Abstract
Transarterial radioembolization is a novel therapy that has gained rapid clinical acceptance for the treatment of hepatocellular carcinoma (HCC). Segmental radioembolization [also termed radiation segmentectomy (RS)] is a technique that can deliver high doses (> 190 Gy) of radiation selectively to the hepatic segment(s) containing the tumor. The aim of this comprehensive review is to provide an illustrative summary of the most relevant imaging findings encountered after radiation segmentectomy. A 62-patient cohort of Child–Pugh A patients with solitary HCC < 5 cm in size was identified. A comprehensive retrospective imaging review was done by interventional radiology staff at our institution. Important imaging findings were reported and illustrated in a descriptive account. For the purposes of completeness, specific patients outside our initial cohort with unique educational imaging features that also underwent segmentectomy were included in this pictorial essay. This review shows that response assessment after RS requires a learning curve with common drawbacks that can lead to false-positive interpretations and secondary unnecessary treatments. It is important to recognize that treatment responses and pathological changes both are time dependent. Findings such as benign geographical enhancement and initial benign pathological enhancement can easily be misinterpreted. Capsular retraction and segmental atrophy are some other examples of unique post-RS response that are not seen in any other treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Hepatocellular carcinoma (HCC) is the most common primary liver cancer. Despite improvements in treatment, it remains the second most common cause of cancer-related mortality [1]. Treatment modalities vary and include liver transplantation, resection, and ablation; these are still regarded as the treatments of choice with prolonged survival [2].
Other options include liver-directed therapies, such as transarterial chemoembolization (TACE) and radioembolization with yttrium-90 (Y90). Treatment choice will depend on many factors, the most important being the patient’s disease stage, tumor burden, and liver functional reserve.
Y90 is a novel therapy that has gained rapid clinical acceptance for the treatment of (HCC) [3]. Segmental radioembolization [also termed radiation segmentectomy (RS)] is a technique which can deliver high doses of radiation selectively to the hepatic segment(s) containing the tumor [4,5,6], sparing the rest of the liver, providing a safe and effective method to target HCC. Patients with lower liver functions that cannot tolerate more extensive and taxing treatments like lobar therapies, TACE, or external beam radiations can be treated safely by reducing the overall radiation burden on the liver. We have observed unique and time-dependent changes in the hepatic parenchyma that affect clinical management and decision making.
Sixty-two patients with HCC who underwent treatment with radiation segmentectomy Y90 over an 11-year period (Dec 2003–Dec 2016) were included in this imaging finding review.
The aim of this pictorial essay is to provide a descriptive account of the most common radiologic imaging findings, from benign to pathologic, encountered after radiation segmentectomy. A review of these imaging findings has not been previously published. This information is of importance for clinical teams including oncologists and radiologists to be aware of these findings as they play a direct role in clinical management and treatment decision making.
Radiation segmentectomy technical considerations
Y90 is a pure beta emitter that decays into stable zirconium. It can be loaded either on a resin (SIR-Spheres®) or on glass [TheraSphere® (BTG)] microspheres [7, 8]. TheraSphere® was used for all patients in this review. Each microsphere ranges from 20 to 30 μm in size. The technical details of Y90 glass microspheres and dosage calculation for radiation segmentectomy have been previously discussed in detail [4].
Pre-treatment planning
Angiography is performed in every patient undergoing RS to assess the abdominal and liver vasculature anatomy, identification of tumor’s blood supply, recognition of any anatomical variants [9, 10] that could lead to non-target deposition of Y90 microspheres, and prophylactic embolization of these vessels [11,12,13]. A technetium-99m macroaggregated albumin (99mTc-MAA) scan is also performed to determine the lung shunt fraction (LSF) and identify any shunting to the gastrointestinal tract [14].
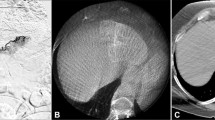
Cone-beam CT is another valuable tool during pre-treatment planning that allows confirmation of complete tumor targeting; the scan is performed during the pre-treatment angiography or before Y90 to collect additional information. It allows improved visualization of the tumor and its supplying arteries which may not be entirely evident with angiography alone [15,16,17,18] (Fig. 1).
