Abstract
Purpose of Review
The purpose of this study is to review the recent literature analyzing the performance of Liver Imaging Reporting and Data System (LI-RADS) v2018 diagnostic and treatment response algorithm (TRA) for initial diagnosis and assessment of hepatocellular carcinoma (HCC) following locoregional therapy (LRT).
Recent Findings
LI-RADS is a comprehensive tool for assessment and reporting of observations in patients at risk of developing HCC. Since HCC is predominantly an imaging diagnosis, it is important to achieve a high sensitivity and specificity for each LR category. Therefore, a multitude of studies have been published over the recent years illustrating the diagnostic yield of both the diagnostic and treatment response algorithms. In addition, the role of abbreviated MRI for screening has also been studied recently.
Summary
LI-RADS diagnostic algorithm has been validated by a number of recent studies that have provided a high diagnostic reliability for categorizing each observation, when using major as well as combination of major and ancillary features. In addition, LI-RADS TRA is being validated by the emerging literature providing promising results for treatment of HCC following ablation and nonradiation-based arterial therapies. However, further insight and in depth research is required to validate the imaging appearance of radiation-based therapies as well as utilization of ancillary features for response assessment after locoregional therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy and third leading cause of cancer-related death worldwide, with a 5-year survival rate of 18% [1]. As the incidence of hepatic cirrhosis and chronic liver disease continues to rise in the USA, there has been a concomitant increase in the frequency of HCC [2, 3, 4•, 5••]. Early detection of HCC is critical for improved overall survival; however, greater than 70% of patients with HCC have advanced disease at diagnosis [2]. Per American Association for the Study of Liver Disease (AASLD) guidelines, the diagnosis of HCC is frequently made based on cross-sectional contrast-enhanced imaging alone, without the need for confirmatory percutaneous biopsy in at-risk populations [2]. Therefore, it is imperative for a radiologist to provide an accurate interpretation of liver imaging in this population, in order to provide appropriate and timely diagnosis that allows for early treatment, as studies show that early detection and improved survival are strongly linked [2, 3].
Initially released in 2011, and with several subsequent revisions, the Liver Imaging Reporting and Data System (LI-RADS) was created to provide standardization of liver imaging, strict diagnostic criteria, a diagnostic algorithm and reporting guidelines for interpretation of observations in at-risk patients, in order to improve radiologist interpretation, reporting [4•] and communication with clinicians. Based on the imaging features, each liver observation is assigned a LI-RADS category which reflects its likelihood of being benign, malignant, or HCC [5••]. Standardization improves communication between radiologists and other clinicians, improves consistency in interpretation and management recommendations [6, 7], and potentially facilitates data collection for research and quality improvement with the long-term goal of positively impacting clinical care, education, and research [8] .
In 2018, LI-RADS was endorsed by and integrated into the AASLD practice guidance for HCC [1]. LI-RADS offers four individual algorithms designed for different clinical contexts: ultrasound (US) LI-RADS for screening and surveillance, CT/MRI LI-RADS algorithm for diagnosis, contrast-enhanced US (CEUS) LI-RADS for diagnosis, and LI-RADS treatment response algorithm (LR-TRA) for response assessment following locoregional therapies (LRT) and resection [4•, 9].
In this article, we review the emerging evidence supporting use of the LI-RADS v2018 CT/MRI diagnostic algorithm. We will also briefly discuss the role of abbreviated MRI for screening at-risk patients. Finally, we will provide a review of emerging evidence supporting the LR-TRA.
Current LI-RADS Imaging Criteria for HCC Diagnosis
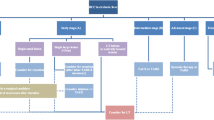
The LI-RADS CT/MRI diagnostic algorithm can only be applied to a specific target population in which the pretest probability of HCC is sufficiently high to attain high specificity (> 95%) of LR-5 (definite HCC) criteria [10]. Each liver observation is categorized from LR-1 (definitely benign) to LR-5, with the categories from 1 to 5 reflecting increasing likelihood of HCC [11••]. LR-1 and LR-2 observations are categorized as definitive or probably benign, respectively [5••]. LR-3 to LR-5 categories correspond to intermediate probability of malignancy, probable HCC, and definite HCC, respectively. The LR-5 category is intended to be 100% specific for HCC, which would preclude the need for percutaneous biopsy for definitive diagnosis [12]. In addition to LR-1 through LR-5, an LR-M (probably or definitely malignant, not HCC specific) category can be assigned to observations that are probably or definitely malignant, but the imaging features are not specific for HCC [13]. While approximately 36% of LR-M observations are HCC, a majority of LR-M represent other malignancies, such as intrahepatic cholangiocarcinoma (iCCA), which may arise in the same at-risk population [14]. The final category, LR-TIV (definite tumor in vein) is used for malignancy that has definite macrovascular invasion on imaging [15]. The assignment of LR-3 to LR-5 categories is based on the combination of major imaging features (i.e., “nonrim arterial phase hyperenhancement [APHE], nonperipheral washout appearance [WO], enhancing capsule appearance, size, and threshold growth [16]) as well as ancillary imaging features, from which a category can be determined by using the CT/MRI diagnostic table [9].
Emerging Evidence on LI-RADS Diagnostic Validation
Since the diagnosis of HCC has predominately become an imaging diagnosis without tissue confirmation, it is critical that the LR-5 category retains a high specificity for HCC. A multitude of studies have investigated the diagnostic accuracy of LI-RADS, including radiology–pathology correlation and outcomes studies (Table 1) [17]. Recently, a large systemic review by van der Pol et al. evaluated the percentage of HCC and malignancy seen in each of the LR categories based on pathology, follow-up imaging analyses, and treatment response [11••]. The review was based on 17 retrospective studies that included 2760 patients, 3556 observations, of which 2482 were HCCs [11••]. Percentage of HCC and other malignancy were 94% and 97% for LR-5, 74% and 80% for LR-4, 38% and 40% for LR-3, 13% and 14% for LR-2, 79% and 92% for LR-TIV, and 36% and 93% for LR-M, respectively [11••]. There were no malignancies found in the LR-1 category, and there was a significant difference in the percentage of confirmed HCCs and overall malignancies (p < 0.00001) in the LR 2–5 categories [11••]. Based on this analysis, the risk of HCC and overall malignancy increases with increasing LR category [11••]. Of note, of 240 LR-M observations, 93% were found to be other malignancy, and 36% were found to be HCC on pathological correlation [11••]. Surprisingly, HCC was found in 14% of LR-2 [11••]. This number is likely elevated as a result of selection bias, however, since the majority of LR-2 observations do not require a biopsy in clinical practice and a subgroup of LR-2 observations necessitating biopsy likely harbor a higher proportion of malignancy than the entire population of LR-2 observations.
