Abstract
Purpose of Review
Heart failure with preserved ejection fraction mainly affects the elderly. The obesity phenotype of heart failure with preserved ejection fraction reflects the coexistence of two highly prevalent conditions in the elderly. Obesity may also lead to heart failure with preserved ejection fraction in middle-aged persons, especially in African American women.
Recent Findings
Obesity is twice as common in middle-aged than in elderly persons with heart failure with preserved ejection fraction. Obese middle-aged persons with heart failure with preserved ejection fraction are less likely to be Caucasian and to have atrial fibrillation or chronic kidney disease as comorbidities than elderly patients with heart failure with preserved ejection fraction. Obesity-associated low-grade systemic inflammation may induce/heighten inflammatory activation of the coronary microvascular endothelium, leading to cardiomyocyte hypertrophy/ stiffness, myocardial fibrosis, and left ventricular diastolic dysfunction.
Summary
Both substantial weight reduction with bariatric surgery and lesser levels of weight reduction with caloric restriction are promising therapeutic approaches to obesity-induced heart failure with preserved ejection fraction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The obesity epidemic is leveling off in men but continues to progress in women [1]. Furthermore, the prevalence of morbid obesity (body mass index [BMI] ≥ 40 kg/m2) is growing at a faster rate than that of obesity as a whole [2]. Obesity and morbid obesity are associated with changes in left ventricular (LV) structure and function, most commonly concentric remodeling and diastolic dysfunction [3,4,5,6]. LV concentric remodeling and diastolic dysfunction (LVDD) are the underpinnings of heart failure with preserved ejection fraction (HFpEF). A likely corollary of the obesity epidemic is the increasing incidence of HFpEF, particularly in women and African Americans [7, 8••, 9]. We review how obesity, female sex, and African American ethnicity may affect the incidence and outcome of HFpEF. We then consider how obesity fosters the development of HFpEF and surgical versus non-surgical approaches to weight reduction.
Obesity and Heart Failure with Preserved Ejection Fraction
Increases in BMI and waist circumference (WC) are associated with increased risk of heart failure (HF). The relative risk of HF incidence is 1.41 for each 5 kg/m2 increment in BMI and 1.29 for each 10 cm increase in WC [10]. The cumulative burden of increasing BMI and blood pressure (BP) from childhood to adulthood is associated with the development of LV hypertrophy (LVH), and the association is stronger for BMI than BP [11]. Young obese women who are otherwise healthy have a greater LV mass and relative wall thickness than non-obese women [12]. After adjustment for age, systolic BP, and type 2 diabetes (T2D), LV mass/height2.7 and LV mass/fat-free mass ratio are greater in obese women than in obese men [13]. The association between obesity and LVDD was first reported 15 years ago [14]. High BMI correlated with greater LV mass, wall thickness, and filling pressure and not with LV ejection fraction (LVEF) in patients free of obstructive coronary artery disease (CAD) [14]. High BMI also increases the risk of LVDD in metabolically healthy obese women free from dyslipidemia, HTN, and T2D [15]. Obesity confers a particularly high risk of HFpEF in women and is more closely associated with the incidence of HFpEF than HF with reduced ejection fraction (HFrEF) in both sexes [8••]. The attributable risk of HFpEF is estimated to range from 44 to 49% when BMI is ≥ 27.5 kg/m2 and WC > 100 cm in men and > 94 cm in women [16]. Of major pathogenic importance, the association between BMI and LVDD is independent of confounding risk factors. Increased BMI was associated with worse LVDD independent of LVH, HTN, T2D, and obstructive sleep apnea (OSA) in 950 participants in the Cardiovascular Abnormalities and Brain Lesions study [17].
Sex and Heart Failure with Preserved Ejection Fraction
Nearly two-thirds of the 40,354 Americans hospitalized for HFpEF from 2005 to 2010 were elderly women with a mean age of 78 years [18]. More recently, 55% of 9957 patients enrolled in the Swedish Heart Failure registry for HFpEF were elderly women (mean age 79 years) [19••]. Women have smaller LV dimensions after adjustment for height than men [20]. Obese women have greater LV mass/height2.7 and LV mass/fat-free mass ratios than non-obese women after adjustment for age, systolic BP, and T2D [13]. The increase in LVDD in women compared with men was unrelated to differences in systolic arterial-ventricular coupling in the prospective comparison of angiotensin receptor neprilysin inhibition (ARNI) with angiotensin receptor blockade (ARB) on the management of Heart FailUre with preserved ejectiOn fracTion (Paramount) trial [21]. However, not all investigators have found that women are at greater risk of developing HFpEF than men [22, 23]. After adjustment for age, BP, BMI, antihypertensive treatment, and previous myocardial infarction, women and men have been shown to be at similar risk for HFpEF [24]. Despite conflicting data, HFpEF is generally believed to be more prevalent, with a nearly twofold greater prevalence, in women than in men [25]. The 2018 statistical report from the American Heart Association indicates that HFpEF is the most common form of HF in women, has a similar incidence in women and men, but is more prevalent in women as they live longer than men [26].
African Americans and Heart Failure with Preserved Ejection Fraction
LV mass and incidence of LVH are significantly greater in African Americans than in Caucasians after adjustment for fat mass, fat-free mass, systolic BP, age, and socioeconomic status [27]. The greater impairment in microvascular endothelial function and increases in arterial wave reflection and stiffness in African Americans compared with Caucasians likely contribute to the higher prevalence of LVH in African Americans [28]. Furthermore, the prevalence of obesity in general and morbid obesity in particular is high in African Americans, reaching 54 and 52.9%, respectively, in patients enrolled in the Jackson Heart Study from 2000 to 2013 [29]. Morbid obesity is particularly more severe in African American than in Caucasian women [30].
The most common form of HF was HFpEF in middle-aged African Americans who, enrolled in the Jackson Mississippi cohort of Atherosclerosis Risk in Communities (ARIC) study, underwent echocardiography in 1993–1995 and were followed for 13.7 years [9]. Obesity was a greater risk for HFpEF in African American than in Caucasian women after adjustment for HTN and T2D in a multi-racial cohort of 42,170 post-menopausal women [31]. However, African Americans and Caucasians had an equal lifetime risk of HFpEF in 2 large prospective cohorts: The cardiovascular health study (CHS) and the multi-ethnic study of atherosclerosis (MESA) [22]. It is important to note that both African American men and women are generally under-represented in multi-racial clinical studies. The percentage of African Americans enrolled is commonly < 20% in multi-racial studies, even when they are conducted in states where African Americans represent ≥ 40% of the population [32, 33]. Thus, whether the abovementioned multi-racial studies accurately capture the clinical profile of HFpEF in African Americans remains to be determined.
