more lateral regions that are known to control orofacial structures were not involved. Before drawing conclusions on this restricted course, one should consider that transcallosal fibers are massively crossed by orthogonal association and projection fibers. The DTI tractography algorithm used is influenced by these crossing fiber populations, and the reconstruction of all callosal connections cannot be easily solved [56]. No diffusion tractography study on stuttering has fully reconstructed transcallosal connections. This may be due to these methodological difficulties. Hence, the current tractogram does not allow ruling out an involvement of fibers terminating in ventral sites of the sensorimotor cortex.
The interpretation of the reduced FA values is not trivial. Particularly, the dorsal stream is affected by crossing fibers from transcallosal as well as corticospinal and corticothalamic connections or other subcortical loops. Whether FA reductions result from a weakened intrahemispheric connectivity, a strengthened interhemispheric connectivity, or both remains to be shown. Ultra-high-field imaging [48] in combination with a sophisticated tracking algorithm [56] might disentangle macro-anatomy-related changes. In contrast, FA is not affected by crossing fibers within the corpus callosum; fibers run exclusively in one direction, reducing the number of variables that influence FA to the degree of myelination, axon diameter distribution, and axon population density. The axons with the largest diameter reside in the posterior midbody of the corpus callosum [57] in healthy human subjects. From this, it follows that the interhemispheric transmission is fastest and most efficient in this area which is capable of transmitting reliable, precisely timed neuronal coupling. Hence, it is plausible that the frequency-specific interhemispheric correlation structure of spontaneous oscillatory neuronal activity is nested in the highest frequency range (32–45 Hz) between the sensorimotor cortices compared to the temporal lobes (4–6 Hz) and the lateral parietal areas (8–23 Hz) [58]. Large-diameter axon fibers may also determine the degree of interhemispheric-correlated fMRI resting-state activity which is again highest in the somatosensory cortices [59]. In stuttering, the reduced FA could be related to either reduced myelination or altered axonal diameter distribution [60] in the affected area. However, these two possibilities could have different outcomes: While reduced myelination would cause a deficient interhemispheric interaction, increased density of large-diameter axon fibers could result in a strengthened interhemispheric interaction. For this reason, it would be desirable to employ advanced methods that better resolve the axon diameter distribution “in vivo” [61, 62].
To summarize, non-invasive in vivo DTI provides the most important insights into connectivity changes of brain networks in stuttering. Short- and long-range widely integrated, parallel, and often redundant neuronal subcircuits supply speech fluency. It is likely that connectivity changes of speech-relevant perisylvian brain areas lead to disruption of speech functions. Our meta-analysis emphasized the important role of left hemisphere corticocortical connections, namely the SLF and the AF, and transcallosal connections of the posterior midbody for fluent speech production. However, right hemisphere connectivity [50, 51, 63••, 64–66] as well as axons of the corticospinal tract [50, 63••, 64–68], thalamic [64, 67], and cerebellar [50, 63••, 64, 65] connections have also been reported to show irregularities in stuttering. Similar to other behavioral and cognitive processes, fluent speech production depends on the embedding of various areas in the human connectome [69••]. The following section of functional imaging changes in stuttering mainly summarizes the altered recruitment of cortical and subcortical areas suggesting irregular input and output operations within the speech-related connectome.
fMRI—Right Frontal Over-activation Characterizes Stuttering While Right Parieto-temporal Co-activation Characterizes Greater Fluency
So far, we have only elaborated on structural imaging, focusing particularly on white matter integrity and thus the connectome. A lot is already known about the underlying function of the connections that come into focus here. Predominantly, left dorsal paths subserve linguistic as well as speech motor functions. The AF, the medial part of the dorsal stream, connects the IFG pars opercularis to the STG and mediates complex syntax [70, 71] and phonology [72•]. Sublexical repetition of speech [73], speech planning [72•], and articulation [74] map to the lateral part of the dorsal stream and the indirect anterior portion of the SLF connecting the precentral gyrus to the SMG and the STG [55]. Articulatory phonetic skills rely on an effective auditory-motor integration partly mediated by the recurrent networks of these dorsal streams [53, 75••, 76].
The functional anatomy underlying stuttering has mostly been studied with positron emission tomography [77–82] and functional magnetic resonance imaging [83–87]. Two activation likelihood meta-analyses on stuttering were recently published [43••, 44••]. The meta-analyses considered 23 functional imaging studies published over the past 30 years; these included 213 [44••] and 222 [43••] persons who stutter and 186 [44••] and 188 [43••] control subjects, and Fig. 1d, e summarizes their outcome.
