Abstract
Objective
The purpose of this study was to evaluate the efficacy of traditional treatment and minimal invasive flexible endoscopy surgery (MIFNES) in the treatment of intraventricular and subarachnoid basal cisterns neurocysticercosis (NCC).
Methods
This was an observational comparative study of two independent series with a total of 140 patients with extremely severe forms of NCC from two different institutions. All 83 patients submitted for traditional treatment series received albendazole, and some of them received additionally praziquantel. Each cycle of both regimens lasted 4 weeks. The majority of these patients had at least one ventriculoperitoneal (VP) shunt. The rest 57 patients were submitted to the MIFNES treatment. The follow-up period was at least 6 months.
Results
In all patients of both series cysticercal cysts disappeared, became calcified, or were removed. Symptoms of 136 patients improved. Four patients died. The average in the quality of life measured using the Karnofsky scale improved from a mean of 52.22 and 52.44 at the beginning to 85.48 and 90.37 at 6 months (p < 0.003), in the traditional treatment and MIFNES series, respectively. From traditional treatment, almost all patients remained with at least one VP shunt, and from the MIFNES series only 12 patients.
Conclusions
The authors postulate that MIFNES is a good alternative for the management of intraventricular and subarachnoid basal cisterns NCC because it allows removal of most of the parasites, rapid recovery of the patients, and removal and placement of shunt under direct vision when necessary. Traditional treatment is a second option where the MIFNES procedure is not available.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neurocysticercosis (NCC) is the most frequent parasitic infection of the central nervous system (CNS) [32]; it is the major cause of seizures and hydrocephalus in adults in endemic regions, including Latin America, Asia, Africa, and Eastern Europe [1]. Recently, it has become more prevalent in North America because of emigration from endemic regions of the world [30]. Hydrocephalus is seen in approximately 30% of all NCC patients due to multiple mechanisms: mechanical cerebrospinal fluid (CSF) pathway obstruction, ventriculitis, and arachnoiditis. The larvae of the tapeworm Taenia solium arrives at the CNS through the choroidal plexus into the ventricular system, it may circulate freely throughout the CSF pathways, reaching the subarachnoid basal cisterns at some point. Cysticercus cellulosae is a form of cyst with a thin-wall measuring 4 to 20 mm in diameter with around 2 to 3 mm immature scolex, usually is located in the brain parenchyma, the subarachnoid, and intraventricular spaces. Cysticercus racemosus is a form multilobular grape-like cluster without scolex which size could rise several centimeters, most frequently located in the basal cisterns, Sylvian fissure, or ventricles. Overall, extraparenchymal NCC has a more aggressive behavior and a higher morbidity and mortality rate than parenchymal form [31]. In the prognostic classification for NCC of Estanol et al. [14], the authors include the ventricular and subarachnoid basal cisterns into the malignant subtypes [13].
Medical and surgical treatments have been proposed for intraventricular and subarachnoid basal cistern NCC. The ideal treatment remains controversial; some authors consider the medical treatment [13, 18, 23, 25, 26] and others, the surgical treatment [3–6, 9, 15, 26, 27, 36, 37]. Albendazole and praziquantel are the drugs used for the medical treatment. Cerebral endoscopy has been proposed by some authors [28, 36] as a very good surgical option for this type of NCC. Flexible cerebral endoscopy permits a full exploration and cyst extraction from the ventricular system and the subarachnoid basal cisterns, including the cisterna magna, and basal cisterns [35] in a minimally invasive manner. Currently, there are no studies that evaluate and compare the efficacy of either traditional treatment and minimally invasive with flexible neuroendoscopic surgery (MIFNES). The purpose of this study is to compare traditional versus neuroendoscopic surgery. For purpose of this paper, traditional treatment refers to the combination of ventriculoperitoneal (VP) shunt and pharmacological treatment.
