Abstract
Primary progressive aphasia (PPA), typically resulting from a neurodegenerative disease such as frontotemporal lobar degeneration or Alzheimer’s disease, is characterized by a progressive loss of specific language functions with relative sparing of other cognitive domains. Three variants of PPA are now recognized: semantic variant, logopenic variant, and nonfluent/agrammatic variant. We discuss recent work characterizing the neurolinguistic, neuropsychological, imaging and pathologic profiles associated with these variants. Improved reliability of diagnoses will be increasingly important as trials for etiology-specific treatments become available. We also discuss the implications of these syndromes for theories of language function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary progressive aphasia (PPA) describes an uncommon syndrome primarily affecting language. The concept of PPA was first described by Pick and Sérieux in the 1890s and re-introduced in the modern literature by Mesulam [1]. It is part of an evolving understanding of neurodegenerative conditions that identifies a range of clinical syndromes affecting cognitive functions other than memory [2]. The diagnosis of PPA requires that an insidiously progressive language impairment be the primary cognitive deficit for approximately 2 years after symptom onset [1]. There should be minimal difficulty in other cognitive functions, including memory, visuospatial skills, and executive abilities. Non-neurodegenerative etiologies, such as tumor or stroke, must be ruled out.
Three subtypes of PPA are currently recognized: semantic variant PPA (svPPA), logopenic variant PPA (lvPPA), and nonfluent/agrammatic variant PPA (navPPA) [3]. These are summarized in Table 1. The classification of PPA into these three subtypes is a relatively recent development, and the terminology and criteria for these subtypes are still somewhat inconsistent across institutions. Clinical evaluations of aphasic patients are challenging because most instruments of cognitive assessment rely extensively on language. Furthermore, differences in testing methods across institutions create an obstacle to making comparisons across studies. Yet the reliability of these clinical diagnoses is of vital importance as the underlying pathologies of PPA subtypes are being elucidated and treatments for these conditions are emerging. The urgent need for more uniform diagnostic methods is underlined by a recent summary of clinical-pathologic correlations in 145 autopsied PPA patients from seven experienced, international institutions. The accuracy of predicting pathology on the basis of a clinical syndromic diagnosis is disappointingly low [4••]. A new consensus on terminology, diagnostic recommendations, and suggested testing procedures is soon to be published (Gorno-Tempini et al., Unpublished data), and hopefully, this will help resolve some of the discrepancies in the descriptions of these patients across institutions. The naming conventions used here reflect this consensus, and the currently recognized characteristics of these conditions are summarized in Table 1. Mesulam et al. [5] have recently proposed a quantitative algorithm for subtyping PPA that may be useful in standardizing diagnoses. Additionally, clinical diagnoses of PPA syndromes can be supplemented with the use of multimodal diagnostic criteria that include combinations of cognitive assessments, genetic biomarkers, cerebrospinal fluid biomarkers, and brain imaging [4••, 6]. In this review, we discuss the three PPA variants and important recent findings for each.
Semantic Variant PPA
SvPPA is also referred to as “semantic dementia” (SD). This is a fluent variant of PPA associated with frontotemporal lobar degeneration (FTLD). The hallmark of svPPA is impaired performance on measures that depend on intact semantics. Thus, patients with svPPA have difficulty with picture and object naming, single word comprehension, category naming fluency, and knowledge of the uses and features of objects [7•]. The impairment is greater for less familiar words and objects. Difficulty with semantics is also evident in spontaneous speech production, which frequently contains indefinite terms such as “that” or “thing” where more precise and meaningful words would be appropriate [8]. Comprehension of speech is negatively affected as well. In an investigation of diagnostic features, Kertesz et al. [9] identified the questioning of word meanings in conversation (e.g., “What is stapler?”) as the primary diagnostic feature of svPPA. In reading, these patients often exhibit surface dyslexia, pronouncing words strictly as they are spelled on the basis of letter-sound correspondence rules (eg, plaid is pronounced as played). In addition to language problems, patients may exhibit behavioral changes similar to those in the behavioral variant of frontotemporal dementia, although these symptoms often have a later onset [9]. Despite these problems, svPPA patients do relatively well on measures of grammar, phonology, episodic memory, visuospatial skills, and number knowledge [7•, 10].