A Superselective angiogram showing hypervascular segment IV lesion (arrow). B Superselective angiogram showing hypervascular right lobe lesion (arrow). C Celiac artery as part of mapping angiography (arrow). D Superselective angiogram showing hypervascular dome lesion (arrow). E Cone-beam CT showing wedge enhancement corresponding to target segment in Figure 1B (arrow)
Radiation segmentectomy patient selection criteria
Patients have to meet the following criteria to be selected as candidates for RS: (1) solitary HCC ≤ 5 cm; (2) liver-only disease (no metastasis and/or vascular invasion); (3) tumor can be isolated angiographically such that no more than two hepatic segments are perfused at the selected treatment location for the segmentectomy to be possible. Baseline characteristics of patients in this review are presented in Table 1.
Follow-up intervals and imaging studies
A universally accepted post-therapeutic imaging follow-up protocol has not been established. These continue to vary by center [19,20,21]; this may in part be due to limited consensus regarding surveillance for each treatment and disease [22, 23]. Imaging follow-up was performed following our institutional standard protocol at 1 month post-treatment and at 3-month intervals after the first evaluation for all patients. Follow-up median time for this pictorial essay was 17 months (range 2–120 months).
Imaging
Imaging was acquired by MRI (our institutional standard) or CT.
-
(a)
Abdominal MRI protocol for liver imaging included transverse and coronal T2-weighted half-Fourier acquisition single-shot turbo spin echo, T2-weighted turbo spin echo with fat suppression. Unenhanced and dynamic gadolinium-enhanced T1-weighted images were acquired by fat-suppressed gradient echo using shared prepulses (arterial/venous phases). Gadopentetate dimeglumine (Magnevist; Bayer HealthCare Pharmaceuticals, Berlin, Germany) was administered at a dose of 0.1 mmol/kg, followed by 20 mL saline flush (2 mL/s) with a power injector (Spectris; Medrad, Inc, Warrendale, PA).
-
(b)
CT imaging included unenhanced arterial and portal venous phases according to our standard HCC protocol for the liver. Contrast-enhanced images were obtained after 40 s in the arterial phase and 70 s in the venous phase after the injection of 125 mL of iohexol (Omnipaque 350; GE Healthcare, Waukesha, WI) at a rate of 5 mL/s.
-
(c)
C-arm CT: imaging was performed using a Siemens Artis zeego Syngo DYNA CT software, non-ionic iodinated contrast agent (omnipaque® = iohexol, GE Healthcare Company, UK), and a high-pressure syringe (Mark V Provis, MERAD.INC, USA). The DYNA CT scan was conducted 6 s after the start of the injection of the contrast agent with the following parameters: acquisition frame rate, 60 frames/s; collection matrix, 1024 × 1024; rotation speed, 30°/s; and acquisition time, 7 s.
Tumor response
Radiological tumor responses were assessed using World Health Organization (WHO) criteria for size, the European Association for the Study of the Liver (EASL) criteria [24, 25], and the modified Response Evaluation Criteria In Solid Tumors (mRECIST) [26] for necrosis (Table 2).
While tumor size remains the main parameter in oncologic treatment response, for Y90 the use of size to evaluate response is not the most accurate method, due to the inherent characteristics of the therapy; size may increase or remain stable after treatment, without any correlation to actual tumor viability. For this reason, the degree of necrosis and enhancing tissue has been accepted as criteria for response. EASL and mRECIST have proven to be better predictors of survival than WHO and RECIST for HCC surveillance after locoregional therapies [27,28,29].
Tumor size was assessed using the WHO criteria [25]. The sum of baseline cross-product (pre-treatment) is compared with the follow-up to determine the percentage change in size by WHO. Tumor necrosis was assessed using both EASL and mRECIST guidelines [24, 26]. EASL recommends measuring the cross-product of the enhancing tissue inside the target tumor and comparing it to the baseline; mRECIST measures the longest diameter of arterially enhancing tissue in the target tumor (Table 2).
In our cohort, mRECIST CR at one-month follow-up was observed in 21 (34%) patients. Response by WHO (CR and PR) was achieved in 65% (40/62) of the patients, which is higher than the average reported response rate with Y90 (20%–40%) [30, 31]; EASL response was achieved in 97% (60/62) of the patients which is in accordance with the reported response rate for overall Y90 [32, 33] (Table 3).