Data regarding HFpEF outcomes in African Americans are also controversial. Observational studies indicate that African Americans have worse outcomes than Caucasians [32, 33]. African Americans had a significantly higher 5-year mortality than Caucasians after adjustment for known risk factors in the cohort of 3303 HFpEF patients from the Duke Cardiovascular Databank [32]. However, analysis of administrative data (nationwide inpatient sample and Medicare) does not support an outcome difference between African Americans and Caucasians with HF/HFpEF [34, 35]. Administrative data are valuable for evaluation of adherence to guidelines and collection of clinical information after hospitalizations, but may be less useful for assessing the clinical course of chronic conditions.
Pathogenesis of Obesity-Induced Heart Failure with Preserved Ejection Fraction
As obesity progresses, visceral adipose tissue (VAT) accumulates, becomes inflamed, and results in low-grade systemic inflammation. Low-grade systemic inflammation then activates inflammatory processes in the coronary microvascular endothelium, leading to impaired cardiomyocyte relaxation, increased myocardial stiffness, and LVDD [36, 37••].
Accumulation of Visceral Adipose Tissue
VAT refers to the intra-abdominal accumulation of mesenteric and omental AT [38]. An enlarged WC clinically signals an increased VAT mass [39]. In the Dallas Heart Study, VAT mass, measured by magnetic resonance imaging (MRI) at the L2-L3 intervertebral level, was associated with LV concentric remodeling [40]. The association between VAT and LV concentric remodeling is independent of age, sex, ethnicity, and obesity status and is stronger in women than in men [41, 42]. Eleven-year follow-up of 1806 MESA participants revealed that VAT and not subcutaneous AT (SAT) mass was independently associated with hospitalization for incident HFpEF [43]. In the Treatment Of Preserved CArdiac function Heart Failure with an aldosterone antagonist (TOPCAT) Trial, men with WC ≥ 102 cm and women with WC ≥ 88 cm were at higher risk of all-cause mortality than patients with normal range WC [44••]. The association of central obesity with fatal outcomes in HFpEF points to a major role for VAT in the pathogenesis of HFpEF.
Visceral Adipose Tissue and Low-Grade Systemic Inflammation
Accumulation of VAT over time leads to VAT inflammation and low-grade systemic inflammation, as evidenced by elevated plasma levels of C-reactive protein (CRP), interleukin (IL)-6, and tumor necrosis factor (TNF)-α [45,46,47]. The association between the amount of VAT and elevated markers of inflammation is independent of other measures of obesity [48]. Accumulation of VAT initially results in an anti-inflammatory response with the release of adipokines and the recruitment of alternatively activated macrophages (M2) that facilitates VAT expansion [49, 50]. Continued adipocyte hypertrophy leads to leptin release, monocyte chemotactic protein 1 (MCP 1) expression, mechanical stress, hypoxia, and cell death, with production of pro-inflammatory adipokines that promote proliferation and AT infiltration of classically activated macrophages (M1) [50,51,52,53]. Circulating markers of inflammation like CRP and IL-6 do not reliably reflect the degree of VAT inflammation or low-grade systemic inflammation. CRP correlates more closely with SAT than with VAT, and only 30% of IL-6 originates from AT [54]. Truncal fat contributes to higher CRP in women than in men [54, 55]. Profiling circulatory cytokines may help assess VAT-related systemic inflammation and metabolic health in obese African American women [56].
Coronary Microvascular Inflammation
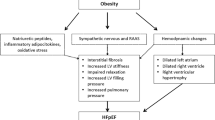
Inflammatory activation of the coronary microvascular endothelium leads to reduced cardiomyocyte elasticity/function and increased myocardial stiffness/fibrosis through the activation of two signaling pathways [37••]. In the first pathway, endothelial expression of adhesion molecules enables infiltration of inflammatory cells, uncouples endothelial nitric oxide synthase {eNOS), reduces NO bioavailability and decreases soluble guanylate cyclase (sGC) stimulation, thereby reducing the activity of cyclic guanosine monophosphate (cGMP) and protein kinase G (PKG) [37••, 57]. Low PKG activity promotes cardiomyocyte hypertrophy and titin hypo-phosphorylation that increase passive LV stiffness (Fig. 1) [36]. The second pathway targets suppression of the unfolded protein response that may lead to interstitial accumulation of destabilized proteins [58]. Microvascular endothelial inflammation is associated with increased expression of inducible NOS (iNOS) and reduces the activity of proteins involved in the unfolding response [58]. In addition to activation of these signaling pathways, coronary microvascular rarefaction and a Sirtuin (SIRT) 3–dependent defect in endothelial cell metabolic programming and angiogenesis may affect the progression of perivascular and myocardial fibrosis in HFpEF [59,60,61].
As obesity progresses, visceral adipose tissue (VAT) inflammation promotes low-grade systemic inflammation as evidenced by elevated plasma levels of C-reactive protein (CRP), interleukin (IL)-6, and tumor necrosis factor (TNF)-α. Systemic inflammation induces/heightens inflammatory activation of the coronary microvascular endothelium with increased expression of vascular cell adhesion molecule (VCAM) and intracellular adhesion molecule (ICAM-1). Enhanced release of transforming growth factor (TGF)-β by monocytes promotes conversion of fibroblasts to myofibroblasts and myocardial deposition of collagen. Increased macrophage expression of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase2 (NOX2) stimulates production of hydrogen peroxide (H2O2). Decreased nitric oxide (NO) bioavailability and oxidative stress reduce soluble guanylate cyclase (sGC) stimulation, thereby lowering cyclic guanosine monophosphate (cGMP) and protein kinase G (PKG) activity. Low PKG activity leads to cardiomyocyte hypertrophy and titin hypo-phosphorylation that, with increased interstitial myocardial collagen deposition, impairs LV relaxation and increases LV stiffness
Obese Phenotypes of Heart Failure with Preserved Ejection Fraction
Obesity and HFpEF are extremely prevalent conditions. As the prevalence of both obesity and HFpEF continues to rise, the two conditions are increasingly likely to coexist in elderly patients, especially in post-menopausal women [7, 62, 63••]. The average BMI of patients in the Irbesartan in heart failure with PRESERVEd ejection fraction (I-PRESERVE) trial was 29.6 kg/m2,, with 41.4% of participants having a BMI > 30 kg/m2 [64]. The average age in I-PRESERVE was 71.6 years, and 29.4% of participants were older than 75 years. The mean age of patients hospitalized for HFpEF from 2005 to 2010 was 78 years, and the mean age of women with HFpEF in the recent Swedish HF registry is 79 years [18, 19••].