There is disagreement over whether or not svPPA should be considered identical to SD, a variant of frontotemporal dementia. Mesulam et al. [11] make a distinction between svPPA and SD. Both syndromes have similar language deficits; the critical difference, according to these authors, is the presence of a visual recognition deficit for objects and faces (associative agnosia and prosopagnosia) in SD that is not prominent in svPPA. Some have pointed out, however, that svPPA patients exhibit mild visual agnosia when sensitive testing methods are used [12]. Furthermore, svPPA patients invariably progress to clear presentations of what Mesulam et al. [11] designate as SD, and both syndromes share a common pathology [13]. Therefore, most authors [3, 4••] have not made a distinction between svPPA and SD.
The defining cognitive deficit for svPPA (and SD) appears to be a deterioration of semantic knowledge [7•, 14]. Investigations of this impairment have important implications for theories of semantic memory, yet the exact nature of the impairment in svPPA is not fully understood. Given the apparent global effects of the semantic deficit in svPPA, some propose a single semantic system that supports conceptual representations in all modalities (eg, verbal, visual) and is referred to as “amodal” [14]. Localized damage to this amodal semantic system would explain the profound semantic impairment in svPPA. However, others point out that svPPA patients exhibit dissociations across conceptual categories. For instance, spared representations of number knowledge and music knowledge are observed [10, 15, 16], demonstrating that if an amodal semantic system exists, it does not support these conceptual domains. Additionally, some find that in svPPA, knowledge of concrete concepts with visual feature associations is often more impaired than knowledge of abstract concepts. Taken together with the relative sparing of number and music knowledge, it is argued that degradation of the visual feature representations of concrete concepts may be the primary semantic deficit in svPPA [17, 18•]. Many of the testing procedures for assessing semantic knowledge in svPPA rely on visual stimuli (eg, picture naming) or the ability to understand words with strong visual associations. The reliance on visually weighted information may hide a relative sparing of semantic information in other categories (eg, abstract concepts). This interpretation is consistent with a theory of semantic memory that is widely supported in the functional neuroimaging literature, which proposes that concrete concepts rely in part on modality-specific feature representations [19]. However, the visual concept relative to abstract concept impairment in svPPA has not been consistently observed [20].
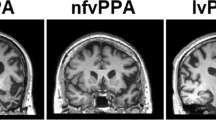
Structural imaging analyses demonstrate that svPPA patients have cortical atrophy affecting ventral and lateral regions of the anterior temporal lobes along with the anterior hippocampus and amygdala [3, 18, 21]. This atrophy is often more pronounced in the left hemisphere than the right. Longitudinal imaging shows atrophy beginning in anterior left temporal cortex, spreading to more posterior and superior temporal regions, and often appearing in anterior right temporal cortex [22, 23]. Those who support the hypothesis that svPPA is characterized by an amodal semantic deficit propose that atrophy of the anterior temporal lobes affects a component of the semantic system that is necessary for integrating conceptual information and that atrophy in this region accounts for the deficit in svPPA [14], whereas those who hypothesize dissociations across conceptual categories in svPPA propose that atrophy of visual association regions in ventral temporal cortex degrades visual feature representations and is critical to the semantic impairment in svPPA [17, 18•]. Consistent with this, a recent study found a correlation between atrophy of ventral temporal cortex and an impairment for words with visual feature associations [18•]. Longitudinal progression of disease may eventually compromise auditory association cortex in dorsal regions of the temporal lobe as well.
Diffusion tensor imaging (DTI) can reveal damage to white matter tracts that may not be observable in standard structural MRI. DTI analyses of svPPA patients have found evidence for damage to the white matter tracts connecting anterior temporal cortex with other brain regions: the inferior longitudinal, arcuate, and uncinate fasciculi [24, 25]. These findings suggest that damage to connections between regions of the language network may contribute to the language impairment in svPPA.
In Grossman’s [4••] summary of the autopsy-confirmed pathology of 145 recent cases of PPA, 36 were diagnosed as having the svPPA phenotype (Table 1). As is found for each of the PPA variants, there is no one-to-one mapping of clinical syndrome to underlying pathology. The most frequent finding in svPPA was FTLD with inclusions immunoreactive to ubiquitin and TDP-43 (FTLD-TDP), observed in 25 of the 36 cases (69%). Alzheimer’s disease (AD) pathology was identified in nine cases (25%), and a minimal number of cases exhibited FTLD with tau-positive immunoreactivity (FTLD-tau; n = 2). In recent imaging investigations, prominent left anterior temporal atrophy in svPPA has been associated with FTLD-TDP pathology at autopsy [21, 26, 27].