Persistent enhancement and residual tumor artifact
Two common and alarming findings in our analysis were the presence of what we called “persistent enhancement” and “residual tumor artifact.”
“Persistent enhancement” refers to a pattern of enhancement that resembles infiltrative disease, an opaque marbled enhancement; “residual tumor artifact” is the presence of enhancement inside the treated lesion, which presents with suspicious characteristics such as nodular enhancement and thick/uneven enhancing capsule [34, 35] (Fig. 2).
A MRI showing baseline lesion (arrow). B MR image at 1-month follow-up after RS showing worrisome enhancement and “residual tumor artifact” in the treated lesion (arrow); also seen near the treated lesion are “parenchymal holes” in no-tumor-bearing areas corresponding to parenchymal changes secondary to treatment (arrowhead). C MR image at 3-month follow-up, the worrisome enhancement seen at 1 month has disappeared, and the lesion now shows complete necrosis (arrow); parenchymal changes have also improved. D MRI at 12 months, showing complete necrosis (arrowhead) and capsular retraction (arrow)
In our review, of the 32 patients that presented with persistent enhancement at 1-month, 20 (63%) patients showed complete resolution at 3-month follow-up, without any local disease progression at subsequent follow-up scans. These persistent findings may be explained by delayed necrosis [35]. (Figs. 3, 4, 5). Additional details on the phenomenon of “persistent enhancement” are provided in Fig. 6.
A Pre-treatment contrast-enhanced MR image showing hyperenhancing lesion in segments V-VIII (arrow). B Post-treatment MR image at 1-month follow-up showing worrisome enhancement, with nodular artifact (arrow). C MR image at 6-month follow-up, showing that the initial worrisome enhancement has resolved on delayed imaging without additional treatment (arrow). Capsular scar is noted (arrowhead). D MR image at 10-month follow-up showing less worrisome enhancement on arterial phase and progressive improvement (arrow). E MR image follow-up at 17-month follow-up showing complete disappearance of worrisome enhancement and complete necrosis, with progressive segment collapse. No local disease progression was seen at any moment of the follow-up (arrow)
A Pre-treatment contrast-enhanced MR image showing hyperenhancing tumor (arrow). B Post-treatment MRI with worrisome enhancement and suspicious nodular residual tissue inside the treated lesion (arrow). C MRI at 3-month follow-up, showing that the initial worrisome enhancement is no longer seen and complete necrosis of the lesion is now evident (arrow). D MRI follow-up 7 years after the initial RS treatment, showing excellent local tumor control and no evidence of local disease (arrow). Atrophy of the treated segment is seen (arrowhead)
A Pre-treatment contrast-enhanced MR image showing arterially hyperenhancing tumor in segment II (arrow). B Contrast-enhanced MRI at 1-month follow-up, showing diffused enhancement in the treated segment that is concerning for infiltrative disease but in reality is related to treatment radiation effect (arrows). C MRI at 1-year follow-up showing continued radiation-enhancing effect in the treated segment, with signs of local disease control. Segment retraction is also seen (arrow)
Local tumor progression
Treatment failure should be differentiated by the cause: (a) lack of technique effectiveness (i.e., incomplete tumor irradiation) and (b) new lesion in the liver. This difference leads to a wide range in the time to progression depending on the cause.
The goal of radiation segmentectomy is to selectively treat the index tumor and at the same time provide a safe margin of radiated area for any small satellite lesions in the treated segment. In this review, local tumor progression was established as any progression or new lesion in the targeted segment determined by imaging follow-up using WHO, RECIST, and mRECIST criteria for all the patients in the cohort. All images were reviewed by 2 board-certified radiologists.
Of the 62 patients in our review, 18% (11/62) of the patients had local tumor progression at some point in their follow-up. The median time to progression (TTP) was 2.4 years (95% CI 2.1–5.7). Only 6% of these 11 patients had imaging findings corresponding to “persistent enhancement” at 1-month follow-up.
Incomplete tumor treatment
Incomplete tumor coverage can lead to a perception of “residual tumor” and subsequent disease progression. Most cases can potentially be avoided if recognized during pre-treatment imaging planning. Performing meticulous angiography and using cone-beam CT can indicate when catheter reposition or vessel embolization is necessary [36].