In 2017, LI-RADS introduced specific criteria for LR-M category, and LI-RADS v2018 updated LR-5 diagnostic criteria in order to align AASLD with LI-RADS [9]. There have been multiple recent studies evaluating the impact of these changes on diagnosis of HCC. For example, Ren et al. [18•] found that LI-RADS v2018 was superior to v2017, increasing the sensitivity of LR-5 for HCC to 81% vs. 71%, negative predictive value (NPV) 70% vs. 61%, and accuracy 84% vs. 78%, respectively, while maintaining the specificity (92% vs 90%, respectively). Similarly, Lee et al. [19] reported an improved sensitivity of v2018 LR-5 criteria compared to those of v2017 (75% vs 62%, respectively), at the expense of minimal decrease in specificity (91% vs 94%, respectively), A recently published study by Smereka et al. [20] compared the diagnostic efficacy of LI-RADS v2018 with v2017 in categorizing newly identified nodules with nonrim APHE in cirrhotic livers. In this study, LR-3 and LR-4 criteria based on v2018 were more accurate in predicting the rate of progression to HCC, as compared to v2017: the rate of progression of LR-3 observations was 38% based on v2017 and 44% based on v2018, and the rate of progression of LR-4 observations was 58% based on v2017 and 70% based on v2018. A study by Chernyak et al. [21] assessed the concordance in categorization and radiologic T-staging using LI-RADS v2017 and v2018 and Organ Procurement and Transplantation Network (OPTN) criteria. In this study, 73/182 (40%) of 641 total observations which were categorized as LR-4 by LI-RADS v2017 were upstaged to LR-5 according to v2018, while only 4/196 (2%) observations categorized as LR-5 were down-staged to LR-4 based on changes in threshold growth definition between the two versions. Furthermore, in 12% (49/398) of patients, T stage was higher using LI-RADS v2018 compared to v2017 [21]. In summary, a number of recent studies indicate that v2018 has increased sensitivity and improved accuracy of LR-5 category for the diagnosis of HCC.
Ko et al. [22] compared the diagnostic performance of LI-RADS v2018 with v2017 for HCC utilizing gadoxetic acid-enhanced MRI, in which 76% (64/84) of observations with threshold growth using v2017 were reclassified as subthreshold growth when using v2018, ultimately downstaging 14% (9/64) of the cases. They also found that v2018 was inferior to v2017 for observations measuring between 10 and 19 mm in size when considering only major features (accuracy 86% versus 80%, p < 0.013) but was comparable after incorporating the ancillary features (accuracy 87% versus 86%, p = 1). Thus, the two versions demonstrated similar diagnostic performance with added value of ancillary features in combination with major features in v2018 for increased detection of HCC.
A number of recent studies evaluating the utility of ancillary features suggest that their use in combination with major features increases sensitivity while preserving high specificity for HCC diagnosis. For example, Cerny et al. [23] compared the performance of major features alone and major and ancillary features in combination on MRI using LI-RADS v2014 criteria. In this study, the authors found that the combination of major and ancillary features resulted in recategorization of 15% of the lesions, with increased sensitivity for HCC diagnosis on a per-lesion level from 76 to 88%, while preserving specificity. In a study by Joo et al. [24], the frequency of recategorization with ancillary features on MRI was similar (18%); all changes were LR-3 upgraded to LR-4. Finally, a more recent study by Ren et al. [25] reported similar results, with 9% recategorization of observations on CT, when combining major and ancillary features as opposed to major features alone. Ren et al. [25] also reported increased specificity, positive predictive value, and accuracy when LR-5 was used a predictor for HCC when applying combination of major and ancillary features as opposed to major features on multiphasic CT (92% versus 90%, 93% versus 92%, and 69% versus 68%, respectively).
The LI-RADS diagnostic algorithm has a high diagnostic performance and also benefits from high inter-reader reliability [26]. Ludwig et al. [27] analyzed the diagnostic performance and inter-reader agreement of LI-RADS v2018 for distinguishing HCC from non-HCC primary hepatic malignancy in patients at risk for HCC and also assessed the impact of changes introduced in the revised 2018 version. They found a high specificity for distinguishing HCC from non-HCC malignancy (89% and 90% for two readers), with moderate inter-reader reliability (IRR) for overall LI-RADS category (k = 0.5) and substantial IRR for LR-5 versus LR-M and LR-TIV (k = 0.64) observations. Multiple additional publications have also reported excellent IRR of all LR categories on MRI when using LI-RADS v2018. For example, Razek et al. [28•] demonstrated an interobserver agreement of k = 0.89 for LR-4 observations and k = 0.84 for LR-5 observations (p = 0.001), with 91% agreement. Stocker et al. [29] compared qualitative and quantitative assessment of APHE and WO to assess diagnostic accuracy and inter-reader agreement of LI-RADS v2018, with results revealing similar performance of LI-RADS on quantitative and qualitative analysis (k = 0.38 and 0.4–0.47). However, there was improved agreement for quantitatively assessed APHE (κ = 0.65 vs. 0.81) when compared to WO (κ = 0.53 vs. 0.78).
Despite the successes of the LI-RADS diagnostic algorithm, the management of LR-3 observations remains an open question. LR-3 category suggests intermediate probability of malignancy, and studies show that 72–93% of LR 3 observations are benign on final pathology [30, 31]. Nevertheless, a non-negligible percentage of LR-3 observations are indeed HCC on pathology, and a substantial proportion of LR-3 observations progress to LR-5. Therefore, it is important to determine the clinical significance of these intermediate probability lesions and their potential to evolve into HCC. For example, a retrospective study utilizing hepatobiliary contrast agent to evaluate LR-3 observations over the period of 11.2 months, found that only 6% of LR-3 observations were upgraded to probable or definite HCC, while 80% remained unchanged and 14% decreased in size or disappeared on follow-up [32]. Shropshire et al. [33] showed that major and ancillary features were not significantly associated with a change in the LR-3 observation category, and instead, this category resulted in longer follow-up times (mean follow-up 20.3 ± 13.4 months, range 2.9–45.2 months), as 40% of lesions remained categorized as LR-3, 50% were down categorized to LR-2 or LR-1, and only 10% progressed to LR-4 or LR-5 on follow-up. Regardless of the low risk of malignancy, the LR-3 observations should be continued to follow. Future studies are needed to better risk-stratify LR-3 observations, to determine which require shorter interval follow-up.