Patients and methods
In the current study, we are reporting the results of the comparison of two independent series of patients with intraventricular and subarachnoid basal cisterns NCC that were treated either with traditional treatment or with MIFNES. Due to the enormous heterogeneity of the disease, we homogenize patient population in both series based on topographic locations by neuroimaging that include patients with intraventricular (lateral, third, and fourth ventricles) cysts, and/or patients with cysts in the basal cisterns (interpeduncular, prepontine, medial and lateral bulbar cisterns, cerebellopontine cisterns, anterior subarachnoid space of the cervical spine, cisterna magna, or posterior subarachnoid space of the cervical spine (Table 1)).
In this observational, retrospective study of two independent series from two different institutions, a total of 140 patients with intraventricular and subarachnoid basal cisterns NCC were seen between November 1995 and January 2005. The information was taken from medical records system, and if the record was incomplete, the patient was taken out of the study. The period of collection of the patients was the same in each clinic. The diagnosis was based on the presence of T. solium antibody in serum and/or CSF and neuroimaging. The traditional treatment group consisted of 83 patients treated at the Neurocysticercosis Clinic in the Hospital de Especialidades, Centro Medico Nacional Siglo XXI, in Mexico City, Mexico. In this institution, all patients were treated pharmacologically and with VP shunt if necessary. The MIFNES group consisted of 57 patients treated at the Neuroendoscopic Clinic, Department of Neurosurgery, Instituto Potosino de Neurociencias A.C. and Medical School of San Luis Potosi, San Luis Potosi, Mexico. This group was treated with a neuroendoscopic procedure only, there were no antiparasitic drugs involved in the management. We decided for this option due to several reasons. First of all, we wanted to evaluate the efficacy of a pure surgical management in this type of NCC where the main morbid-mortality is related to hydrocephalus. Second, it is very unclear the role of antiparasitic drugs in extraparenchymal NCC, and even more, there is evidence that the use of these drugs will exacerbate symptoms due to inflammatory response [21, 28].
The traditional group consisted of 42 men and 41 women 41 to 74 years of age (mean, 44.29). The MIFNES group consisted of 32 men and 25 women 9 to 79 years of age (mean, 39.37). The locations of the cysts are summarized in Table 1. The patient’s quality of life was measured by Karnofsky scale, with scores from 0 (which indicates death) to 100 (which indicate functional independence and lack of symptoms).
Results
Traditional treatment series
From the 83 patients of this group, 72 presented with intracranial hypertension, 68 of them due to hydrocephalus, and four due to mass effect. Thirteen patients had seizures, four of them as the unique symptom. From the total 83 patients, 58 had at least one VP shunt at some point. Thirty-three of them were shunted only once. Nineteen patients had one VP shunt placed in other hospital that failed, requiring one revision. Three patients had two previous VP shunt revision, requiring a third one. Other three patients had four, five, and six VP shunt revision requiring a new revision at the time they were seen in our clinic.
Minimally invasive with flexible neuroendoscopic surgery (MIFNES) series
From the total 57 patients, 55 had symptoms and signs of intracranial hypertension due to hydrocephalus. Ten patients had seizures. Twenty-one patients had a VP shunt at some point. Sixteen patients required one shunt revision, six patients required two shunt revisions, other two patients with three and four shunt revision each one; these VP shunt revision were done before the patients presented to our neuroendoscopic clinic. This group of patients never received medical treatment.
Imaging studies and laboratory tests
Diagnosis was made by computed tomography (CT) and/or magnetic resonance imaging (MRI; Fig. 1). The presence of antibodies against T. solium in serum and CSF was determined by Western blot analysis in all patients. Follow-up scanning with CT (Fig. 2) and/or MRI was performed at 2 and 6 months or until the lesions disappeared or became calcified. The criteria for treatment success based on imaging in the traditional group were evidence of the disappearance and/or calcification of the lesions and no hydrocephalus, and the criteria for treatment success in the MIFNES group was evidence of complete removal active cyst and no hydrocephalus.