Persistent enhancement, when seen in controls, cannot be assessed independently, and they should always be reviewed retrospectively with all the patients’ studies ruling out an incomplete tumor irradiation. Our data show that most persistent enhancement will disappear or show evidence of improvement by the third month after treatment; because of this, continued imaging follow-up is advised, unless there is evidence suggesting incomplete treatment (Fig. 7).
A Pre-treatment contrast-enhanced MRI, showing arterial hyperenhancing lesion in segment VI (arrow). B Cone-beam CT, showing crescentic unenhanced tissue (arrow) predicting viable disease after treatment due to incomplete targeting. C Contrast-enhanced MRI at 1-month follow-up showing thick crescent-shaped residual tissue concerning for residual tumor (arrow). This is directly correlated to the pre-treatment cone-beam CT in Fig. 6 B. This is an untreated disease and is not a post-Y90 effect. D Second Y90 angiogram, showing enhancing tissue corresponding to the residual tumor (arrow). E 1-year follow-up after the second Y90, showing complete necrosis (arrow)
Rim enhancement
Rim enhancement constitutes a benign finding related to capsular fibrosis suggesting a favorable response to treatment [37] and not viable tumor as demonstrated in previous studies [30, 38]. It may persist for months, with no time-dependent pathologic correlation.
In our review, 34% (21/62) of the patients had rim enhancement in their imaging follow-ups (Table 4).
Non-tumor imaging findings
Benign findings like ascites and perihepatic edema can also be seen after RS. They are related to local inflammatory response following treatment and radiation exposure to the liver capsule. In the case of RS, because of the smaller area of radiation treatment, perihepatic fluid, and ascites are not as frequent as compared to instances of Y90 to broader territories. They have no clinical significance and are transient (Table 4).
Capsular retraction and segment atrophy
Radiation segmentectomy commonly leads to retraction of the capsule and atrophy of the treated segment; this capsular retraction has been previously hypothesized as having to do with the tumor necrosis and treated tissue fibrosis and scarring [39]. It is also important to understand that the lack of capsular retraction and segment atrophy, although rare, does not imply a failure of treatment (Figs. 8, 9).
In our review, segment atrophy and capsular retraction were seen in 90% (56/62) and 89% (55/62) of the patients, respectively (Table 4).
Geographical enhancement: correlating with cone-beam CT
Cone-beam CT has taken a major role in guaranteeing a successful treatment, by providing additional information during response assessments [16, 36].
A common finding, characteristic for Y90, is the presence of geographical enhancement with an extension greater than the treated tumor; this could be confused with active infiltrative tumor disease [40]. In RS, geographical enhancement usually presents with a characteristic wedge pattern. This wedge can be correlated perfectly to the pre-treatment cone-beam CT, reaffirming the relation of the enhancement with the treated segment. This enhancement may persist for months and, in some cases, will not completely disappear, but the overall segment will progressively scar and contract. It is a benign finding specific to Y90 and does not correspond to an adverse treatment reaction or tumor progression (Figs. 10, 11). “Geographical enhancement” was seen in 94% (58/62) of the patients in our review (Table 4).
A Pre-treatment contrast-enhanced MR image showing arterial hyperenhancing lesion (arrow). B Pre-treatment cone-beam CT that shows good targeting of the segment (arrows). C MR image at 1-month follow-up showing wedge-shaped geographical enhancement that correlates 1:1 with pre-treatment cone-beam CT (arrows). D 7-month image follow-up showing persistent geographical enhancement, scar formation, and segmental retraction corresponding to the treated area. (arrow)
Adverse events
Radiation segmentectomy is regarded as a generally well-tolerated procedure that is associated with few adverse events, possibly due to the selective nature of the treatment. Previous studies have reported fatigue as the most common presenting symptom (52%) [4].
In our 62-patient cohort, no major adverse event was seen. Only one patient presented with a post-treatment biliary injury that resolved itself without any intervention. Low incidence of complications and adverse reactions can be correlated with the high tolerance of radiation segmentectomy when compared to other transarterial therapies.
