Abstract
Neurocysticercosis is an important cause of seizures worldwide and is endemic in most of Latin America, Sub-Saharan Africa, Southeast Asia, India, and China. Neurocysticercosis has profoundly different disease manifestations varying from asymptomatic presentation to life-threatening hydrocephalus. Clinical manifestations, pathogenesis, diagnostic methods, and optimal treatment vary with the location, number of lesions, and host response. Diagnosis is based on a combination of clinical presentation, neuroimaging findings, history of exposure, and serologic testing. Initial therapy should be focused on symptom management including seizure control and management of increased intracranial pressure. Emerging data are demonstrating that the optimal management approach varies with stage. Single enhancing or cystic lesions should be treated with albendazole and steroids. Patients with more than two cystic lesions should be treated with combination therapy with albendazole and praziquantel, whereas patients with hydrocephalus benefit from surgical management, especially with minimally invasive approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
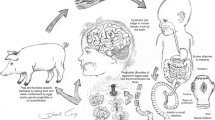
Neurocysticercosis (NCC), the nervous system infection by the larval stage of the pork worm Taenia solium, is endemic in many low-income countries and is a frequent cause of seizures worldwide. T. solium is endemic in most Latin American countries, Sub-Saharan Africa, Southeast Asia, India, and China [1, 2]. In endemic regions, it is an important cause of seizures, disability, and neurologic hospital admissions. Approximately, 29 % percent of patients with seizures in endemic countries are due to NCC [3]. The disease affects mostly immigrant population in the USA where there are over 2000 cases diagnosed each year [4••, 5••].
Humans are the only definitive host for the adult tapeworm, and both pigs and humans can carry the larval form. T. solium tapeworms develop when the larval cysts are ingested with contaminated pork. The scolex contained within the cystic lesion evaginates and attaches to the intestinal wall and produces segments that grow into the tapeworm. Tapeworm segments (proglottids) and fertile eggs are excreted into the environment in feces. Pigs can ingest infected stool or progrottids after which the eggs release embryos, which cross intestinal mucosa into the bloodstream, and are carried to peripheral tissues including the brain where they become cysticerci. Human cysticercosis is acquired from a tapeworm carrier, usually a household contact.
Clinical Manifestations and Staging
The clinical presentation of NCC is variable depending on the localization of lesions, stage of the parasite (viable, degenerating, calcified), and the host response. The presentation can vary from asymptomatic infection to life-threatening intracranial hypertension. The most common presentations are seizures, increased intracranial pressure, and headache. Other less common neurologic manifestations include radiculopathies, strokes, visual changes, mass lesions, altered mental status, and meningitis. The clinical evaluation should include a thorough history, physical exam, and epidemiology, including travel history. Cysticercosis typically has a latent period of a few years so distant travel history should be included. Patients should be asked about contact with pork-raising areas among family members, access to safe water and sanitation, and known contact with tapeworm carriers.
In patients from endemic areas with a classical presentation of seizures and a typical single lesion on imaging, the diagnosis can be made on clinical grounds. In cases where diagnosis is less certain, additional diagnostic tools can be used. Definitive histologic diagnosis is rarely made and brain biopsy is warranted only when the diagnosis cannot be made with non-invasive testing.
Neurocysticercosis is pleomorphic with different forms defined by the appearance of the organism (cystic versus calcified), the location, and the host response [6]. The differences are so profound that neurocysticercosis should be thought of as a group of diseases caused by a single organism, T. solium (Table 1). The main clinical manifestations, pathogenesis, diagnostic methods, and optimal treatment vary with the location, number of cysticerci, and the host response.
Single Enhancing Lesions
The most common presentation in hospital-based series from the USA and India is with seizures and a single enhancing lesion [7–9]. This form of disease is sometimes referred to as solitary cysticercal granuloma. Patients generally present with one or more seizures. The seizures are often focal with secondary generalization, but may be described as either focal or generalized. On CT scan, the lesions may be cystic or focal areas of enhancement with surrounding edema. Cases can be diagnosed based on clinical and neuroradiologic appearance [10]. Clinically, the patients present with seizures, without focal findings on physical examination, and with no symptoms or signs of other diseases. For example, fever, hilar or cervical adenopathy, or weight loss would suggest an alternative diagnosis. On neuroimaging, the lesions are round lesions, less than 20 mm in diameter, with no midline shift.
