Abstract
Management of acute right ventricular failure, both with and without coexisting pulmonary hypertension, is a common challenge encountered in the intensive care setting. Both right ventricular dysfunction and pulmonary hypertension portend a poor prognosis, regardless of the underlying cause and are associated with significant morbidity and mortality. The right ventricle is embryologically distinct from the left ventricle and has unique morphologic and functional properties. Management of right ventricular failure and pulmonary hypertension in the intensive care setting requires tailored hemodynamic management, pharmacotherapy, and often mechanical circulatory support. Unfortunately, our understanding of the management of right ventricular failure lags behind that of the left ventricle. In this review, we will explore the underlying pathophysiology of the failing right ventricle and pulmonary vasculature in patients with and without pulmonary hypertension and discuss management strategies based on evidence-based studies as well as our current understanding of the underlying physiology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Right ventricular (RV) failure is a common complication of pulmonary hypertension (PH) and is the major determinant of morbidity and mortality among patient inflicted with the condition [1]. Early RV dysfunction, manifesting as elevated right-sided filling pressures with right-sided congestion and fluid retention can occur with pulmonary hypertension of any etiology (Table 1) according to the World Health Organization classification [2•]. Advanced RV failure, manifesting as low cardiac output, elevated intracardiac filling pressures, and cardiogenic shock are more commonly seen in patients with pulmonary arterial hypertension (WHO group 1) and chronic thromboembolic pulmonary hypertension (WHO group 4) [2•, 3]. RV failure can also occur independently from changes in the pulmonary vasculature, occurring in patients with RV myocardial infarctions, myocarditis, cardiomyopathies, or right-sided valvular dysfunction.
Traditional management in the intensive care unit is often focused on optimization of the left ventricle. The right ventricle is morphologically distinct from the left ventricle and has differing adaptive responses to changes in preload and afterload [4, 5]. Unlike the thick-walled, conical ellipse shape characteristic of the left ventrical, the right ventricle is a thin-walled crescent-shaped structure designed to deliver blood into the low-resistance, high-compliance pulmonary vasculature [6]. Furthermore, there is an interplay between the left ventricle and right ventricle as both ventricles share an interventricular septum and are continued by the same pericardium [7]. Thus, management options that prioritize optimization of the left ventricle often come at the expense of right ventricular function. Unfortunately, given the clinical severity of the patient population, there are no randomized control trials studying different management options in patients with acute RV failure. Instead, management of RV failure relies heavily on consensus opinion obtained from extrapolation of evidence-based data from less sick patient populations, as well as understanding the underlying physiologic principles of the disease state. Here, we review the underlying pathophysiology of right ventricular failure and its relationship to pulmonary hypertension as well as the pharmacomechanical management strategies currently utilized in the intensive care unit.
Pathophysiology of Right Ventricular Failure
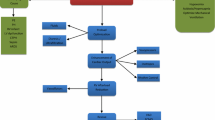
Right ventricular cardiac output is dependent on RV contractility, preload in the form of venous return back to the right ventricle, and afterload in the form of pulmonary vascular resistance. Whereas the right ventricle is able to accommodate acute changes in preload quite well, acute increases in afterload, such as after a pulmonary embolism, are poorly tolerated. Initial adaptive changes attempt to maintain RV stroke volume. In response to an acute increase in afterload or decrease in contractility, the right ventricle dilates in an effort to increase RV end-diastolic volume and improve cardiac output by the Frank-Starling mechanism. However, RV dilation can lead to functional tricuspid regurgitation from stretching of the tricuspid annulus [8]. With more severe dilation, the contractile sarcomere can become disrupted leading to hemodynamic collapse [9]. The rising RV end-diastolic volume and pressure leads to increased RV wall stress and reduced RV stroke volume [10]. As a result of the increase in RV volume and pressure, the interventricular septum shifts toward the LV, further distorting RV morphology and contractile efficiency (Fig. 1).
When RV afterload increases more gradually, such as with pulmonary hypertension, the RV compensates with myocardial hypertrophy. In animal models, RV hypertrophy is observed as soon as 96 h after an acute insult [11]. Myocardial hypertrophy reduces wall stress in the face of rising RV end-diastolic volume and pressure according to Leplace’s law thereby maintaining adequate stroke volume [12, 13]. Despite the adaptive remodeling, the compensatory mechanism of the RV can be overwhelmed with minor perturbations in RV demand or afterload. The RV hypertrophy comes at a cost as RV hypertrophy directly leads to increased myocardial demand setting up a supply-demand mismatch [9]. In patients with advanced PH, acute RV failure can be triggered by disease progression despite appropriate therapy but more commonly occurs following an inciting event such as medication noncompliance, systemic infection, upper respiratory infection, anemia, arrhythmias, pulmonary embolism, or changes in overall volume status (volume overload/depletion) (Table 2) [14]. In addition, the stresses accompanied with elective and nonelective surgery can trigger right ventricular failure [15].