Additional assessment tools
Post-Y90 imaging changes may take time to appear; response by size or necrosis may not be evident in every patient. For these unclear cases, alternative diagnostic tools like diffusion-weighted MRI to measure diffusion coefficient, FDG PET to measure metabolic activity, volume changes, and angiographic response should be used [39]. Having all proven to be of great utility [41], these should be considered for unclear cases. They were not included in this review.
Clinical and laboratory correlations with imaging follow-up are always necessary. Overall patient condition, liver function panel, and tumor marker changes need to be assessed in every follow-up as they provide important additional information, although it is important to remember that tumor marker changes do not always correlate with imaging response [42, 43].
Some important limitations in our review are the retrospective nature of the study and the cohort from only a single center. Important strengths include the homogeneity of this cohort and the single treatment modality. Long follow-up time also strengthens our findings; imaging modality was heterogeneous using both CT and MRI contrast-enhanced studies.
Miscellaneous cases
For completeness sake, we have included some additional cases that highlight the other manifestations of RS that can also be encountered (Figs. 12, 13, 14, 15, 16, 17, 18, 19, 20) These additional examples illustrate cases of lesions abutting the falciform ligament, gallbladder, inferior vena cava, liver surface, as well as central segmentectomy, multisegmental injections, and radiation segmentectomy in portal vein thrombosis.
A Pre-treatment contrast-enhanced MR image, showing arterial hyperenhancing lesion in segment II/III (arrow). B Cone-beam CT (arrow) of branch perfusing around the planned treated territory (arrowheads), confirming that the lesion was perfused by a different branch. C Contrast-enhanced MRI at 1-month follow-up with worrisome enhancement and thick and irregular borders around target lesion (arrow). D 6-month follow-up image demonstrates the resolution of worrisome enhancement seen, showing no evidence of local tumor progression and complete segment retraction (arrow)
A Pre-treatment contrast-enhanced MRI showing hyperenhancing lesion (arrow) abutting the falciform ligament (arrowhead), a difficult area to ablate. B Contrast-enhanced MRI at 1-month follow-up showing worrisome enhancement (arrow) and peritumoral radiation effect (arrowhead). C 6-month follow-up MRI showing persistent radiation effect (arrowhead) and improving worrisome enhancement (arrow). D 17-month follow-up image showing persistent radiation effect, complete necrosis of lesion, and no evidence of local disease progression (arrow)
A Contrast-enhanced MRI showing arterial hyperenhancing lesion (arrow) indenting the gallbladder wall (arrowhead). B 1-month follow-up image showing complete necrosis (arrow) and no injury to the gallbladder. Geographic enhancement is also seen (arrowheads). Patient did not experience adverse events
A Pre-treatment MRI showing lesion in segment IV (arrow) touching the left portal vein (arrowhead); this will require a “central segmentectomy.” B MRI at 1-month follow-up showing complete necrosis (arrow) and geographical enhancement (arrowhead). C 6-month follow-up image showing capsular retraction and segment IV atrophy (arrow). This is termed “central segmentectomy”
A Pre-treatment MRI showing arterial hyperenhancing lesion (arrow) abutting the inferior vena cava (arrowhead). B Pre-treatment angiogram showing hypervascular lesion (arrow). C Pre-treatment in a different projection showing hypervascular lesion (arrow). D 1-month follow-up image demonstrating necrosis (arrow). E 6-month follow-up image showing wedge-shaped and perivascular radiation effect (arrows). F 6-month follow-up image confirming radiation effect in wedge shape (arrowhead) with capsular retraction (arrow)
A Pre-treatment MRI showing surface lesion in segment II (arrow). B MRI 1 month after RS showing enhancing lesion with worrisome enhancement (arrow). C MRI at 6 months showing persistent geographical radiation effect (arrowhead) and worrisome enhancement within the target lesion and adjacent parenchyma (arrow). D 10-month follow-up image showing improving necrosis (arrow) and persistent parenchymal enhancement (arrowhead). E MRI 40 months after RS, showing good local tumor control, with no disease progression (arrow). Patient underwent only one treatment