Viable Parenchymal Cysticerci
Studies suggest that cystic lesions with cyst fluid isodense with CSF are usually viable and this neuroradiologic finding is typically used to define viable parenchymal cysticerci. This is the most common presentation in hospital-based series from Latin America. Patients usually present with seizures, often more than a single episode. On neuroimaging studies, patients present with one or more cystic lesions often associated with some degree of contrast enhancement and surrounding edema.
Calcified Parenchymal Cysticercosis
The most common abnormality on imaging studies done on inhabitants of endemic villages is calcified lesions [11]. Most of those with calcified lesions are asymptomatic. However, a substantial proportion of patients have recurrent seizures and chronic epilepsy [12]. Seizures are accompanied by perilesional edema or enhancement in about half of cases [13], which is thought to be caused by breakdown of the granuloma releasing parasite antigen.
Ventricular Neurocysticercosis
About 10–20 % of cases of neurocysticercosis in hospital-based series present with cysticerci in the ventricles [14]. The most common presentations include chronic headaches and/or symptoms of obstructive hydrocephalus. In some cases, patients can present with abrupt or intermittent symptoms of obstructive hydrocephalus (Bruns’ syndrome). Untreated, this form is associated with a high case fatality rate.
Subarachnoid Neurocysticercosis
The term refers to neurocysticercosis that involves the subarachnoid space of the basilar cisterns and/or Sylvian fissures. Patients typically present with multiple cysticerci, often also with cysticerci in the brain parenchyma (viable or calcified) or ventricles. This form of neurocysticercosis is unusual but, due to its severity, is an important clinical problem. The clinical manifestations are also quite broad. Patients may present with headaches, meningeal signs, hydrocephalus, or focal neurologic findings. Patients may have seizures from associated parenchymal lesions or obstructive hydrocephalus from ventricular cysticerci. Cysticerci in the basilar cisterns are also frequently associated with involvement of the spinal subarachnoid space, which may or may not be symptomatic.
Spinal Neurocysticercosis
Neurocysticercosis of the spine is usually found in the subarachnoid space, but can also involve the spinal medulla [15, 16]. When spinal MRI is performed on patients with subarachnoid neurocysticercosis, there is a high frequency of asymptomatic involvement [15]. Symptoms may include radicular pain or myelopathy.
Ocular Disease
Cysticerci may also involve the orbits or eye [17, 18]. Involvement of the orbital muscles may lead to ocular palsies. Subconjunctival cysts may be visible on the surface of the eye. Ocular involvement may include cysticerci in the anterior chamber, vitreous, or subretinal location. All are associated with decreased visual acuity.
Diagnosis
Diagnosis of cysticercosis can be difficult. The parasite can occasionally be identified by direct visualization in the eye, but most cases depend on a combination of neuroimaging findings, history of exposure, and serologic confirmation [6].
Risk Factors
The accuracy of diagnostic tests depends on the prior probability of disease. Most US clinicians limit questioning of risk to country of origin. However, the risk is not uniform in all regions of endemic countries. For example, in Peru, transmission primarily occurs in rural, pig-raising areas. By contrast, most cases in Lima are linked to immigration to the city from rural communities [19]. Similarly, most US cases have had contact with pig-raising areas of rural Latin America [20]. While neurocysticercosis has been described among travelers, nearly all cases have had prolonged exposure to rural areas [21]. Thus, residence in endemic regions rather than exposure is the real risk factor.