These meta-analyses highlight the neurofunctional hallmark signs of persistent chronic stuttering. What is striking, is the consistent over-activation of the frontal motor areas of the right hemisphere encompassing the primary motor cortex, the premotor cortex, the pre-supplementary motor area (pre-SMA), the supplementary motor area (SMA), the IFG, the insula, and the frontal and the rolandic operculum [43••, 44••] (Fig. 1d). An opposite pattern of cortical activity emerges in the left hemisphere. Here, frontal regions show no over-activation but instead a reduced activation of the M1 larynx area combined with reduced activity in the planum temporale and the middle temporal gyrus. The left cerebellar vermis and the left red nucleus also display robust imaging changes that emerge from a comparison of speech-related hemodynamic differences between persons who stutter and fluent speakers. The only region that shows a higher activation is the right parietal lobe. Stronger activations are located in the anterior intraparietal sulcus and in the IPL PFcm. The remarkable right hemisphere over-activation in stuttering suggests an imbalanced hemispheric lateralization [28, 29]. It is not yet clear whether this imbalance causes stuttering, whether it is the result of impeded left fronto-parieto-temporal signal processes, or if it reflects compensatory mechanisms [31, 78, 84, 88–90].
Every investigation of stuttering tries to find out how fluency of speech production can be attained. Therefore, imaging contrasts that relate brain activations to greater fluency in persons who stutter are of special interest. In the right hemisphere, such contrasts show a shift of activation patterns to parietal areas spanning several loci in the IPL, heavy involvement of the temporal lobe (Heschl’s gyrus, planum temporale, and STG) and the cerebellum. Greater fluency is associated with the recruitment of superior temporal and inferior parietal regions in both hemispheres, whereas severe stuttering is associated with dysfunction of a distributed network of classical motor areas engaging sensorimotor regions amongst the central sulcus including the left and right somatosensory cortex, the left larynx motor cortex, extended regions of the IFG including the left pars opercularis, the left pars triangularis and right pars orbitalis, bilateral SMA, and the cerebellum (Fig. 1e). Fluency-related activations in unimodal and heteromodal association areas of the parietal and right temporal lobe, the right pars opercularis, and the posterior ventral part of right Broca’s region strongly suggest an important role of internal models and feedforward- and feedback-relevant control mechanisms during speaking. In fluent speakers, lateralization of speech production seems to start in the left temporal and parietal regions [91], namely the somatosensory cortex, the auditor cortex, and the planum temporale which might be the source of the early sound feature-related cortical entrainment observed in left Broca’s area and the left premotor cortex even ahead of external speech production [40]. Equivalent studies in stuttering are missing, leaving the question open as to whether right lateralization already occurs in the planning stage. However, one TMS study has indeed observed missing lateralization at an early stage.
TMS Indicates a Restricted Range of Neuronal Dynamics at the Level of the Primary Motor Cortex in Stuttering
Both DTI and fMRI elucidate the spatial distribution of large-scale neuronal dysfunction in persistent stuttering, but its physiological basis remains unclear. Nonlinear neuronal dynamics consist of excitatory and inhibitory activation, but these cannot be discriminated with in vivo neuroimaging. The only non-invasive technique that allows to measure excitatory and inhibitory brain function in healthy humans is TMS [92]. A TMS pulse induces currents in conductive tissue such as the human cortex. When applied to the motor cortex, neurons are stimulated and evoke motor potentials (MEP) serving as a readout measure of excitability dynamics of local circuits. State-dependent excitability regulation is quantified by comparing baseline MEP amplitudes with amplitudes resulting under test conditions. Fortunately, the primary motor cortex is the final overarching cortical output region [93] that generates speech behavior. Almost all dysfunctional computations accumulate at this site, making it an attractive target for stuttering research even in the nonspeech domain [94–96].
Paired-pulse TMS protocols are suitable for testing intracortical inhibitory and excitatory circuits [97]. Compared to single-pulse responses, MEP amplitudes are reliably reduced when a subthreshold pulse is followed by a superthreshold pulse with a short interstimulus interval of 2 to 3 ms. This inhibition is likely caused by excited GABAergic interneurons [98, 99]. In stuttering, ipsilateral and contralateral tongue representations in the left and right hemisphere showed a delayed inhibition of intracortical circuits [100]. The opposite phenomenon, intracortical facilitation, can be generated when applying paired pulses with longer interstimulus intervals of 10 to 15 ms. In this case, MEP amplitudes are amplified driven by the sensory input on excitatory motor circuits [101••]. In stuttering, this facilitation is remarkably reduced in the primary motor tongue area of both hemispheres [100]. Thus, intracortical excitability regulation is hampered in an area that controls one of the main effectors of articulation. The combined reductions of intracortical inhibition and facilitation indicate a restricted range of neuronal dynamics at rest.
Although orofacial midline muscles such as the tongue are bilaterally innervated from corticobulbar projections of both hemispheres, speech motor plans are primarily encoded in the left hemisphere motor cortex. However, this functional asymmetry towards the left orofacial motor cortex is missing in stuttering [102••], suggesting that a lack of a speech-motor-planning-induced facilitation of the left orofacial motor cortex is a major pathophysiological cause of disfluent speech production. This lack might be related to the under-activation of this area [32] as frequently reported in neuroimaging studies [44••] implicating a fallible transmission or integration of speech-planning-related feedforward signals [20, 33, 103•]. Conversely, given the regularly reported over-activation of the right primary motor cortex in stuttering [77, 85, 87, 104–106], one might expect to see a speech-planning-induced facilitation of this site, but this pattern was not noted [102••].