Following a sudden insult, the adaptive changes of both acute and acute on chronic RV failure often lead to a destructive cycle of RV dysfunction begetting further RV dysfunction that is often nonrecoverable. The RV dilation following an acute rise in afterload or reduction in RV contractility can lead to worsening tricuspid regurgitation as described above. Furthermore, the rise in both volume and pressure leads to elevated right atrial pressures and dilation, predisposing to atrial arrhythmias and an increase in previously inconsequential right to left interatrial shunts through a patent foramen ovale. Atrial arrhythmias and hypoxemia from a right to left shunt further worsen the already tenous supply-demand mismatch established by the increased RV wall tension and hypertrophy. As compensatory mechanisms become overwhelmed, RV stroke volume decreases leading to underfilling the LV and a drop in systemic blood pressure. The drop in systemic blood pressure decreases the aortic perfusion pressure, further leading to a reduction in right coronary artery perfusion and worsening RV ischemia. The RV ischemia further worsens RV function (Fig. 1). Effective management of RV failure must therefore utilize pharmacologic and mechanical therapies that attempt to break this vicious cycle while at the same time addressing the underlying disease process that led to the decompensation.
Pharmacotherapy in Acute Right Ventricular Failure
The management of acute RV failure, especially with concomitant pulmonary hypertension, is complex. Patients with evidence of multiorgan dysfunction should ideally be referred to a quarternary care facility with experts in PH and RV management. Initial management should focus on hemodynamic stabalization with simultaneous identification of precipitating causes of RV decompensation. Common precipitating causes and their therapies are outlined in Table 2. Tailored pharmacotherapy to improve RV function and systemic perfusion should focus on optimizing ventilatory support and tissue oxygenation, preload, contractility, RV perfusion, afterload, and rhythm control.
Ventilatory Support and Tissue Oxygenation
Adequate oxygenation is of vital importance in all cases of shock; however, the issue is even more paramount in RV failure. As described above, the failing RV is constantly struggling with a supply-demand mismatch and even subtle tissue hypoxia in the RV can exacerbate the vicious cycle described in Fig. 1. Furthermore, alveolar hypoxia and hypoxemia in pulmonary arterial blood cause hypoxic pulmonary vasoconstriction, further increasing pulmonary vascular resistance and RV afterload as well as inducing RV diastolic dysfunction [16]. Supplemental oxygen should be applied to maintain near-normal systemic oxygen saturations. Tissue hypoxia can also be minimized by maintaining an adequate hemoglobin level. Hypoxia worsens left ventricular oxygen delivery and performance, and it is often extrapolated that hypoxia has similar effects on the right ventricle [17]. Although never prospectively studied, many centers prefer a hemoglobin concentration of at least 10 g/dl [18]. However, it should be noted that transfusion of stored blood or red blood cells does not always result in significant improvement in oxygen-carrying capacity.
Endotracheal intubation should be avoided unless it is absolutely clinically indicated as patients often respond poorly to the sedatives needed for intubation leading to systemic hypoperfusion and hemodynamic collapse [19]. Positive pressure ventilation also increases intrathoracic pressure and impedes right ventricular preload. If intubation is unavoidable, vasoactive agents should be started in advance to maintain vascular tone. Induction agents that maintain vascular tone and contractility such as etomidate are often preferred [20]. Positive pressure ventilation with high tidal volumes, plateau pressures, and positive end-expiratory pressures can similarly lead to increased RV afterload as can the hypercapnea that occurs with low tidal volume ventilation [21, 22]. Therefore, ventilatory settings should be set to maintain adequate oxygenation and ventilation with the lowest increase in intrathoracic pressure. This can usually be achieved with the use of low to moderate tidal volumes (6–8 cc/kg) and moderate levels of positive end-expiratory pressures (<12 cm H2O) [22].
Optimization of Preload
Finding the optimal fluid balance is paramount in patients with RV failure, as both hypovolemia and hypervolemia can impair RV function and organ perfusion. Early canine models of RV failure suggested that volume loading improved both RV stroke volume and systemic perfusion [23]. Earlier studies and clinical experience suggested that aggressive volume loading may have improved hemodynamics in acute pulmonary embolism and RV infarct [24, 25]. However, it soon became apparent that overaggressive and unmonitored volume administration had detrimental effects. More contemporary studies support volume loading only in patients with underfilled right ventricles with low central venous pressures [26]. Volume status can often be difficult to assess by jugular venous assessment, as many patients have high-grade tricuspid regurgitation and a large “a” waves from atrial contraction against a noncompliant RV. Central venous pressure monitoring and/or pulmonary artery catheters can be used to obtain a more accurate assessment of intracardiac filling pressures; however, caution must be used when using prolonged pulmonary artery catheters in this population owing to the higher rates of right ventricular arrhythmia and pulmonary hemorrhage [27]. The majority of patients with RV failure will present with fluid overload, and net fluid removal with loop diuretics with or without thiazide diuretics is often needed [13]. Generally, an intermediate central venous pressure goal of between 6 and 12 mmHg is targeted.
Right Ventricular Contractility and Perfusion
In the setting of cardiogenic shock or systemic hypoperfusion, augmentation of cardiac contractility with inotropic support is necessary. Several intravenous medications are available to increase contractility, all with varying affects on systemic vascular resistance, pulmonary vascular resistance, and chronotropy. When selecting an inotropic agent, care must be taken to avoid systemic hypotension which can further impair right ventricular perfusion as well as to avoid undue tachycardia which can increase RV workload and impair RV filling [28].