Neuroimaging
Neurocysticercosis is usually initially suggested from neuroimaging studies. Most patients presenting to US hospitals with neurologic findings usually undergo non-contrast CT scans. Non-contrast CT scans are a sensitive method for detecting calcified or intraparenchymal cystic lesions, but they usually miss subarachnoid or intraventricular involvement. MRI is also more sensitive for small lesions, subarachnoid or intraventricular lesions, visualization of the scolex, and demonstration of edema or enhancements surrounding calcified lesions. However, MRI is not sensitive for demonstration of calcified lesions. Thus, it is preferable that both modalities be used for staging. Viable cysts are seen as small round lesions, with cyst fluid usually isodense with cerebrospinal fluid. On high-resolution CT or MRI, the scolex may appear as a nodule attached to the cyst wall. The scolex is typically 1–2 mm in diameter. It appears as slightly more dense than brain parenchyma on CT or MRI T1 sequences. Definitive demonstration of a scolex within a cysticercus is thought to be diagnostic of neurocysticercosis. Degenerating cysts are seen as poorly defined lesions with surrounding edema and can have ring enhancement. Calcified cysticerci are seen as hyperdense nodules on CT scans. The nodules are usually 1–10 mm in diameter and solid. Diffusion weighted images improve visualization of the scolex which may not be visible in regular MRI imaging or CT scans [21].
Subarachnoid cysticerci within sulci behave as parenchymal cysts and can present with similar findings. Cystic lesions within Sylvian fissures or basal CSF cisterns can grow and create a mass effect. Subarachnoid NCC can be associated with increased intracranial pressure through the occlusion of ventricular foramina (by cystic lesions or from host inflammatory proteins), CSF outflow obstruction, or through mass effect.
Recent data have highlighted the role of newer MRI sequences. Fluid Attenuation Inversion Recovery (FLAIR) augments identification of areas of edema. It also can improve visualization of the scolex. Three-dimensional imaging sequences such as (Fast Imaging Employing Steady-state Acquisition (FIESTA), Spoiled Gradient Recalled Echo (SPGR), and constructive interference in steady state (3D CISS) improve sensitivity for detection of intraventricular and subarachnoid lesions [22–24].
Serology
Serologic testing can be an important aid when diagnosis is uncertain with clinical findings and radiologic imaging. Enzyme-linked immunoelectrotransfer blot (EITB) assay which detects antibodies to T. solium in serum through lentil lectin purified glycoprotein antigens is the test of choice for antibody detection. Sensitivity is excellent for patients with two or more cystic parasites in the nervous system. However, the sensitivity of EITB in patients with one lesion is only 50–60 % so can allow for false negatives in patients with neurocysticercosis. The specificity is high for T. solium infection. However, in endemic regions, results can be positive from muscular or subcutaneous involvement and rare false-positive results have been reported in those with no history of exposure [25]. ELISA assays for detection of antibody are widely available, but due to poor sensitivity and specificity, most experts do not recommend them [26].
A number of assays have been developed that employ monoclonal antibodies to detect Taenia antigens [27, 28]. While less sensitive than antibody assays, specificity is reported to be high. Serum antigen testing is positive in the serum of patients with viable parasitic tissue, but can be negative in patients with few live cysts. An advantage of this test is that levels decrease rapidly after antiparasitic treatment and may help detect a response to treatment [29, 30].
Management
There are important roles for neurosurgery, anti-inflammatory drugs, anti-epileptic drugs, and antiparasitic drugs in the management of neurocysticercosis. T. solium does not usually proliferate in the human host and most symptoms result from the host response rather than infection per se [31, 32]. Thus, antiparasitic therapy is never an emergency and should generally be deferred until patients are stabilized. Instead, the initial focus of therapy should be on symptomatic therapy. For patients with seizures, the initial focus should be on controlling seizures with anti-epileptic drugs (Table 2). For patients with elevated intracranial pressure, the focus should be on identifying the cause and treating the increased pressure. In the case of diffuse cerebral edema or mass effect from edema surrounding a large cysticercus, corticosteroids are the mainstay of initial therapy. By contrast, hydrocephalus usually requires surgical management for either removal of a cysticercus causing obstruction or by CSF diversion.
Since management strategies depend on disease classification and involvement, appropriate classification of disease and staging is an important step of initial management. Parenchymal lesions causing seizures respond well to antiepileptics; however, antiparasitic therapy has shown a role in reducing seizure recurrence. For extraparenchymal manifestations, it is important to manage intracranial hypertension. Overall management can include a range of symptom management with antiepileptics, antiparasitic therapy, corticosteroids, and occasionally surgery.