The right hemisphere is known to play a dominant role in prosody perception and production [107–110]. One theory on stuttering suggests a misalignment of segmental (phonemic) and suprasegmental (prosodic) phonetic features [111]. While consonantal voice onsets and offsets act on a fast temporal scale with a resolution of 20 to 50 ms [112], features such as rhythm, stress, and melody patterns span the temporal frame of a whole utterance. The underlying auditory-to-articulatory alignment requires a precise temporal coupling at multiple timescales. Fast auditory signals are preferentially integrated in the left auditory cortex, while slow auditory signals are preferentially integrated in the right auditory cortex [113]. Supposing the sensorimotor control of slower suprasegmental features to be lateralized to the right hemisphere, and slow auditory targets such as melody and stress mainly arise from the right frontal motor regions. This would suggest speech-planning-induced facilitation of the right larynx area rather than the right tongue area. Especially prosodic features are regulated at the laryngeal level, and notably the right primary motor larynx area shows increased hemodynamic responses in persons who stutter [44••].
Conclusion and Outlook
Speech is regulated by co-activated neuronal circuits of large-scale dynamic networks [114] and their dysfunction results in persistent stuttering. Reduced speech-related dynamics in the left hemisphere and augmented right hemisphere involvement are cardinal neuronal signs possibly caused by imbalanced wiring. This review lacks a detailed description of subcortical contributions to stuttering behavior, although there is converging evidence for cerebellar, thalamic, as well as basal ganglia irregularities [23, 50, 65, 86, 115–118]. We attach importance to the cortical dynamics within the speech-related connectome as a result of new meta-analyses offering a condensed view of imaging changes associated with chronic persistent stuttering. This is by no means intended to scale down the importance of neuroimaging findings derived from every individual study. Quite the contrary is true; it elucidates that current methods are not sensitive enough to fully disentangle the brain dynamics of stuttering. However, our review provides a focused view on the brain deficits of persons affected with persistent stuttering, which might open the gate for a rethinking of how best to proceed. Future studies employing TMS, deep brain stimulation [118], sophisticated neuroimaging techniques [119, 120], and selected animal studies [121, 122•, 123•, 124•] may advance mechanistic models [75] and may eventually guide success in therapeutic efforts aiming to facilitate fluency. The following questions are of particular interest: What are the interhemispheric interactions that allow fluent speech production and how do they change in stuttering? Which brain dynamics characterize single acts of stuttering and would it be possible to interfere with those sudden interruptions of the integrity of the speech motor network? Is it possible to employ special hearing aids to facilitate the maturation of temporo-parieto-frontal interactions necessary for stable sensorimotor integration? Which neuromodulatory interventions could strengthen the left fronto-parieto-temporal network to overcome the problem that only fluency-enhancing techniques such as chorus speaking or speaking to the rhythm of a metronome unburden the computational load of the frontal motor network [116] and bypass the IFG, precentral gyrus, insula, putamen, nucleus caudatus, and globus pallidus?
Abbreviations
- AF:
-
Arcuate fasciculus
- ALE:
-
Activation likelihood estimation
- DTI:
-
Diffusion tensor imaging
- FA:
-
Fractional anisotropy
- FDR:
-
False discovery rate
- IFG:
-
Inferior frontal gyrus
- IPL:
-
Inferior parietal lobe
- M1:
-
Primary motor cortex
- MEP:
-
Motor evoked potential
- MFG:
-
Middle frontal gyrus
- MTG:
-
Middle temporal gyrus
- SLF:
-
Superior longitudinal fasciculus
- SMA:
-
Supplementary motor area
- SMG:
-
Supramarginal gyrus
- SPL:
-
Superior parietal lobe
- STG:
-
Superior temporal gyrus
- TBSS:
-
Tract-based spatial statistics
- TMS:
-
Transcranial magnetic stimulation
- VBS:
-
Voxel-based statistics
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Yairi E, Ambrose NG. Early childhood stuttering. Austin: Pro-Ed; 2005.
Howell P, Davis S. Predicting persistence of and recovery from stuttering by the teenage years based on information gathered at age 8 years. J Dev Behav Pediatr. 2011;32:196–205.
Dworzynski K, Remington A, Rijsdijk F, Howell P, Plomin R. Genetic etiology in cases of recovered and persistent stuttering in an unselected longitudinal sample of young twins. Am J Speech-Lang Pathol. 2007;16:169.
Craig A. Epidemiology of stuttering in the community across the entire life span. J Speech Lang Hear Res. 2002;45:1097–105.