Obesity and Heart Failure with Preserved Ejection Fraction in Elderly Patients
Obesity has been shown to increase height-adjusted right ventricle (RV) dimensions in women with increased RV ejection fractions and in men with increased RV volumes [65]. Extremely obese patients are more likely to develop impaired systolic and diastolic cardiac function and pulmonary hypertension [66]. RV prominence, interventricular interaction and increased systemic inflammation characterize the coexistence of obesity and HFpEF i.e. the obese phenotype of HFpEF in the elderly [67, 68].
Obesity and Heart Failure with Preserved Ejection Fraction in Middle-Aged Patients
The prevalence of obesity was much greater in middle-aged (age < 55 years) than in older (age ≥ 75 years) HFpEF patients in the multinational registry of Asian patients with HF (ASIAN-HF): 36 versus 16% [69••]. Middle-aged patients with HFpEF were more likely to be obese and less likely to be Caucasian in the Candesartan Cilexetil in heart failure assessment and reduction of mortality and morbidity (CHARM Preserved), TOPCAT, and in I-PRESERVE trials [70••]. In the USA, African Americans have a high prevalence of obesity [30, 71]. The prevalence of obesity was 59% in African American women versus 41% in women of other ethnic backgrounds in the New York HFpEF registry [72]. The clinical characteristics of patients with HFpEF were similar in the Urban Baltimore community, with a prevalence of 84% in women and 76% in African Americans and a mean BMI of 37 kg/m2 [73]. HFpEF most often occurs in elderly patients who have multiple comorbidities, particularly atrial fibrillation and chronic kidney disease that contribute to functional impairment [74]. Middle-aged patients with HFpEF have fewer comorbidities and are twice as likely to be obese than elderly patients with HFpEF [69••, 70••]. The consistent association of middle age and obesity with HFpEF in 11 Asian regions and in the USA suggests that obesity hastens the development of HFpEF; i.e., there is an obesity-induced phenotype of HFpEF.
Weight Reduction in Obesity-Induced Heart Failure with Preserved Ejection Fraction
To establish the central role of obesity in the pathobiology of HFpEF in middle-aged patients, it will be necessary to demonstrate improvement/reversal of LVDD and increased functional capacity after substantial weight loss. Bariatric surgery is the most reliable intervention for weight loss. It is approved by the FDA for the treatment of patients with BMI ≥ 40 kg/m2 or ≥ 35 kg/m2 with an obesity-associated comorbidity. The mean age of patients undergoing bariatric surgery is 46 years, and recent data indicate that, while associated with a moderate increase in the risk of complications, bariatric surgery is a safe surgical procedure in elderly patients [75]. However, elderly patients lose less weight than middle-aged patients after bariatric surgery. In patients unwilling to undergo an invasive procedure, mild weight reduction with caloric restriction (CR) is a promising approach.
Bariatric Surgery
Bariatric surgery significantly reduces incident HF (HFpEF and HFrEF) in morbidly obese patients [76,77,78]. Combined analysis of data from the Swedish registry of patients treated with intensive lifestyle interventions and the Scandinavian obesity surgery registry showed that gastric bypass (GB) surgery decreases HF incidence by 50% more than lifestyle management [77]. Since obesity is a major risk factor for HF and diet, exercise and pharmacotherapy are relatively ineffective for weight loss, bariatric surgery is now considered to be the treatment of choice for obese HFrEF patients who are resistant to guideline-directed medical therapy for their HFrEF and may become eligible for cardiac transplantation after substantial weight loss [79, 80]. Bariatric surgery may be even more beneficial for the treatment of HFpEF than HFrEF. Weight loss, either dietary or surgical, markedly decreases LV mass in obese patients with LV concentric remodeling and preserved EF [81,82,83]. The effects of bariatric surgery on LV structure and function are most commonly assessed by 2D-Doppler echocardiography [84]. Trans-mitral valve pulmonary veins and tissue Doppler of LV diastolic function including septal and lateral mitral annular velocities and LV diastolic strain all improve after bariatric surgery [85,86,87,88]. LV mass index (LVMI, indexed to height) decreased from 44.0 to 38.4 g/m2.7 (p < 0.01) after GB surgery in 354 patients with morbid obesity but did not change in 249 counterparts who sought bariatric surgery but could not afford it [89]. Two meta-analyses confirmed that bariatric surgery reduces LV mass, improves LVDD, and reduces left atrial size in patients with LV concentric remodeling and preserved EF [90, 91]. Of note, bariatric surgery reduces LVMI independent of obesity-related co-morbidities like OSA [92]. Furthermore, LVMI continues to decline at a linear rate for 24 months after bariatric surgery, while rates of weight loss and loss of lean mass and fat mass plateau at 9 months [93]. In addition to reversing LV concentric remodeling, GB surgery has been shown to improve quality of life and functional capacity in patients with HFpEF, as evidenced by a lower score on Minnesota living with HF questionnaire and an improved NYHA functional class [94].
Bariatric surgery consistently reduces the amount of VAT by 40–70% in obese patients [95,96,97,98,99]. VAT begins to decrease as early as 1 month after surgery and continues to decrease for 24 months, then stabilizes [96, 99]. Bariatric surgery reduces VAT mass to a greater extent in women than men [100]. The effects of Roux-en-Y GB surgery on circulating markers of inflammation have been well studied. Blood levels of CRP and IL-6 are consistently lower after GB surgery, while TNF-α levels are lower or unchanged [101,102,103,104,105]. Sleeve gastrectomy and GB surgery may have similar effects on CRP, IL-6, and TNF-α levels [104, 106]. Two meta-analyses have measured inflammatory markers after bariatric surgery [107, 108]. The first showed that levels of CRP and IL-6 were significantly lower after bariatric surgery, while the change in TNF-α did not reach statistical significance. In the second meta-analysis, CRP, IL-6, and TNF-α levels decreased significantly after bariatric surgery. Thus, bariatric surgery appears to be a reliable intervention to reduce both VAT mass and circulating markers of inflammation.
Caloric Restriction
Moderate weight reduction is associated with a metabolic adaptation that may attenuate low-grade systemic inflammation and reduce inflammation in the coronary microvascular endothelium. Reduced inflammation in the coronary microvasculature may alleviate LVDD and improve the functional capacity of HFpEF patients.