Logopenic Variant PPA
LvPPA, also referred to as “logopenic progressive aphasia” or “progressive mixed aphasia,” is characterized by slowed spontaneous speech output with frequent word-finding pauses and phonemic paraphasias. Grammar and motor control of speech remain intact [3, 28••]. These patients perform poorly on measures of auditory-verbal short-term memory, including sentence repetition, digit span, word span, and letter span. Comprehension of speech is impaired and does not improve for speech that is syntactically simple. LvPPA is frequently associated with the clinical characteristics of AD. Thus, these patients tend to have poorer episodic memory than patients with the other PPA variants. However, whereas AD patients have difficulty with single word meaning, it is claimed that lvPPA patients, early in the course of their condition, perform well on single word comprehension and other measures of semantics. The core impairment in lvPPA appears to be to the phonological loop function, a function of auditory-verbal short-term memory [28••]. This is said to account for the difficulty with repetition and speech comprehension, despite preserved grammar and single word comprehension. Nevertheless, as these patients progress, deficits with single word comprehension and sentence-level grammar do emerge; thus the term “progressive mixed aphasia” has been used to characterize these individuals [4••, 5].
The lvPPA subtype was first characterized in detail by Gorno-Tempini et al. [3]. Although lvPPA cases had previously been reported [1, 29], the Gorno-Tempini et al. [3] study initiated the consistent use of the term “logopenic” and established the identification of lvPPA (referred to at that time as “logopenic progressive aphasia”) as a third subtype of PPA. The lvPPA classification may correspond to earlier reports of progressive aphasia, including PPA with no specified subtype [30] and nonfluent variant PPA [31, 32]. As pointed out by Gorno-Tempini et al. [28••], the discrepancies in identifying and labeling lvPPA and similar syndromes stem partly from discrepancies in how the labels “fluent” and “nonfluent” have been used in the literature. Whereas lvPPA patients often fall into the “fluent” category in formal aphasia tests owing to spared articulation and grammar, clinicians not relying on formal aphasia tests may label them as “nonfluent” given their slowed speech and frequent word-finding pauses. Consistency in the PPA literature can be improved by reserving the label “nonfluent/agrammatic” for patients with speech sound production and grammar deficits and using the label “logopenic” (meaning “lack of words”) for patients with slowed speech but few speech errors or grammar deficits, as in the current recommendations (Gorno-Tempini et al., Unpublished data).
Several investigations have now demonstrated that lvPPA is associated with atrophy of posterior perisylvian and inferior parietal regions, typically more pronounced in the left hemisphere [3, 4••, 28••, 33]. This pattern of atrophy is consistent with the hypothesized impairment of phonological-loop functions, which are thought to be carried out in inferior parietal cortex [28••, 34].
Of the autopsy-confirmed cases summarized by Grossman (Table 1) [4••], 24 cases were diagnosed as having the lvPPA phenotype. The most frequent finding in lvPPA at autopsy was AD pathology, observed in 12 of 24 cases (50%). FTLD-TDP pathology was identified in nine cases (38%), and a small number of cases exhibited FTLD-tau pathology (n = 3). Consistent with the common association with AD pathology, many of the clinical features of lvPPA resemble language impairments observed in AD [35].
Nonfluent/Agrammatic Variant PPA
NavPPA is also referred to as “nonfluent progressive aphasia” or “progressive nonfluent aphasia.” This syndrome is most often associated with FTLD and marked by dysfluent and effortful speech, with hesitations, retakes, and errors in the production of speech sounds [3, 36, 37]. Speech rate (words per minute) in navPPA is one third to one half that of healthy speakers [37, 38••]. The average length of utterances is reduced in the connected speech of navPPA patients because their sentences are often less complex in structure, with a reduction in the use of dependent clauses and phrases compared with healthy speakers. NavPPA patients also make grammatical errors. For example, they may omit required determiners, and they may fail to produce appropriate subject-verb agreement [39]. Dysfluency may also stem in part from dysarthria and speech production errors known as apraxia of speech (AOS), which is defined as an impairment of articulatory planning [40, 41]. Over time, patients may reach the point of producing little more than minimally connected content words; eventually they may become mute. They also have difficulty with grammatical comprehension, primarily for complex grammatical constructions [42, 43], which, considered along with their poor grammatical production, suggests that a grammar deficit may be central to the syndrome of navPPA. Difficulty in naming is a feature commonly found in navPPA [37, 38••], although this is not as severe as in the semantic and logopenic variants of PPA, and it may be related to difficulty in the phonologic assembly of words [44]. Executive resources, notably working memory, may be impaired. However, single word and object comprehension are relatively spared [45].