Bloodstein O, Ratner NB. A handbook on stuttering. 6th ed. Clifton Park: Delmar Learning; 2008.
Wingate ME. A standard definition of stuttering. J Speech Hear Disord. 1964;29:484.
Yaruss JS. Assessing quality of life in stuttering treatment outcomes research. J Fluen Disord. 2010;35:190–202.
Kraft SJ, Yairi E. Genetic bases of stuttering: the state of the art, 2011. Folia Phoniatr Logop. 2012;64:33–46.
Suresh R, Ambrose N, Roe C, Pluzhnikov A, Wittke-Thompson JK, Ng MC-Y, et al. New complexities in the genetics of stuttering: significant sex-specific linkage signals. Am J Hum Genet. 2006;78:554–63.
Riaz N, Steinberg S, Ahmad J, Pluzhnikov A, Riazuddin S, Cox NJ, et al. Genomewide significant linkage to stuttering on chromosome 12. Am J Hum Genet. 2005;76:647–51.
Yairi E, Ambrose N. Epidemiology of stuttering: 21st century advances. J Fluen Disord. 2013;38:66–87. This article is a comprehensive review on epidemiological factors in stuttering also critically evaluating the current biological research of gene identification.
Kraft SJ. Genome-wide association study of persistent developmental stuttering. University of Illinois at Urbana- Champaign; 2010
Damsté PH, Zwaan EJ, Schoenaker TJ. Learning principles applied to the stuttering problem. Folia Phoniatr Logop. 1968;20:327–41.
Riper CV. The nature of stuttering. Prentice-Hall; 1971
Smith A, Kelly E. Stuttering: a dynamic multifactorial model. In: Curlee R, Siegel G, editors. Nature and treatment of stuttering: new directions. 2nd ed. Needham Heights, MA: Allyn & Bacon; 1997. p. 204–17.
Starkweather CW, Gottwald SR. The demands and capacities model II: clinical applications. J Fluen Disord. 1990;15:143–57.
Coulter CE, Anderson JD, Conture EG. Childhood stuttering and dissociations across linguistic domains: a replication and extension. J Fluen Disord. 2009;34:257–78.
Howell P. Assessment of some contemporary theories of stuttering that apply to spontaneous speech. Contemp Issues Commun Sci Disord. 2004;31:122–39.
Postma A, Kolk H. The covert repair hypothesis: prearticulatory repair processes in normal and stuttered disfluencies. J Speech Hear Res. 1993;36:472–87.
Civier O, Tasko SM, Guenther FH. Overreliance on auditory feedback may lead to sound/syllable repetitions: simulations of stuttering and fluency-inducing conditions with a neural model of speech production. J Fluen Disord. 2010;35:246–79.
Namasivayam AK, van Lieshout P, McIlroy WE, De Nil L. Sensory feedback dependence hypothesis in persons who stutter. Hum Mov Sci. 2009;28:688–707.
Van Lieshout P, Hulstijn W, Peters H. Searching for the weak link in the speech production chain of people who stutter: a motor skill approach. In: Maassen B, Kent R, Peters HFM, van Lieshout P, Hulstijn W, editors. Speech motor control in normal and disordered speech. New York: Oxford University Press; 2004.
Alm PA. Stuttering and the basal ganglia circuits: a critical review of possible relations. J Commun Disord. 2004;37:325–69.
Büchel C, Sommer M. What causes stuttering? PLoS Biol. 2004;2:e46.
Etchell AC, Johnson BW, Sowman PF. Beta oscillations, timing, and stuttering. Front Hum Neurosci. 2015;8:1036.
Kent RD. Research on speech motor control and its disorders: a review and prospective. J Commun Disord. 2000;33:391–428.
Ludlow CL. Stuttering: dysfunction in a complex and dynamic system. Brain. 2000;123:1983–4.
Travis LE. The cerebral dominance theory of stuttering: 1931–1978. J Speech Hear Disord. 1978;43:278–81.
Orton ST, Travis L. Studies in stuttering: IV. Studies of action currents in stutterers. Arch NeurPsych. 1929;21:61–8.
Foundas AL, Bollich AM, Corey DM, Hurley M, Heilman KM. Anomalous anatomy of speech-language areas in adults with persistent developmental stuttering. Neurology. 2001;57:207–15.
Sommer M, Koch MA, Paulus W, Weiller C, Büchel C. Disconnection of speech-relevant brain areas in persistent developmental stuttering. Lancet. 2002;360:380–3.
Ludlow CL, Loucks T. Stuttering: a dynamic motor control disorder. J Fluen Disord. 2003;28:273–95.
Salmelin R, Schnitzler A, Schmitz F, Freund H-J. Single word reading in developmental stutterers and fluent speakers. Brain. 2000;123:1184–202.
Hickok G, Houde J, Rong F. Sensorimotor integration in speech processing: computational basis and neural organization. Neuron. 2011;69:407–22.