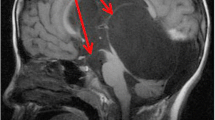
Intraventricular neurocysticercosis. A 42-year-old male patient with a cysticercal cyst in third ventricle (black arrow). a Axial gadolinium-enhanced spin-echo T1-weighted magnetic resonance imaging (MRI) scan before cerebral endoscopy. b An endoscopic view of the cysticercal cyst (black arrow). c An endoscopic view of the removal time of the cyst (white arrow) with a grasping forceps. d Axial MRI 1 month after the neuroendoscopic cyst removal
Subarachnoid basal cisterns neurocysticercosis. A 69-year-old male patient with unknown etiology hydrocephalus. a, d Contrast-enhanced axial computed tomography (CT) scans with lateral and third ventricle enlargement and transependymal edema. b, e Endoscopic views of the exploratory procedure. b Small cysticercal cyst (black arrow) next to the cerebellar tonsils (white arrowhead). e Shows a large parasite (black arrow) coming from the interpeduncular cistern through a third ventriculostomy (black arrowhead). c, f A contrast-enhanced axial CT scan after 6 months from the endoscopic procedure with normal size ventricles
Traditional treatment
Eighty-three patients received at least one course of albendazole at a dose of 15 mg per kilogram of body weight per day for 4 weeks. Intravenous dexamethasone was initiated at a dose of 8 mg every eight 8 h and tapered down until the patient had received full antiparasitic treatment. Thirty-one patients received two cycles; six received three cycles. The criterion for giving a patient an additional course of albendazole was a partial or incomplete response. Fourteen patients who did not have a response to albendazole received one course of 100 mg of praziquantel per kilogram per day in two divided doses accompanied by the same time of corticosteroid treatment. Fifty-eight of these patients developed hydrocephalus requiring a VP shunt. Other five patients required emergent craniotomy for giant supratentorial cyst resection before medical treatment.
MIFNES treatment
All patients from the MIFNES group were operated at the time they presented in the emergency room with shunt malfunction and/or hydrocephalus. At this point, patients had enlarged ventricles, making the ventricular endoscopic navigation easy to perform.
Operative procedure
Under general anesthesia, the patient was placed supine with the head bent slightly at 30°. The access was through a pre-coronal burr-hole in the right midpupillary line, identification of this point is critical to prevent damage to the structures while approaching the third ventricle and the cerebral aqueduct. The dura was opened in a cruciate fashion. The ventricle was cannulated with a ventricular needle; CSF sample was taken for antibody analysis. A plastic sheath “peel away” (Codman, Johnson & Johnson, Austin, TX, USA) was placed to allow CSF flow out of the ventricles in order to avoid intracranial hypertension due to continuous irrigation and to protect the brain parenchyma from multiple scope insertions. Ventricular exploration began in the anterior horn of the lateral ventricle. Cysticercal cysts in the right lateral ventricle were found and extracted in 23 patients. Navigating into the contralateral ventricle through a septum pellucidum fenestration (septostomy), cysts in this other lateral ventricle were found and extracted in 18 patients. After a full exploration of the lateral ventricles, the scope was passed through the foramen of Monro into the third ventricle; at this point, cysts were found and extracted in 22 patients. Once the cysts from the third ventricle were removed, the fourth ventricle was approached through a transaqueductal approach; cysticercal cysts were found and extracted in 19 patients (Fig. 3a). During the transaqueductal approach, cysticercal cysts were found in the cerebral aqueduct in nine patients. Great care was taken to avoid injury to the ventricular surfaces with the endoscope or instruments, a delicate and slow clockwise rotation maneuver was performed to introduce the flexible endoscope through the cerebral aqueduct; 12 patients had aqueductal stenosis that required aqueductoplasty. Once in the fourth ventricle, the endoscope was advanced following the midline to reach the cisterna Magna through the Magendie foramen. In nine patients, cysts were removed from cisterna magna (Fig. 3b, d) through the Magendie foramen (Fig. 3e). Magendie foramen was occluded in ten patients, a surgical opening (Magendie foraminoplasty) was performed in such cases. In case the cysts were small (<5 mm), they were removed en bloc, larger cysts were deflated and collapsed in order to extract them from this narrow locations. Most of the cysts were removed through the working channel; in some cases, the entire endoscope needed to be removed. When withdrawing the endoscope from the fourth ventricle through the cerebral aqueduct, a counterclockwise rotation maneuver was done to avoid damage to the surrounding structures. In all patients, all cysticercal cysts that were visualized with the endoscope were removed. Continuous irrigation with lactate Ringer’s solution was used during the entire procedure to clear the cloudiness of the CSF.