The LR-M category was designed to include malignancies of non-hepatocellular origin in order to preserve the high specificity of LR-5 category for diagnosing HCC. Up to 28–36% of HCCs do not demonstrate “classic” imaging features and are thus characterized as LR-M observations [11••, 34]. An LR-M category is assigned when at least one targetoid LR-M feature (i.e., rim APHE, peripheral WO, delayed central enhancement, targetoid restricted diffusion, or targetoid appearance on transitional or hepatobiliary phase) is present [9]. LR-M category is also assigned to observations not meeting LR-5 or LR-TIV criteria [9] and with nontargetoid LR-M features (infiltrative appearance, severe ischemia/necrosis, markedly restricted diffusion). Kim et al. compared the diagnostic performance of LR-5 and LR-M in LI-RADS v2018 for distinguishing HCC from other malignancy (OM) [35•]. In this study, imaging findings were evaluated from 55 patients with OM (histologically proven cases of iCCA (n = 16), combined HCC and cholangiocarcinoma (cHCC-CCA) (n = 31), metastatic adenocarcinoma (n = 7) and angiosarcoma (n = 1)) and 165 histologically proven HCC [35•]. The LR-M category was found to have a sensitivity and specificity of 86% and 48% for the diagnosis of OM, respectively [35•]. On the other hand, 11% (6/55) of the patients with OM satisfied the LR-5 criteria [35•]. The LR-5 category showed a high specificity (89%) for the diagnosis of HCC and was significantly more accurate than LR-M (78% versus 58%, p < 0.01) [35•]. The low specificity of LR-M category for other malignancy was explained by high number of biopsy-proven HCC (86/165) exhibiting imaging features of LR-M in this retrospective study [35•].
Given that a third of LR-M lesions are ultimately HCC on pathology, Park et al. assessed different imaging features to identify if there is a way to differentiate HCC categorized as LR-M from non-HCC malignancies on gadoxetic acid enhancement MRI using LI-RADS v2018 [36]. The authors devised a classification tree analysis model that had a sensitivity of 64%, specificity of 90%, and accuracy of 81% for differentiating HCC categorized as LR-M from non-HCC malignancy [36]. Results of the study revealed a total of 36 (28%) HCC and 70 (78%) non-HCC malignancies categorized as LR-M, with features such as enhancing capsule (p = 0.0293), blood products in the mass (p = 0.0393), and nontargetoid restriction (p = 0.018) as independent predictors of HCC, compared to other malignancies. A recent study by Kim et al. in which 165 high-risk patients for HCC had pathology confirmed primary liver cancer showed a high rate of sensitivity of the LR-M category for diagnosing malignancy, with sensitivities of 88–93% [37].
Role of Abbreviated MR
While US is an accepted modality for HCC screening and surveillance, its performance can be limited in some patients, particularly in the setting of steatosis and severe cirrhosis [38]. In some patients, CT or MRI serve as both surveillance and diagnosis tools. Although MRI is reported to have the highest accuracy for HCC diagnosis of all available modalities [39, 40], its cost and length of exam may be prohibitive from widespread utilization. Consequently, there has been a recent surge in literature evaluating the use of an abbreviated MRI (aMRI) protocol for screening and surveillance of HCC (Table 1). In all published studies to date, a radiologist interprets both the full and abbreviated MRI to compare the sensitivity of each. A recent study by Khatri et al. retrospectively compared the sensitivity of a complete MRI to aMRI (coronal T2-weighted and axial dynamic post-contrast T1-weighted fat-suppressed sequences). In this study, in which 93 patients with 121 liver observations met inclusion criteria, they reported a sensitivity and specificity of aMRI at 93 and 89% compared to complete MRI at 94 and 88%, respectively, suggesting that an abbreviated protocol screening MRI is not only interchangeable, but equivalent to a complete diagnostic MRI in cirrhotic patients for HCC detection [41•]. In another study, cirrhotic patients who underwent gadoxetic acid-enhanced abbreviated MRI utilizing axial DWI with apparent diffusion coefficient (ADC) maps, axial T2-weighted single-shot fast spin echo, and axial T1-weighted three-dimensional gradient echo fat-suppressed hepatobiliary phase imaging revealed that the abbreviated MRI had a sensitivity of 92% and specificity of 91% for detection of HCC [42]. A third recent study with 188 patients investigated the role of abbreviated non-contrast MRI protocol, utilizing axial T2-weighted sequence, axial T1-weighted Dixon sequence (in-phase, opposed-phase, fat and water), DWI with ADC maps, as a screening alternative in patients with poor-quality screening ultrasounds. They too reported a high specificity (93%) and negative predictive value (97%) with a sensitivity of 85% when compared to that of ultrasound and gadoxetic acid contrast-enhanced MRI [38]. Thus, early data suggests that an abbreviated MRI protocol can be used for screening and surveillance of HCC.
Treatment of HCC
Approximately 80% of cirrhotic patients diagnosed with HCC are non-surgical candidates, resulting in increasing use of locoregional therapies (LRT). Over the past few years, the number of LRT options for patients with HCC have increased; these include thermal ablation (e.g., microwave, radiofrequency, cryoablation), percutaneous ethanol injection (PEI), transarterial chemoembolization (TACE), transarterial bland embolization (TAE), transarterial radioembolization (TARE), and stereotactic body radiotherapy (SBRT) [43, 44••, 45, 46]. LRT can be used alone, in combination with each other, or in combination with systemic therapy, and has been shown to improve disease-free and overall survival in non-surgical candidates [47,48,49]. In addition, LRT can help prolong time to progression, palliate symptoms, prevent lesions from progressing outside of Milan criteria in order to maintain liver transplant candidacy (bridge to transplant), and convert nontransplant candidates to transplant candidates based on Milan criteria (downstage to transplant) [50,51,52]. Treatment decisions are usually made by a multidisciplinary tumor board and depend on patient factors, including tumor location, size and multiplicity, disease stage, liver function, performance status, technical feasibility, and potential for future transplant candidacy [53, 54].
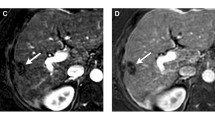
Following treatment with LRT, it is important to provide accurate treatment response assessment to help guide clinical management. The imaging appearance of HCC following LRT depends on type of LRT and is distinct for each treatment modality. In general, complete loss of APHE is suggestive of nonviable disease. However, post-treatment imaging evaluation is complicated after radiation-based therapies, in which there can be intra-tumoral APHE or nodular or geographic peri-tumoral enhancement that can persist for up to 1 year after therapy and often longer [55••, 56]. Thus, it is imperative to be aware of the different types of LRTs, as well as their expected post-treatment imaging appearance. HCC response assessment can be performed according to various response assessment guidelines, such as the European Association for the Study of Liver Disease (EASL) [57] and modified Response Evaluation Criteria in Solid Tumors (mRECIST) [58], which are patient-level response systems. Alternatively, LI-RADS TRA [45••] can be used to assess response on a per-lesion level.