As is often the case, patients with right ventricular failure and pulmonary hypertension present with systemic hypotension which limits the ability to use inodilator therapies such as dobutmine and milrinone in isolation. Norepinephrine is a catecholamine vasopressor that increases cardiac contractility by activating β-1 receptors while at the same time acts as vasocontrictor via α-1 receptors [29]. By acting as a vasocontrictor, norepinephrine is often better tolerated in patients with relative hypotension. Epinephrine and dopamine are alternative catecholamine vasopressors that can be used; however, these agents tend to cause more tachycardia which is often poorly tolerated. A major limitation of catecholamine vasopressors is that they tend to increase pulmonary vascular resistance at higher doses [30]. However, these studies have not been pursued in PH patients specifically.
Dobutamine is a strong β-1 and β-2 agonist which increases myocardial contractility and also reduces both right ventricular and left ventricular afterload by reducing pulmonary vascular resistance and systemic vascular resistance, respectively [29]. A major limitation of dobutamine is tachycardia and hypotension, both which may be poorly tolerated especially among patients with coexisting pulmonary hypertension. Milrinone, a phosphodiesterase-3 inhibitor, works downstream from the β-adrenergic receptor and acts by increasing cyclic AMP levels. Milrinone has less of a chronotropic response than dobutamine while still augmenting cardiac contractility and vascular vasodilation [13, 29]. Milrinone is useful in patients with preserved systemic pressures but must be used with caution or concomitantly with a systemic vasopressive agent in patients with low systemic blood pressures, as the systemic vasodilation caused by milrinone may exacerbate right ventricular ischemia.
An ideal vasopressor will increase systemic vascular resistance more than pulmonary vascular resistance, thereby still supporting right ventricular perfusion. Vasopressin acts predominantly through the V 1a receptor in vascular smooth muscle leading to vasoconstriction and elevation in systemic vascular resistance [29]. Rat studies suggest that vasopressin may actually lower pulmonary vascular resistance via the localized release of nitric oxide [31]. An added benefit of vasopressin is that its efficacy remains intact in the presence of profound hypoxia and acidemia, both of which are common in advanced RV failure [29]. Phenylephrine is another pure vasopressor which acts on α-adrenergic receptors in the periphery. Phenylephrine has been shown to increase aortic and right ventricular coronary driving pressure in both animal studies and humans and may promote RV perfusion allowing for recovery of the right ventricle [32, 33]. Vasopressin or phenylephrine can be used in combination with inotropic agents in patients with RV failure and hypotension.
Afterload Reduction
The right ventricle responds poorly to even minimal, acute increases in afterload, and function can recover quite rapidly with an acute reduction in RV afterload in select patients such as after pulmonary endarterectomy or lung transplantation [34, 35]. Selective pulmonary vasodilators can be used in the acute setting to reduce RV afterload; however, caution must be used to avoid systemic hypotension. In general, pulmonary vasodilators should only be used after optimization of RV perfusion and cardiac output. Also, caution must be used when initiating vasodilators in the setting of elevated pulmonary capillary wedge pressures to avoid pulmonary edema [28]. Agents can be subdivided according to their mechanism of action and route of administration.
Inhaled nitric oxide (NO) is a powerful pulmonary vasodilator that works by increasing production of cyclic guanosine monophosphate (cGMP). NO is rapidly inactivated by hemoglobin and therefore has a short half-life meaning that it is easy to titrate. The short half-life does necessitate the need for a continuous delivery system [36]. Inhaled NO improves hemodynamics and RV performance in a variety of clinical settings including postsurgical pulmonary hypertension, acute pulmonary embolism, RV myocardial infarction, acute RV failure following left ventricular assist device implantation, and cardiac transplantation [37–41]. Prolonged use of NO can lead to accumulation of toxic metabolites, reactive nitrogen species, and methemoglobinemia [42].
Prostacyclins, including epoprostenol, treprostinil, and iloprost, increase the synthesis of cyclic adenosine monophsophate (cAMP) leading to vasodilation. Prostacyclins, both in their intravenous form and in their inhaled form, can improve RV performance by reducing pulmonary vascular resistance. Whereas inhaled prostacyclins are selective and only lead to vasodilation in aerated lung segments, intravenous prostacyclins are less selective leading to a global reduction in systemic vascular resistance and can lead to worsening hypoxia from ventilatory-perfusion mismatch in patients with pulmonary disease [43]. Inhaled epoprostenol is generally the preferred agent in the ICU setting given its short half-life and ease of use [28]. Inhaled epoprostenol improves pulmonary artery pressures and cardiac index comparably to inhaled nitric oxide in patients with right heart failure after heart and lung transplantation and may be more effective at reducing afterload in patients with pulmonary hypertension [44–46]. Inhaled prostacyclins are generally more cost-effective than inhaled nitric oxide and have become the preferred inhaled pulmonary vasodilator in many centers.
