Abstract
Primary progressive aphasia (PPA) is a dementia syndrome associated with several neuropathologic entities, including Alzheimer’s disease (AD) and all major forms of frontotemporal lobar degeneration (FTLD). It is classified into subtypes defined by the nature of the language domain that is most impaired. The asymmetric neurodegeneration of the hemisphere dominant for language (usually left) is one consistent feature of all PPA variants. This feature offers unique opportunities for exploring mechanisms of selective vulnerability in neurodegenerative diseases and the neuroanatomy of language. This chapter reviews some of the current trends in PPA research as well as the challenges that remain to be addressed on the nosology, clinicopathologic correlations, and therapy of this syndrome.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Primary progressive aphasia (PPA) is a major syndrome of frontotemporal lobar degeneration (FTLD) and accounts for nearly 25% of all FTLD cases [1]. Approximately 60% of PPA is associated with FTLD and the remaining 40% with the neuropathology of Alzheimer’s disease (AD). Information on PPA prevalence is limited. One study from the UK suggests an approximate prevalence of 3–4/100,000, a level comparable to what has been reported for ALS [1]. The one common denominator for all PPA, whether caused by FTLD or AD, is the preferential degeneration of the language network, usually located in the left hemisphere of the brain. Current research on primary progressive aphasia is evolving in multiple directions. For one, the variety of the aphasic disturbances continues to fuel discussion on nomenclature and clinical classification. Second, the selective dissolution of individual language domains is offering new paradigms for exploring the functional anatomy of language, a pursuit that has already prompted modifications of classic models. Third, the multiplicity of the underlying degenerative diseases is generating new insights on the heterogeneity of dementias, the probabilistic relationship of syndrome to pathology, and the mechanisms of selective vulnerability. Fourth, there is lively interest in formulating personalized interventions aimed not only at the nature of the language disturbance but also at the biology of the underlying disease entity. These are some of the current trends that will be reviewed in this chapter. Given the constraints of space and the vast literature on PPA, the account will be selective and based predominantly on the PPA research programs at Northwestern University where a cohort of 235 PPA patients have been enrolled, 97 of whom have come to brain autopsy.
Diagnosis, Nomenclature, and Subtyping
The existence of progressive language disorders had been known for more than 100 years. Pick, Sérieux, Dejerine, Franceschi, and Rosenfeld were among the first to report such patients during the late nineteenth and early twentieth centuries [2,3,4,5,6,7]. However, this topic did not attract much, if any, attention during most of the twentieth century. The current resurgence of interest in this condition can be traced to the 1982 report of six patients who experienced a slowly progressive aphasia without other cognitive or behavioral impairments [8]. The syndrome was named “primary progressive aphasia,” and diagnostic criteria were formulated [9, 10]. The following decades witnessed a rapidly expanding literature on PPA and on overlapping entities designated progressive nonfluent aphasia (PNFA) and semantic dementia (SD) [11]. For a number of years, research on PNFA and SD developed in parallel to research on PPA. In 2011, an international group of investigators presented classification guidelines that incorporated PNFA and SD under the PPA umbrella [12]. This unitary approach stimulated rapid progress in this field.
Three features define PPA: (1) adult-onset and progressive impairment of language (not just speech), (2) absence of other consequential behavioral or cognitive deficits for approximately the first 2 years, and (3) neurodegenerative disease as the only cause of impairment [10]. These criteria help to filter out patients where progressive aphasias arise in conjunction with equally prominent speech apraxia, behavioral disturbances, loss of memory for recent events, associative agnosias, or visuospatial deficits. In the course of diagnostic evaluation, patients may show subtle impairments in non-language tasks, especially those related to memory and executive function. Such abnormalities of test performance do not by themselves preclude a PPA diagnosis unless they are associated with limitations of daily life in the corresponding non-language domains.
Many neuropsychological tests require verbal responses and verbal instructions. The clinician needs to consider the influence of the aphasia on these aspects of performance. For example, a patient with PPA who cannot name a famous face is not necessarily prosopagnosic, a patient who cannot verbalize the nature of an object does not necessarily lack knowledge of the object, and a patient who cannot learn a word list is not necessarily amnestic. Conversely, patients who cannot produce words because of articulation deficits, those who cannot repeat language because of general working memory limitations, those who misname objects or faces they do not recognize, or those who have impoverished speech because of abulia or impaired executive function are not necessarily aphasic. As in the case of many other syndromes, the diagnosis of PPA relies on the judgment and experience of the clinician. While clear-cut cases do exist, there are also cases where the salience and primacy of the aphasia will generate debate, especially if the patient is examined a few years after symptom onset. In some patients, the aphasia will remain the only salient feature for over a decade [13]. Other patients, however, may first come to a specialty clinic at a time when the disease has progressed to encompass other cognitive domains. The term “PPA plus” (PPA+) can be used to designate such patients, based on the assumption that the disease had started as PPA, but that it had since spread beyond the language network [14].
In contrast to many other dementias, where the patient has little insight into the predicament, patients with PPA are usually the first to notice and report the difficulty. At those stages of the disease, MRI and metabolic positron emission tomography (PET) scans may be negative. The absence of positive neurodiagnostic tests, combined with lack of recognition of these symptoms in general practice, may lead to unwarranted referrals to otolaryngologists or psychiatrists [15]. Patients and families often ask whether the diagnosis is PPA or AD. When AD biomarkers (such as amyloid and phospho-tau in cerebrospinal fluid [CSF] or amyloid PET scans) are positive, the clinician will have to explain that the patient has both PPA and AD, that PPA refers to the symptoms that bring the patient to the clinic, and that AD refers to the abnormal amyloid and tau proteins in the brain that attack the language centers. There was a time when PPA was underdiagnosed. There are now instances where it seems to be overdiagnosed, probably because language impairments can be so prominent during the office evaluation that other equally substantial cognitive and behavioral impairments become overlooked. This issue comes up most commonly in patients with prominent apraxia of speech or executive dysfunction who are also aphasic. We give these patient descriptive diagnoses such as “apraxia of speech with aphasia” or “aphasic frontal syndrome.”
Language impairment can encompass word retrieval, object naming, sentence construction, or language comprehension, either singly or in combination. Once the PPA diagnosis is established, the subtyping exercise can be initiated. At the time of writing, the 2011 guidelines dominate this process [12]. They help to classify PPA into nonfluent/agrammatic, logopenic, and semantic variants. Although this system has been immensely influential and is even frequently mandated during the review of manuscripts submitted for publication, it has widely recognized shortcomings [16,17,18]. For one, a strict adherence to the 2011 guidelines entails arduous assessment of nearly a dozen separate aspects of language. Second, even if the guidelines are strictly applied, approximately one-third of the patients will fail to be classified into any of the three variants. Third, there are certain feature clusters that allow the same patient to simultaneously fit the designation of both nonfluent/agrammatic and logopenic PPA. Yet another challenge is posed by the evolution over time, so that a patient who fits the logopenic subtype initially may fit criteria for one of the other two subtypes as the disease progresses.