LI-RADS Treatment Assessment of HCC
LI-RADS treatment response algorithm was released in 2017, to provide a step by step approach for assessment of lesions following LRT [5••, 59]. LI-RADS TRA, modeled after mRECIST, not only includes APHE as a criterion to detect viable tumor but also incorporates imaging findings of washout appearance or treatment-specific enhancement pattern [5••]. Another distinguishing characteristic of LI-RADS TRA is that it provides assessment at a lesion level, which is important for clinical management, as individual lesions may be treated by different LRT over time, with distinct responses.
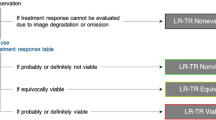
The LI-RADS treatment response categories include LR-TR nonviable, LR-TR equivocal, LR-TR viable, and LR-TR nonevaluable. A unique aspect of LI-RADS TRA is the inclusion of the category of LR-TR equivocal, which allows reporting of lesions in which the imaging appearance does not meet criteria for viable or nonviable disease. Treated tumors are characterized as equivocal when enhancement is atypical for treatment-specific enhancement pattern and not meeting criteria for probably or definitely viable tumor. In this scenario, short-term follow-up imaging is recommended, as opposed to immediate retreatment [1, 5••, 12, 13]. The LR-TR equivocal category is particularly important for tumors treated with intra-arterial and radiation-based therapies which target not only the tumor itself but also the hepatic parenchyma adjacent to the treated tumor, resulting in perfusional alterations visible on post-treatment imaging. In such instances, use of the LR-TR equivocal category helps to prevent unnecessary retreatment. While there is a risk that viable tumor is left untreated, HCC is generally a slow-growing tumor with a doubling time of 86–117 days [60,61,62], thus a wait and watch approach with a 3-month interval imaging may be a safe approach to differentiate residual viable disease from benign parenchymal perfusional alterations.
Current Evidence Validating LI-RADS TRA
Since LR-TRA is relatively novel, only a few publications have assessed its performance thus far (Table 2). Chaudhry et al. [64] and Shropshire et al. [65] both reported that following TACE and TAE, 81–86% and 67–71% lesions categorized as LR-TR nonviable demonstrate 100% necrosis on pathology. This study supports the LR-TR nonviable category for prediction of treatment response.
Both Chaudhry et al. [64] and Shropshire et al. [65] have reported high sensitivity and specificity of the LR-TR viable category. Chaudry et al. [64] reported that 73% of treated lesions categorized as LR-TR viable, were viable on pathology while Shropshire et al. [65] reported 60–65% of LR-TR viable observations were similarly viable on pathology, defined as < 99% pathologic necrosis.
Interestingly, most LI-RADS TRA validation studies have shown high rates of viable disease in the LR-TR equivocal category. In these studies, 71% of tumors treated with TAE and 83% of tumors treated with thermal ablation, which were categorized as LR-TR equivocal, demonstrated viable disease at pathology [64, 65]. This high rate of pathologic viability is likely the result of microscopically viable tumor which cannot be seen on imaging, but can be detected pathologically. Despite these early reports, the LR-TR equivocal category may still serve a role, especially in patients treated with radioembolization or SBRT, to avoid early and repeat retreatment, particularly for those lesions which are truly nonviable.
Several studies have evaluated inter-reader agreement of LR-TRA response categorization. These show moderate inter-reader agreement in assigning LR-TR categories following ablation and nonradiation arterial based therapies for HCC. Cool et al. [66] and Chaudhary et al. [64] have shown an inter-reader agreement of 90% and 95% with kappa of 0.75 and 0.71, respectively, following thermal ablation. Seo et al. evaluated HCC tumors treated with TACE, which were imaged with either CT or MRI and reported kappa values of 0.69 and 0.56, respectively [56]. Shropshire et al. [65] reported kappas of 0.55, in which all tumors were treated with TAE and imaged with MRI. Lower inter-reader agreement in patients treated with intra-arterial therapy, as compared to thermal ablation, is likely related to the complex imaging features seen after transcatheter arterial based therapies, in which abnormal parenchymal enhancement may contribute to uncertainty in imaging findings early post-treatment. A recent study by Abdel Razak et al. [67] evaluated the reproducibility of LI-RADS TRA for HCC after thermal ablation and TACE. They found excellent inter-reader agreement of all LI-RADS TRA categories, 97% for overall treated nonviable HCC (k = 0.94), 98% agreement for LR-TR viable disease (k = 0.96) and 97% for LR-TR equivocal HCC (k = 0.7) [58].
Unlike the LI-RADS diagnostic algorithm, the LI-RADS TRA currently does not use ancillary criteria for imaging assessment. However, new studies evaluating the role of ancillary features suggest increasing sensitivities to detect viable tumor. For example, one recent study [68•] evaluated LR-TRA on MRI with or without the addition of ancillary features to the existing enhancement-based criteria. Using pathologic necrosis confirmed by liver explant or surgical resection as their reference standard in 138 patients with HCC, the authors found that the use of ancillary features increased the sensitivity to detect viable disease on MRI from 76 to 84%, without sacrificing specificity (80% vs 83%). These results are comparable to a recent study by Kim et al., who found an increase in sensitivity of detecting viability from 40–67 to 83–87%, when adding the ancillary features [69]. In both studies, the ancillary features which were used included hepatobiliary phase (HBP) hypointensity, restricted diffusion, and intermediate signal intensity on T2-weighted imaging (WI). In both studies, HBP hypointensity restricted diffusion and intermediate signal intensity on T2-WI were seen in nearly all cases where the readers upgraded an LR-TR equivocal category to LR-TR viable. All three ancillary features resulted in improved sensitivities, (81%, 79%, and 83%, respectively) for detection of viability when compared to MRI alone without ancillary features (76%). Conversely, HBP isointensity was treated as an ancillary feature favoring nonviable tumor and may prove useful when arterial phase hyperenhancement is seen at the margin of an otherwise necrotic treated HCC. In these instances, isointensity on HBP may help increase a radiologist’s confidence that the observed arterial phase hyperenhancement represents perfusional abnormality and not viable tumor.
Of note, both studies revealed a marked reduction in use of the LR-TR equivocal category when incorporating ancillary features into treatment response interpretation. In Park et al., strict adherence to the LR-TR algorithm resulted in the use of LR-TR Equivocal in 9 and 12 cases out of 138 on MRI, by two readers, but incorporation of ancillary features reduced LR-TR Equivocal categorization to 2 and 0 cases, respectively [68•]. In this study, all up-categorized cases had pathologic confirmation of viable tumor [68•]. Similarly, Kim et al. [69] showed that the use of ancillary features reduced the use of the LR-TR equivocal category, from 29 and 59 assignments out of 183 cases by two radiologists, to only 2 and 14 cases, respectively. Both studies suggest improved sensitivities for detection of viable HCC when using ancillary features by correctly changing a majority of LR-TR equivocal assignments to LR-TR viable. They have also reported an excellent inter-reader agreement for LR-TR categories when using ancillary features, with a weighted kappa statistic of 0.75, which is comparable to the use of the original LR-TR algorithm [69]. These early studies support incorporation of ancillary features into the LR-TRA, as it seems to improve response assessment accuracy without apparent downside.