NavPPA has many similarities to Broca’s aphasia, a syndrome resulting from stroke in the left hemisphere and characterized by effortful, dysfluent speech. However, agrammatism and phonological processing are typically worse in Broca’s aphasia than in navPPA. Moreover, unlike navPPA, executive resources are often spared in Broca’s aphasia [46]. Differences between these nonfluent syndromes may be related in part to differences in their underlying pathologies. Stroke produces an abrupt and focal loss of tissue that also significantly injures white matter areas; this contrasts with the insidiously progressive tissue loss affecting more extensive regions of gray matter in neurodegenerative disease.
NavPPA has some features in common with lvPPA, including impaired repetition of phrases and sentences, speech sound errors, and spared single word comprehension and object knowledge. Little work has investigated whether there are qualitative differences between navPPA and lvPPA in the nature of the difficulties encountered during sentence repetition and speech-sound error production. Descriptions of the two syndromes consistently remark on the presence of agrammatism and simplified grammatical structures in the speech of navPPA, contrasted with the relative sparing of grammar and the impaired word retrieval exhibited in lvPPA. Some authors group logopenic patients under the navPPA classification [37], but currently most view the logopenic and nonfluent variants as distinct syndromes. This differentiation is supported to the extent that a differentiation of underlying pathology has been established (Table 1).
There are differing views on whether the language difficulties in navPPA primarily reflect a motor impairment from AOS or a nonmotor language impairment. In a recent study of speech errors in navPPA, the large majority of speech sound errors (82%) were substitutions, insertions, deletions, or transpositions, all of which contain actual speech sounds of American English and likely represent incorrect retrieval of the desired sound [38••]. Only the remaining 18% of errors consisted of sounds that were not well-formed phonemes and thus are likely to represent a motor impairment in which the speech articulators are not optimally positioned or activated to produce a proper speech sound of English. Neither type of error correlated in frequency with speech rate or syntactic complexity, but both types of error correlated with grammatical correctness, consistent with other reports [37]. These findings suggest that AOS may occur within navPPA, but it is not the central cause of the slowed, effortful speech that characterizes this syndrome. Rather, a nonmotor language impairment, perhaps in phonological retrieval, seems to play a greater role in navPPA patients’ dysfluency. This may accompany a syntactic deficit affecting the sentence construction aspect of fluency.
Cortical atrophy in navPPA occurs predominantly in the left hemisphere in an anterior perisylvian distribution, involving inferior, opercular, and insular regions [3, 42]. As the disease progresses, atrophy extends to left dorsolateral prefrontal cortex and left superior temporal cortex. It extends medially to involve orbital and anterior cingulate regions and spreads posteriorly along the sylvian fissure into the parietal lobe [6]. The impaired grammatical comprehension that is characteristic of navPPA has been found to correlate with atrophy in left inferior frontal cortex, in accordance with considerable evidence that this brain region contributes to sentence processing [42, 47]. Impaired fluency in terms of words per minute of spontaneous speech has been associated with atrophy in left interior frontal and superior temporal cortex [39, 47]. This association may reflect the role of a syntactic deficit in impairing the flow of speech. Speech errors associated with AOS in stroke patients have been localized to the anterior portion of the left insula in one study [48]. A contrasting study found a correlation of AOS with damage to left Broca’s area in acute stroke patients [49]. However, these findings in stroke patients may not be generalizable to a neurodegenerative condition such as PPA.