The following modifications have helped us address some of these concerns [16]. (1) The relative preservation of both grammar and comprehension is made to be a core feature of the logopenic variant. This prevents the double assignment problem. (2) In contrast to the 2011 guidelines, repetition impairment is not considered an obligatory core feature of the logopenic variant. This practice reduces the number of unclassifiable patients. (3) Patients with combined impairments of grammar and word comprehension even early in the disease, and who would therefore remain unclassifiable by the 2011 guidelines, make up a fourth variant of “mixed” PPA. (4) The semantic variant is diagnosed when poor word comprehension is the principal feature. When additional and equally prominent impairments of object or face recognition (not just naming) are detected, a diagnosis of semantic dementia (SD) is made [11]. This recommendation is at odds with the 2011 guidelines, which would diagnose semantic PPA even in patients with significant face and object recognition impairment (i.e., visual associative agnosia). The justification for the distinction of PPA from SD is summarized in the section on the anatomy of language.
The modifications listed above lead to a classification method based on a template where the Y-axis represents worsening impairment in the grammaticality of sentence construction and the X-axis represents worsening impairment in single word comprehension [15]. Each of the four PPA subtypes will cluster within a different quadrant of this template. The nonfluent/agrammatic PPA patients, for example, will cluster in the upper left quadrant (impaired grammar but spared comprehension); the semantic PPA patients will cluster in the lower right quadrant (impaired comprehension but spared grammar); the mixed PPA patients will cluster in the lower left quadrant (combined impairments of grammar and comprehension); and the logopenic PPA patients will cluster in the upper right quadrant (relatively spared grammar and comprehension). The logopenic group would have met the PPA criteria through impairments of word retrieval, naming, and spelling. Specific tests for assessing grammaticality of sentence construction and word comprehension and their normative values have been reported [15]. As patterns of agrammatism vary greatly from language to language, considerable attention is being directed to the adaptation of grammar tests for languages other than English [19].
Some logopenic patients maintain fluency as they circumvent word finding failures through circumlocution; others pause after word retrieval failures and produce halting nonfluent speech that appears similar to what is seen in patients with nonfluent/agrammatic PPA. Word finding impairments and paraphasias may make it impossible to gauge a sentence grammaticality. The delineation of logopenic from agrammatic PPA can thus be quite challenging [17]. Quantitative analyses of speech samples show that the nonfluent/agrammatic patients make word finding pauses that are longer before verbs, whereas logopenic patients make pauses that are longer before nouns [20]. Furthermore, patients with nonfluent/agrammatic PPA display a preferential impairment of verb rather than object naming, whereas the converse may be seen in logopenic PPA [21]. When research objectives necessitate such distinctions, these features may help to establish a quantitative differentiation of nonfluent/agrammatic from logopenic forms of PPA. Subtyping need not become an end onto itself. For purposes of both research and treatment, the emphasis could also be on single parameters, such as grammar or naming, across all subjects and regardless of subtype.
The 2011 guidelines did not prescribe acronyms for the three variants. At present, non-fluent variant (nfvPPA) , logopenic variant (lvPPA) , and semantic variant (svPPA) are the most popular choices. Alternative acronyms such as naPPA, agPPA, PPA-NFV, LPA, and PPA-SV have also been used, albeit more rarely [22,23,24,25]. The “nfv” prefix is particularly problematic because it appears to overlook grammar, which is the single most characteristic impairment of this subtype. The choice of “nfv” was probably based on experience derived from stroke aphasia where low fluency can be used as a proxy for agrammatism. In PPA, grammar and fluency can be dissociated, especially in logopenic patients where long word finding pauses diminish fluency but without grammatical impairment [26]. Based on these considerations and also in order to underscore the primacy of the PPA diagnosis, we have used the alternative acronyms of PPA-G, PPA-L, PPA-S and PPA-M for the nonfluent/agrammatic, logopenic, semantic and mixed variants, respectively. It may take another collective international effort to determine whether the 2011 consensus guidelines should be modified along the lines listed above and whether the acronyms can be harmonized.
Clinical progression patterns vary by subtype and are likely to reflect the differential anatomical trajectories of disease spread. In PPA-S, the spread of atrophy from the anterior temporal lobe to orbitofrontal, insular, or contralateral temporal lobe can lead to the additional face and object recognition impairments of SD, and to the behavioral abnormalities seen in behavioral variant frontotemporal dementia (bvFTD). In PPA-G, spread of atrophy from the inferior frontal gyrus (IFG) to other premotor and frontal cortices can lead to the abnormalities seen in apraxia of speech, corticobasal syndrome, supranuclear ophthalmoplegia, and frontal-type executive dysfunction. In PPA-L, spread of atrophy from the temporoparietal junction (TPJ) to surrounding cortices can lead to additional impairments of explicit memory and constructions. For all subtypes, the spread of atrophy tends to be more pronounced in the left hemisphere, and there are substantial interindividual differences in the speed and trajectory of progression [27].
Contributions to the Anatomy of Language
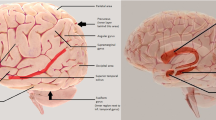
The classic Wernicke-Lichtheim-Geschwind model of language revolved around two epicenters, namely Broca’s area in the inferior frontal gyrus (IFG) and Wernicke’s area in the temporoparietal junction (TPJ), a region that can be said to encompass parts of the inferior parietal lobule and the posterior segments of the superior and middle temporal gyri [28] (Fig. 1). The former has been linked to fluency and grammar and the latter to language comprehension. The literature of the past 150 years displays greater agreement on the location and function of Broca’s area than of Wernicke’s area [28]. These two epicenters are connected through the arcuate fasciculus, which is thought to play a critical role in language repetition [29]. This basic model has undergone major revisions through investigations with functional imaging, event-related potentials, and sophisticated neuropsychological assessments [30,31,32].
Major components of the left hemisphere language network – ATL: The acronym ATL will be used to refer to the anterior third of the temporal lobe including the temporal pole; CS (the central sulcus) is shown as a reference point, IFG-B (the inferior frontal gyrus) contains Broca’s area, IPL (inferior parietal) lobule, MTL (the middle third of the temporal lobe), TPJ-W (the temporoparietal junction) contains the posterior third of the temporal lobe and the immediately adjacent parts of the inferior parietal lobule. Although the exact site of Wernicke’s area remains ambiguous, it is usually considered to be located within the TPJ-W and adjacent parts of the MTL
Each of these approaches has advantages and disadvantages. Cerebrovascular lesions cause sudden and irreversible destruction of the core lesion site. However, the damage usually extends into deep white matter. The exact contribution of the damaged cortical region to the ensuing language impairment is therefore difficult to specify. Functional mapping approaches based on MRI and electrical recordings, on the other hand, can reveal activity confined to the cerebral cortex but cannot differentiate areas that are critical for a function from those that have collateral participatory roles.