Foundas AL, Bollich AM, Feldman J, Corey DM, Hurley M, Lemen LC, et al. Aberrant auditory processing and atypical planum temporale in developmental stuttering. Neurology. 2004;63:1640–6.
Ackermann H. Cerebellar contributions to speech production and speech perception: psycholinguistic and neurobiological perspectives. Trends Neurosci. 2008;31:265–72.
Goldstein L, Pouplier M. The temporal organization of speech. In: Goldrick M, Ferreira VS, Miozzo M, editors. The Oxford Handbook of Language Production. Oxford University Press; 2014.
Batliner A, Möbius B, Möhler G, Schweitzer A, Nöth E. Prosodic models, automatic speech understanding, and speech synthesis: towards the common ground. In: Proc. 7th Europ. Conf. on Speech Communication and Technology, 2001;2285-8.
Vry M-S, Saur D, Rijntjes M, Umarova R, Kellmeyer P, Schnell S, et al. Ventral and dorsal fiber systems for imagined and executed movement. Exp Brain Res. 2012;219:203–16.
Magrassi L, Aromataris G, Cabrini A, Annovazzi-Lodi V, Moro A. Sound representation in higher language areas during language generation. PNAS. 2015;112:1868–73.
Wymbs NF, Ingham RJ, Ingham JC, Paolini KE, Grafton ST. Individual differences in neural regions functionally related to real and imagined stuttering. Brain Lang. 2013;124:153–64.
Rosenfield DB. Neural anomaly and reorganization in speakers who stutter: a short-term intervention study. Neurology. 2013;80:1538–8.
Budde KS, Barron DS, Fox PT. Stuttering, induced fluency, and natural fluency: a hierarchical series of activation likelihood estimation meta-analyses. Brain Lang. 2014;139:99–107. Together with the ALE meta-analysis by Belyk and colleagues this article concentrates the most robust findings of functional neuroimaging in stuttering of the last 30 years.
Belyk M, Kraft SJ, Brown S. Stuttering as a trait or state—an ALE meta-analysis of neuroimaging studies. Eur J Neurosci. 2015;41:275–84. Together with the ALE meta-analysis by Budde and colleagues this article concentrates the most robust findings of functional neuroimaging in stuttering of the last 30 years.
Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2007;34:51–61.
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–505.
Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–26.
Heidemann RM, Anwander A, Feiweier T, Knösche TR, Turner R. k-space and q-space: combining ultra-high spatial and angular resolution in diffusion imaging using ZOOPPA at 7 T. NeuroImage. 2012;60:967–78.
Jeon H-A, Anwander A, Friederici AD. Functional network mirrored in the prefrontal cortex, caudate nucleus, and thalamus: high-resolution functional imaging and structural connectivity. J Neurosci. 2014;34:9202–12.
Connally EL, Ward D, Howell P, Watkins KE. Disrupted white matter in language and motor tracts in developmental stuttering. Brain Lang. 2014;131:25–35.
Cieslak M, Ingham RJ, Ingham JC, Grafton ST. Anomalous white matter morphology in adults who stutter. J Speech Lang Hear Res. 2015;58:268–77.
Chang S-E, Horwitz B, Ostuni J, Reynolds R, Ludlow CL. Evidence of left inferior frontal–premotor structural and functional connectivity deficits in adults who stutter. Cereb Cortex. 2011;21:2507–18.
Friederici AD, Gierhan SM. The language network. Curr Opin Neurobiol. 2013;23:250–4.
Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo DT-MRI study. Cereb Cortex. 2005;15:854–69.
Martino J, Hamer PCDW, Berger MS, Lawton MT, Arnold CM, de Lucas EM, et al. Analysis of the subcomponents and cortical terminations of the perisylvian superior longitudinal fasciculus: a fiber dissection and DTI tractography study. Brain Struct Funct. 2012;218:105–21.
Schreiber J, Riffert T, Anwander A, Knösche TR. Plausibility tracking: a method to evaluate anatomical connectivity and microstructural properties along fiber pathways. NeuroImage. 2014;90:163–78.
Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992;598:143–53.
Hipp JF, Hawellek DJ, Corbetta M, Siegel M, Engel AK. Large-scale cortical correlation structure of spontaneous oscillatory activity. Nat Neurosci. 2012;15:884–90.
Stark DE, Margulies DS, Shehzad ZE, Reiss P, Kelly AMC, Uddin LQ, et al. Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. J Neurosci. 2008;28:13754–64.
Barazany D, Basser PJ, Assaf Y. In vivo measurement of axon diameter distribution in the corpus callosum of rat brain. Brain. 2009;132:1210–20.
Assaf Y, Blumenfeld-Katzir T, Yovel Y, Basser PJ. Axcaliber: a method for measuring axon diameter distribution from diffusion MRI. Magn Reson Med. 2008;59:1347–54.