Single Enhancing Lesions due to Cysticercosis
Patients with single enhancing lesions usually present with seizures and should be treated with anti-epileptic drugs. However, with just anti-epileptic drugs, there is a 16 % rate of recurrent seizures while on anti-epileptic drugs and another 15 % developed seizures after withdrawal of anti-epileptic drugs [33]. However, there is no added benefit of continuing antiparasitic drugs beyond 6 months unless there is residual parasite material or calcifications [34]. Recent meta-analyses of placebo-controlled trials support the use of albendazole combined with steroids to decrease seizure recurrence and hasten lesion resolution [35, 36]. Albendazole is typically dosed at 15 mg/kg per day in two daily doses up to 1200 mg per day and continued for 1–2 weeks [37]. The choice of dose and duration of corticosteroids is not well studied, but could include a regimen such as 1 mg/kg of prednisone beginning a day before initiation of albendazole and continuing throughout the course.
Viable Parenchymal Cysticerci
In the past, there was considerable controversy on the role of antiparasitic drugs for viable parenchymal cysticerci. However, two placebo-controlled trials have now demonstrated unequivocally improved radiologic resolution with albendazole therapy [38, 39]. In addition, both studies demonstrated a reduction in the number of seizures in those treated with albendazole (15 mg/kg/day for 7–10 days) that was not statistically significant, but a significant reduction in generalized seizures [38, 40]. While the response to albendazole monotherapy was better than placebo, the response rates remained poor in those with multiple lesions. Serum levels of albendazole sulfoxide (the active metabolite of albendazole) are variable, but levels are higher and more consistent when the two drugs are combined [41]. Two recent studies have examined the effect of combination therapy with albendazole and praziquantel on parasiticidal efficacy. Both studies demonstrated improved cysticidal activity, primarily in those with more than two cysticerci [42••, 43•]. Thus, combination therapy is preferred for those with more than two lesions.
Anti-inflammatory drugs are recommended along with antiparasitic drugs in the treatment of viable parenchymal cysticerci. A recent study suggested that doses may need to be increased (for example 8 mg/d of dexamethasone) and prolonged (for 28 days followed by a taper) to optimally prevent seizure recurrence [44•]. However, the optimal drug, dose, and duration have yet to be defined.
There are also limited data on optimal anti-epileptic drugs in the setting of viable parenchymal cysticerci [34, 45]. Generally, drug recommendations are similar to recommendations for patients with chronic epilepsy. There are clearly drug interactions between some of the common anti-epileptic drugs such as phenytoin and carbamazepine and antiparasitic drugs. There are limited data on which patients may safely be tapered off anti-epileptic drugs. However, persistent lesions or calcifications on imaging studies are associated with seizure recurrence [46].
Calcified Parenchymal Neurocysticercosis
Calcified lesions from cysticercosis are often associated with ongoing inflammation [47, 48], but do not contain viable parasites. Thus, there is no reason to treat them with antiparasitic drugs. In many cases, there is evidence of perilesional edema. Some have treated patients with perilesional edema with anti-inflammatory drugs. However, a recent study suggested that corticosteroid withdrawal may actually precipitate clinical symptoms [49]. Thus, corticosteroids should generally be avoided in these cases.
Recent studies have highlighted that calcified lesions can be associated with hippocampal sclerosis and/or refractory epilepsy [50–52]. There are anecdotes of improvement with removal of the epileptic focus. However, the focus does not always correlate with calcified lesions on CT.
Ventricular Neurocysticercosis
Patients with cysticerci in the cerebral ventricles often present with obstructive hydrocephalus. While there are small case series that reported favorable outcome with just medical therapy, there are also reports of disastrous outcomes [14]. Recent data have emphasized treatment with minimally invasive surgery (neuroendoscopy) [14, 53, 54]. When cysticerci can be removed by neuroendoscopy, the outcomes are generally favorable. Inflamed and adherent cysts are more difficult to remove and are associated with higher rates of complications. Thus, antiparasitic drugs should not be used prior to neuroendoscopy. While there are reports of successful removal of fourth ventricular cysticerci by neuroendoscopy via a transaqueductal approach, this approach poses risks for significant morbidity in inexperienced hands. The role of antiparasitic drugs after successful removal of parasites is controversial. With the current generation of MRI scans and improved imaging techniques, the likelihood of benefit is small.