Investigations based on focal cortical atrophy can circumvent some of these shortcomings. Regions where the magnitude of cortical thinning correlates with the magnitude of impairment can be said to have critical (rather than participatory) roles in maintaining the integrity of that function. Consequently, PPA has offered new tools for investigating the cortical anatomy of the language network without the deep white matter problem of stroke or the collateral activation dilemma of functional brain mapping. Nonetheless, clinicoanatomical correlations in PPA are not without caveats. For one, the slow evolution of the lesion is likely to trigger compensatory plasticity that may complicate the interpretation of correlations. Second, even areas of peak atrophy may contain residual neurons that could sustain some functionality of that region [33]. Third, each neuropathologic entity may trigger a different pattern of cortical injury. For example, the neurofibrillary tangles of AD have a predilection for deep cortical layers whereas the opposite is the case for Pick’s disease.
Despite these potential complications, clinicoanatomical investigations on PPA have generated new insights into the functional anatomy of language. Each PPA variant is associated with a characteristic location of peak atrophy, for instance, Broca’s area (IFG) in PPA-G, Wernicke’s area (TPJ) in PPA-L, and the anterior half of the temporal lobe (ATL) in PPA-S [34,35,36]. The anatomical correlate of PPA-G is in keeping with prevailing models of language, which give Broca’s area a critical role in the maintenance of fluency and grammar [37]. The relationships in PPA-L and PPA-S, however, are in conflict with classic aphasiology and also with most contemporary models of language. For one, traditional models of language exclude the ATL. For example, an influential review published at the height of twentieth-century aphasiology states that the probability that a lesion would impair comprehension is “very high in or near the first temporal gyrus, and fades out with different gradients (varying among individuals) toward the poles. And by the time it gets to any pole (occipital, temporal, or frontal) the probability is essentially zero” [38]. Research on PPA-S has contradicted this statement by showing that damage to the left ATL, including the temporal pole and anterior fusiform gyrus, causes severe impairments of word comprehension. Based on this finding, a proposal has been made that this region should be considered a core component of the language network [28].
This proposal has generated considerable debate. The disagreement revolves around the alternative characterization of ATL as an amodal hub for all semantic knowledge, verbal and non-verbal. Consequently, ATL damage should cause more than a language impairment (i.e., aphasia) and should give rise to a universal loss of semantic knowledge not only for words but also for faces and objects [39]. Based on this point of view, the syndrome of ATL damage was designated semantic dementia (SD), a syndrome defined by the combination of semantic aphasia (word comprehension deficit) with visual associative agnosia (loss of face and object recognition) [11, 39]. Such patients would not fit the diagnostic criteria for PPA since the aphasia would no longer constitute the dominant feature.
The disagreement on the nature of the syndrome caused by ATL damage can be resolved by considering the influence of hemispheric specialization [28, 40, 41]. Clinical observations and specially designed experimental tasks show that PPA-S is a selective aphasic syndrome of the left anterior temporal lobe, whereas the SD syndrome reflects a wider deficit with a more bilateral anatomical substrate [42,43,44,45]. The patients with left ATL damage may not be able to name objects and faces but are generally cognizant of their identity and nature [46]. It should be pointed out, however, that many PPA-S patients may also have minor atrophy in the right anterior temporal lobe, and that further spread of neurodegeneration within the right hemisphere may lead some, but not all, to eventually develop the additional face and object recognition deficits of SD. It is not surprising, therefore, that some authors have considered PPA-S and SD to be the two sides of the same coin [40, 41]. The question is whether syndromic designations should be based on clinical presentation at disease onset, as we advocate, or based on possible progression trajectories (Fig. 2). When ATL atrophy is predominantly right-sided, the patient may present with one of three syndromes, SD, non-aphasic associative agnosia, or bvFTD [47, 48].
PPA-S versus SD . Figure 2a shows the MRI scan of a right-handed man with symptom onset at the age of 59. On examination, 7 years later, the clinical pattern was PPA-S and atrophy was much more prominent in the left anterior temporal lobe (ATL). At that time, he had severe word comprehension impairments but no difficulty with non-verbal object recognition either in testing or in everyday life. In comparison, Fig. 2b shows the MRI scan of a right-handed man with symptom onset at the age of 65. Three years later, at his initial visit, ATL atrophy was bilateral. He had prominent word comprehension and object recognition impairments. This combination led to a subsequent diagnosis of semantic dementia (SD)
Exactly how the left ATL contributes to word comprehension is a topic of active investigation. Resting state functional imaging experiments show that the left ATL has left-sided asymmetric functional connectivity patterns that support its inclusion within the language network [49]. In our cohort, all right-handed patients with severe word comprehension impairment have also had substantial left ATL atrophy extending all the way into the pole. However, some patients with such a location of atrophy may have severe anomia in the absence of word comprehension impairment. In these patients, the distinctive comprehension impairment of PPA-S emerges as the atrophy extends posteriorly from the anterior tip of the left temporal lobe into adjacent parts of the middle portion of the temporal lobe (MTL), especially the middle temporal gyrus (MTG) [28]. In keeping with this observation, functional MRI studies in PPA and clinicoanatomical correlations in stroke have shown that the connectivity of the mid-to-posterior parts of the MTG with ATL and other parts of the language network may have important roles in sustaining word comprehension [50, 51]. In our experience, isolated atrophy of the middle parts of the temporal lobe in PPA has not been associated with impairment of this function [28]. Damage to the left ATL may therefore be necessary but not always sufficient for word recognition impairment. Posterior expansion of damage into the middle parts of the temporal lobe may also be required.
Patients with PPA-S have severe naming impairments principally because they do not understand the meaning of the word that denotes the object they are asked to name [46]. The impairment initially undermines the comprehension of a word at its specific level of meaning (does the word denote a strawberry or a cherry) but later generalizes to the generic meaning of the word (does the word denote a fruit or an animal) [52]. Based on these observations in PPA-S, the left ATL can be conceptualized as a transmodal region of cortex where sensory word form information is linked to the multimodal associations that collectively encode the meaning of the word [28, 53]. Word recognition at a specific level of meaning requires more extensive associative elaboration and would therefore be more vulnerable to early stages of neurodegeneration.
Another unexpected outcome of research on PPA was the finding that patients with the logopenic variant have normal single word comprehension despite peak atrophy sites that encompass Wernicke’s area as defined above. In fact, regression analyses in 73 PPA patients showed no correlation between atrophy in Wernicke’s area and impairment of word comprehension [28, 54]. In addition to clinicoanatomical correlations in PPA-L, which have shown that severe cortical degeneration of Wernicke’s area does not impair single word comprehension, investigations on PPA-S have shown that an intact Wernicke’s area is not sufficient to sustain word comprehension if the ATL is damaged. The body of work on PPA therefore leads to the conclusion that the cortex of Wernicke’s area is neither necessary nor sufficient for word comprehension. This conclusion can be reconciled with classic aphasiology by keeping in mind that nearly all reports linking Wernicke’s area to word comprehension are based on cerebrovascular lesions. Such lesions include not only the cortex of Wernicke’s area but also deep white matter axons, such as those in the middle longitudinal fasciculus [55], that are likely to carry projections of otherwise intact distal posterior and contralateral cortices. The resultant additional cortical disconnections may explain why stroke in Wernicke’s region impairs word comprehension while neurodegeneration in Wernicke’s cortex does not [54].