Of the autopsy-confirmed cases summarized by Grossman (Table 1) [4••], 85 cases were diagnosed as having the navPPA phenotype. The most frequent finding in navPPA was FTLD-tau, observed in 44 of the 85 cases (52%). AD pathology was found in 21 cases (25%), and FTLD-TDP was identified in 16 cases (19%). A minimal number of cases exhibited dementia with Lewy bodies (n = 2), dementia lacking distinct histopathology (n = 1), or mixed pathology (n = 1). Inferior frontal and superior temporal atrophy is seen in navPPA with tau-positive disease confirmed by autopsy [6, 21]. Parietal cortical atrophy on MRI and reduced functioning in parietal cortex on positron emission tomography or single photon emission computed tomography are seen in navPPA patients who have AD pathology [50].
Conclusions
The classification of PPA into semantic, logopenic, and nonfluent/agrammatic variants is now recommended for PPA diagnosis (Gorno-Tempini et al., Unpublished data). In the past, these subtypes of PPA have often been recognized under different names (Table 1). The systematic identification and investigation of these variants will be improved through adoption of the new recommendations and nomenclature.
Much work has already demonstrated that the PPA variants have distinct clinical profiles. Despite this, the accuracy of predicting pathology on the basis of clinical diagnosis is disappointingly low. In a recent summary of clinical-pathologic correlations, even the strongest correlation—between svPPA and FTLD-TDP pathology—was only 69% [4••]. The ability to accurately predict pathology will be necessary as trials for etiology-specific treatments become available. To this end, further work is needed on the development of multimodal diagnostic methods, combining neuroimaging, neuropsychological measures, and molecular markers from biofluids. These methods have the potential to significantly improve pathology prediction. For example, recent work demonstrates that combining neuroimaging with quantitative neuropsychological measures yields a powerful multimodal predictor for AD pathology in nonfluent forms of PPA [6].
In addition to characterizing PPA phenotypes, investigations of PPA have considerable impact on theories of language function. The nature of the semantic impairment in svPPA has important implications for theories of semantic memory. Similarly, investigations of navPPA inform our understanding of syntactic, phonological, and motor processes in the brain, and lvPPA patients may be an informative population for investigations of auditory working memory functions. There are still many open questions about the nature of the language impairments in each of the PPA variants. Future work can provide a more detailed understanding of these language impairments and their relationships to the underlying disease processes.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mesulam MM: Primary progressive aphasia. Ann Neurol 2001, 49:425–432.
Mesulam MM: Primary progressive aphasia--a language-based dementia. N Engl J Med 2003, 349:1535–1542.
Gorno-Tempini ML, Dronkers NF, Rankin KP, et al.: Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 2004, 55:335–346.
• Grossman M: Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol 2010, 6:88–97. This paper summarizes the autopsy-confirmed pathology results from 145 PPA patients across seven institutions. The current findings for neuropsychological, neuroimaging, genetic, and cerebrospinal fluid biomarkers of the PPA variants are reviewed.
Mesulam M, Wieneke C, Rogalski E, et al.: Quantitative template for subtyping primary progressive aphasia. Arch Neurol 2009, 66:1545–1551.
Hu WT, McMillan C, Libon D, et al.: Multi-modal predictors for Alzheimer’s disease in non-fluent primary progressive aphasia. Neurology 2010, 75:595–602.
• Hodges JR, Patterson K: Semantic dementia: a unique clinicopathological syndrome. Lancet Neurol 2007, 6:1004–1014. This paper provides an overview of SD. Clinical and pathologic features are summarized. The authors discuss neuropsychological findings and considerations in choosing testing methods for these patients.
Meteyard L, Patterson K: The relation between content and structure in language production: an analysis of speech errors in semantic dementia. Brain Lang 2009, 110:121–134.
Kertesz A, Jesso S, Harciarek M, et al.: What is semantic dementia?: a cohort study of diagnostic features and clinical boundaries. Arch Neurol 2010, 67:483–489.
Halpern C, Glosser G, Clark R, et al.: Dissociation of numbers and objects in corticobasal degeneration and semantic dementia. Neurology 2004, 62:1163–1169.
Mesulam M, Rogalski E, Wieneke C, et al.: Neurology of anomia in the semantic variant of primary progressive aphasia. Brain 2009, 132(Pt 9):2553–2565.
Adlam AL, Patterson K, Rogers TT, et al.: Semantic dementia and fluent primary progressive aphasia: two sides of the same coin? Brain 2006, 129(Pt 11):3066–3080.