Subarachnoid Neurocysticercosis
Cysticercosis involving the subarachnoid space of the basilar cisterns carries a poor prognosis. In an older series of patients treated with CSF diversion alone, most died within 5 years [55]. In addition, most patients do not respond to antiparasitic drug regimens developed for parenchymal neurocysticercosis. In a placebo-controlled trial, there were more deaths among patients with extraparenchymal disease treated with placebo than with albendazole, although the numbers were small and not statistically significant [39]. Proaño and colleagues demonstrated that patients with giant cysticerci (mostly in the basilar cisterns or Sylvian fissure) seemed to have a better prognosis when treated with multiple 1-month courses of antiparasitic drugs [56]. Most experts now recommend prolonged therapy typically with albendazole [6]. However, based on studies demonstrating improved parasitic activity, some experts now treat patients with combination therapy with albendazole and praziquantel.
Inflammation plays a key role in the pathogenesis of subarachnoid neurocysticercosis. Thus, anti-inflammatory drugs are a critical part of management. There are no good data on the optimal choice of agent, dose, or duration of therapy. Most authorities recommend continuing anti-inflammatory therapy throughout the course of antiparasitic drugs, even with prolonged therapy. Low-dose methotrexate has been used as a steroid-sparing agent [57].
Other Management Issues
Cysticercosis of the retina or vitreous may worsen with antiparasitic therapy. Thus, a fundoscopic examination (ideally indirect fundoscopy with dilation of the pupil) should be performed prior to institution of antiparasitic therapy. Prolonged courses of corticosteroids may also increase the risks of tuberculosis and strongyloidiasis, which are more prevalent in populations at risk for neurocysticercosis.
Since neurocysticercosis is acquired from tapeworm carriers, some have advocated screening patients and their close contacts for tapeworms. Overall, the yield of screening is very low. However, selected patients such as cases acquired outside of endemic areas should be screened. Stool microscopy only detects a minority of infections. Copro-antigen tests and serologic tests for tapeworm-stage antibodies are more sensitive, but are currently only available in research laboratories.
Prevention
Garcia and colleagues recently demonstrated that it is possible to eliminate transmission of T. solium infection by the combination of mass treatment for tapeworm carriage, mass treatment of pigs, and vaccination of pigs [58••]. This study demonstrates the feasibility of eradication of this important disease. However, we are unaware of any large-scale efforts to apply this approach.
Conclusions
NCC includes a wide presentation of diseases. Diagnosis requires clinical suspicion and is often made through the combination of imaging, clinical manifestations and serologic studies. Symptoms in NCC are a manifestation of host response rather than active parasites, and initial treatment should focus on symptom management such as seizure control, management of cerebral edema, and reduction of intracranial pressure. When viable cysticerci are present, two placebo-controlled studies have shown the benefit of albendazole in reducing lesions on imaging, and have shown a reduction in generalized seizures. Those with more than two lesions benefit from combination therapy. There is limited data on optimal duration of anti-epileptic drugs and optimal duration of anti-inflammatory drugs.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Coyle CM, Mahanty S, Zunt JR, et al. Neurocysticercosis: neglected but not forgotten. PLoS Negl Trop Dis. 2012;6(5), e1500.
Garcia H, Coyle C, White Jr AC. Cysticercosis. In: Guerrant R, Walker D, Weller P, editors. Tropical Infectious Diseases, Principles, Pathogens and Practice. 3rd ed. Philadelphia: Elsevier-Saunders; 2011. p. 815–23.
Ndimubanzi PC, Carabin H, Budke CM, et al. A systematic review of the frequency of neurocyticercosis with a focus on people with epilepsy. PLoS Negl Trop Dis. 2010;4(11), e870.
•• O’Keefe KA, Eberhard ML, Shafir SC, Wilkins P, Ash LR, Sorvillo FJ. Cysticercosis-related hospitalizations in the United States, 1998–2011. Am J Trop Med Hyg. 2015;92(2):354–9. This study independantly evaluated national databases of hospital discharges for Neurocysticercosis admissions in the United State. This document over 2,000 cases admitted to hospitals per year.