Caloric restriction (CR) is a dietary intervention that aims at a 25% reduction of metabolic requirements from baseline values [109]. The Comprehensive Assessment of the Long-term Effects of Reducing Intake of Energy (CALERIE 1) study showed that 6 months of CR decreased body weight by 10 (0.8)% in overweight, healthy, sedentary middle-aged men, and pre-menopausal women [109]. CR appears to induce metabolic adaptation as the decrease in energy expenditure exceeds that expected from the loss of fat-free mass. The CALERIE 2 study investigated the effects of CR for 2 years on BP, CRP, plasma lipids, fasting insulin, and insulin resistance in young and middle-aged (21–51 years) healthy non-obese (BMI 22.0–27.5 kg/m2) men and women. The mean caloric intake and weight reduction were − 11.9% and − 7.5 kg respectively at 2 years. All conventional cardiometabolic risk factors were reduced at 2 years after controlling for relative weight loss [110••]. Two years of CR at levels achieved in CALERIE 2 is well tolerated and safe providing close monitoring of bone loss and red blood cells [111]. An ancillary study of CALERIE 2 confirmed that CR for 2 years induces metabolic adaptation and reduces thyroid axis activity and reactive oxygen species production in 34 non-obese middle-aged healthy men and women [112••]. Another ancillary study of CALERIE 2 showed that, in addition to reductions in BP, total cholesterol, and cardiovascular risk, a 9% reduction on body weight is associated with a significant decrease in VAT mass at 12 and 24 months in non-obese middle-aged healthy men and women [113]. The Calorie Restriction in Overweight SeniorS: Response of Older Adults to a Dieting Study (CROSSROADS) trial randomized elderly (age > 65 years) and obese (BMI 30–40 kg/m2) patients who were receiving at ≥ 1 medication for HTN, T2D, or dyslipidemia, to CR, exercise, or diet modification for 6 months [114]. Per protocol, the body weight of patients randomized to exercise and dietary modification was to be kept constant. Patients randomized to CR lost 4.1% of body weight and no VAT mass. However, exercise that has been reported to decrease VAT mass by 6.1% in the absence of change in body weight did not alter VAT mass in CROSSROADS [115, 116]. Observational data suggest that CR for several years may slow down age-related deterioration in LV diastolic function [117]. Twenty weeks of CR and CR plus exercise training resulted in moderate improvement in peak aerobic capacity but did not enhance the quality of life in obese elderly patients with HFpEF [118].
In summary, the pathobiology and treatment of the obese phenotype of HFpEF may differ in middle-aged and elderly patients. In the elderly, obesity and HFpEF are not closely related and bariatric surgery and CR are unlikely to improve LVDD and HFpEF, although bariatric surgery is likely to improve patients’ sense of well-being and cardiovascular disease risk. In the middle-aged, bariatric surgery and possibly CR may improve LVDD and HFpEF. Thus, treatment needs to focus on weight reduction in middle-aged patients with obesity-induced HFpEF.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–91. https://doi.org/10.1001/jama.2016.6458.
Jackson SE, Llewellyn CH, Smith L. The obesity epidemic - nature via nurture: a narrative review of high-income countries. SAGE Open Med. 2020;8:2050312120918265. https://doi.org/10.1177/2050312120918265.
Turer AT, Hill JA, Elmquist JK, Scherer PE. Adipose tissue biology and cardiomyopathy: translational implications. Circ Res. 2012;111(12):1565–77. https://doi.org/10.1161/CIRCRESAHA.111.262493.
Aurigemma GP, de Simone G, Fitzgibbons TP. Cardiac remodeling in obesity. Circ Cardiovasc Imaging. 2013;6(1):142–52. https://doi.org/10.1161/CIRCIMAGING.111.964627.
Szczepaniak LS, Victor RG, Orci L, Unger RH. Forgotten but not gone: the rediscovery of fatty heart, the most common unrecognized disease in America. Circ Res. 2007;101(8):759–67. https://doi.org/10.1161/CIRCRESAHA.107.160457.
Turkbey EB, McClelland RL, Kronmal RA, Burke GL, Bild DE, Tracy RP, et al. The impact of obesity on the left ventricle: the multi-ethnic study of atherosclerosis (MESA). JACC Cardiovasc Imaging. 2010;3(3):266–74. https://doi.org/10.1016/j.jcmg.2009.10.012.
Tsao CW, Lyass A, Enserro D, Larson MG, Ho JE, Kizer JR, et al. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail. 2018;6(8):678–85. https://doi.org/10.1016/j.jchf.2018.03.006.
•• Savji N, Meijers WC, Bartz TM, Bhambhani V, Cushman M, Nayor M, et al. The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Heart Fail. 2018;6(8):701–9. https://doi.org/10.1016/j.jchf.2018.05.018The data from 4 community-based cohorts clearly establishthat obesity is a risk for HFpEF especially in women.
Gupta DK, Shah AM, Castagno D, Takeuchi M, Loehr LR, Fox ER, et al. Heart failure with preserved ejection fraction in African Americans: the ARIC (atherosclerosis risk in communities) study. JACC Heart Fail. 2013;1(2):156–63. https://doi.org/10.1016/j.jchf.2013.01.003.
Aune D, Sen A, Norat T, Janszky I, Romundstad P, Tonstad S, et al. Body mass index, abdominal fatness, and heart failure incidence and mortality: a systematic review and dose-response meta-analysis of prospective studies. Circulation. 2016;133(7):639–49. https://doi.org/10.1161/CIRCULATIONAHA.115.016801.
Yan Y, Li S, Guo Y, Fernandez C, Bazzano L, He J, et al. Life-course cumulative burden of body mass index and blood pressure on progression of left ventricular mass and geometry in midlife: the Bogalusa heart study. Circ Res. 2020;126(5):633–43. https://doi.org/10.1161/CIRCRESAHA.119.316045.
Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, Barzilai B, et al. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol. 2004;43(8):1399–404. https://doi.org/10.1016/j.jacc.2003.10.062.
De Simone G, Devereux RB, Chinali M, Roman MJ, Barac A, Panza JA, et al. Sex differences in obesity-related changes in left ventricular morphology: the strong heart study. J Hypertens. 2011;29(7):1431–8. https://doi.org/10.1097/HJH.0b013e328347a093.
Powell BD, Redfield MM, Bybee KA, Freeman WK, Rihal CS. Association of obesity with left ventricular remodeling and diastolic dysfunction in patients without coronary artery disease. Am J Cardiol. 2006;98(1):116–20. https://doi.org/10.1016/j.amjcard.2006.01.063.
Rozenbaum Z, Topilsky Y, Khoury S, Pereg D, Laufer-Perl M. Association of body mass index and diastolic function in metabolically healthy obese with preserved ejection fraction. Int J Cardiol. 2019;277:147–52. https://doi.org/10.1016/j.ijcard.2018.08.008.
Campbell DJ, Gong FF, Jelinek MV, Castro JM, Coller JM, McGrady M, et al. Threshold body mass index and sex-specific waist circumference for increased risk of heart failure with preserved ejection fraction. Eur J Prev Cardiol. 2019;26(15):1594–602. https://doi.org/10.1177/2047487319851298.
Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, et al. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. J Am Coll Cardiol. 2011;57(12):1368–74. https://doi.org/10.1016/j.jacc.2010.10.042.
Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126(1):65–75. https://doi.org/10.1161/CIRCULATIONAHA.111.080770.