Knibb JA, Xuereb JH, Patterson K, et al.: Clinical and pathological characterization of progressive aphasia. Ann Neurol 2006, 59:156–165.
Patterson K, Nestor PJ, Rogers TT: Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci 2007, 8:976–987.
Weinstein J, Koenig P, Gunawardena D, et al.: Preserved musical semantic memory in semantic dementia. Arch Neurol 2010 (in press).
Hailstone JC, Omar R, Warren JD: Relatively preserved knowledge of music in semantic dementia. J Neurol Neurosurg Psychiatry 2009, 80:808–809.
Yi HA, Moore P, Grossman M: Reversal of the concreteness effect for verbs in semantic dementia. Neuropsychology 2007, 21:9–19.
• Bonner MF, Vesely L, Price C, et al.: Reversal of the concreteness effect in semantic dementia. Cogn Neuropsychol 2009, 26:568–579. This paper examines the proposed deficit in visual semantic features in SD. The authors find that atrophy in ventral temporal cortex correlates with a deficit in concrete word knowledge.
Martin A: The representation of object concepts in the brain. Annu Rev Psychol 2007, 58:25–45.
Jefferies E, Patterson K, Jones RW, et al.: Comprehension of concrete and abstract words in semantic dementia. Neuropsychology 2009, 23:492–499.
Rohrer JD, Warren JD, Modat M, et al.: Patterns of cortical thinning in the language variants of frontotemporal lobar degeneration. Neurology 2009, 72:1562–1569.
Avants B, Anderson C, Grossman M, et al.: Spatiotemporal normalization for longitudinal analysis of gray matter atrophy in frontotemporal dementia. Med Image Comput Comput Assist Interv 2007, 10(Pt 2):303–310.
Rohrer JD, McNaught E, Foster J, et al.: Tracking progression in frontotemporal lobar degeneration: serial MRI in semantic dementia. Neurology 2008, 71:1445–1451.
Duda JT, Avants BB, Asmuth JC, et al.: A fiber tractography based examination of neurodegeneration on language-network neuroanatomy. Med Image Comput Comput Assist Interv 2008, 12:191–198.
Agosta F, Henry RG, Migliaccio R, et al.: Language networks in semantic dementia. Brain 2010, 133:286–299.
Josephs KA, Whitwell JL, Duffy JR, et al.: Progressive aphasia secondary to Alzheimer disease vs FTLD pathology. Neurology 2008, 70:25–34.
Grossman M, Libon DJ, Forman MS, et al.: Distinct antemortem profiles in patients with pathologically defined frontotemporal dementia. Arch Neurol 2007, 64:1601–1609.
•• Gorno-Tempini ML, Brambati SM, Ginex V, et al.: The logopenic/phonological variant of primary progressive aphasia. Neurology 2008, 71:1227–1234. This paper characterizes the language impairments and neuroimaging data in six lvPPA patients. This is one of only a handful of studies characterizing this patient group. The results are discussed in light of the proposed phonological-loop deficit for lvPPA.
Kertesz A, Davidson W, McCabe P: Primary progressive aphasia: diagnosis, varieties, evolution. J Int Neuropsychol Soc 2003, 9:710–719.
Sonty SP, Mesulam M, Thompson CK, et al.: Primary progressive aphasia: PPA and the language network. Ann Neurol 2003, 53:35–49.
Galton CJ, Patterson K, Xuereb JH, et al.: Atypical and typical presentations of Alzheimer’s disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain 2000, 123:484–498.
Mendez MF, Clark DG, Shapira JS, et al.: Speech and language in progressive nonfluent aphasia compared with early Alzheimer’s disease. Neurology 2003, 61:1108–1113.
Rohrer JD, Ridgway GR, Crutch SJ, et al.: Progressive logopenic/phonological aphasia: erosion of the language network. Neuroimage 2010, 49:984–993.
Baldo JV, Dronkers NF: The role of inferior parietal and inferior frontal cortex in working memory. Neuropsychology 2006, 20:529–538.
Grossman M, Mega M, Cummings J, et al.: The aphasias and related disturbances. In Clinical Neurology. Edited by Baker AB, Joynt RJ. Philadelphia: Lippincott Williams and Wilkins; 2004:1–83.