While tumor response assessment after thermal ablation and nonradiation intra-arterial therapy seems relatively straight-forward, HCC treated with radiation-based therapies have a unique and complex post-treatment imaging appearance and thus categorization based on LI-RADS TRA is much more challenging. There is limited data on radiologic-pathologic correlation following radiation-based therapy using LI-RADS TRA. Mendiratta-Lala et al. [55••] evaluated 10 stereotactic body radiation (SBRT)-treated HCC, in which all tumors were necrotic on explant pathology at 12 months, out of which 40% exhibited persistent APHE and 90% exhibited WO on imaging prior to transplant. Although only a very small cohort, this study suggests that persistent arterial enhancement post-SBRT can be an expected post-treatment imaging finding. Further radiology–pathology studies in HCC treated with SBRT will be needed in order to validate the application of the LI-RADS TRA.
Likewise, there is limited radiology–pathology data for HCC treated with TARE. In a study evaluating 37 lesions post-TARE, Riaz et al. [70] compared the degree of tumor necrosis at pathologic diagnosis to the imaging appearance, albeit utilizing EASL tumor response categories, in which a complete response was seen in 32% of lesions on histopathology at a median time of 34 days. Another study by Vouche et al. [71] compared EASL, mRECIST, WHO, and RECIST tumor response categories by comparing imaging to explant pathology to determine degree of tumor necrosis. Sixteen HCC treated with TARE were evaluated and complete pathological necrosis could not be predicted by WHO, RECIST, EASL, or mRECIST. Furthermore, response to treatment was categorized as equivocal in all tumors when using EASL and mRECIST, as neither could reliably predict complete tumor necrosis. This may be explained by the persistent enhancement seen in TARE-treated HCC limiting the utility of enhancement-based response assessment guidelines (EASL, mRECIST). Further evaluation with outcome data such as time to progression, disease-free survival, and overall survival will be needed in conjunction with radiology and pathology data. To date, no studies have been performed evaluating the reproducibility of the LI-RADS TRA categories or their diagnostic performance compared to explant pathology for radiation-based therapies.
Limitations, Knowledge Gaps, and Controversies
Though there is early promising data on the sensitivity/specificity on LRT, further validation studies are needed given the increasing number of different LRTs, as well as in patients receiving combination LRT and systemic therapies. LI-RADS TRA after radiation-based therapies for HCC may also require modifications of the current algorithm. Furthermore, longitudinal follow-up studies are needed to determine the utility of the LR-TR equivocal category, particularly since many recent studies have suggested high frequency of viable tumor within this category after locoablative and nonradiation embolotherapies. Secondly, LI-RADS TRA is not yet applicable to patients on systemic and/or biologic treatments and further research comparing outcomes using the different treatment response guidelines such as RECIST, mRECIST, and LI-RADS TRA are needed. Lastly, post-treatment imaging follow-up recommendations and standardized reporting templates need to be developed.
Conclusion
Diagnostic LI-RADS CT/MR is a validated tool for diagnosis of HCC, improving characterization of lesions in at-risk patients and improving communication between radiologists and clinicians. Since its introduction in 2011, LI-RADS has clarified communications between radiology researchers with multiple studies leading to refinements in LI-RADS categories over time. LI-RADS TRA is a relatively new but promising approach for response assessment following LRT, which needs further validation and refinement to be applicable to radiation-based therapies.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–50. https://doi.org/10.1002/hep.29913.
Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–80. https://doi.org/10.1002/hep.29086.
Rich NE, Parikh ND, Singal AG. Overdiagnosis: An understudied issue in hepatocellular carcinoma surveillance. Semin Liver Dis. 2017;37(4):296-304. Epub 2017/12/22. https://doi.org/10.1055/s-0037-1608775.
• Elsayes KM, Kielar AZ, Chernyak V, Morshid A, Furlan A, Masch WR, et al. LI-RADS: a conceptual and historical review from its beginning to its recent integration into AASLD clinical practice guidance. J Hepatocell Carcinoma. 2019;6:49-69. Epub 2019/02/05. https://doi.org/10.2147/JHC.S186239. This article provides an important review of major LI-RADS updates since its inception in 2011 and also discusses the currently published LI-RADS algorithms.
•• American College of Radiology 2018 Liver Imaging Reporting and Data Systems Version 2018 Manual. 2018. ACR LI-RADS manual is an extremely important document that provides standardized reporting of HCC on CT/MR in at risk population and has been integrated into the American Association for the study of liver diseases (AASLD) 2018 hepatocellular carcinoma clinical practice guideline.
Corwin MT, Lee AY, Fananapazir G, Loehfelm TW, Sarkar S, Sirlin CB. Nonstandardized terminology to describe focal liver lesions in patients at risk for hepatocellular carcinoma: implications regarding clinical communication. AJR Am J Roentgenol. 2018;210(1):85–90. Epub 2017/10/12. https://doi.org/10.2214/AJR.17.18416.
Flusberg M, Ganeles J, Ekinci T, Goldberg-Stein S, Paroder V, Kobi M, et al. Impact of a structured report template on the quality of CT and MRI reports for hepatocellular carcinoma diagnosis. J Am Coll Radiol. 2017;14(9):1206–11. Epub 2017/05/06. https://doi.org/10.1016/j.jacr.2017.02.050.
Goldberg-Stein S, Chernyak V. Adding value in radiology reporting. J Am Coll Radiol. 2019;16(9 Pt B):1292–8. https://doi.org/10.1016/j.jacr.2019.05.042.
Chernyak V, Fowler KJ, Kamaya A, Kielar AZ, Elsayes KM, Bashir MR, et al. Liver Imaging Reporting and Data System (LI-RADS) version 2018: Imaging of hepatocellular carcinoma in at-risk patients. Radiology. 2018;289(3):816–30. Epub 2018/09/25. https://doi.org/10.1148/radiol.2018181494.
Tang A, Hallouch O, Chernyak V, Kamaya A, Sirlin CB. Epidemiology of hepatocellular carcinoma: target population for surveillance and diagnosis. Abdom Radiol (NY). 2018;43(1):13–25. https://doi.org/10.1007/s00261-017-1209-1.
•• van der Pol CB, Lim CS, Sirlin CB, McGrath TA, Salameh JP, Bashir MR, et al. Accuracy of the liver imaging reporting and data system in computed tomography and magnetic resonance image analysis of hepatocellular carcinoma or overall malignancy-a systematic review. Gastroenterology. 2019;156(4):976-986. Epub 2018/11/13. https://doi.org/10.1053/j.gastro.2018.11.020. Large systemic analysis that demonstrates the percentage of HCC and other malignancy in each LI-RADS category.