•• Stolfo D, Uijl A, Vedin O, Strömberg A, Faxén UL, Rosano GMC, et al. Sex-Based Differences in Heart Failure Across the Ejection Fraction Spectrum: Phenotyping, and Prognostic and Therapeutic Implications. JACC Heart Fail. 2019;7(6):505–15. https://doi.org/10.1016/j.jchf.2019.03.011Recent data from the Swedish Heart Failure Registry confirm that in contrast to HFrEF HFpEF affect predominantly women.
Salton CJ, Chuang ML, O'Donnell CJ, Kupka MJ, Larson MG, Kissinger KV, et al. Gender differences and normal left ventricular anatomy in an adult population free of hypertension. A cardiovascular magnetic resonance study of the Framingham Heart Study Offspring cohort. J Am Coll Cardiol. 2002;39(6):1055–60. https://doi.org/10.1016/s0735-1097(02)01712-6.
Gori M, Lam CS, Gupta DK, Santos AB, Cheng S, Shah AM, et al. Sex-specific cardiovascular structure and function in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16(5):535–42. https://doi.org/10.1002/ejhf.67.
Pandey A, Omar W, Ayers C, LaMonte M, Klein L, Allen NB, et al. Sex and race differences in lifetime risk of heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. Circulation. 2018;137(17):1814–23. https://doi.org/10.1161/CIRCULATIONAHA.117.031622.
Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14(10):591–602. https://doi.org/10.1038/nrcardio.2017.65.
Ho JE, Enserro D, Brouwers FP, Kizer JR, Shah SJ, Psaty BM, et al. Predicting heart failure with preserved and reduced ejection fraction: The International Collaboration on Heart Failure Subtypes. Circ Heart Fail. 2016;9(6). https://doi.org/10.1161/CIRCHEARTFAILURE.115.003116.
Tadic M, Cuspidi C, Plein S, Belyavskiy E, Heinzel F, Galderisi M. Sex and heart failure with preserved ejection fraction: from pathophysiology to clinical studies. J Clin Med. 2019;8(6). https://doi.org/10.3390/jcm8060792.
Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke Statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492. https://doi.org/10.1161/CIR.0000000000000558.
Drazner MH, Dries DL, Peshock RM, Cooper RS, Klassen C, Kazi F, et al. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension. 2005;46(1):124–9. https://doi.org/10.1161/01.HYP.0000169972.96201.8e.
Morris AA, Patel RS, Binongo JN, Poole J, Al Mheid I, Ahmed Y, et al. Racial differences in arterial stiffness and microcirculatory function between Black and White Americans. J Am Heart Assoc. 2013;2(2):e002154. https://doi.org/10.1161/JAHA.112.002154.
Krishnamoorthy A, Greiner MA, Bertoni AG, Eapen ZJ, O'Brien EC, Curtis LH, et al. The obesity and heart failure epidemics among African Americans: insights from the Jackson Heart Study. J Card Fail. 2016;22(8):589–97. https://doi.org/10.1016/j.cardfail.2016.03.004.
Buffington CK, Marema RT. Ethnic differences in obesity and surgical weight loss between African-American and Caucasian females. Obes Surg. 2006;16(2):159–65. https://doi.org/10.1381/096089206775565258.
Eaton CB, Pettinger M, Rossouw J, Martin LW, Foraker R, Quddus A, et al. Risk factors for incident hospitalized heart failure with preserved versus reduced ejection fraction in a multiracial cohort of postmenopausal women. Circ Heart Fail. 2016;9(10). https://doi.org/10.1161/CIRCHEARTFAILURE.115.002883.
East MA, Peterson ED, Shaw LK, Gattis WA, O'Connor CM. Racial differences in the outcomes of patients with diastolic heart failure. Am Heart J. 2004;148(1):151–6. https://doi.org/10.1016/j.ahj.2004.01.017.
Lekavich CL, Barksdale DJ. A critical evaluation of the representation of black patients with heart failure and preserved ejection fraction in clinical trials: a literature review. J Cardiovasc Nurs. 2016;31(3):202–8. https://doi.org/10.1097/JCN.0000000000000237.
Goyal P, Paul T, Almarzooq ZI, Peterson JC, Krishnan U, Swaminathan RV, et al. Sex- and race-related differences in characteristics and outcomes of hospitalizations for heart failure with preserved ejection fraction. J Am Heart Assoc. 2017;6(4). https://doi.org/10.1161/JAHA.116.003330.
Vivo RP, Krim SR, Liang L, Neely M, Hernandez AF, Eapen ZJ, et al. Short- and long-term rehospitalization and mortality for heart failure in 4 racial/ethnic populations. J Am Heart Assoc. 2014;3(5):e001134. https://doi.org/10.1161/JAHA.114.001134.
Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–71. https://doi.org/10.1016/j.jacc.2013.02.092.
•• Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschöpe C, et al. Myocardial Microvascular Inflammatory Endothelial Activation in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2016;4(4):312–24. https://doi.org/10.1016/j.jchf.2015.10.007Human and experimental data that link HFpEF to coronary microvascular endothelial activation and oxidative stress.
Després JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126(10):1301–13. https://doi.org/10.1161/CIRCULATIONAHA.111.067264.
Rubin R. Postmenopausal women with a “normal” BMI might be overweight or even obese. JAMA. 2018;319(12):1185–7. https://doi.org/10.1001/jama.2018.0423.
Neeland IJ, Gupta S, Ayers CR, Turer AT, Rame JE, Das SR, et al. Relation of regional fat distribution to left ventricular structure and function. Circ Cardiovasc Imaging. 2013;6(5):800–7. https://doi.org/10.1161/CIRCIMAGING.113.000532.
Abbasi SA, Hundley WG, Bluemke DA, Jerosch-Herold M, Blankstein R, Petersen SE, et al. Visceral adiposity and left ventricular remodeling: the multi-ethnic study of atherosclerosis. Nutr Metab Cardiovasc Dis. 2015;25(7):667–76. https://doi.org/10.1016/j.numecd.2015.03.016.
de Simone G, Izzo R, De Luca N, Gerdts E. Left ventricular geometry in obesity: is it what we expect? Nutr Metab Cardiovasc Dis. 2013;23(10):905–12. https://doi.org/10.1016/j.numecd.2013.06.012.
Rao VN, Zhao D, Allison MA, Guallar E, Sharma K, Criqui MH, et al. Adiposity and incident heart failure and its subtypes: MESA (multi-ethnic study of atherosclerosis). JACC Heart Fail. 2018;6(12):999–1007. https://doi.org/10.1016/j.jchf.2018.07.009.