•• O’Neal SE, Flecker RH. Hospitalization frequency and charges for neurocysticercosis, United States, 2003–2012. Emerg Infect Dis. 2015;21(6):969–76. This study independantly evaluated national databases of hospital discharges for Neurocysticercosis admissions in the United State. This document over 2,000 cases admitted to hospitals per year.
Garcia HH, Nash TE, Del Brutto OH. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol. 2014;13(12):1202–15.
García HH, Gonzalez AE, Rodriguez S, et al. Neurocysticercosis: unraveling the nature of the single cysticercal granuloma. Neurology. 2010;75(7):654–8.
Serpa JA, Graviss EA, Kass JS, White AC. Neurocysticercosis in Houston, Texas: an update. Medicine (Baltimore). 2011;90(1):81–6.
Serpa JA, White Jr, AC. Neurocysticercosis in the United States. Pathog Glob Health. 2012;106(5):256–60.
Rajshekhar V, Chandy MJ. Validation of diagnostic criteria for solitary cerebral cysticercus granuloma in patients presenting with seizures. Acta Neurol Scand. 1997;96(2):76–81.
Villarán MV, Montano SM, Gonzalvez G, et al. Epilepsy and neurocysticercosis: an incidence study in a Peruvian rural population. Neuroepidemiology. 2009;33(1):25–31.
Moyano LM, Saito M, Montano SM, et al. Neurocysticercosis as a cause of epilepsy and seizures in two community-based studies in a cysticercosis-endemic region in Peru. PLoS Negl Trop Dis. 2014;8(2), e2692.
Nash TE, Pretell EJ, Lescano AG, et al. Perilesional brain oedema and seizure activity in patients with calcified neurocysticercosis: a prospective cohort and nested case-control study. Lancet Neurol. 2008;7(12):1099–105.
Rangel-Castilla L, Serpa J, Gopinath S, Graviss E, Diaz-Marchan P, White Jr, AC. Contemporary neurosurgical approaches to neurocysticercosis. Am J Trop Med Hyg. 2009;80(3):373–8.
Callacondo D, Garcia HH, Gonzales I, Escalante D, Nash TE, et al. High frequency of spinal involvement in patients with basal subarachnoid neurocysticercosis. Neurology. 2012;78(18):1394–400.
Cárdenas G, Guevara-Silva E, Romero F, et al. Spinal Taenia solium cysticercosis in Mexican and Indian patients: a comparison of 30-year experience in two neurological referral centers and review of literature. Eur Spine J. 2016;25(4):1073–81.
Rath S, Honavar SG, Naik M, et al. Orbital cysticercosis: clinical manifestations, diagnosis, management, and outcome. Ophthalmology. 2010;117(3):600–5. 5.e1.
Madigubba S, Vishwanath K, Reddy G, Vemuganti GK. Changing trends in ocular cysticercosis over two decades: an analysis of 118 surgically excised cysts. Indian J Med Microbiol. 2007;25(3):214–9.
Gonzales I, Miranda JJ, Rodriguez S, et al. Seizures, cysticercosis and rural-to-urban migration: the PERU MIGRANT study. Trop Med Int Health. 2015;20(4):546–52.
del la Garza Y, Graviss EA, Daver NG, et al. Epidemiology of neurocysticercosis in Houston, Texas. Am J Trop Med Hyg. 2005;73(4):766–70.
Del Brutto OH. Neurocysticercosis among international travelers to disease-endemic areas. J Travel Med. 2012;19(2):112–7.
Carrillo Mezo R, Lara García J, Arroyo M, Fleury A. Relevance of 3D magnetic resonance imaging sequences in diagnosing basal subarachnoid neurocysticercosis. Acta Trop. 2015;152:60–5.
Neyaz Z, Patwari SS, Paliwal VK. Role of FIESTA and SWAN sequences in diagnosis of intraventricular neurocysticercosis. Neurol India. 2012;60(6):646–7.
Mont’Alverne Filho FE, Machado Lo R, Lucato LT, Leite CC. The role of 3D volumetric MR sequences in diagnosing intraventricular neurocysticercosis: preliminar results. Arq Neuropsiquiatr. 2011;69(1):74–8.