Once stabilized with either intravenous or inhaled pulmonary vasodilators, patients can be transitioned to oral agents including phosphodiesterase type 5 (PDE5)-inhibitors such as sildenafil, or tadalafil, endothelin receptor antagonists such as ambrisentan, bosentan, or macitentan or in select patients with pulmonary arterial hypertension or inoperable chronic thromboembolic pulmonary hypertension, the novel soluble guanylate cyclase stimulator, riociguat [47–49, 50•, 51•]. It must be emphasized that these agents have only been studied in patients with chronic disease, and limited data is available on their use in the setting of acute RV failure. Small studies suggest that PDE5 inhibitors may facilitate NO weaning and minimize rebound pulmonary hypertension following discontinuation of inhaled pulmonary vasodilators [52]. PDE5 inhibitors can also improve RV performance and pulmonary vascular resistance in patients with RV failure following left ventricular assist device implantation [53].
Rhythm Control
The failing RV is extremely sensitive to atrial tachyarrhythmias and atrioventricular dyssynchrony. Animal studies suggest that augmentation of right atrial contraction to enhance RV filling is an important adaptive response to improve RV function [54]. Accordingly, loss of atrioventricular synchrony is poorly tolerated. Patients who regain atrioventricular synchrony after placement of temporary dual-chamber or atrial pacing that leads following right ventricular infarct have improved hemodynamics and occasionally complete reversal of hypotension and shock [55, 56]. The tachycardia associated with the majority of atrial tachyarrhythmias is poorly tolerated as this increases RV workload and further worsens the constant supply demand mismatch present with RV failure. Beta blockers and calcium channel blockers should be avoided in acute RV failure as they impair ventricular contraction. Digitalis can be considered for rate control; however, rate control alone is often not enough, especially in patients with coexisting pulmonary hypertension, and a rhythm control strategy must often be utilized [13]. Aggressive use of antiarrhythmic medications, most commonly amiodarone and/or early electrical cardioversion, is often needed. Radiofrequency ablation of easily targeted atrial arrhythmias such as isthmus-dependent atrial flutter can also be considered; however, peri-procedural morbidity and mortality are high, and atrial arrhythmias tend to recur [57, 58].
Percutaneous and Surgical Interventions in Acute Right Ventricular Failure
When right ventricular failure is refractory to pharmacotherapy, percutaneous and surgical interventions can be considered to unload the failing right ventricle for palliation, as a bridge to recovery, or in select patients, as a bridge to heart or simultaneous heart-lung transplantation [59, 60].
Controlled Right to Left Shunts
As described above, the struggling right ventricle responds poorly to increases in afterload. Early observational studies suggested that patients with patent foramen ovale had improved survival in the setting of pulmonary hypertension [61]. Accordingly, considerable attention has been given to both percutaneous and surgical creation of controlled right to left shunts that can instantaneously reduce right ventricular afterload. In patients with advanced pulmonary arterial hypertension, balloon atrial septostomy leads to a decrease in right ventricular end-diastolic pressure, increase in systemic arterial oxygen saturation, increase in cardiac index, and improved functional status [62]. In select patients with pulmonary hypertension, atrial septostomy can improve symptoms and serve as a bridge to heart-lung transplantation [63]. While limited studies have suggested that balloon atrial septostomy may be useful in patients with pulmonary hypertension and preserved RV function, its role in patients with RV failure appears less certain and can lead to systemic hypoxemia and RV ischemia [64]. Similarly, closing a patent foramen ovale in the setting of acute right ventricular failure is contraindicated as the shunt acts as a compensatory pressure offload for the right ventricle. Alternatively, the Potts shunt, a communication between the pulmonary artery and descending aorta has been shown to improve RV function and functional status in patients with congenital transposition of the great arteries and RV failure [65]. In pulmonary arterial hypertension, both surgical and percutaneous creation of the Potts shunt has been shown to improve functional status and prolong survival [66, 67].
Right Ventricular Support Devices
Mechanical circulatory support for the failing right ventricle can serve as an important bridging strategy in patients for whom there is hope of right ventricular recovery or who are candidates for transplantation. Mechanical circulatory support comes in many different forms, but all serve to unload the right ventricle, thereby decreasing the workload of the right ventricle leading to a more favorable supply-demand profile allowing the ventricle to rest and recover. Right ventricular assist devices (RVAD) in the form of centrifugal pumps can be surgically implanted (CentriMag, Thoratec Corporation, Pleasanton, CA) or percutaneously inserted (TandemHeart, Cardiac Assist, Pittsburg, PA) with or without an oxygenator and divert blood from the vena cava or right atrium to the pulmonary artery or left atrium, effectively bypassing the right ventricle. Right ventricular assist devices have been successfully used in patients with RV failure following right ventricular infarct, postcartiotomy shock, cardiac transplantation, and left ventricular assist device implantation [68–71].
The Impella RP (Abiomed Inc, Danvers, MA) is a novel percutaneous, axial flow, investigational device designed to support the right ventricle with a single vascular access. The Impella RP is introduced through the femoral vein and positioned across the tricuspid valve and pulmonic valve and can provide greater than 4 l of flow from the right atrium to the pulmonary artery [72]. In patients who develop RV failure within 48 h after left ventricular assist device implantation, postcardiotomy shock or after myocardial infarction, implantation of the Impella RP was associated with a 73 % 30-day survival [73]. A randomized control trial is still needed to better evaluate the efficacy of the Impella RP.
Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) drains deoxygenated blood from the venous circulation, runs it through an oxygenator, and returns oxygenated blood to the arterial system. VA-ECMO can be implanted either surgically or percutaneously and bypasses both ventricles and thus provides biventricular support. By avoiding the pulmonary circulation, VA-ECMO is often preferred in patients with pulmonary hypertension and pulmonary emboli or in pulmonary hypertension with hypoxia from a reversable cause as a bridge to recovery [74]. Major limitations to VA-ECMO include high rates of bleeding, vascular complications, thromboembolism, and immobility.
Conclusion
Right ventricular failure and decompensated pulmonary hypertension are common challenges in the intensive care unit that require prompt recognition and tailored therapies. Although limited by a paucity of large-scale randomized control trials, current treatment strategies, based on evidence-based studies and physiologic principles, target RV support and recovery with pharmacologic agents and/or mechanical unloading of the right ventricle. Ultimately, recovery depends on timely identification and treatment of the underlying cause of decompensation. When recovery is not possible, heart or heart-lung transplantation remains an option in selected patients.
Abbreviations
- RV:
-
Right ventricle
- LV:
-
Left ventricle
- PH:
-
Pulmonary hypertension
- ICU:
-
Intensive care unit
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115(5):343–9.
Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34–41. doi:https://doi.org/10.1016/j.jacc.2013.10.029. The Fifth World Symposium on pulmonary hypertension was held in 2013 in Nice, France and is the most current update on the classification of pulmonary hypertension. Compared to prior iterations, this update aimed better define group I pulmonary hypertension and also to create a common, comprehensive classification for both adult and pediatric patients.
Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34(6):1219–63. doi:https://doi.org/10.1183/09031936.00139009.
Redington AN, Rigby ML, Shinebourne EA, Oldershaw PJ. Changes in the pressure-volume relation of the right ventricle when its loading conditions are modified. Br Heart J. 1990;63(1):45–9.
Chin KM, Kim NH, Rubin LJ. The right ventricle in pulmonary hypertension. Coron Artery Dis. 2005;16(1):13–8.
Greyson CR. The right ventricle and pulmonary circulation: basic concepts. Rev Esp Cardiol. 2010;63(1):81–95.
Santamore WP, Dell’Italia LJ. Ventricular interdependence: significant left ventricular contributions to right ventricular systolic function. Prog Cardiovasc Dis. 1998;40(4):289–308.
Green EM, Givertz MM. Management of acute right ventricular failure in the intensive care unit. Curr Heart Fail Rep. 2012;9(3):228–35. doi:https://doi.org/10.1007/s11897-012-0104-x.
Matthews JC, McLaughlin V. Acute right ventricular failure in the setting of acute pulmonary embolism or chronic pulmonary hypertension: a detailed review of the pathophysiology, diagnosis, and management. Curr Cardiol Rev. 2008;4(1):49–59. doi:https://doi.org/10.2174/157340308783565384.
Mebazaa A, Karpati P, Renaud E, Algotsson L. Acute right ventricular failure—from pathophysiology to new treatments. Intensive Care Med. 2004;30(2):185–96. doi:https://doi.org/10.1007/s00134-003-2025-3.
Dias CA, Assad RS, Caneo LF, Abduch MC, Aiello VD, Dias AR, et al. Reversible pulmonary trunk banding. II. An experimental model for rapid pulmonary ventricular hypertrophy. J Thorac Cardiovasc Surg. 2002;124(5):999–1006.
Chen EP, Akhter SA, Bittner HB, Koch WJ, Davis RD, Van Trigt P. Molecular and functional mechanisms of right ventricular adaptation in chronic pulmonary hypertension. Ann Thorac Surg. 1999;67(4):1053–8.
Hoeper MM, Granton J. Intensive care unit management of patients with severe pulmonary hypertension and right heart failure. Am J Respir Crit Care Med. 2011;184(10):1114–24. doi:https://doi.org/10.1164/rccm.201104-0662CI.
Sztrymf B, Souza R, Bertoletti L, Jaïs X, Sitbon O, Price LC, et al. Prognostic factors of acute heart failure in patients with pulmonary arterial hypertension. Eur Respir J. 2010;35(6):1286–93. doi:https://doi.org/10.1183/09031936.00070209.
Gille J, Seyfarth HJ, Gerlach S, Malcharek M, Czeslick E, Sablotzki A. Perioperative anesthesiological management of patients with pulmonary hypertension. Anesthesiol Res Pract. 2012;2012:356982. doi:https://doi.org/10.1155/2012/356982.
Naeije R. Physiology of the pulmonary circulation and the right heart. Curr Hypertens Rep. 2013;15(6):623–31. doi:https://doi.org/10.1007/s11906-013-0396-6.
Apstein CS, Lorell BH. The physiological basis of left ventricular diastolic dysfunction. J Card Surg. 1988;3(4):475–85.
Ruiter G, Lankhorst S, Boonstra A, Postmus PE, Zweegman S, Westerhof N, et al. Iron deficiency is common in idiopathic pulmonary arterial hypertension. Eur Respir J. 2011;37(6):1386–91. doi:https://doi.org/10.1183/09031936.00100510.
Myles PS, Hall JL, Berry CB, Esmore DS. Primary pulmonary hypertension: prolonged cardiac arrest and successful resuscitation following induction of anesthesia for heart-lung transplantation. J Cardiothorac Vasc Anesth. 1994;8(6):678–81.