Alexander DC, Hubbard PL, Hall MG, Moore EA, Ptito M, Parker GJM, et al. Orientationally invariant indices of axon diameter and density from diffusion MRI. NeuroImage. 2010;52:1374–89.
Chang S-E, Zhu DC, Choo AL, Angstadt M. White matter neuroanatomical differences in young children who stutter. Brain. 2015;138:694–711. This article reports the neuroanatomical connectivity changes in developmental stuttering measured with diffusion MRI in the youngest and largest cohort published to date.
Chang S-E, Erickson KI, Ambrose NG, Hasegawa-Johnson MA, Ludlow CL. Brain anatomy differences in childhood stuttering. NeuroImage. 2008;39:1333–44.
Watkins KE, Smith SM, Davis S, Howell P. Structural and functional abnormalities of the motor system in developmental stuttering. Brain. 2008;131:50–9.
Cai S, Tourville JA, Beal DS, Perkell JS, Guenther FH, Ghosh SS. Diffusion imaging of cerebral white matter in persons who stutter: evidence for network-level anomalies. Front Hum Neurosci. 2014;8:54.
Civier O, Kronfeld-Duenias V, Amir O, Ezrati-Vinacour R, Ben-Shachar M. Reduced fractional anisotropy in the anterior corpus callosum is associated with reduced speech fluency in persistent developmental stuttering. Brain Lang. 2015;143:20–31.
Kronfeld-Duenias V, Amir O, Ezrati-Vinacour R, Civier O, Ben-Shachar M. The frontal aslant tract underlies speech fluency in persistent developmental stuttering. Brain Struct Funct. 2014;1–17.
Friederici AD, Singer W. Grounding language processing on basic neurophysiological principles. Trends Cogn Sci. 2015. doi:10.1016/j.tics.2015.03.012. This review is in line with a current paradigm shift in cognitive neuroscience emphasizing the view that cognitive functions depend on distributed computations in specialized cortical areas forming large-scale dynamic recurrent networks.
Wilson SM, Galantucci S, Tartaglia MC, Rising K, Patterson DK, Henry ML, et al. Syntactic processing depends on dorsal language tracts. Neuron. 2011;72:397–403.
Friederici AD. The cortical language circuit: from auditory perception to sentence comprehension. Trends Cogn Sci. 2012;16:262–8.
Sarubbo S, De Benedictis A, Merler S, Mandonnet E, Balbi S, Granieri E, et al. Towards a functional atlas of human white matter. Hum Brain Mapp. 2015. doi:10.1002/hbm.22832. This original paper reports results from cortical and subcortical electrostimulations in 130 patients under awake surgery for glioma, providing comprehensive subcortical functional maps of left and right hemisphere connections.
Bizzi A, Nava S, Ferrè F, Castelli G, Aquino D, Ciaraffa F, et al. Aphasia induced by gliomas growing in the ventrolateral frontal region: assessment with diffusion MR tractography, functional MR imaging and neuropsychology. Cortex. 2012;48:255–72.
Duffau H, Gatignol P, Denvil D, Lopes M, Capelle L. The articulatory loop: study of the subcortical connectivity by electrostimulation. Neuroreport. 2003;14:2005–8.
Guenther FH, Hickok G. Chapter 9 - Role of the auditory system in speech production. In: Aminoff MJ, Boller F, Swaab DF, editors. Handbook of clinical neurology. Elsevier; 2015. p. 161–75. Guenther and Hickok set out to advance mechanistic models of speech production summarizing and comparing their approaches for the first time in this most recent article.
Rauschecker JP, Scott SK. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat Neurosci. 2009;12:718–24.
Fox PT, Ingham RJ, Ingham JC, Hirsch TB, Downs JH, Martin C, et al. A PET study of the neural systems of stuttering. Nature. 1996;382:158–62.
Braun AR, Varga M, Stager S, Schulz G, Selbie S, Maisog JM, et al. Altered patterns of cerebral activity during speech and language production in developmental stuttering. An H2(15)O positron emission tomography study. Brain. 1997;120:761–84.
Ingham RJ, Fox PT, Costello Ingham J, Zamarripa F. Is overt stuttered speech a prerequisite for the neural activations associated with chronic developmental stuttering? Brain Lang. 2000;75:163–94.
Ingham RJ, Grafton ST, Bothe AK, Ingham JC. Brain activity in adults who stutter: similarities across speaking tasks and correlations with stuttering frequency and speaking rate. Brain Lang. 2012;122:11–24.
De Nil LF, Kroll RM, Kapur S, Houle S. A positron emission tomography study of silent and oral single word reading in stuttering and nonstuttering adults. J Speech Lang Hear Res. 2000;43:1038–53.
De Nil LF, Kroll RM, Lafaille SJ, Houle S. A positron emission tomography study of short- and long-term treatment effects on functional brain activation in adults who stutter. J Fluen Disord. 2003;28:357–80.