Endoscopic images of different kinds of neurocysticercosis. a A cysticercal cyst extraction from the cerebral aqueduct. b A large cysticercal cyst (large black arrow) in the cisterna magna evolving the right posteroinferior cerebellar artery (PICA; arrowhead) removed with a grasping forceps (white arrow). c A cysticercal cyst in the interpeduncular cistern viewed from the third ventricle through an ETV. d Three small and one large cysticercal cysts in the cisterna magna. e A single giant cysticercal cyst (large arrow) behind the right PICA (arrowhead) seen through the Magendie foramen with inflammatory adherences (small arrow). f A typical racemosus cysticercus in the prepontine cistern
Interventions performed during the endoscopy
To achieve a full exploration of the ventricles and subarachnoid space, other endoscopic procedures were performed. By far, the most common was endoscopic third ventriculostomy (ETV), which was performed in 54 patients (Fig. 3c). The objectives of ETV were open an access to remove parasites of the anterior basal cisterns and re-establish the CSF circulation in case of aqueductal stenosis. In three cases, an ETV was not technically possible to perform, a lamina terminalis fenestration was done instead. In 21 patients, cysts were extracted from the subarachnoid basal cisterns (Fig. 3f). A careful exploration of the basal cisterns was done, starting at the interpeduncular cistern and extending caudally to the upper levels of the cervical spine subarachnoid space. (Figs. 1, 2, and 3).
Clinical follow-up
The follow-up period for the clinical and surgical series was 6 months, although some patients were evaluated 12 and 24 months after the treatment.
Traditional treatment group
One patient died as a consequence of fourth ventricle exploration by open craniotomy due to trapped fourth ventricle 4 months after the second course of albendazole. During the first 6 months after the first albendazole treatment, seven patients required a VP shunt placement and/or revision. Other complications were VI cranial nerve palsy in two patients, visual impairment in 12 patients most likely due to intracranial hypertension, and two patients experienced hemiparesis due to stroke, possible related to cysticercal vasculitis.
MIFNES group
Three patients died: a 79-year-old man secondary to postoperative myocardial infarction and a 54- and 37-year-old man secondary to VP shunt-related meningitis. Twenty-one patients of this group had a VP shunt at the time they were seen in our neuroendoscopic clinic for the first time; in 15 of them, VP shunt was removed definitely after the neuroendoscopic intervention. Other six patients required a new VP shunt in the fourth ventricle to treat trapped fourth ventricle. Ten patients of the surgical series had seizures with good response to antiepileptic treatment. The sequels of the MIFNES series were VI cranial nerve palsy in one patient and bilateral partial optic atrophy in other patient. One patient developed hemiparesis due to previous stroke secondary to cysticercal vasculitis.
Karnofsky scale evaluation
The quality of life was evaluated with the Karnofsky scale [16] (Fig. 4) in all patients before and 6 months after the treatment. In the medical treatment group and MIFNES group, the Karnofsky scale mean was 52.44 (SD ± 14.62) and 52.22 (SD ± 11.97), respectively, before treatment, and 85.48 (SD ± 9.95) and 90.37 (SD ± 8.89) after treatment (p < 0.003). Some patients had a longer follow-up period. In the medical group, 79 patients were evaluated at 12 months and 59 at 24 months; in the MIFNES group, 47 patients were evaluated at 12 months and 17 at 24 months.