Pritts CD, Pearl RG. Anesthesia for patients with pulmonary hypertension. Curr Opin Anaesthesiol. 2010;23(3):411–6. doi:https://doi.org/10.1097/ACO.0b013e32833953fb.
Balanos GM, Talbot NP, Dorrington KL, Robbins PA. Human pulmonary vascular response to 4 h of hypercapnia and hypocapnia measured using Doppler echocardiography. J Appl Physiol (1985). 2003;94(4):1543–51. doi:https://doi.org/10.1152/japplphysiol.00890.2002.
Jardin F, Vieillard-Baron A. Right ventricular function and positive pressure ventilation in clinical practice: from hemodynamic subsets to respirator settings. Intensive Care Med. 2003;29(9):1426–34. doi:https://doi.org/10.1007/s00134-003-1873-1.
Sarnoff SJ. Myocardial contractility as described by ventricular function curves; observations on Starling’s law of the heart. Physiol Rev. 1955;35(1):107–22.
Cohn JN, Guiha NH, Broder MI, Limas CJ. Right ventricular infarction. Clinical and hemodynamic features. Am J Cardiol. 1974;33(2):209–14.
Mercat A, Diehl JL, Meyer G, Teboul JL, Sors H. Hemodynamic effects of fluid loading in acute massive pulmonary embolism. Crit Care Med. 1999;27(3):540–4.
Piazza G, Goldhaber SZ. The acutely decompensated right ventricle: pathways for diagnosis and management. Chest. 2005;128(3):1836–52. doi:https://doi.org/10.1378/chest.128.3.1836.
Evans DC, Doraiswamy VA, Prosciak MP, Silviera M, Seamon MJ, Rodriguez Funes V, et al. Complications associated with pulmonary artery catheters: a comprehensive clinical review. Scand J Surg. 2009;98(4):199–208.
Price LC, Wort SJ, Finney SJ, Marino PS, Brett SJ. Pulmonary vascular and right ventricular dysfunction in adult critical care: current and emerging options for management: a systematic literature review. Crit Care. 2010;14(5):R169. doi:https://doi.org/10.1186/cc9264.
Overgaard CB, Dzavík V. Inotropes and vasopressors: review of physiology and clinical use in cardiovascular disease. Circulation. 2008;118(10):1047–56. doi:https://doi.org/10.1161/CIRCULATIONAHA.107.728840.
Kwak YL, Lee CS, Park YH, Hong YW. The effect of phenylephrine and norepinephrine in patients with chronic pulmonary hypertension*. Anaesthesia. 2002;57(1):9–14.
Russ RD, Walker BR. Role of nitric oxide in vasopressinergic pulmonary vasodilatation. Am J Physiol. 1992;262(3 Pt 2):H743–7.
Vlahakes GJ, Turley K, Hoffman JI. The pathophysiology of failure in acute right ventricular hypertension: hemodynamic and biochemical correlations. Circulation. 1981;63(1):87–95.
Rich S, Gubin S, Hart K. The effects of phenylephrine on right ventricular performance in patients with pulmonary hypertension. Chest. 1990;98(5):1102–6.
D’Armini AM, Zanotti G, Ghio S, Magrini G, Pozzi M, Scelsi L, et al. Reverse right ventricular remodeling after pulmonary endarterectomy. J Thorac Cardiovasc Surg. 2007;133(1):162–8. doi:https://doi.org/10.1016/j.jtcvs.2006.08.059.
Kramer MR, Valantine HA, Marshall SE, Starnes VA, Theodore J. Recovery of the right ventricle after single-lung transplantation in pulmonary hypertension. Am J Cardiol. 1994;73(7):494–500.
Griffiths MJ, Evans TW. Inhaled nitric oxide therapy in adults. N Engl J Med. 2005;353(25):2683–95. doi:https://doi.org/10.1056/NEJMra051884.
Inglessis I, Shin JT, Lepore JJ, Palacios IF, Zapol WM, Bloch KD, et al. Hemodynamic effects of inhaled nitric oxide in right ventricular myocardial infarction and cardiogenic shock. J Am Coll Cardiol. 2004;44(4):793–8. doi:https://doi.org/10.1016/j.jacc.2004.05.047.
Rich GF, Murphy GD, Roos CM, Johns RA. Inhaled nitric oxide. Selective pulmonary vasodilation in cardiac surgical patients. Anesthesiology. 1993;78(6):1028–35.
Schenk P, Mittermayer C, Ratheiser K. Inhaled nitric oxide in a patient with severe pulmonary embolism. Ann Emerg Med. 1999;33(6):710–4.
Macdonald PS, Keogh A, Mundy J, Rogers P, Nicholson A, Harrison G, et al. Adjunctive use of inhaled nitric oxide during implantation of a left ventricular assist device. J Heart Lung Transplant. 1998;17(3):312–6.
Carrier M, Blaise G, Bélisle S, Perrault LP, Pellerin M, Petitclerc R, et al. Nitric oxide inhalation in the treatment of primary graft failure following heart transplantation. J Heart Lung Transplant. 1999;18(7):664–7.
Christenson J, Lavoie A, O’Connor M, Bhorade S, Pohlman A, Hall JB. The incidence and pathogenesis of cardiopulmonary deterioration after abrupt withdrawal of inhaled nitric oxide. Am J Respir Crit Care Med. 2000;161(5):1443–9. doi:https://doi.org/10.1164/ajrccm.161.5.9806138.