•• Tsujimoto T, Kajio H. Abdominal obesity is associated with an increased risk of all-cause mortality in patients with HFpEF. J Am Coll Cardiol. 2017;70(22):2739–49. https://doi.org/10.1016/j.jacc.2017.09.1111Analysis of TOPCAT indicates that the amount of abdominal obesity; i.e., visceral adipose tiissue plays an essential role in the clinical outcome of HFpEF.
Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–7. https://doi.org/10.1038/nature05488.
Blackburn P, Després JP, Lamarche B, Tremblay A, Bergeron J, Lemieux I, et al. Postprandial variations of plasma inflammatory markers in abdominally obese men. Obesity (Silver Spring). 2006;14(10):1747–54. https://doi.org/10.1038/oby.2006.201.
Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282(22):2131–5. https://doi.org/10.1001/jama.282.22.2131.
Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116(11):1234–41. https://doi.org/10.1161/CIRCULATIONAHA.107.710509.
Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20(1):103–18. https://doi.org/10.1016/j.cmet.2014.05.005.
Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–84. https://doi.org/10.1172/JCI29881.
Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13(11):633–43. https://doi.org/10.1038/nrendo.2017.90.
Lee YS, Wollam J, Olefsky JM. An integrated view of Immunometabolism. Cell. 2018;172(1–2):22–40. https://doi.org/10.1016/j.cell.2017.12.025.
Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, et al. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014;19(1):162–71. https://doi.org/10.1016/j.cmet.2013.11.017.
Schlecht I, Fischer B, Behrens G, Leitzmann MF. Relations of visceral and abdominal subcutaneous adipose tissue, body mass index, and waist circumference to serum concentrations of parameters of chronic inflammation. Obes Facts. 2016;9(3):144–57. https://doi.org/10.1159/000443691.
Khera A, Vega GL, Das SR, Ayers C, McGuire DK, Grundy SM, et al. Sex differences in the relationship between C-reactive protein and body fat. J Clin Endocrinol Metab. 2009;94(9):3251–8. https://doi.org/10.1210/jc.2008-2406.
Denis GV, Sebastiani P, Bertrand KA, Strissel KJ, Tran AH, Slama J, et al. Inflammatory signatures distinguish metabolic health in African American women with obesity. PLoS One. 2018;13(5):e0196755. https://doi.org/10.1371/journal.pone.0196755.
DeBerge M, Shah SJ, Wilsbacher L, Thorp EB. Macrophages in heart failure with reduced versus preserved ejection fraction. Trends Mol Med. 2019;25(4):328–40. https://doi.org/10.1016/j.molmed.2019.01.002.
Paulus WJ. Unfolding discoveries in heart failure. N Engl J Med. 2020;382(7):679–82. https://doi.org/10.1056/NEJMcibr1913825.
Mohammed SF, Hussain I, AbouEzzeddine OF, Abou Ezzeddine OF, Takahama H, Kwon SH, et al. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation. 2014;130(25):2310–20. https://doi.org/10.1161/CIRCULATIONAHA.113.008461.
Zeng H, Chen JX. Microvascular rarefaction and heart failure with preserved ejection fraction. Front Cardiovasc Med. 2019;6:15. https://doi.org/10.3389/fcvm.2019.00015.
Bajaj NS, Osborne MT, Gupta A, Tavakkoli A, Bravo PE, Vita T, et al. Coronary microvascular dysfunction and cardiovascular risk in obese patients. J Am Coll Cardiol. 2018;72(7):707–17. https://doi.org/10.1016/j.jacc.2018.05.049.
Zylke JW, Bauchner H. The unrelenting challenge of obesity. JAMA. 2016;315(21):2277–8. https://doi.org/10.1001/jama.2016.6190.
•• Maslov PZ, Kim JK, Argulian E, Ahmadi A, Narula N, Singh M, et al. Is Cardiac Diastolic Dysfunction a Part of Post-Menopausal Syndrome? JACC Heart Fail. 2019;7(3):192–203. https://doi.org/10.1016/j.jchf.2018.12.018Post-menopausal women gain a lot of weight that, in addition to estrogen deficiency, leads to the development and progression of HFpEF.
Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massie BM, et al. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail. 2011;4(3):324–31. https://doi.org/10.1161/CIRCHEARTFAILURE.110.959890.
Foppa M, Arora G, Gona P, Ashrafi A, Salton CJ, Yeon SB, et al. Right ventricular volumes and systolic function by cardiac magnetic resonance and the impact of sex, age, and obesity in a longitudinally followed cohort free of pulmonary and cardiovascular disease: the Framingham Heart Study. Circ Cardiovasc Imaging. 2016;9(3):e003810. https://doi.org/10.1161/CIRCIMAGING.115.003810.
McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation. 2001;104(23):2797–802. https://doi.org/10.1161/hc4801.100076.
Reddy YNV, Lewis GD, Shah SJ, Obokata M, Abou-Ezzedine OF, Fudim M, et al. Characterization of the obese phenotype of heart failure with preserved ejection fraction: a RELAX trial ancillary study. Mayo Clin Proc. 2019;94(7):1199–209. https://doi.org/10.1016/j.mayocp.2018.11.037.
Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136(1):6–19. https://doi.org/10.1161/CIRCULATIONAHA.116.026807.
•• Tromp J, MacDonald MR, Tay WT, Teng TK, Hung CL, Narasimhan C, et al. Heart failure with preserved ejection fraction in the young. Circulation. 2018;138(24):2763–73. https://doi.org/10.1161/CIRCULATIONAHA.118.034720The distinctive features of HFpEF in middle-aged patients are outlined for the first time. Middle-aged patients were twice more likely to be obese than elderly patients with HFpEF in the Asian sudden death in heart failure (ASIAN-HF) registry.
•• Tromp J, Shen L, Jhund PS, Anand IS, Carson PE, Desai AS, et al. Age-related characteristics and outcomes of patients with heart failure with preserved ejection fraction. J Am Coll Cardiol. 2019;74(5):601–12. https://doi.org/10.1016/j.jacc.2019.05.052Younger patients with HFpEF were also more likely to be obese than elderly patients in the I-PRESERVE (Irbesartan in heart failure with preserved systolic function and CHARM (Candesartan cilexetil in heart failure assessment of reduction of mortality and morbidity) trials.
Powell-Wiley TM, Ngwa J, Kebede S, Lu D, Schulte PJ, Bhatt DL, et al. Impact of body mass index on heart failure by race/ethnicity from the get with the guidelines-heart failure (GWTG-HF) registry. JACC Heart Fail. 2018;6(3):233–42. https://doi.org/10.1016/j.jchf.2017.11.011.
Klapholz M, Maurer M, Lowe AM, Messineo F, Meisner JS, Mitchell J, et al. Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: results of the New York heart failure registry. J Am Coll Cardiol. 2004;43(8):1432–8. https://doi.org/10.1016/j.jacc.2003.11.040.
Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, et al. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49(2):198–207. https://doi.org/10.1016/j.jacc.2006.08.050.
Samson R, Jaiswal A, Ennezat PV, Cassidy M, Le Jemtel TH. Clinical phenotypes in heart failure with preserved ejection fraction. J Am Heart Assoc. 2016;5(1). doi:https://doi.org/10.1161/JAHA.115.002477.
Susmallian S, Raziel A, Barnea R, Paran H. Bariatric surgery in older adults: should there be an age limit? Medicine (Baltimore). 2019;98(3):e13824. https://doi.org/10.1097/MD.0000000000013824.
Persson CE, Björck L, Lagergren J, Lappas G, Giang KW, Rosengren A. Risk of heart failure in obese patients with and without bariatric surgery in Sweden-a registry-based study. J Card Fail. 2017;23(7):530–7. https://doi.org/10.1016/j.cardfail.2017.05.005.
Sundström J, Bruze G, Ottosson J, Marcus C, Näslund I, Neovius M. Weight loss and heart failure: a nationwide study of gastric bypass surgery versus intensive lifestyle treatment. Circulation. 2017;135(17):1577–85. https://doi.org/10.1161/CIRCULATIONAHA.116.025629.
Benotti PN, Wood GC, Carey DJ, Mehra VC, Mirshahi T, Lent MR, et al. Gastric bypass surgery produces a durable reduction in cardiovascular disease risk factors and reduces the long-term risks of congestive heart failure. J Am Heart Assoc. 2017;6(5). https://doi.org/10.1161/JAHA.116.005126.
Kindel TL, Strande JL. Bariatric surgery as a treatment for heart failure: review of the literature and potential mechanisms. Surg Obes Relat Dis. 2018;14(1):117–22. https://doi.org/10.1016/j.soard.2017.09.534.
Vest AR. Has the time come to be more aggressive with bariatric surgery in obese patients with chronic systolic heart failure? Curr Heart Fail Rep. 2018;15(3):171–80. https://doi.org/10.1007/s11897-018-0390-z.
de las Fuentes L, Waggoner AD, Mohammed BS, Stein RI, Miller BV, Foster GD, et al. Effect of moderate diet-induced weight loss and weight regain on cardiovascular structure and function. J Am Coll Cardiol. 2009;54(25):2376–81. https://doi.org/10.1016/j.jacc.2009.07.054.
Rider OJ, Francis JM, Ali MK, Petersen SE, Robinson M, Robson MD, et al. Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. J Am Coll Cardiol. 2009;54(8):718–26. https://doi.org/10.1016/j.jacc.2009.02.086.
Le Jemtel TH, Samson R, Jaiswal A, Lewine EB, Oparil S. Regression of left ventricular mass after bariatric surgery. Curr Hypertens Rep. 2017;19(9):68. https://doi.org/10.1007/s11906-017-0767-5.
Grapsa J, Tan TC, Paschou SA, Kalogeropoulos AS, Shimony A, Kaier T, et al. The effect of bariatric surgery on echocardiographic indices: a review of the literature. Eur J Clin Investig. 2013;43(11):1224–30. https://doi.org/10.1111/eci.12162.
Alpert MA, Omran J, Mehra A, Ardhanari S. Impact of obesity and weight loss on cardiac performance and morphology in adults. Prog Cardiovasc Dis. 2014;56(4):391–400. https://doi.org/10.1016/j.pcad.2013.09.003.
Ashrafian H, le Roux CW, Darzi A, Athanasiou T. Effects of bariatric surgery on cardiovascular function. Circulation. 2008;118(20):2091–102. https://doi.org/10.1161/CIRCULATIONAHA.107.721027.
Kurnicka K, Domienik-Karłowicz J, Lichodziejewska B, Bielecki M, Kozłowska M, Goliszek S, et al. Improvement of left ventricular diastolic function and left heart morphology in young women with morbid obesity six months after bariatric surgery. Cardiol J. 2018;25(1):97–105. https://doi.org/10.5603/CJ.a2017.0059.
Leung M, Xie M, Durmush E, Leung DY, Wong VW. Weight loss with sleeve gastrectomy in obese type 2 diabetes mellitus: impact on cardiac function. Obes Surg. 2016;26(2):321–6. https://doi.org/10.1007/s11695-015-1748-x.
Owan T, Avelar E, Morley K, Jiji R, Hall N, Krezowski J, et al. Favorable changes in cardiac geometry and function following gastric bypass surgery: 2-year follow-up in the Utah obesity study. J Am Coll Cardiol. 2011;57(6):732–9. https://doi.org/10.1016/j.jacc.2010.10.017.
Aggarwal R, Harling L, Efthimiou E, Darzi A, Athanasiou T, Ashrafian H. The effects of bariatric surgery on cardiac structure and function: a systematic review of cardiac imaging outcomes. Obes Surg. 2016;26(5):1030–40. https://doi.org/10.1007/s11695-015-1866-5.
Cuspidi C, Rescaldani M, Tadic M, Sala C, Grassi G. Effects of bariatric surgery on cardiac structure and function: a systematic review and meta-analysis. Am J Hypertens. 2014;27(2):146–56. https://doi.org/10.1093/ajh/hpt215.
Garza CA, Pellikka PA, Somers VK, Sarr MG, Collazo-Clavell ML, Korenfeld Y, et al. Structural and functional changes in left and right ventricles after major weight loss following bariatric surgery for morbid obesity. Am J Cardiol. 2010;105(4):550–6. https://doi.org/10.1016/j.amjcard.2009.09.057.
Algahim MF, Lux TR, Leichman JG, Boyer AF, Miller CC, Laing ST, et al. Progressive regression of left ventricular hypertrophy two years after bariatric surgery. Am J Med. 2010;123(6):549–55. https://doi.org/10.1016/j.amjmed.2009.11.020.
Mikhalkova D, Holman SR, Jiang H, Saghir M, Novak E, Coggan AR, et al. Bariatric surgery-induced cardiac and lipidomic changes in obesity-related heart failure with preserved ejection fraction. Obesity (Silver Spring). 2018;26(2):284–90. https://doi.org/10.1002/oby.22038.
Gaborit B, Jacquier A, Kober F, Abdesselam I, Cuisset T, Boullu-Ciocca S, et al. Effects of bariatric surgery on cardiac ectopic fat: lesser decrease in epicardial fat compared to visceral fat loss and no change in myocardial triglyceride content. J Am Coll Cardiol. 2012;60(15):1381–9. https://doi.org/10.1016/j.jacc.2012.06.016.
Lehmann S, Linder N, Retschlag U, Schaudinn A, Stange R, Garnov N, et al. MRI assessment of changes in adipose tissue parameters after bariatric surgery. PLoS One. 2018;13(11):e0206735. https://doi.org/10.1371/journal.pone.0206735.