Karnofsky scale (KS) evaluation. Before treatment, the KS was similar on both series, 52.44 and 52.22 for the medical and surgical series, respectively. Six months after treatment, the KS was 85.48 and 90.37 for the medical and surgical series, respectively, (p < 0.003). Twelve months after, the KS was 82.95 and 94.02 for the medical and series, respectively. Twenty-four months after, the KS was 87.23 and 95.86 for the medical and surgical series, respectively. Note: not all the patients were evaluated after 12 and 24 months
Discussion
Extraparenchymal NCC refers primarily to infection of the ventricles and subarachnoid spaces. Clinical presentation and management differ from parenchymal infection, and generally, the prognosis of these patients is worse. Anti-helminthic therapy may have some benefit in the treatment of these conditions [24, 25]. Primary medical treatment of extraparenchymal NCC has not gained universal acceptance. The most persuasive argument against treatment with anti-helminthic therapy alone is that, until the cyst disappears, the patient is still at risk of life-threatening complications [5]. Nash et al. [22], Agapejev et al. [1], Proano et al. [24, 25], Allcut and Coulthard [2], Joubert and van As [18], and Del Brutto et al. [13] had reported good results for the pesticide treatment with different albendazole and praziquantel regiments for intraventricular and subarachnoid cysticercosis. Neurosurgical procedures for NCC are still part of the armamentarium when treating this disease. Colli et al. [7, 8], Stern [34], Soto Hernandez et al. [33], and Madrazo et al. [20] among others, had published good results for open craniotomy and rigid endoscopic surgery in patients with intraventricular and subarachnoid basal cistern NCC. Other author’s reports as Citow et al. [6], Cuetter and Andrews [10], Gravori et al. [15], Psarros et al. [26, 27], Zymberg et al. [37], Anandh et al. [3], Bergsneider and Bergsneider et al. [4, 5], Cudlip et al. [9], and Rangel-Castilla et al. [28] are related to minimally invasive neurological surgery through cerebral endoscopy, focus on technical aspects, and report only a few anecdotal cases, each one of them. Some of these authors concluded that future studies need to be undertaken to assess the efficacy of this procedure [28]. Currently, there is no study that compares MIFNES treatment versus traditional treatment for intraventricular and basal cisterns NCC in order to determine which of both procedures is the most recommendable.
To our knowledge, we present the largest comparison of traditional treatment and cerebral endoscopy for the treatment of intraventricular and basal subarachnoid cisterns NCC. Both series show a similar distribution in the means of the Karnofsky quality of life scale before treatments. After 6 months of the follow-up period, better outcome was seen in the MIFNES series with statistical significance (p < 0.003).
Success in avoiding a CSF shunt in the MIFNES group
There is ample evidence that external shunts are prone to complications in such patients, and many of them die due to repeated shunt revisions and ventricular ependymitis [19]. In our study, an important difference between groups was that in the MIFNES series, from 21 patients that had a VP shunt at admission time, only six of them remained with them; in the other 15 patients, the VP shunt was definitely removed after the neuroendoscopic procedure. When shunts are associated with hydrocephalus from cysticercosis, the complications are even higher [19]. In a report by Colli et al. [7], 82% of his patients needed a revision due to malfunction. Our experience confirms that of other, the cyst itself can get sucked into the ventricular catheter, thereby obstructing it. Moreover, it appeared that a VP shunt could actually draw cysts from the posterior fossa into the supratentorial ventricular system. The endoscopic exploration of the ventricles also revealed the existence of a lacy, mucoid material surrounding degenerating cysts. We suspect that this material and the debris could account for some of the VP shunt obstruction in patients undergoing a shunt placement without cyst removal; most likely, this was the case on those patients that were referred to our institution after they had a VP shunt in other institution.