The large-scale network model posits that each network node mediates critical (or essential) as well as ancillary (or sustaining) functions related to its principal cognitive domain [56, 57]. While damage to a given node may not cause fixed impairments of its ancillary functionalities, the overall computational flexibility of the network for mediating that task may be compromised. These principles apply to the role of Wernicke’s area in language comprehension. For example, agrammatic and logopenic PPA patients whose atrophy encompasses Wernicke’s area but not the ATL, and who have normal word comprehension in standard tests and daily life, display abnormally prolonged semantic interference effects and loss of the N400 semantic incongruence potential [52, 58]. Furthermore, functional magnetic resonance imaging (fMRI) investigations using synonym identification tasks revealed activations not only in the anterior temporal lobe but also in regions overlapping Wernicke’s area [59, 60]. The cerebral cortex within Wernicke’s area therefore serves an ancillary role in word comprehension. Multiple lines of evidence show that Wernicke’s area plays a critical role in language repetition, a finding that is in keeping with observations in stroke aphasia [54]. This area is important for language repetition presumably because it links phonologic word form codes to their articulatory sequences [61,62,63].
An additional contribution of PPA to the anatomy of language comes through the discovery of the aslant tract, a pathway that connects the core language network with dorsal premotor cortex and appears to play a major role in sustaining fluency [64]. Patients with PPA may also show patterns of aphasia that have not been observed in other settings. For example, some patients may show a preferential inability to name objects orally but not in writing and fail to understand words they hear but not those they read [65]. These patients do not fit the pattern seen in pure word deafness because they are anomic and they do not fit the pattern of auditory agnosia because they can match objects to their characteristic sounds. Investigations on this small group of patients have helped to explore the functionality of a putative “auditory word form area” that sits at the confluence of modality-specific pathways for word comprehension and language repetition.
The totality of these investigations on PPA depicts a large-scale language network built upon the interactive functionalities of dorsal and ventral (rather than anterior and posterior) streams of processing [31]. The dorsal route mediates phonological encoding, repetition, articulatory programming, fluency, word retrieval and also the sequencing of morphemes and words into grammatically correct sentences. The ventral route mediates the lexicosemantic processes of object naming and word comprehension. Word finding in speech is a joint function of both routes and therefore the most common presenting complaint in PPA.
Asymmetry of Neuropathology and Genetics
In our group of 97 consecutive autopsies, the primary neuropathology was FTLD with tauopathy (FTLD-tau) in 29%, FTLD with transactive response DNA-binding protein 43 (FTLD-TDP) in 25%, and AD in 44%. All three major neuropathologic forms of FTLD-tau (Pick’s disease, corticobasal degeneration [CBD], progressive supranuclear palsy [PSP]), and all three major forms of FTLD-TDP (types A, B and C) were represented. There were some disease-specific preferential patterns of atrophy. For example, AD almost always led to peak atrophy that included the temporoparietal junction; TDP-C almost always led to severe anterior temporal atrophy; Pick’s disease routinely caused combined atrophy of anterior temporal and prefrontal cortex; and PSP and CBD tended to be associated with surprisingly modest cortical atrophy, usually in dorsal premotor or inferior frontal cortex. The one common denominator of nearly all cases is the leftward asymmetry of the atrophy (Figs. 3 and 4). What is surprising is that the asymmetry is almost always maintained up to the time of death. The initial predilection of the language-dominant left hemisphere is therefore not a random event at disease onset but a core biological feature of the syndrome.
Asymmetry of neurodegeneration. Postmortem examination of a right-handed woman with symptom onset at the age of 72 and findings of agrammatic PPA with prominent word finding impairments. Death occurred 6 years later. The primary neuropathology was found to be FTLD-tau of the CBD type. The top figures show the profound asymmetry of atrophy. There is an almost cystic area of atrophy around the left inferior frontal gyrus (IFG) but no comparable atrophy of the right. The photomicrographs at the bottom, based on phosphotau immunostaining in the same patient, show the tauopathy to be more intense in the left IFG than in the right
Correspondences of pathology, atrophy, and syndrome. Quantitative MRI morphometry in three right-handed patients who had come to postmortem brain autopsy. Areas of significant cortical thinning compared to controls are shown in red and yellow. (a) Onset of PPA-G was at the age of 65. The scan was obtained 2 years after onset. At postmortem, the primary pathology was FTLD-TDP type A. (b) Onset of PP-G was at the age of 57. The scan was obtained 5 years after onset. At postmortem, the primary pathology was Pick’s disease. (c) Onset of PPA-S was at the age of 62. The scan was obtained 5 years after onset. At postmortem, the primary pathology was Pick’s disease. Despite the differences in neuropathology and clinical syndrome, the one common denominator is the profound leftward asymmetry of atrophy. Abbreviations: ATL anterior third of the temporal lobe, IFG-B inferior frontal gyrus where Broca’s area is located, MTL middle third of the temporal lobe, TPJ-W temporoparietal junction where Wernicke’s area is located
There was nearly equal representation of males and females in our autopsy cohort. Age of onset varied from 41 to 80 with a mean of 61 ± 8 years. Survival from symptom onset to death varied from 2 to 23 years with a mean of 9.69 ± 3.93. Survival tended to be the longest for those with AD (10.8 ± 4.4) and FTLD-TDP type C (12.4 ± 2.6) and shortest for those with FTLD-TDP types A and B (5.8 ± 2.2). In keeping with these different rates of progression, FTLD-TDP aggregates extracted from subjects with type A pathology were shown to be more cytotoxic than aggregates from subjects with type C pathology [66].
The relationship of PPA variants to the underlying neuropathologic entity is probabilistic rather than absolute [67]. Autopsy data show that the vast majority of PPA-S cases have had TDP-C pathology but approximately 20% have had Pick’s disease; the majority of PPA-G cases have had FTLD-tau (all types) but approximately 30% have had FTLD-TDP or AD; the majority of PPA-L cases have had AD but 30% have shown FTLD-tau or FTLD-TDP. Figure 4 illustrates the clinicopathologic heterogeneity of PPA, namely that the same neuropathologic entity can cause more than one aphasic variant and that the same PPA variant may be caused by more than one neuropathologic entity. As shown in Fig. 4a and b, FTLD-TDP type A and Pick’s disease cause nearly identical peak atrophy patterns that extend into the frontal components of the language network known to underlie grammar and fluency, giving rise to the concordant syndrome of PPA-G. Figure 4b and c raise challenging questions. They show atrophy patterns in two different patients with Pick’s disease at autopsy, one with PPA-G (Fig. 4b), the other with PPA-S (Fig. 4c). As explained in the section on the anatomy of language, the semantic aphasia associated with Fig. 4c could be attributed to the posterior expansion of atrophy from ATL into more middle sections of the temporal lobe. However, it is difficult to understand why the patient in Fig. 4c was not also agrammatic since the frontal atrophy is nearly as extensive as in the other two cases with PPA-G. Perhaps this discrepancy can be blamed on vagaries of cortical morphometry performed on single subjects or, alternatively, on individual variations in the functional anatomy of the language network.