Tang A, Bashir MR, Corwin MT, Cruite I, Dietrich CF, Do RKG, et al. Evidence supporting LI-RADS major features for CT- and MR imaging-based diagnosis of hepatocellular carcinoma: a systematic review. Radiology. 2018;286(1):29–48. Epub 2017/11/21. https://doi.org/10.1148/radiol.2017170554.
Tang A, Singal AG, Mitchell DG, Hecht EM, Fowler KJ, Kulik L, et al. Introduction to the liver imaging reporting and data system for hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2019;17(7):1228–38. Epub 2018/10/13. https://doi.org/10.1016/j.cgh.2018.10.014.
Fowler KJ, Potretzke TA, Hope TA, Costa EA, Wilson SR. LI-RADS M (LR-M): definite or probable malignancy, not specific for hepatocellular carcinoma. Abdom Radiol (NY). 2018;43(1):149–57. https://doi.org/10.1007/s00261-017-1196-2.
Santillan C, Chernyak V, Sirlin C. LI-RADS categories: concepts, definitions, and criteria. Abdom Radiol (NY). 2018;43(1):101–10. https://doi.org/10.1007/s00261-017-1334-x.
Thölking G, Schuette-Nuetgen K, Kentrup D, Pawelski H, Reuter S. Imaging-based diagnosis of acute renal allograft rejection. World J Transplant. 2016;6(1):174–82. https://doi.org/10.5500/wjt.v6.i1.174.
Furlan A. New Progress toward Validation of LI-RADS Version 2018. Radiology. 2019;291(1):81–2. Epub 2019/01/29. https://doi.org/10.1148/radiol.2019182890.
• Ren AH, Zhao PF, Yang DW, Du JB, Wang ZC, Yang ZH. Diagnostic performance of MR for hepatocellular carcinoma based on LI-RADS v2018, compared with v2017. J Magn Reson Imaging. 2019;50(3):746-755. Epub 2019/01/15. https://doi.org/10.1002/jmri.26640. This is an important study comparing the diagnostic performance of LI-RADS v2018 and 2017 with greater sensitivity, negative predictive value and accuracy of v2018 especially when adopting LR-5 as a predictor of HCC.
Lee SM, Lee JM, Ahn SJ, Kang HJ, Yang HK, Yoon JH. LI-RADS version 2017 versus version 2018: diagnosis of hepatocellular carcinoma on gadoxetate disodium-enhanced MRI. Radiology. 2019;292(3):655–63. Epub 2019/07/16. https://doi.org/10.1148/radiol.2019182867.
Smereka P, Doshi AM, Lavelle LP, Shanbhogue K. New arterial phase enhancing nodules on MRI of cirrhotic liver: risk of progression to hepatocellular carcinoma and implications for LI-RADS classification. AJR Am J Roentgenol. 2020:1–8. Epub 2020/05/20. https://doi.org/10.2214/AJR.19.22033.
Chernyak V, Flusberg M, Berman J, Fruitman KC, Kobi M, Fowler KJ, et al. Liver imaging reporting and data system version 2018: impact on categorization and hepatocellular carcinoma staging. Liver Transpl. 2019;25(10):1488–502. Epub 2019/09/09. https://doi.org/10.1002/lt.25614.
Ko A, Park HJ, Lee ES, Park SB, Kim YK, Choi SY, et al. Comparison of the diagnostic performance of the 2017 and 2018 versions of LI-RADS for hepatocellular carcinoma on gadoxetic acid enhanced MRI. Clin Radiol. 2020;75(4):319.e1-319.e9. Epub 2019/12/16. https://doi.org/10.1016/j.crad.2019.11.004.
Cerny M, Bergeron C, Billiard JS, Murphy-Lavallée J, Olivié D, Bérubé J, et al. LI-RADS for MR Imaging Diagnosis of Hepatocellular Carcinoma: Performance of Major and Ancillary Features. Radiology. 2018;288(1):118–28. Epub 2018/04/10. https://doi.org/10.1148/radiol.2018171678.
Joo I, Lee JM, Lee DH, Ahn SJ, Lee ES, Han JK. Liver imaging reporting and data system v2014 categorization of hepatocellular carcinoma on gadoxetic acid-enhanced MRI: Comparison with multiphasic multidetector computed tomography. J Magn Reson Imaging. 2017;45(3):731-740. Epub 2016/07/30. https://doi.org/10.1002/jmri.25406.
Ren AH, Du JB, Yang DW, Zhao PF, Wang ZC, Yang ZH. The role of ancillary features for diagnosing hepatocellular carcinoma on CT: based on the Liver Imaging Reporting and Data System version 2017 algorithm. Clin Radiol. 2020;75(6):478.e25–35. Epub 2020/02/20. https://doi.org/10.1016/j.crad.2019.08.031.
Fowler KJ, Tang A, Santillan C, Bhargavan-Chatfield M, Heiken J, Jha RC, et al. Interreader reliability of LI-RADS version 2014 algorithm and imaging features for diagnosis of hepatocellular carcinoma: A large international multireader study. Radiology. 2018;286(1):173–85. Epub 2017/11/01. https://doi.org/10.1148/radiol.2017170376.
Ludwig DR, Fraum TJ, Cannella R, Ballard DH, Tsai R, Naeem M, et al. Hepatocellular carcinoma (HCC) versus non-HCC: accuracy and reliability of Liver Imaging Reporting and Data System v2018. Abdom Radiol (NY). 2019;44(6):2116–32. https://doi.org/10.1007/s00261-019-01948-x.
• Abdel Razek AAK, El-Serougy LG, Saleh GA, Abd El-Wahab R, Shabana W. Interobserver agreement of magnetic resonance imaging of liver imaging reporting and data system version 2018. J Comput Assist Tomogr. 2020;44(1):118–23. https://doi.org/10.1097/RCT.0000000000000945A retrospective analysis showing excellent agreement in LR-5 and LR-M categories on MRI with LI-RADS v2018.
Stocker D, Becker AS, Barth BK, Skawran S, Kaniewska M, Fischer MA, et al. Does quantitative assessment of arterial phase hyperenhancement and washout improve LI-RADS v2018-based classification of liver lesions?. Eur Radiol. 2020. Epub 2020/02/04. https://doi.org/10.1007/s00330-019-06596-9.
Choi D, Mitchell DG, Verma SK, Bergin D, Navarro VJ, Malliah AB, et al. Hepatocellular carcinoma with indeterminate or false-negative findings at initial MR imaging: effect on eligibility for curative treatment initial observations. Radiology. 2007;244(3):776-783. Epub 2007/08/09. https://doi.org/10.1148/radiol.2443061355.