Otto M, Färber J, Haneder S, Michaely H, Kienle P, Hasenberg T. Postoperative changes in body composition--comparison of bioelectrical impedance analysis and magnetic resonance imaging in bariatric patients. Obes Surg. 2015;25(2):302–9. https://doi.org/10.1007/s11695-014-1382-z.
Meyer-Gerspach AC, Peterli R, Moor M, Madörin P, Schötzau A, Nabers D, et al. Quantification of liver, subcutaneous, and visceral adipose tissues by MRI before and after bariatric surgery. Obes Surg. 2019;29(9):2795–805. https://doi.org/10.1007/s11695-019-03897-2.
Toro-Ramos T, Goodpaster BH, Janumala I, Lin S, Strain GW, Thornton JC, et al. Continued loss in visceral and intermuscular adipose tissue in weight-stable women following bariatric surgery. Obesity (Silver Spring). 2015;23(1):62–9. https://doi.org/10.1002/oby.20932.
Korner J, Punyanitya M, Taveras C, McMahon DJ, Kim HJ, Inabnet W, et al. Sex differences in visceral adipose tissue post-bariatric surgery compared to matched non-surgical controls. Int J Body Compos Res. 2008;6(3):93–9.
Illán-Gómez F, Gonzálvez-Ortega M, Orea-Soler I, Alcaraz-Tafalla MS, Aragón-Alonso A, Pascual-Díaz M, et al. Obesity and inflammation: change in adiponectin, C-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obes Surg. 2012;22(6):950–5. https://doi.org/10.1007/s11695-012-0643-y.
Schmatz R, Bitencourt MR, Patias LD, Beck M, da C Alvarez G, Zanini D et al. Evaluation of the biochemical, inflammatory and oxidative profile of obese patients given clinical treatment and bariatric surgery. Clin Chim Acta 2017;465:72–79. doi:https://doi.org/10.1016/j.cca.2016.12.012.
Williams DB, Hagedorn JC, Lawson EH, Galanko JA, Safadi BY, Curet MJ, et al. Gastric bypass reduces biochemical cardiac risk factors. Surg Obes Relat Dis. 2007;3(1):8–13. https://doi.org/10.1016/j.soard.2006.10.003.
Viana EC, Araujo-Dasilio KL, Miguel GP, Bressan J, Lemos EM, Moyses MR, et al. Gastric bypass and sleeve gastrectomy: the same impact on IL-6 and TNF-α. prospective clinical trial. Obes Surg. 2013;23(8):1252–61. https://doi.org/10.1007/s11695-013-0894-2.
Freitas WR, Oliveira LVF, Perez EA, Ilias EJ, Lottenberg CP, Silva AS, et al. Systemic inflammation in severe obese patients undergoing surgery for obesity and weight-related diseases. Obes Surg. 2018;28(7):1931–42. https://doi.org/10.1007/s11695-017-3104-9.
Chiappetta S, Schaack HM, Wölnerhannsen B, Stier C, Squillante S, Weiner RA. The impact of obesity and metabolic surgery on chronic inflammation. Obes Surg. 2018;28(10):3028–40. https://doi.org/10.1007/s11695-018-3320-y.
Rao SR. Inflammatory markers and bariatric surgery: a meta-analysis. Inflamm Res. 2012;61(8):789–807. https://doi.org/10.1007/s00011-012-0473-3.
Askarpour M, Khani D, Sheikhi A, Ghaedi E, Alizadeh S. Effect of bariatric surgery on serum inflammatory factors of obese patients: a systematic review and meta-analysis. Obes Surg. 2019;29(8):2631–47. https://doi.org/10.1007/s11695-019-03926-0.
Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295(13):1539–48. https://doi.org/10.1001/jama.295.13.1539.
•• Kraus WE, Bhapkar M, Huffman KM, Pieper CF, Krupa Das S, Redman LM, et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(9):673–83. https://doi.org/10.1016/S2213-8587(19)30151-2First demonstration of the beneficial effect of caloric restriction for 2 years on cardiometabolic risk factors in healthy, non-obese, young and middle-aged persons.
Romashkan SV, Das SK, Villareal DT, Ravussin E, Redman LM, Rochon J, et al. Safety of two-year caloric restriction in non-obese healthy individuals. Oncotarget. 2016;7(15):19124–33. https://doi.org/10.18632/oncotarget.8093.
•• Redman LM, Smith SR, Burton JH, Martin CK, Il'yasova D, Ravussin E. Metabolic slowing and reduced oxidative damage with sustained caloric restriction support the rate of living and oxidative damage theories of aging. Cell Metab. 2018;27(4):805–15.e4. https://doi.org/10.1016/j.cmet.2018.02.019Sustained caloric restriction reduces oxidative damage and is thought to induce a slowing of energy metabolism that may delay aging and possibly increase life expectancy.
Most J, Gilmore LA, Smith SR, Han H, Ravussin E, Redman LM. Significant improvement in cardiometabolic health in healthy nonobese individuals during caloric restriction-induced weight loss and weight loss maintenance. Am J Physiol Endocrinol Metab. 2018;314(4):E396–405. https://doi.org/10.1152/ajpendo.00261.2017.
Ard JD, Gower B, Hunter G, Ritchie CS, Roth DL, Goss A, et al. Effects of calorie restriction in obese older adults: the CROSSROADS randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2017;73(1):73–80. https://doi.org/10.1093/gerona/glw237.
Verheggen RJ, Maessen MF, Green DJ, Hermus AR, Hopman MT, Thijssen DH. A systematic review and meta-analysis on the effects of exercise training versus hypocaloric diet: distinct effects on body weight and visceral adipose tissue. Obes Rev. 2016;17(8):664–90. https://doi.org/10.1111/obr.12406.
Ruiz JR, Lavie CJ, Ortega FB. Exercise versus pharmacological interventions for reducing visceral adiposity and improving health outcomes. Mayo Clin Proc. 2019;94(2):182–5. https://doi.org/10.1016/j.mayocp.2018.12.018.
Meyer TE, Kovács SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol. 2006;47(2):398–402. https://doi.org/10.1016/j.jacc.2005.08.069.
Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2016;315(1):36–46. https://doi.org/10.1001/jama.2015.17346.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Disclosures
The authors report no financial relationships or conflicts of interest regarding the content herein. No funding sources to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hypertension and Obesity
Rights and permissions
About this article
Cite this article
Ayinapudi, K., Samson, R., Le Jemtel, T.H. et al. Weight Reduction for Obesity-Induced Heart Failure with Preserved Ejection Fraction. Curr Hypertens Rep 22, 47 (2020). https://doi.org/10.1007/s11906-020-01074-w
Published:
DOI: https://doi.org/10.1007/s11906-020-01074-w