During life, cortical thinning (i.e., atrophy) and hypometabolism are the two most conspicuous markers of asymmetric neurodegeneration. Considerable progress has been made in exploring the potential cellular substrates of the asymmetrical atrophy (Fig. 3). For example, neurofibrillary tangles (NFT) (but not the amyloid plaques) of AD, tauopathy of CBD/PSP, Pick bodies, abnormal TDP-43 deposits of FTLD-TDP, activated microglia, and the extent of neuronal atrophy/loss tend to be more prominent in the left hemisphere than in the right hemisphere and also more prominent in language-related than other cortical areas of the left hemisphere [68,69,70,71,72,73]. In one left-handed PPA patient with documented right hemisphere language dominance and FTLD-TDP neuropathology, cortical atrophy and neurodegeneration markers were more prominent in the right hemisphere [74]. In at least some PPA patients with AD neuropathology, NFT may be more numerous in the language-related cortices of the left hemisphere than in the medial temporal areas, a distribution that deviates from the Braak and Braak pattern of neuropathology and underlies the atypical preservation of episodic memory in these patients [71, 73].
Quantitative investigations have also looked into the concordance of PPA subtypes with regional variations of neurodegeneration markers. A study of four right-handed PPA patients with FTLD-TDP type A neuropathology showed that the two patients with PPA-G displayed the highest density of TDP-43 precipitates in the frontal components of the language network, whereas the two with PPA-L displayed the highest density of precipitates in the temporoparietal components of the language network [69]. The cellular pathology in PPA can therefore asymmetrically target parts of the language-dominant hemisphere in a way that also mirrors the anatomical predilection patterns of the specific PPA variant. In the future, it would be useful to conduct similar analyses based on synaptic density. Some patients, especially those with PPA-G and FTLD-tau, may have no detectable cortical atrophy in the initial years of disease. These patients display abnormalities of functional connectivity, suggesting that physiological perturbations of the language network may precede atrophy [75]. In this group of patients, the neurodegeneration may be particularly prominent in subcortical white matter [76]. It is important to keep in mind that the identity of the disease marker that shows the best correlation with clinical dysfunction can change over time. Inclusions are likely to reflect leading indicators and would be expected to show the best correlation with clinical patterns in early disease stages, whereas neuronal death is likely to represent a trailing indicator more closely aligned with clinical patterns late in the disease.
In our autopsy cohort of 97 cases, a third of TDP-A cases had granulin (GRN) mutations. No other disease-causing mutations were encountered. Other studies have also shown that mutations in the GRN gene constitute the most common genetic correlate of familial PPA [77]. In such GRN families, some members may have PPA and others bvFTD [78, 79]. Rarely, all affected members of a GRN family will have PPA [80]. Even then, the type of aphasia may differ from one sibling to another and there is considerable heterogeneity of PPA subtypes associated with GRN mutations [81, 82]. The literature also contains rare associations of PPA with mutations in the presenilin (PSEN1), tau (MAPT), and C9orf72 genes [83,84,85]. The most common clinical variants associated with dominantly inherited diseases are PPA-G and PPA-L, but rare cases of PPA-S have been reported [82]. The cellular neuropathology is FTLD-TDP type A in GRN mutations, FTLD-TDP type B in C9orf72 mutations, and any one of the major FTLD-tau types in MAPT mutations. FTLD-TDP type C is very rarely, if ever, associated with known disease-causing mutations [86,87,88].
The heterogeneity of phenotypes encountered within GRN families shows that molecular underpinnings alone are not sufficient to account for the patterns of selective vulnerability and their clinical manifestation. The biological mechanisms underlying the selective and asymmetric involvement of the language-dominant hemisphere in PPA remain to be elucidated. One line of investigation has focused on the significantly higher frequency of learning disabilities, including dyslexia, in PPA patients and their first-degree relatives compared to control populations and patients with other dementias [89,90,91]. Follow-up research has replicated this association and raised the possibility that it may be peculiar to PPA-L [92]. Some families of PPA probands have strikingly high prevalence of developmental dyslexia in siblings or children [89]. We saw one family where seven of nine siblings of a PPA patient had findings indicative of developmental dyslexia [93]. As a group, the dyslexic siblings in this family had decreased functional connectivity within the language network although none had any findings of PPA. These observations led to the speculation that at least some cases of PPA could be arising on a developmentally or genetically based vulnerability of the left hemisphere language network. In some family members, this vulnerability would interfere with the acquisition of language and lead to dyslexia, while in others, it would make the language network a locus of least resistance for the effects of an independently arising neurodegenerative process, leading to PPA [33]. So far, linkage studies addressing this hypothesis have not detected an association between PPA and known dyslexia genes [77]. Given the polygenic nature of dyslexia, negative results may reflect an insufficient number of cases.
Therapeutic Interventions
The heterogeneity of PPA highlights the need to individualize therapeutic approaches. Interventions in individual patients should target the underlying disease as well as the symptom complex. The former step requires the use of in vivo biomarkers. There are excellent CSF and PET biomarkers for detecting PPA patients with AD neuropathology and blood-based biomarkers may be on the horizon. However, current tau ligands for PET do not yet offer reliable identification of non-AD tauopathies associated with CBD, PSP, and Pick’s disease [94]. When such biomarkers become available, they will enable the identification of PPA patients with FTLD-tau and, by exclusion, those with FTLD-TDP. The goal of these diagnostic investigations is to prescribe approved medications (e.g., cholinesterase inhibitors if AD) and to channel the patient to relevant disease-specific clinical trials. Although clinical examination is rarely sufficient to specify the underlying disease entity, we have found that prominent single word comprehension deficits that arise as the most salient feature of PPA are never associated with AD. The presence of this feature may therefore be used to forego AD biomarker testing.
The nonpharmacologic interventions aimed at the language impairment include speech therapy and brain stimulation modalities such as transcranial magnetic stimulation (TMS) or transcranial direct current stimulation (tDCS) [95]. Promising effects have been reported following left hemisphere tDTS in PPA-S [96]. If confirmed, this may well be the first time that brain stimulation will be shown to have therapeutic effects in an FTLD syndrome. Evidence for the effectiveness of speech-language therapy in PPA is emerging [97,98,99]. Utilization of this intervention modality is low in part due to the misconception that speech-language therapy is not appropriate for neurodegenerative syndromes where worsening is inevitable [100, 101]. An additional barrier is the lack of familiarity of speech-language pathologists with neurodegenerative conditions. Speech-language therapy in PPA requires personalization to fit the pattern of impairment and its evolution over time. For example, there are patients with modality-selective impairments of naming and word comprehension who could benefit from treatments emphasizing the relatively spared channels of language processing [65]. Additional questions to be resolved in the course of speech-language therapy include the relative usefulness of multicomponent, impairment-based, or compensatory approaches and the comparative benefits of group, dyadic, or patient-only approaches [102]. In each case, ecologically meaningful and statistically robust outcome measures will need to be devised.