Furrows S, McCroddan J, Bligh W, Chiodini P. Lack of specificity of a single positive 50-kDa band in the electroimmunotransfer blot (EITB) assay for cysticercosis. Clin Microbiol Infect. 2006;12(5):459–62.
Carod JF, Randrianarison M, Razafimahefa J, et al. Evaluation of the performance of 5 commercialized enzyme immunoassays for the detection of Taenia solium antibodies and for the diagnosis of neurocysticercosis. Diagn Microbiol Infect Dis. 2012;72(1):85–9.
Gabriël S, Blocher J, Dorny P, et al. Added value of antigen ELISA in the diagnosis of neurocysticercosis in resource poor settings. PLoS Negl Trop Dis. 2012;6(10), e1851.
Deckers N, Dorny P. Immunodiagnosis of Taenia solium taeniosis/cysticercosis. Trends Parasitol. 2010;26(3):137–44.
Gonzalez AE, Bustos JA, Garcia HH, et al. Successful antiparasitic treatment for cysticercosis is associated with a fast and marked reduction of circulating antigen levels in a naturally infected pig model. Am J Trop Med Hyg. 2015;93(6):1305–10.
Fleury A, Garcia E, Hernández M, et al. Neurocysticercosis: HP10 antigen detection is useful for the follow-up of the severe patients. PLoS Negl Trop Dis. 2013;7(3), e2096.
Gonzales I, Rivera JT, Garcia HH, Peru CWGI. Pathogenesis of Taenia solium taeniasis and cysticercosis. Parasite Immunol. 2016;38(3):136–46.
Nash TE, Mahanty S, Loeb JA, et al. Neurocysticercosis: a natural human model of epileptogenesis. Epilepsia. 2015;56(2):177–83.
Rajshekhar V, Jeyaseelan L. Seizure outcome in patients with a solitary cerebral cysticercus granuloma. Neurology. 2004;62(12):2236–40.
Sharma M, Singh T, Mathew A. Antiepileptic drugs for seizure control in people with neurocysticercosis. Cochrane Database Syst Rev. 2015;10, CD009027.
Otte WM, Singla M, Sander JW, Singh G. Drug therapy for solitary cysticercus granuloma: a systematic review and meta-analysis. Neurology. 2013;80(2):152–62.
Zhao BC, Jiang HY, Ma WY, et al. Albendazole and corticosteroids for the treatment of solitary cysticercus granuloma: a network meta-analysis. PLoS Negl Trop Dis. 2016;10(2), e0004418.
Singh G, Murthy JM. Solitary cysticercus granuloma-treatment with albendazole: what is the optimal duration? Neurol India. 2010;58(4):507–8.
Garcia HH, Pretell EJ, Gilman RH, et al. A trial of antiparasitic treatment to reduce the rate of seizures due to cerebral cysticercosis. N Engl J Med. 2004;350(3):249–58.
Carpio A, Kelvin E, Bagiella E, et al. Effects of albendazole treatment on neurocysticercosis: a randomised controlled trial. J Neurol Neurosurg Psychiatry. 2008;79(9):1050–5.
Romo ML, Wyka K, Carpio A, et al. The effect of albendazole treatment on seizure outcomes in patients with symptomatic neurocysticercosis. Trans R Soc Trop Med Hyg. 2015;109(11):738–46.
Garcia HH, Lescano AG, Lanchote VL, et al. Pharmacokinetics of combined treatment with praziquantel and albendazole in neurocysticercosis. Br J Clin Pharmacol. 2011;72(1):77–84.
•• Garcia HH, Gonzales I, Lescano AG, et al. Efficacy of combined antiparasitic therapy with praziquantel and albendazole for neurocysticercosis: a double-blind, randomised controlled trial. Lancet Infect Dis. 2014;14(8):687–95. This welll-designed randomized clinical trial demonstrated the efficacy of combination antiparasitic therapy in patients with multiple parenchymal cysticerci.