Physiological diversionary procedures such as ETV, aqueductoplasty, septostomy, and Monro and Magendie foraminoplasty are not associated with overdrainage, slit ventricles syndrome, intracranial hypotension, subdural hematomas, craniosynostosis, and microcephaly, which can often accompany the VP shunt. The ease of performing ETV make us consider that it should be done in all cases with intraventricular and basal subarachnoid cisterns NCC subjected to endoscopic surgery, because of the possibility of future inflammation and scarring with stenosis of the cerebral aqueduct and/or Luschka and Magendie foramen leading to hydrocephalus. Extraction of cysts through the cerebral aqueduct in patients with NCC from the fourth ventricle may be criticize by some non-familiarized neurosurgeons with the flexible scope; in reality, the aqueduct is widened in these patients making it safe to navigate and retrieve the cysts from this region. When the diameter of the cysticercal cyst overpass the diameter of the cerebral aqueduct, we evacuated the content using puncture needles and suction cannulas before attempting to remove the cyst, despite this rupture, postoperatively, ventricular ependymitis was not seen. Continuous irrigation during the endoscopic exploration helps in removing the debris and provides clear vision. It is true that using flexible endoscope provides enhanced navigation at cost of image quality; in our experience, this was not an issue when the ventricular anatomy is perfectly well known and the neuroendoscopist is well oriented. As published before in Husain et al. [17], transaqueductal passage of flexible endoscope provides a better view of the fourth ventricle and the cyst in situ and, as previously mentioned, an experienced neuroendoscopist with the flexible and steerable endoscope, the risk of brain damage is minimal. Schroeder et al. described an exploration across the aqueduct with a 2.5-mm flexible neuroendoscope in a scope-in-scope technique with the same outcome [29]. Also, in our experience, the instruments for the flexible endoscope have the appropriate characteristics to extract cysts, specially from difficult regions including the basal cisterns and fourth ventricle. Fourth ventricle trapped is a common condition in patients with NCC and inflammatory conditions [12, 23, 27]; based on this, we also found an additional benefit seen in the current study was the possibility of placement under direct vision a new VP shunt in the fourth ventricle in order to treat trapped fourth ventricle in the same surgery time, as seen in six cases of the MIFNES series.
All patients in the traditional series remained with VP shunt during and after the treatment. Five patients of the medical series required a single VP shunt change and, in two patients, a new VP shunt was placed during treatment, because hydrocephalus developed during this period. This is another important difference between the two series: hydrocephalus remains as a life-threatening condition due to high VP shunt dysfunction rate, and we have to remark that hydrocephalus had been reported as the most ominous condition for long outcome of life and functional prognosis [11, 19, 21, 28].
Complications may arise when an endoscopic procedure is performed. The major risk is hemorrhage caused by a vessel injury, specially the basilar artery, or one of its branches. In patients with hydrocephalus, the floor of the third ventricle usually becomes thin and is bowed caudally and the floor appears thin and translucent when observed with the endoscope, making easy the identification of the basilar artery. It is important to have in mind that the presence of cysticercal cyst in the interpeduncular cisterns may distort the regular anatomy. Intraoperative bradycardia is not uncommon, especially when an endoscopic dissection is performed and cysts from the prepontine cistern are being extracted, bradycardia responds immediately after the scope is withdrawn from this region. Hypothalamic dysfunction can cause transient high fever unrelated to infection, it can be relieved easily with the administration of acetaminophen. Other uncommon complications that have been reported include infection, transient inappropriate antidiuretic hormone secretion, midbrain injury, and transient nerve palsy. All these complications can be avoided by meticulous attention to technique and detailed knowledge of the endoscopic anatomy of the ventricles and subarachnoid basal cisterns.
In previous reports, a rigid scope was used to approach the lateral and the third ventricle; however, the authors stated that the assessment of the occipital horn and the posterior portion of the third ventricle including the entrance of the cerebral aqueduct were challenging, and a flexible endoscope would be more appropriate to explore the entire ventricular system [28].
Conclusions
Considering that previous good results have been reported in this extremely malignant form of intraventricular and subarachnoid basal cisterns NCC in series for cysticidal treatment and anecdotal cases for MIFNES treatment, the results of our current study, comparing both therapeutic procedures, demonstrate that our present large series of patients submitted to MIFNES had a better outcome after a follow-up period of 6 months with a reasonable complication and mortality rates, let us to recommend this surgical option as the treatment of choice but is restricted to high specialty hospitals where there is a formal service of neuroendoscopy and an expert surgical team. Neuroendoscopic surgery is the modality of choice for the treatment of intraventricular and subarachnoid basal cisterns NCC with and without hydrocephalus; it restores CSF, lead to complete removal of the cysts, and an additional benefit is to have a shunt-free patient. Intraoperative rupture of the cysts has no negative sequel on the final outcome. In places where this hi-tech surgical option is not available, as it occurs in the large proportion of the endemic developing countries, traditional treatment still remains as a good alternative treatment of choice.