Recent developments in telemedicine raise the possibility of delivering speech-language therapy in the home of the individual living with PPA [103, 104]. Communication Bridge, for example, is a two-arm, randomized control trial of speech-language intervention delivered through video chat for individuals with PPA [104]. The experimental arm uses a client-informed, dyadic approach for individuals with PPA and their communication partner. Impairment-based exercises using personalized stimuli and compensatory strategies are utilized to address real-world communication difficulties. The trial includes an individually tailored web application with native practice exercises and education materials that participants rehearse between treatment sessions. To evaluate whether treatment gains are relevant to the daily functions of the participant, outcomes are measured using a communication confidence rating scale and goal attainment scores. This method allows the targeting of individualized goals of high relevance to participants. In the future, transcranial stimulation could be combined with speech-language therapy to attain even more effective benefits [95].
Conclusions
Despite its relative rarity, PPA has led to conceptual advances in understanding the heterogeneity of dementia, the principles of selective brain vulnerability, and the neuroanatomy of the language network. PPA was arguably the first entity to show that there is more to dementia than memory loss, that the same clinical syndrome can be caused by multiple neuropathologies, that the same neuropathology can cause multiple syndromes, and that the relationship of syndrome to neuropathology is probabilistic rather than deterministic. Future work on PPA is likely to shed new light on the anatomical tropisms of neurodegenerative diseases and on the internal architecture of the language network.
References
Coyle-Gilchrist TS, Dick KM, Patterson K, Rodriquez PV, Wehmann E, Wilcox A et al (2016) Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology 86:1736–1743
Rosenfeld M (1909) Die partielle Grosshirnatrophie. J Psychol Neurol 14:115–130
Pick A (1892) Ueber die Beziehungen der senilen Hirnatrophie zur Aphasie. Prager Medizinische Wochenschrift 17:165–167
Pick A (1904) Zur Symptomatologie der linksseitigen Schlaffenlappenatrophie. Monatsschr Psychiatr Neurol 16:378–388
Franceschi F (1908) Gliosi perivasculare in un caso de demenza afasica. Ann Neurol 26:281–290
Sérieux P (1893) Sur un cas de surdité verbale pure. Revue de Medecine 13:733–750
Dejerine J, Sérieux P (1897) Un cas de surdité verbale pure terminée par aphasie sensorielle, suivie d’autopsie. Comptes Rendues des Séances de la Société de Biologie 49:1074–1077
Mesulam MM (1982) Slowly progressive aphasia without generalized dementia. Ann Neurol 11(6):592–598
Mesulam MM (1987) Primary progressive aphasia – differentiation from Alzheimer’s disease [editorial]. Ann Neurol 22(4):533–534
Mesulam M-M (2001) Primary progressive aphasia. Ann Neurol 49:425–432
Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S et al (1998) Frontotemporal lobar degeneration. A consensus on clinical diagnostic criteria. Neurology 51:1546–1554
Gorno-Tempini ML, Hillis A, Weintraub S, Kertesz A, Mendez MF, Cappa SF et al (2011) Classification of primary progressive aphasia and its variants. Neurology 76:1006–1014
Weintraub S, Rubin NP, Mesulam MM (1990) Primary progressive aphasia. Longitudinal course, neuropsychological profile, and language features. Arch Neurol 47(12):1329–1335
Mesulam M-M, Weintraub S (2008) Primary progressive aphasia and kindred disorders. In: Duyckaerts C, Litvan I (eds) Handbook of clinical neurology. Elsevier, New York, pp 573–587
Mesulam M-M, Wieneke C, Thompson C, Rogalski E, Weintraub S (2012) Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain 135:1537–1553
Mesulam M, Weintraub S (2014) Is it time to revisit the classification of primary progressive aphasia? Neurology 82:1108–1109
Sajjadi SA, Patterson K, Arnold RJ, Watson PC, Nestor PJ (2012) Primary progressive aphasia: a tale of two syndromes and the rest. Neurology 78:1670–1677
Wicklund MR, Duffy JR, Strand EA, Machulda MM, Whitwell JL, Josephs KA (2014) Quantitative application of the primary progressive aphasia consensus criteria. Neurology 82(13):1119–1126
Canu E, Agosta F, Imperiale F, Ferraro PM, Fontana A, Magnani G et al (2019) Northwestern anagram test-Italian (NAT-I) for primary progressive aphasia. Cortex 119:497–510
Mack JE, Chandler SD, Meltzer-Asscher A, Rogalski E, Weintraub S, Mesulam M-M et al (2015) What do pauses in narrative production reveal about the nature of word retrieval deficits in PPA. Neuropsychologia 77:211–222
Hillis AE, Tuffiash E, Caramazza A (2002) Modality-specific deterioration in naming verbs in nonfluent primary progressive aphasia. J Cog Neurosci 14:1099–1108
Mendez MF, Sabadash V (2015) Clinical amyloid imaging in logopenic progressive aphasia. Alzheimer Dis Assoc Disord 29:94–96
Tree J, Kay J (2014) Longitudinal assessment of short-term memory deterioration in logopenic variant primary progressive aphasia with post-mortem confirmed Alzheimer’s disease pathology. J Neuropsychol 9:184–202
Josephs KA, Duffy J, Strand EA, Machulda MM, Senjem ML, Lowe VJ et al (2013) Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology 81:337–345
Grossman M (2012) The non-fluent/agrammatic variant of primary progressive aphasia. Lancet Neurol 11:545–555
Thompson CK, Cho S, Hsu C-J, Wieneke C, Rademaker A, Weitner BB et al (2012) Dissociations between fluency and agrammatism in primary progressive aphasia. Aphasiology 26:20–43
Rogalski E, Cobia D, Martersteck AC, Rademaker A, Wieneke CA, Weintraub S et al (2014) Asymmetry of cortical decline in subtypes of primary progressive aphasia. Neurology 83:1184–1191
Mesulam M-M, Thompson CK, Weintraub S, Rogalski EJ (2015) The Wernicke conundrum and the anatomy of language comprehension in primary progressive aphasia. Brain 138:2423–2437
Catani M, Mesulam M (2008) The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex 44:953–961
Friederici AD (2011) The brain basis of language processing: from structure to function. Physiol Rev 91:1357–1392
Hickok G, Poeppel D (2007) The cortical organization of speech processing. Nat Rev Neurosci 8:293–402
Hagoort P (2013) MUC (memory, unification, control) and beyond. Front Psychol 4:1–13
Mesulam M-M, Rogalski E, Wieneke C, Hurley RS, Geula C, Bigio E et al (2014) Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol 10:554–569
Hodges JR, Patterson K, Oxbury S, Funnell E (1992) Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain 115:1783–1806
Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ et al (2004) Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 55:335–346