Neumann K, Preibisch C, Euler HA, von Gudenberg AW, Lanfermann H, Gall V, et al. Cortical plasticity associated with stuttering therapy. J Fluen Disord. 2005;30:23–39.
Preibisch C, Neumann K, Raab P, Euler HA, von Gudenberg AW, Lanfermann H, et al. Evidence for compensation for stuttering by the right frontal operculum. NeuroImage. 2003;20:1356–64.
Nil LFD, Beal DS, Lafaille SJ, Kroll RM, Crawley AP, Gracco VL. The effects of simulated stuttering and prolonged speech on the neural activation patterns of stuttering and nonstuttering adults. Brain Lang. 2008;107:114–23.
Giraud A-L, Neumann K, Bachoud-Levi A-C, von Gudenberg AW, Euler HA, Lanfermann H, et al. Severity of dysfluency correlates with basal ganglia activity in persistent developmental stuttering. Brain Lang. 2008;104:190–9.
Chang S-E, Kenney MK, Loucks TMJ, Ludlow CL. Brain activation abnormalities during speech and non-speech in stuttering speakers. NeuroImage. 2009;46:201–12.
Neef NE, Jung K, Rothkegel H, Pollok B, von Gudenberg AW, Paulus W, et al. Right-shift for non-speech motor processing in adults who stutter. Cortex. 2011;47:945–54.
Kikuchi Y, Ogata K, Umesaki T, Yoshiura T, Kenjo M, Hirano Y, et al. Spatiotemporal signatures of an abnormal auditory system in stuttering. NeuroImage. 2011;55:891–9.
Krishnan G, Nair RP, Tiwari S. Clinical evidence for the compensatory role of the right frontal lobe and a novel neural substrate in developmental stuttering: a single case study. J Neurolinguistics. 2010;23:501–10.
Kell CA, Morillon B, Kouneiher F, Giraud A-L. Lateralization of speech production starts in sensory cortices—a possible sensory origin of cerebral left dominance for speech. Cereb Cortex. 2011;21:932–7.
Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–99.
Weiler N, Wood L, Yu J, Solla SA, Shepherd GMG. Top-down laminar organization of the excitatory network in motor cortex. Nat Neurosci. 2008;11:360–6.
Busan P, D’Ausilio A, Borelli M, Monti F, Pelamatti G, Pizzolato G, et al. Motor excitability evaluation in developmental stuttering: a transcranial magnetic stimulation study. Cortex. 2013;49:781–92.
Alm PA, Karlsson R, Sundberg M, Axelson HW. Hemispheric lateralization of motor thresholds in relation to stuttering. PLoS ONE. 2013;8:e76824.
Sommer M, Knappmeyer K, Hunter EJ, Gudenberg AW, Neef N, Paulus W. Normal interhemispheric inhibition in persistent developmental stuttering. Mov Disord. 2009;24:769–73.
Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–19.
Fisher RJ, Nakamura Y, Bestmann S, Rothwell JC, Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp Brain Res. 2002;143:240–8.
Hanajima R, Furubayashi T, Iwata NK, Shiio Y, Okabe S, Kanazawa I, et al. Further evidence to support different mechanisms underlying intracortical inhibition of the motor cortex. Exp Brain Res. 2003;151:427–34.
Neef NE, Paulus W, Neef A, von Gudenberg AW, Sommer M. Reduced intracortical inhibition and facilitation in the primary motor tongue representation of adults who stutter. Clin Neurophysiol. 2011;122:1802–11.
Cash RFH, Isayama R, Gunraj CA, Ni Z, Chen R. The influence of sensory afferent input on local motor cortical excitatory circuitry in humans. J Physiol. 2015;593:1667–84. This work suggests that the sensory input on excitatory motor cortical circuitry plays an important role in sensorimotor integration and motor control.
Neef NE, Hoang TNL, Neef A, Paulus W, Sommer M. Speech dynamics are coded in the left motor cortex in fluent speakers but not in adults who stutter. Brain. 2015;138:712–25. Neef et al. verify the proposed uncoupling of motor output cells from motor plan cells in left primary motor cortex in fluent speech, and reveal its disruption in stuttering.
Civier O, Bullock D, Max L, Guenther FH. Computational modeling of stuttering caused by impairments in a basal ganglia thalamo-cortical circuit involved in syllable selection and initiation. Brain Lang. 2013;126:263–78. Civier et al. employ the most sophisticated mechanistic model of speech production to simulate stuttering and at the same time accounting for brain imaging findings.
Sakai N, Masuda S, Shimotomai T, Mori K. Brain activation in adults who stutter under delayed auditory feedback: an fMRI study. Int J Speech Lang Pathol. 2009;11:2–11.
den Ouden D-B, Montgomery A, Adams C. Simulating the neural correlates of stuttering. Neurocase. 2014;20:434–45.
Kell CA, Neumann K, von Kriegstein K, Posenenske C, von Gudenberg AW, Euler H, et al. How the brain repairs stuttering. Brain. 2009;132:2747–60.