Holland AE, Hecht EM, Hahn WY, Kim DC, Babb JS, Lee VS, et al. Importance of small (< or = 20-mm) enhancing lesions seen only during the hepatic arterial phase at MR imaging of the cirrhotic liver: evaluation and comparison with whole explanted liver. Radiology. 2005;237(3):938–44. https://doi.org/10.1148/radiol.2373041364.
Choi JY, Cho HC, Sun M, Kim HC, Sirlin CB. Indeterminate observations (liver imaging reporting and data system category 3) on MRI in the cirrhotic liver: fate and clinical implications. AJR Am J Roentgenol. 2013;201(5):993–1001. https://doi.org/10.2214/AJR.12.10007.
Shropshire E, Mamidipalli A, Wolfson T, Allen BC, Jaffe TA, Igarashi S, et al. LI-RADS ancillary feature prediction of longitudinal category changes in LR-3 observations: an exploratory study. Abdom Radiol (NY). 2020. Epub 2020/02/12. https://doi.org/10.1007/s00261-020-02429-2.
Kim DH, Choi SH, Park SH, Kim KW, Byun JH, Kim SY, et al. Liver imaging reporting and data system category M: A systematic review and meta-analysis. Liver Int. 2020;40(6):1477-1487. Epub 2020/03/15. https://doi.org/10.1111/liv.14420.
• Kim YY, Kim MJ, Kim EH, Roh YH, An C. Hepatocellular carcinoma versus other hepatic malignancy in cirrhosis: performance of LI-RADS version 2018. Radiology. 2019;291(1):72-80. Epub 2019/01/29. https://doi.org/10.1148/radiol.2019181995. LR-5 and LR-M in LI-RADS v2018 can effectively differentiate HCC from other malignancy in patients with liver cirrhosis.
Park HJ, Kim YK, Cha DI, Ko SE, Kim S, Lee ES, et al. Targetoid hepatic observations on gadoxetic acid-enhanced MRI using LI-RADS version 2018: emphasis on hepatocellular carcinomas assigned to the LR-M category. Clin Radiol. 2020. Epub 2020/02/05. https://doi.org/10.1016/j.crad.2020.01.002.
Kim MY, Joo I, Kang HJ, Bae JS, Jeon SK, Lee JM. LI-RADS M (LR-M) criteria and reporting algorithm of v2018: diagnostic values in the assessment of primary liver cancers on gadoxetic acid-enhanced MRI. Abdom Radiol (NY). 2020. Epub 2020/05/07. https://doi.org/10.1007/s00261-020-02545-z.
Chan MV, McDonald SJ, Ong YY, Mastrocostas K, Ho E, Huo YR, et al. HCC screening: assessment of an abbreviated non-contrast MRI protocol. Eur Radiol Exp. 2019;3(1):49. Epub 2019/12/18. https://doi.org/10.1186/s41747-019-0126-1.
Roberts LR, Sirlin CB, Zaiem F, Almasri J, Prokop LJ, Heimbach JK, et al. Imaging for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. Hepatology. 2018;67(1):401-421. Epub 2017/11/29. https://doi.org/10.1002/hep.29487.
Hanna RF, Miloushev VZ, Tang A, Finklestone LA, Brejt SZ, Sandhu RS, et al. Comparative 13-year meta-analysis of the sensitivity and positive predictive value of ultrasound, CT, and MRI for detecting hepatocellular carcinoma. Abdom Radiol (NY). 2016;41(1):71–90. https://doi.org/10.1007/s00261-015-0592-8.
• Khatri G, Pedrosa I, Ananthakrishnan L, de Leon AD, Fetzer DT, Leyendecker J, et al. Abbreviated-protocol screening MRI vs. complete-protocol diagnostic MRI for detection of hepatocellular carcinoma in patients with cirrhosis: An equivalence study using LI-RADS v2018. J Magn Reson Imaging. 2020;51(2):415-425. https://doi.org/10.1002/jmri.26835. An important study indicating abbreviated MRI is comparable and equivalent to complete diagnostic MRI for HCC detection in cirrhotic patients.
Brunsing RL, Chen DH, Schlein A, Wolfson T, Gamst A, Mamidipalli A, et al. Gadoxetate-enhanced abbreviated MRI for hepatocellular carcinoma surveillance: preliminary experience. Radiology: Imaging Cancer. 2019;1:2.
Sawrie SM, Fiveash JB, Caudell JJ. Stereotactic body radiation therapy for liver metastases and primary hepatocellular carcinoma: normal tissue tolerances and toxicity. Cancer Control. 2010;17(2):111–9. https://doi.org/10.1177/107327481001700206.
•• Kielar A, Fowler KJ, Lewis S, Yaghmai V, Miller FH, Yarmohammadi H, et al. Locoregional therapies for hepatocellular carcinoma and the new LI-RADS treatment response algorithm. Abdom Radiol (NY). 2018;43(1):218–30. https://doi.org/10.1007/s00261-017-1281-6An important review article providing insight into the mechanism of different liver-directed therapies, illustrate the typical expected imaging appearance of HCC on CT and MRI after locoregional therapy and discuss the LI-RADS treatment response algorithm.
Narsinh KH, Duncan DP, Newton IG, Minocha J, Rose SC. Liver-directed therapy for hepatocellular carcinoma. Abdom Radiol (NY). 2018;43(1):203–17. https://doi.org/10.1007/s00261-017-1435-6.
Inchingolo R, Posa A, Mariappan M, Spiliopoulos S. Locoregional treatments for hepatocellular carcinoma: Current evidence and future directions. World J Gastroenterol. 2019;25(32):4614–28. https://doi.org/10.3748/wjg.v25.i32.4614.
Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37(2):429–42. https://doi.org/10.1053/jhep.2003.50047.
Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30(1):6–25. https://doi.org/10.1007/s00270-006-0062-3.
Meyer T, Kirkwood A, Roughton M, Beare S, Tsochatzis E, Yu D, et al. A randomised phase II/III trial of 3-weekly cisplatin-based sequential transarterial chemoembolisation vs embolisation alone for hepatocellular carcinoma. Br J Cancer. 2013;108(6):1252-1259. Epub 2013/02/28. https://doi.org/10.1038/bjc.2013.85.
Molla N, AlMenieir N, Simoneau E, Aljiffry M, Valenti D, Metrakos P, et al. The role of interventional radiology in the management of hepatocellular carcinoma. Curr Oncol. 2014;21(3):e480–92. https://doi.org/10.3747/co.21.1829.
Heckman JT, Devera MB, Marsh JW, Fontes P, Amesur NB, Holloway SE, et al. Bridging locoregional therapy for hepatocellular carcinoma prior to liver transplantation. Ann Surg Oncol. 2008;15(11):3169–77. Epub 2008/08/12. https://doi.org/10.1245/s10434-008-0071-3.