Channick RN, Hoch RC, Newhart JW, Johnson FW, Smith CM. Improvement in pulmonary hypertension and hypoxemia during nitric oxide inhalation in a patient with end-stage pulmonary fibrosis. Am J Respir Crit Care Med. 1994;149(3 Pt 1):811–4. doi:https://doi.org/10.1164/ajrccm.149.3.8118653.
Khan TA, Schnickel G, Ross D, Bastani S, Laks H, Esmailian F, et al. A prospective, randomized, crossover pilot study of inhaled nitric oxide versus inhaled prostacyclin in heart transplant and lung transplant recipients. J Thorac Cardiovasc Surg. 2009;138(6):1417–24. doi:https://doi.org/10.1016/j.jtcvs.2009.04.063.
Hoeper MM, Olschewski H, Ghofrani HA, Wilkens H, Winkler J, Borst MM, et al. A comparison of the acute hemodynamic effects of inhaled nitric oxide and aerosolized iloprost in primary pulmonary hypertension. German PPH study group. J Am Coll Cardiol. 2000;35(1):176–82.
Hoeper MM, Schwarze M, Ehlerding S, Adler-Schuermeyer A, Spiekerkoetter E, Niedermeyer J, et al. Long-term treatment of primary pulmonary hypertension with aerosolized iloprost, a prostacyclin analogue. N Engl J Med. 2000;342(25):1866–70. doi:https://doi.org/10.1056/NEJM200006223422503.
Sastry BK, Narasimhan C, Reddy NK, Raju BS. Clinical efficacy of sildenafil in primary pulmonary hypertension: a randomized, placebo-controlled, double-blind, crossover study. J Am Coll Cardiol. 2004;43(7):1149–53. doi:https://doi.org/10.1016/j.jacc.2003.10.056.
Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358(9288):1119–23. doi:https://doi.org/10.1016/S0140-6736(01)06250-X.
Ghofrani HA, Simonneau G, Rubin LJ. PATENT-1 AoC-a. Riociguat for pulmonary hypertension. N Engl J Med. 2013;369(23):2268. doi:https://doi.org/10.1056/NEJMc1312903.
Ghofrani HA, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369(4):319–29. doi:https://doi.org/10.1056/NEJMoa1209657. Riociguat represents a new class of drugs, the soluble guanylate cyclase stimulators, which was shown to be beneficial in patients with chronic thromboembolic pulmonary hypertension. In patients with inoperable chronic thromboembolic pulmonary hypertension or persistent pulmonary hypertension after pulmonary embolectomy, riociguat led to a significant improvement in 6-minute walk distance, decrease in pulmonary vascular resistance, improvement in NT-proBNP and imrovement in WHO functional class.
Ghofrani HA, Galiè N, Grimminger F, Grünig E, Humbert M, Jing ZC, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369(4):330–40. doi:https://doi.org/10.1056/NEJMoa1209655. In this randomized, double-blind study, compared to placebo, patients with pulmonary arterial hypertension treated with riociguat had improved 6-minute walk distance, pulmonary vascular resistance, NT-proBNP, WHO functional class, time to clinical worsening and Borg dyspnea score.
Trachte AL, Lobato EB, Urdaneta F, Hess PJ, Klodell CT, Martin TD, et al. Oral sildenafil reduces pulmonary hypertension after cardiac surgery. Ann Thorac Surg. 2005;79(1):194–7. doi:https://doi.org/10.1016/j.athoracsur.2004.06.086. discussion −7.
Tedford RJ, Hemnes AR, Russell SD, Wittstein IS, Mahmud M, Zaiman AL, et al. PDE5A inhibitor treatment of persistent pulmonary hypertension after mechanical circulatory support. Circ Heart Fail. 2008;1(4):213–9. doi:https://doi.org/10.1161/CIRCHEARTFAILURE.108.796789.
Goldstein JA, Harada A, Yagi Y, Barzilai B, Cox JL. Hemodynamic importance of systolic ventricular interaction, augmented right atrial contractility and atrioventricular synchrony in acute right ventricular dysfunction. J Am Coll Cardiol. 1990;16(1):181–9.
Love JC, Haffajee CI, Gore JM, Alpert JS. Reversibility of hypotension and shock by atrial or atrioventricular sequential pacing in patients with right ventricular infarction. Am Heart J. 1984;108(1):5–13.
Topol EJ, Goldschlager N, Ports TA, Dicarlo LA, Schiller NB, Botvinick EH, et al. Hemodynamic benefit of atrial pacing in right ventricular myocardial infarction. Ann Intern Med. 1982;96(5):594–7.
Bradfield J, Shapiro S, Finch W, Tung R, Boyle NG, Buch E, et al. Catheter ablation of typical atrial flutter in severe pulmonary hypertension. J Cardiovasc Electrophysiol. 2012;23(11):1185–90. doi:https://doi.org/10.1111/j.1540-8167.2012.02387.x.
Garlitski AC, Mark Estes NA. Ablation of atrial flutter in severe pulmonary hypertension: pushing the outside of the envelope. J Cardiovasc Electrophysiol. 2012;23(11):1191–2. doi:https://doi.org/10.1111/j.1540-8167.2012.02401.x.