Weintraub S, Mesulam M, Kramer L. Disturbances in prosody—a right-hemisphere contribution to language. Arch Neurol. 1981;38:742–4.
Wildgruber D, Ackermann H, Klose U, Kardatzki B, Grodd W. Functional lateralization of speech production at primary motor cortex: a fMRI study. Neuroreport. 1996;7:2791–5.
Friederici AD, Alter K. Lateralization of auditory language functions: a dynamic dual pathway model. Brain Lang. 2004;89:267–76.
Meyer M, Alter K, Friederici AD, Lohmann G, von Cramon DY. FMRI reveals brain regions mediating slow prosodic modulations in spoken sentences. Hum Brain Mapp. 2002;17:73–88.
Karniol R. Stuttering, language, and cognition: a review and a model of stuttering as suprasegmental sentence plan alignment (SPA). Psychol Bull. 1995;117:104–24.
Giraud A-L, Poeppel D. Cortical oscillations and speech processing: emerging computational principles and operations. Nat Neurosci. 2012;15:511–7.
Giraud A-L, Kleinschmidt A, Poeppel D, Lund TE, Frackowiak RSJ, Laufs H. Endogenous cortical rhythms determine cerebral specialization for speech perception and production. Neuron. 2007;56:1127–34.
Duffau H. Stimulation mapping of white matter tracts to study brain functional connectivity. Nat Rev Neurol. 2015;11:255–65.
Wu JC, Maguire G, Riley G, Lee A, Keator D, Tang C, et al. Increased dopamine activity associated with stuttering. Neuroreport. 1997;8:767–70.
Toyomura A, Fujii T, Kuriki S. Effect of external auditory pacing on the neural activity of stuttering speakers. NeuroImage. 2011;57:1507–16.
Toyomura A, Fujii T, Kuriki S. Effect of an 8-week practice of externally triggered speech on basal ganglia activity of stuttering and fluent speakers. NeuroImage. 2015;109:458–68.
Bhatnagar S, Buckingham H. Neurogenic stuttering: its reticular modulation. Curr Neurol Neurosci Rep. 2010;10:491–8.
Wald LL. The future of acquisition speed, coverage, sensitivity, and resolution. NeuroImage. 2012;62:1221–9.
Huber L, Goense J, Kennerley AJ, Trampel R, Guidi M, Reimer E, et al. Cortical lamina-dependent blood volume changes in human brain at 7 T. NeuroImage. 2015;107:23–33.
Kubikova L, Bosikova E, Cvikova M, Lukacova K, Scharff C, Jarvis ED. Basal ganglia function, stuttering, sequencing, and repair in adult songbirds. Sci Rep. 2014;4:6590. doi:10.1038/srep06590.
Fukushima M, Margoliash D. The effects of delayed auditory feedback revealed by bone conduction microphone in adult zebra finches. Sci Rep. 2015;5:8800. doi:10.1038/srep08800. This work demonstrates that a transient stuttering period can be induced by delayed auditory feedback (DAF) and that neither DAF-induced stuttering nor its recovery occurs instantaneously but depends on long-term tuning of involved circuits.
Palmer LM, Schulz JM, Murphy SC, Ledergerber D, Murayama M, Larkum ME. The cellular basis of GABAB-mediated interhemispheric inhibition. Science. 2012;335:989–93. Palmer et al. discovered a mechanistic principle of how interhemispheric inhibition could be implemented in the brain.
Manita S, Suzuki T, Homma C, Matsumoto T, Odagawa M, Yamada K, et al. A top-down cortical circuit for accurate sensory perception. Neuron. 2015;86:1–13. doi:10.1016/j.neuron.2015.05.006. Manita et al. demonstrate the impact of recurrent interaction between primary and secondary motor and somatosensory circuits on perception.
Fillard P, Pennec X, Arsigny V, Ayache N. Clinical DT-MRI estimation, smoothing, and fiber tracking with log-Euclidean metrics. IEEE Trans Med Imaging. 2007;26:1472–82.
Cykowski MD, Fox PT, Ingham RJ, Ingham JC, Robin DA. A study of the reproducibility and etiology of diffusion anisotropy differences in developmental stuttering: a potential role for impaired myelination. NeuroImage. 2010;52:1495–504.
Acknowledgments
Deutsche Forschungsgemeinschaft (NE1841/1-1) supported Nicole E. Neef.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Nicole E. Neef, Alfred Anwander, and Angela D. Friederici declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Neuroimaging
Rights and permissions
About this article
Cite this article
Neef, N.E., Anwander, A. & Friederici, A.D. The Neurobiological Grounding of Persistent Stuttering: from Structure to Function. Curr Neurol Neurosci Rep 15, 63 (2015). https://doi.org/10.1007/s11910-015-0579-4
Published:
DOI: https://doi.org/10.1007/s11910-015-0579-4