References
Agapejev S, Da Silva MD, Ueda AK (1996) Severe forms of neurocysticercosis: treatment with albendazole. Arq Neuropsiquiatr 54:82–93
Allcut DA, Coulthard A (1991) Neurocysticercosis: regression of a fourth ventricular cyst with praziquantel. J Neurol Neurosurg Psychiatry 54:461–462
Anandh B, Mohanty A, Sampath S, Praharaj SS, Kolluri S (2001) Endoscopic approach to intraventricular cysticercal lesions. Minim Invasive Neurosurg 44:194–196
Bergsneider M (1999) Endoscopic removal of cysticercal cysts within the fourth ventricle. Technical note. J Neurosurg 91:340–345
Bergsneider M, Holly LT, Lee JH, King WA, Frazee JG (2000) Endoscopic management of cysticercal cysts within the lateral and third ventricles. J Neurosurg 92:14–23
Citow JS, Johnson JP, McBride DQ, Ammirati M (2002) Imaging features and surgery-related outcomes in intraventricular neurocysticercosis. Neurosurg Focus 12:e6
Colli BO, Martelli N, Assirati JA Jr, Machado HR, de Vergueiro FS (1986) Results of surgical treatment of neurocysticercosis in 69 cases. J Neurosurg 65:309–315
Colli BO, Martelli N, Assirati Junior JA, Machado HR, Salvarani CP, Sassoli VP, Forjaz SV (1994) Cysticercosis of the central nervous system. I. Surgical treatment of cerebral cysticercosis: a 23 years experience in the Hospital das Clinicas of Ribeirao Preto Medical School. Arq Neuropsiquiatr 52:166–186
Cudlip SA, Wilkins PR, Marsh HT (1998) Endoscopic removal of a third ventricular cysticercal cyst. Br J Neurosurg 12:452–454
Cuetter AC, Andrews RJ (2002) Intraventricular neurocysticercosis: 18 consecutive patients and review of the literature. Neurosurg Focus 12:e5
DeFeo D, Foltz EL, Hamilton AE (1975) Double compartment hydrocephalus in a patient with cysticercosis meningitis. Surg Neurol 4:247–251
DeFeo D, Foltz EL, Hamilton AE (1975) Double compartment hydrocephalus in a patient with cysticercosis meningitis. Surg Neurol 4:247–251
Del Brutto OH, Sotelo J, Aguirre R, Diaz-Calderon E, Alarcon TA (1992) Albendazole therapy for giant subarachnoid cysticerci. Arch Neurol 49:535–538
Estanol B, Corona T, Abad P (1986) A prognostic classification of cerebral cysticercosis: therapeutic implications. J Neurol Neurosurg Psychiatry 49:1131–1134
Gravori T, Steineke T, Bergsneider M (2002) Endoscopic removal of cisternal neurocysticercal cysts. Technical note. Neurosurg Focus 12:e7
Grieco A, Long CJ (1984) Investigation of the Karnofsky performance status as a measure of quality of life. Health Psychol 3:129–142
Husain M, Jha DK, Rastogi M, Husain N, Gupta RK (2007) Neuro-endoscopic management of intraventricular neurocysticercosis (NCC). Acta Neurochir (Wien) 149:341–346
Joubert J, van As AD (1990) Rapid and complete resolution of giant cysticercal cysts after administration of praziquantel. A report of 4 cases. S Afr Med J 77:154–157
Kelley R, Duong DH, Locke GE (2002) Characteristics of ventricular shunt malfunctions among patients with neurocysticercosis. Neurosurgery 50:757–761
Madrazo I, Garcia-Renteria JA, Sandoval M, Lopez Vega FJ (1983) Intraventricular cysticercosis. Neurosurgery 12:148–152
Nash TE, Singh G, White AC, Rajshekhar V, Loeb JA, Proano JV, Takayanagui OM, Gonzalez AE, Butman JA, DeGiorgio C, Del Brutto OH, Delgado-Escueta A, Evans CA, Gilman RH, Martinez SM, Medina MT, Pretell EJ, Teale J, Garcia HH (2006) Treatment of neurocysticercosis: current status and future research needs. Neurology 67:1120–1127