Grossman M, Ash S: Primary progressive aphasia: a review. Neurocase 2004, 10:3–18.
Knibb JA, Woollams AM, Hodges JR, et al.: Making sense of progressive non-fluent aphasia: an analysis of conversational speech. Brain 2009, 132(Pt 10):2734–2746.
•• Ash S, McMillan C, Gunawardena D, et al.: Speech errors in progressive non-fluent aphasia. Brain Lang 2010, 113:13–20. This paper provides a detailed, quantitative study of speech sound errors in the connected speech of navPPA patients, with linguistic, neuropsychological, and neural correlates. It is argued that speech errors in navPPA mainly arise within the language system and are not caused by a motor impairment.
Ash S, Moore P, Vesely L, et al.: Non-fluent speech in frontotemporal lobar degeneration. J Neurolinguistics 2009, 22:370–383.
Josephs KA, Duffy JR, Strand EA, et al.: Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain 2006, 129(Pt 6):1385–1398.
Ogar JM, Dronkers NF, Brambati SM, et al.: Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer Dis Assoc Disord 2007, 21:S23–S30.
Peelle JE, Troiani V, Gee J, et al.: Sentence comprehension and voxel-based morphometry in progressive nonfluent aphasia, semantic dementia, and nonaphasic frontotemporal dementia. J Neurolinguistics 2008, 21:418–432.
Peelle JE, Cooke A, Moore P, et al.: Syntactic and thematic components of sentence processing in progressive nonfluent aphasia and nonaphasic frontotemporal dementia. J Neurolinguistics 2007, 20:482–494.
Weintraub S, Rubin NP, Mesulam MM: Primary progressive aphasia: longitudinal course, neuropsychological profile, and language features. Arch Neurol 1990, 47:1329–1335.
Libon DJ, Xie SX, Moore P, et al.: Patterns of neuropsychological impairment in frontotemporal dementia. Neurology 2007, 68:369–375.
Patterson K, Graham NL, Ralph MA, et al.: Progressive non-fluent aphasia is not a progressive form of non-fluent (post-stroke) aphasia. Aphasiology 2006, 20:1018–1034.
Gunawardena D, Ash S, McMillan CT, et al.: Why are progressive non-fluent aphasics non-fluent? Neurology 2010, 75:588–594.
Dronkers NF: A new brain region for coordinating speech articulation. Nature 1996, 384:159–161.
Hillis AE, Work M, Barker PB, et al.: Re-examining the brain regions crucial for orchestrating speech articulation. Brain 2004, 127(Pt 7):1479–1487.
Nestor PJ: Nuclear imaging can predict pathologic diagnosis in progressive nonfluent aphasia. Neurology 2007, 68:238–239.
Acknowledgments
Michael F. Bonner has received grant support from the US National Institutes of Health (NIH). Sharon Ash has received grants from NIH (AG15116, AG17586, NS44266, and NS53488). Murray Grossman has received grants from NIH (NS054575, AG15116, AG17586, NS44266, NS53488, and AG32953).
Disclosure
Murray Grossman is a member and a medical advisor for the board of the Association for Frontotemporal Dementias; he has been a consultant for Pfizer, Forest, and Allon; he has received honoraria for Ground Rounds, Penn State; and has received expenses for travel/accommodations for Grand Rounds, UCSF, and McGill. Sharon Ash has received royalties from Mouton de Gruyter for The Atlas of North American English: Phonetics, Phonology and Sound Change, published in 2006.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonner, M.F., Ash, S. & Grossman, M. The New Classification of Primary Progressive Aphasia into Semantic, Logopenic, or Nonfluent/Agrammatic Variants. Curr Neurol Neurosci Rep 10, 484–490 (2010). https://doi.org/10.1007/s11910-010-0140-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-010-0140-4
Keywords
- Primary progressive aphasia (PPA)
- Semantic variant PPA (svPPA)
- Semantic dementia (SD)
- Semantic PPA (PPA-S)
- Logopenic variant PPA (lvPPA)
- Logopenic progressive aphasia (LPA)
- Logopenic PPA (PPA-L)
- Nonfluent/agrammatic variant PPA (navPPA)
- Progressive nonfluent aphasia (PNFA)
- Agrammatic PPA (PPA-G)
- Frontotemporal dementia (FTD, FTLD)
- Alzheimer’s disease (AD)