Gamblin TC, Geller DA. Downstaging hepatocellular carcinoma prior to liver transplantation. Liver Transpl. 2005;11(12):1466–8. https://doi.org/10.1002/lt.20528.
Mora RA, Ali R, Gabr A, Abouchaleh N, Asadi AA, Kallini JR, et al. Pictorial essay: imaging findings following Y90 radiation segmentectomy for hepatocellular carcinoma. Abdom Radiol (NY). 2018;43(7):1723–38. https://doi.org/10.1007/s00261-017-1391-1.
Galun D, Basaric D, Zuvela M, Bulajic P, Bogdanovic A, Bidzic N, et al. Hepatocellular carcinoma: From clinical practice to evidence-based treatment protocols. World J Hepatol. 2015;7(20):2274–91. https://doi.org/10.4254/wjh.v7.i20.2274.
•• Mendiratta-Lala M, Gu E, Owen D, Cuneo KC, Bazzi L, Lawrence TS, et al. Imaging findings within the first 12 months of hepatocellular carcinoma treated With stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2018;102(4):1063-1069. Epub 2017/08/24. https://doi.org/10.1016/j.ijrobp.2017.08.022. This study correlates the imaging findings of HCC treated with SBRT to explant pathology. This is important since post treatment imaging appearance of HCC following treatment with radiation based therapy is different with persistent APHE that can last upto a year or more when compared to other non-radiation based treatments and does not necessarily mean treatment failure.
Mendiratta-Lala M, Masch W, Shankar PR, Hartman HE, Davenport MS, Schipper MJ, et al. Magnetic resonance imaging evaluation of hepatocellular carcinoma treated with stereotactic body radiation therapy: long term imaging follow-Up. Int J Radiat Oncol Biol Phys. 2019;103(1):169-179. Epub 2018/09/10. https://doi.org/10.1016/j.ijrobp.2018.09.004.
Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35(3):421–30. https://doi.org/10.1016/s0168-8278(01)00130-1.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52-60. Epub 2010/02/19. https://doi.org/10.1055/s-0030-1247132.
Elsayes KM, Hooker JC, Agrons MM, Kielar AZ, Tang A, Fowler KJ, et al. 2017 version of LI-RADS for CT and MR imaging: an update. Radiographics. 2017;37(7):1994–2017. https://doi.org/10.1148/rg.2017170098.
Ebara M, Hatano R, Fukuda H, Yoshikawa M, Sugiura N, Saisho H. Natural course of small hepatocellular carcinoma with underlying cirrhosis. A study of 30 patients. Hepatogastroenterology. 1998(45 Suppl 3):1214–20.
Kubota K, Ina H, Okada Y, Irie T. Growth rate of primary single hepatocellular carcinoma: determining optimal screening interval with contrast enhanced computed tomography. Dig Dis Sci. 2003;48(3):581–6. https://doi.org/10.1023/a:1022505203786.
An C, Choi YA, Choi D, Paik YH, Ahn SH, Kim MJ, et al. Growth rate of early-stage hepatocellular carcinoma in patients with chronic liver disease. Clin Mol Hepatol. 2015;21(3):279-286. Epub 2015/09/30. https://doi.org/10.3350/cmh.2015.21.3.279.
Seo N, Kim MS, Park MS, Choi JY, Do RKG, Han K, et al. Evaluation of treatment response in hepatocellular carcinoma in the explanted liver with Liver Imaging Reporting and Data System version 2017. Eur Radiol. 2020;30(1):261–71.
Chaudhry M, McGinty KA, Mervak B, Lerebours R, Li C, Shropshire E, et al. The LI-RADS version 2018 mri treatment response algorithm: evaluation of ablated hepatocellular carcinoma. Radiology. 2020;294(2):320-326. Epub 2019/12/17. https://doi.org/10.1148/radiol.2019191581.
Shropshire EL, Chaudhry M, Miller CM, Allen BC, Bozdogan E, Cardona DM, et al. LI-RADS treatment response algorithm: performance and diagnostic accuracy. Radiology. 2019;292(1):226-234. Epub 2019/04/30. https://doi.org/10.1148/radiol.2019182135.
Cools KS, Moon AM, Burke LMB, McGinty KA, Strassle PD, Gerber DA. Validation of the liver imaging reporting and data system treatment response criteria after thermal ablation for hepatocellular carcinoma. Liver Transpl. 2020;26(2):203–14. Epub 2019/12/20. https://doi.org/10.1002/lt.25673.
Abdel Razek AAK, El-Serougy LG, Saleh GA, Shabana W, Abd El-Wahab R. Reproducibility of LI-RADS treatment response algorithm for hepatocellular carcinoma after locoregional therapy. Diagn Interv Imaging. 2020. Epub 2020/04/03. https://doi.org/10.1016/j.diii.2020.03.008.
• Park S, Joo I, Lee DH, Bae JS, Yoo J, Kim SW, et al. Diagnostic performance of LI-RADS treatment response algorithm for hepatocellular carcinoma: adding ancillary features to MRI compared with enhancement patterns at CT and MRI. Radiology. 2020:192797. Epub 2020/07/21. https://doi.org/10.1148/radiol.2020192797. Recently published article discussing the importance of incorporating ancillary features to the LI-RADS treatment response algorithm in prediction of pathologic tumor viability in patients with HCC treated with loco-regional therapy.
Kim SW, Joo I, Kim HC, Ahn SJ, Kang HJ, Jeon SK, et al. LI-RADS treatment response categorization on gadoxetic acid-enhanced MRI: diagnostic performance compared to mRECIST and added value of ancillary features. Eur Radiol. 2020;30(5):2861-2870. Epub 2020/01/31. https://doi.org/10.1007/s00330-019-06623-9.
Riaz A, Kulik L, Lewandowski RJ, Ryu RK, Giakoumis Spear G, Mulcahy MF, et al. Radiologic-pathologic correlation of hepatocellular carcinoma treated with internal radiation using yttrium-90 microspheres. Hepatology. 2009;49(4):1185–93. https://doi.org/10.1002/hep.22747.
Vouche M, Kulik L, Atassi R, Memon K, Hickey R, Ganger D, et al. Radiological-pathological analysis of WHO, RECIST, EASL, mRECIST and DWI: Imaging analysis from a prospective randomized trial of Y90 ± sorafenib. Hepatology. 2013;58(5):1655–66. Epub 2013/10/01. https://doi.org/10.1002/hep.26487.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hepatic Cancer
Rights and permissions
About this article
Cite this article
Aslam, A., Do, R.K.G., Chernyak, V. et al. LI-RADS Imaging Criteria for HCC Diagnosis and Treatment: Emerging Evidence. Curr Hepatology Rep 19, 437–447 (2020). https://doi.org/10.1007/s11901-020-00546-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-020-00546-6