Nash TE, Singh G, White AC, Rajshekhar V, Loeb JA, Proano JV, Takayanagui OM, Gonzalez AE, Butman JA, DeGiorgio C, Del Brutto OH, Delgado-Escueta A, Evans CA, Gilman RH, Martinez SM, Medina MT, Pretell EJ, Teale J, Garcia HH (2006) Treatment of neurocysticercosis: current status and future research needs. Neurology 67:1120–1127
Prasad S, MacGregor RR, Tebas P, Rodriguez LB, Bustos JA, White AC Jr (2006) Management of potential neurocysticercosis in patients with HIV infection. Clin Infect Dis 42:e30–e34
Proano JV, Madrazo I, Garcia L, Garcia-Torres E, Correa D (1997) Albendazole and praziquantel treatment in neurocysticercosis of the fourth ventricle. J Neurosurg 87:29–33
Proano JV, Madrazo I, Avelar F, Lopez-Felix B, Diaz G, Grijalva I (2001) Medical treatment for neurocysticercosis characterized by giant subarachnoid cysts. N Engl J Med 345:879–885
Psarros T, Zouros A, Coimbra C (2003) Bromocriptine-responsive akinetic mutism following endoscopy for ventricular neurocysticercosis. Case report and review of the literature. J Neurosurg 99:397–401
Psarros TG, Krumerman J, Coimbra C (2003) Endoscopic management of supratentorial ventricular neurocysticercosis: case series and review of the literature. Minim Invasive Neurosurg 46:331–334
Rangel-Castilla L, Serpa JA, Gopinath SP, Graviss EA, az-Marchan P, White AC Jr (2009) Contemporary neurosurgical approaches to neurocysticercosis. Am J Trop Med Hyg 80:373–378
Schroeder HW, Oertel J, Gaab MR (2004) Endoscopic aqueductoplasty in the treatment of aqueductal stenosis. Childs Nerv Syst 20:821–827
Schultz TS, Ascherl GF Jr (1978) Cerebral cysticercosis: occurrence in the immigrant population. Neurosurgery 3:164–169
Shandera WX, Kass JS (2006) Neurocysticercosis: current knowledge and advances. Curr Neurol Neurosci Rep 6:453–459
Shandera WX, White AC Jr, Chen JC, Diaz P, Armstrong R (1994) Neurocysticercosis in Houston, Texas. A report of 112 cases. Medicine (Baltimore) 73:37–52
Soto Hernandez JL, Ostrosky ZL, Tavera G, Gomez AA (1996) Neurocysticercosis and HIV infection: report of two cases and review. Surg Neurol 45:57–61
Stern WE (1981) Neurosurgical considerations of cysticercosis of the central nervous system. J Neurosurg 55:382–389
Torres-Corzo J, Vecchia RR, Rangel-Castilla L (2005) Observation of the ventricular system and subarachnoid space in the skull base by flexible neuroendoscopy: normal structures. Gac Med Mex 141:165–168
Torres-Corzo J, Rodriguez-della VR, Rangel-Castilla L (2006) Bruns syndrome caused by intraventricular neurocysticercosis treated using flexible endoscopy. J Neurosurg 104:746–748
Zymberg ST, Paiva Neto MA, Gorgulho AA, Cavalheiro S (2003) Endoscopic approach to fourth ventricle cysticercosis. Arq Neuropsiquiatr 61:204–207
Acknowledgments
We are indebted to Dr. Israel Grijalva for his critical reading of the manuscript and to Margarita Jimenez and Olga Elias for their invaluable assistance in the statistical analysis of the data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Proaño, J.V., Torres-Corzo, J., Rodríguez-Della Vecchia, R. et al. Intraventricular and subarachnoid basal cisterns neurocysticercosis: a comparative study between traditional treatment versus neuroendoscopic surgery. Childs Nerv Syst 25, 1467–1475 (2009). https://doi.org/10.1007/s00381-009-0933-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-009-0933-4