Rogalski E, Cobia D, Harrison TM, Wieneke C, Weintraub S, Mesulam M-M (2011) Progression of language impairments and cortical atrophy in subtypes of primary progressive aphasia. Neurology 76:1804–1810
Hagoort P (2014) Nodes and networks in the neural architecture for language: Broca’s region and beyond. Curr Opin Neurobiol 28:136–141
Bogen JE, Bogen GM (1976) Wernicke’s region-where is it? Ann N Y Acad Sci 280:834–843
Patterson K, Nestor P, Rogers TT (2007) Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci 8:976–988
Adlam A-LR, Patterson K, Rogers TT, Nestor PJ, Salmond CH, Acosta-Cabronero J et al (2006) Semantic dementia and fluent primary progressive aphasia: two sides of the same coin? Brain 129:3066–3080
Bright P, Moss ME, Stamatakis EA, Tyler LK (2008) Longitudinal studies of semantic dementia: the relationship between structural and functional changes over time. Neuropsychologia 46:2177–2188
Lambon Ralph MA, Cipolotti L, Manes F, Patterson K (2010) Taking both sides: do unilateral anterior temporal lobe lesions disrupt semantic memory? Brain 133:3243–3255
Mesulam M-M, Rogalski E, Wieneke C, Cobia D, Rademaker A, Thompson C et al (2009) Neurology of anomia in the semantic subtype of primary progressive aphasia. Brain 132:2553–2565
Gefen T, Wieneke C, Martersteck AC, Whitney K, Weintraub S, Mesulam M-M et al (2013) Naming vs knowing faces in primary progressive aphasia. A tale of two hemispheres. Neurology 81:658–664
Hurley RS, Mesulam M-M, Sridhar J, Rogalski E, Thompson CK (2018) A nonverbal route to conceptual knowledge involving the right anterior temporal lobe. Neuropsychologia 117:92–101
Mesulam M-M, Wieneke C, Hurley RS, Rademaker A, Thompson CK, Weintraub S et al (2013) Words and objects at the tip of the left temporal lobe in primary progressive aphasia. Brain 136:601–618
Nakachi R, Muramatsu T, Kato M, Akiyama T, Saito F, Yoshino F et al (2007) Progressive prosopagnosia at a very early stage of frontotemporal lobar degeneration. Psychogeriatrics 7:155–162
Snowden J, Harris JM, Thompson JC, Kobylecki C, Jones M, Richardson AMT et al (2018) Semantic dementia and the left and right temporal lobes. Cortex 107:188–203
Hurley RS, Bonakdarpour B, Wang X, Mesulam M-M (2015) Asymmetric connectivity between the anterior temporal lobe and the language network. J Cog Neurosci 27:464–473
Bonakdarpour B, Hurley RS, Wang A, Fereira HR, Basu A, Chatrathi A et al (2019) Perturbations of language network connectivity in primary progressive aphasia. Cortex 121:468–480
Turken AU, Dronkers NF (2011) The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analysis. Front Syst Neurosci 5:1–20
Hurley RS, Paller K, Rogalski E, Mesulam M-M (2012) Neural mechanisms of object naming and word comprehension in primary progressive aphasia. J Neurosci 32:4848–4855
Seckin M, Mesulam MM, Voss JL, Huang W, Rogalski EJ, Hurley RS (2016) Am I looking at a cat or a dog? Gaze in semantic variant of primary progressive aphasia is subject to excessive taxonomic capture. J Neurolinguistics 37:68–81
Mesulam M-M, Rader B, Sridhar J, Nelson MJ, Hyun J, Rademaker A et al (2019) Word comprehension in temporal cortex and Wernicke area: a PPA perspective. Neurology 92:e224–e233
Luo C, Makaretz S, Stepanivic M, Papadimitrou G, Quimby M, Palanivelu S et al (2020) Middle longitudinal fasciculus is associated with semantic processing deficits in primary progressive aphasia. NeuroImage 25:1–7
Mesulam M-M (1990) Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol 28(5):597–613
Mesulam M-M (1998) From sensation to cognition. Brain 121:1013–1052
Thompson C, Cho S, Rogalski E, Wieneke C, Weintraub S, Mesulam M-M (2012) Semantic interference during object naming in agrammatic and logopenic primary progressive aphasia (PPA). Brain Lang 120:237–250
Gitelman DR, Nobre AC, Sonty S, Parrish TB, Mesulam M-M (2005) Language network specializations: an analysis with parallel task design and functional magnetic resonance imaging. NeuroImage 26:975–985
Sonty SP, Mesulam M-M, Weintraub S, Johnson NA, Parrish TP, Gitelman DR (2007) Altered effective connectivity within the language network in primary progressive aphasia. J Neurosci 27:1334–1345
Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A et al (2008) The logopenic/phonological variant of primary progressive aphasia. Neurology 71:1227–1234
Binder JR (2015) The Wernicke area. Neurology 85:1–6
Binder J (2017) Current controversies on Wernicke’s area and its role in language. Curr Neurol Neurosci Rep 17:1–10
Catani M, Mesulam M-M, Jacobsen E, Malik F, Martersteck A, Wieneke C et al (2013) A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain 136:2619–2628
Mesulam M-M, Nelson MJ, Hyun J, Rader B, Hurley RS, Rademakers R et al (2019) Preferential disruption of auditory word representations in primary progressive aphasia with the neuropathology of FTLD-TDP type A. Cogn Behav Neurol 32(1):46–53
Laferriére F, Maniecka Z, Pérez-Berlanga M, Hruska-Plochan M, Gilhrspy L, Hock E-M et al (2019) TDP-43 extracted from frontotemporal lobar degeneration subject brains displays distinct aggregate assemblies and neurotoxic effects reflecting disease progression rates. Nat Neurosci 22:65–77
Mesulam M-M, Weintraub S, Rogalski EJ, Wieneke C, Geula C, Bigio EH (2014) Asymmetry and heterogeneity of Alzheimer and frontotemporal pathology in primary progressive aphasia. Brain 137:1176–1192
Gliebus G, Bigio E, Gasho K, Mishra M, Caplan D, Mesulam M-M et al (2010) Asymmetric TDP-43 distribution in primary progressive aphasia with progranulin mutation. Neurology 74:1607–1610
Kim G, Ahmadian SS, Peterson M, Parton Z, Memon R, Weintraub S et al (2016) Asymmetric pathology in primary progressive aphasia with progranulin mutations and TDP inclusions. Neurology 86:627–636
Kim G, Bolbolan K, Gefen T, Weintraub S, Bigio E, Rogalski E et al (2018) Atrophy and microglial distribution in primary progressive aphasia with transactive response DNA-binding protein-43 kDa. Ann Neurol 83(6):1096–1104
Gefen T, Gasho K, Rademaker A, Lalehzari M, Weintraub S, Rogalski E et al (2012) Clinically concordant variations of Alzheimer pathology in aphasic versus amnestic dementia. Brain 135:1554–1565
Giannini LAA, Xie SX, McMillan CT, Liang M, Williams A, Jester C et al (2019) Divergent patterns of TDP-43 and tau pathologies in primary progressive aphasia. Ann Neurol 85:630–643
Ohm DT, Fought AJ, Rademaker A, Kim G, Sridhar J, Coventry C et al (2019) Neuropathologic basis of in vivo cortical atrophy in the aphasic variant of Alzheimer’s disease. Brain Pathol 30:332–344
Kim G, Vahedi S, Gefen T, Weintraub S, Bigio E, Mesulam M-M et al (2018) Asymmetric TDP pathology in primary progressive aphasia with right hemisphere language dominance. Neurology 90:e396–e403