• Garcia HH, Lescano AG, Gonzales I, et al. Cysticidal efficacy of combined treatment with praziquantel and albendazole for parenchymal brain cysticercosis. Clin Infect Dis. 2016;62(11):1375–9. This study confirms the improved cysticidal activity of combination antiparasitic therapy.
• Garcia HH, Gonzales I, Lescano AG, et al. Enhanced steroid dosing reduces seizures during antiparasitic treatment for cysticercosis and early after. Epilepsia. 2014;55(9):1452–9. This randomized trial demonstrated an improved clinical response when antiparasitic drugs were accompanied by higher doses of corticosteroids.
Bustos JA, García HH, Del Brutto OH. Antiepileptic drug therapy and recommendations for withdrawal in patients with seizures and epilepsy due to neurocysticercosis. Expert Rev Neurother 2016: 1–7.
Carpio A, Hauser WA. Prognosis for seizure recurrence in patients with newly diagnosed neurocysticercosis. Neurology. 2002;59(11):1730–4.
Nash TE, Bartelt LA, Korpe PS, Lopes B, Houpt ER. Calcified neurocysticercus, perilesional edema, and histologic inflammation. Am J Trop Med Hyg. 2014;90(2):318–21.
Fujita M, Mahanty S, Zoghbi SS, et al. PET reveals inflammation around calcified Taenia solium granulomas with perilesional edema. PLoS One. 2013;8(9), e74052.
Mejia R, Nash TE. Corticosteroid withdrawal precipitates perilesional edema around calcified Taenia solium cysts. Am J Trop Med Hyg. 2013;89(5):919–23.
Bianchin MM, Velasco TR, Wichert-Ana L, et al. Neuroimaging observations linking neurocysticercosis and mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsy Res. 2015;116:34–9.
Del Brutto OH, Salgado P, Lama J, et al. Calcified neurocysticercosis associates with hippocampal atrophy: a population-based study. Am J Trop Med Hyg. 2015;92(1):64–8.
Rathore C, Thomas B, Kesavadas C, Abraham M, Radhakrishnan K. Calcified neurocysticercosis lesions and antiepileptic drug-resistant epilepsy: a surgically remediable syndrome? Epilepsia. 2013;54(10):1815–22.
Proaño JV, Torres-Corzo J, Rodríguez-Della Vecchia R, Guizar-Sahagun G, Rangel-Castilla L. Intraventricular and subarachnoid basal cisterns neurocysticercosis: a comparative study between traditional treatment versus neuroendoscopic surgery. Childs Nerv Syst. 2009;25(11):1467–75.
Torres-Corzo J, Rodriguez-della Vecchia R, Rangel-Castilla L. Bruns syndrome caused by intraventricular neurocysticercosis treated using flexible endoscopy. J Neurosurg. 2006;104(5):746–8.
Sotelo J, Marin C. Hydrocephalus secondary to cysticercotic arachnoiditis. A long-term follow-up review of 92 cases. J Neurosurg. 1987;66(5):686–9.
Proano JV, Madrazo I, Avelar F, Lopez-Felix B, Diaz G, Grijalva I. Medical treatment for neurocysticercosis characterized by giant subarachnoid cysts. N Engl J Med. 2001;345(12):879–85.
Mitre E, Talaat KR, Sperling MR, Nash TE. Methotrexate as a corticosteroid-sparing agent in complicated neurocysticercosis. Clin Infect Dis. 2007;44(4):549–53.
•• Garcia HH, Gonzalez AE, Tsang VC, et al. Elimination of Taenia solium transmission in Northern Peru. N Engl J Med. 2016;374(24):2335–44. This study demonstrates the feasibility of elimination of Taenia solium transmission in a region in northern Peru using mass chemotherapy for human tapeworm carriers, mass chemotherapy of pigs, and vaccination of pigs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Drs Webb & White have no conflicts of interests to declare
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Additional information
This article is part of the Topical Collection on Central Nervous System Infections
Rights and permissions
About this article
Cite this article
Webb, C.M., White, A.C. Update on the Diagnosis and Management of Neurocysticercosis. Curr Infect Dis Rep 18, 44 (2016). https://doi.org/10.1007/s11908-016-0547-4
Published:
DOI: https://doi.org/10.1007/s11908-016-0547-4