Fang JC, DeMarco T, Givertz MM, Borlaug BA, Lewis GD, Rame JE, et al. World Health Organization Pulmonary Hypertension group 2: pulmonary hypertension due to left heart disease in the adult—a summary statement from the Pulmonary Hypertension Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2012;31(9):913–33. doi:https://doi.org/10.1016/j.healun.2012.06.002.
Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol. 2013;62(25 Suppl):D22–33. doi:https://doi.org/10.1016/j.jacc.2013.10.027.
Rozkovec A, Montanes P, Oakley CM. Factors that influence the outcome of primary pulmonary hypertension. Br Heart J. 1986;55(5):449–58.
Sandoval J, Gaspar J, Pulido T, Bautista E, Martínez-Guerra ML, Zeballos M, et al. Graded balloon dilation atrial septostomy in severe primary pulmonary hypertension. A therapeutic alternative for patients nonresponsive to vasodilator treatment. J Am Coll Cardiol. 1998;32(2):297–304.
Reichenberger F, Pepke-Zaba J, McNeil K, Parameshwar J, Shapiro LM. Atrial septostomy in the treatment of severe pulmonary arterial hypertension. Thorax. 2003;58(9):797–800.
Rich S, Dodin E, McLaughlin VV. Usefulness of atrial septostomy as a treatment for primary pulmonary hypertension and guidelines for its application. Am J Cardiol. 1997;80(3):369–71.
Blanc J, Vouhé P, Bonnet D. Potts shunt in patients with pulmonary hypertension. N Engl J Med. 2004;350(6):623. doi:https://doi.org/10.1056/NEJM200402053500623.
Baruteau AE, Serraf A, Lévy M, Petit J, Bonnet D, Jais X, et al. Potts shunt in children with idiopathic pulmonary arterial hypertension: long-term results. Ann Thorac Surg. 2012;94(3):817–24. doi:https://doi.org/10.1016/j.athoracsur.2012.03.099.
Esch JJ, Shah PB, Cockrill BA, Farber HW, Landzberg MJ, Mehra MR, et al. Transcatheter Potts shunt creation in patients with severe pulmonary arterial hypertension: initial clinical experience. J Heart Lung Transplant. 2013;32(4):381–7. doi:https://doi.org/10.1016/j.healun.2013.01.1049.
Kaul TK, Kahn DR. Postinfarct refractory right ventricle: right ventricular exclusion. A possible option to mechanical cardiac support, in patients unsuitable for heart transplant. J Cardiovasc Surg (Torino). 2000;41(3):349–55.
Moazami N, Pasque MK, Moon MR, Herren RL, Bailey MS, Lawton JS, et al. Mechanical support for isolated right ventricular failure in patients after cardiotomy. J Heart Lung Transplant. 2004;23(12):1371–5. doi:https://doi.org/10.1016/j.healun.2003.09.022.
Furukawa K, Motomura T, Nosé Y. Right ventricular failure after left ventricular assist device implantation: the need for an implantable right ventricular assist device. Artif Organs. 2005;29(5):369–77. doi:https://doi.org/10.1111/j.1525-1594.2005.29063.x.
Klima U, Ringes-Lichtenberg S, Warnecke G, Lichtenberg A, Strüber M, Haverich A. Severe right heart failure after heart transplantation. A single-center experience. Transpl Int. 2005;18(3):326–32. doi:https://doi.org/10.1111/j.1432-2277.2004.00059.x.
Cheung A, Freed D, Hunziker P, Leprince P. TCT-371 first clinical evaluation of a novel percutaneous right ventricular assist device: the Impella RP. J Am Coll Cardiol. 2012;60(17_S). doi:https://doi.org/10.1016/j.jacc.2012.08.399.
O’Neil WW. A prospective multicenter study to evaluate a new percutaneous ventricular assist device for right ventricular failure: the RECOVER right study. Presented at the Cardiovascular Research Foundation’s annual Transcatheter Cardiovascular Therapeutics 2014 scientific meeting in Washington, DC. 2014
Belohlavek J, Rohn V, Jansa P, Tosovsky J, Kunstyr J, Semrad M, et al. Veno-arterial ECMO in severe acute right ventricular failure with pulmonary obstructive hemodynamic pattern. J Invasive Cardiol. 2010;22(8):365–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest The University of Chicago receives research grant support from Actelion, Gilead, Novartis, Medtronic, Lung Biotechnology, and Reata for Dr. Gomberg-Maitland to be a principal investigator on research grants. Dr. Gomberg-Maitland has served as a consultant for Actelion, Bayer, Gilead, Medtronic, Merck, Bellerophon (formerly known as Ikaria), and United Therapeutics as a member of steering committees and DSMB/event committees. Jonathan Grinstein declares no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pulmonary Hypertension
Rights and permissions
About this article
Cite this article
Grinstein, J., Gomberg-Maitland, M. Management of Pulmonary Hypertension and Right Heart Failure in the Intensive Care Unit. Curr Hypertens Rep 17, 32 (2015). https://doi.org/10.1007/s11906-015-0547-z
Published:
DOI: https://doi.org/10.1007/s11906-015-0547-z