Bonakdarpour B, Rogalski E, Wang A, Sridhar J, Mesulam M-M, Hurley RS (2017) Functional connectivity is reduced in early-stage primary progressive aphasia when atrophy is not prominent. Alzheimer Dis Assoc Disord 31:101–106
Caso F, Mandelli ML, Henry ML, Gesierich B, Bettcher BM, Ogar J et al (2014) In vivo signatures of nonfluent/agrammatic primary progressive aphasia caused by FTLD pathology. Neurology 82:239–247
Ramos EM, Dokuru ER, Van Berlo V, Wojta K, Wang Q, Huang AY et al (2019) Genetic screen in a large series of patients with primary progressive aphasia. Alzheimers Dement 15:553–560
Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C et al (2006) Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442:916–919
Gass J, Cannon A, Mackenzie I, Boeve B, Baker M, Adamson J et al (2006) Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet 15(20):2988–3001
Mesulam M, Johnson N, Krefft TA, Gass JM, Cannon AD, Adamson JL et al (2007) Progranulin mutations in primary progressive aphasia. Arch Neurol 64:43–47
Coppola C, Oliva M, Saracino D, Pappata S, Zampella E, Cimini S et al (2019) One novel GRN null mutation, two different aphasia phenotypes. Neurobiol Age 87:e9–e14
LeBer I, Camuzat A, Hannequin D, Pasquier F, Guedj E, Rovelet-Lecrux A et al (2008) Phenotype variability in progranulin mutation carriers: a clinical, neuropsychological, imaging and genetic study. Brain 131:732–746
Simón-Sánchez J, Dopper EGP, Cohn-Hokke PE, Hukema RK, Nicolau N, Seelar H et al (2012) The clinical and pathological phenotype of C9ORF72 hexanucleotide repeat expansions. Brain 135:723–735
Munoz DG, Ros R, Fatas M, Bermejo F, de Yebenes J (2007) Progressive nonfluent aphasia associated with a new mutation V363I in tau gene. Am J Alzheimers Dis Other Dement 22:294–299
Godbolt AK, Beck JA, Collinge J, Garrard P, Warren JD, Fox NC et al (2004) A presenilin 1 R278I mutation presenting with language impairment. Neurology 63:1702–1704
Josephs K, Whitwell JL, Murray ME, Parisi JE, Graff-Radford N, Knopman D et al (2013) Corticospinal tract degeneration associated with TDP-43 type C pathology and semantic dementia. Brain 136:455–470
Lee EB, Porta S, Michael Baer G, Xu Y, Suh E, Kwong LK et al (2017) Expansion of the classification of FTLD-TDP: distinct pathology associated with rapidly progressive frontotemporal degeneration. Acta Neuropathol 134:65–78
Rohrer JD, Lashley T, Schott JM, Warren JE, Mead S, Isaacs AM et al (2011) Clinical and neuroanatomical signatures of tissue pathology in frontotemporal lobal degeneration. Brain 134:2565–2581
Rogalski E, Johnson N, Weintraub S, Mesulam M-M (2008) Increased frequency of learning disability in patients with primary progressive aphasia and their first degree relatives. Arch Neurol 65:244–248
Mesulam M-M, Weintraub S (1992) Primary progressive aphasia: sharpening the focus on a clinical syndrome. In: Boller F, Forette F, Khachaturian Z, Poncet M, Christen Y (eds) Heterogeneity of Alzheimer’s disease. Springer-Verlag, Berlin, pp 43–66
Rogalski EJ, Rademaker A, Wieneke C, Bigio EH, Weintraub S, Mesulam M-M (2014) Association between the prevalance of learning disabilities and primary progressive aphasia. JAMA Neurol 71:1576–1577
Miller ZA, Mandelli MA, Rankin KP, Henry ML, Babiak MC, Frazier DT et al (2013) Handedness and language learning disability differentially distribute in progressive aphasia variants. Brain 136(Pt 11):3461–3473
Weintraub S, Rader B, Coventry C, Sridhar J, Wood J, Guillaume K et al (2020) Familial languge network vulnerability in primary progressive aphasia. Neurology 22:1–9
Marquié M, Normandin MD, Vanderburg CR, Costantino IM, Bien EA, Rycyna LG et al (2015) Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol 78:787–800
Cotelli M, Manenti R, Ferrari C, Gobbi E, Macis A, Cappa SF (2020) Effectiveness of language training and non-invasive brain stimulation on oral and written naming performance in primary progressive aphasia: a meta-analysis and systematic review. Neurosci Biobehav Rev 108:498–525
Teichmann M, Lesoil C, Godard J, Vernet M, Bertrand A, Levy R et al (2016) Direct current stimulation over the anterior temporal areas boosts semantic processing in primary progressive aphasia. Ann Neurol 80:693–707
Carthery-Goulart MT, da Silveira AC, Machado TH, Mansur LL, Parente MM, Senaha MLH et al (2013) Interventions for cognitive impairments following primary progressive aphasia. Dement Neuropsychol 7:121–131
Volkmer A, Spector A, Meitanis V, Warren JD, Beeke S (2019) Effects of functional communication interventions for people with primary progressive aphasia and their caregivers: a systematic review. Aging Ment Health 28:1–13
Henry ML, Hubbard HI, Grasso SM, Mandelli ML, Wilson SM, Sathishkumar MT et al (2018) Retraining speech production and fluency in nonfluent/agrammatic primary progressive aphasia. Brain 141:1799–1814
Riedl L, Last D, Danek A, Diehl-Schmid J (2014) Long-term follow-up in primary progressive aphasia: clinical course and health care utilization. Aphasiology 28:981–992
Taylor C, Kingma RM, Croot K, Nickels L (2009) Speech pathology services for primary progressive aphasia: exploring an emerging area of preactice. Aphasiology 23:161–174
Jokel R, Meltzer J (2017) Group intervention for individuals with primary progressive aphasia and their spouses: who comes first? J Commun Disord 66:51–64
Meyer AM, Getz HR, Brennan DM, Hu TM, Friedman RB (2016) Telerehabilitation of anomia in primary progressive aphasia. Aphasiology 30:483–507
Rogalski E, Saxon M, McKenna H, Wieneke C, Rademaker A, Corden M et al (2016) Communication bridge: a pilot feasibility study of internet-based speech-language therapy for individuals with primary progressive aphasia. Alzheimers Dement (N Y) 2:213–221
Acknowledgments
SUPPORT: R01 DCOO8552 and K23 DC014303 from the National Institute of Deafness and Communication Disorders; P30 AG013854 and R01 AG056258 from the National Institute on Aging, R01 NS085770 from the National Institute of Neurological Disorders and Stroke, the Davee Foundation and the Jeanine Jones Fund.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mesulam, M.M. et al. (2021). Nosology of Primary Progressive Aphasia and the Neuropathology of Language. In: Ghetti, B., Buratti, E., Boeve, B., Rademakers, R. (eds) Frontotemporal Dementias . Advances in Experimental Medicine and Biology, vol 1281. Springer, Cham. https://doi.org/10.1007/978-3-030-51140-1_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-51140-1_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-51139-5
Online ISBN: 978-3-030-51140-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)