Surface lesion and pre-treatment coil. A MRI showing lesion in segment IVa (arrow). B Pre-treatment angiogram showing middle hepatic artery (arrow) and falciform artery (arrowhead). C Pre-treatment angiogram showing middle hepatic artery after embolization of the falciform artery (arrow). D MRI 1 month after treatment showing complete necrosis, rim enhancement (arrow), and geographical enhancement (arrowheads). E 3-month follow-up image showing a decrease in lesion dimension, no tumor progression (arrow), and persistent radiation effect (arrowheads). F 6-month follow-up image showing a progressive decrease in lesion dimension (arrow), no tumor progression, and persistent radiation effect (arrowheads)
Multisegmental segmentectomy: A Pre-treatment MRI showing lesion in segments II-III (arrow). B Pre-treatment MRI showing the second lesion in segment IV (arrow). C MRI at 1-month follow-up showing good treatment response in segment II-III lesion and rim enhancement (arrow). D MRI at 1-month follow-up showing good treatment response in segment IV lesion and rim enhancement (arrow). E Image at 8 months demonstrating a continued decrease in lesion size and persistent necrosis (arrow). F Image at 8 months demonstrating a continued decrease in lesion size and persistent necrosis (arrow). G A new lesion seen in segment VI 12 months after first treatment (arrow). H 1-month follow-up image after Y90 to new segment VI lesion, showing complete necrosis (arrow) and geographic enhancement (arrowheads). I 1-month follow-up image after Y90 to new segment VI lesion, persistent complete necrosis previously treated segment II-III and IV lesions (arrows)
A Pre-treatment MRI showing lesion in segments VI-VII and right portal vein invasion (arrow). B 1-month follow-up image after radiation segmentectomy to the posterior sector of the right lobe. C 3-month follow-up image showing retraction of the portal vein tumor and lack of enhancement (arrow). D 5-month follow-up image showing significant atrophy (arrow). E 9-month follow-up image demonstrating parenchymal enhancement secondary to radiation (arrows). F 12-month follow-up image showing continued atrophy of the treated segment (arrowheads) and continued response of the treated lesion (arrow)
Conclusion
Oncologic imaging response assessment is a complex task, which continues to evolve as new treatments are being developed. It plays a major role, as it is the method by which treatment success, recurrent disease, and complications are assessed. This evaluation would affect subsequent patient management. Response assessment after RS requires a learning curve with common pitfalls that can lead to false-positive interpretations and secondary unnecessary treatments. It is important to recognize that treatment responses and pathological changes both are time dependent. Findings such as benign geographical enhancement and initial benign pathological enhancement can easily be misinterpreted. Capsular retraction and segmental atrophy are some other examples of unique post-RS response that are not seen in any other treatment.
References
El-Serag HB (2012) Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142(6):1264–1273.e1
Mazzaferro V, et al. (1996) Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 334(11):693–699
Padia SA, et al. (2017) Segmental Yttrium-90 radioembolization versus segmental chemoembolization for localized hepatocellular carcinoma: results of a single-center, retrospective, propensity score-matched study. J Vasc Interv Radiol. https://doi.org/10.1016/j.jvir.2017.02.018
Riaz A, et al. (2011) Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys 79(1):163–171
Gaba RC, et al. (2009) Radiation lobectomy: preliminary findings of hepatic volumetric response to lobar yttrium-90 radioembolization. Ann Surg Oncol 16(6):1587–1596
Vouche M, et al. (2014) Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology 60(1):192–201
Salem R, Thurston KG (2006) Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol 17(8):1251–1278
Nordion M (2004) TheraSphere yttrium-90 microspheres package insert. Kanata: MDS Nordion
Hiatt JR, Gabbay J, Busuttil RW (1994) Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg 220(1):50–52
Michels NA (1966) Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg 112(3):337–347
Abdelmaksoud MH, et al. (2011) Consolidation of hepatic arterial inflow by embolization of variant hepatic arteries in preparation for yttrium-90 radioembolization. J Vasc Interv Radiol 22(10):1364–1371.e1
Karunanithy N, et al. (2011) Embolization of hepatic arterial branches to simplify hepatic blood flow before yttrium 90 radioembolization: a useful technique in the presence of challenging anatomy. Cardiovasc Intervent Radiol 34(2):287–294
Campbell AM, Bailey IH, Burton MA (2000) Analysis of the distribution of intra-arterial microspheres in human liver following hepatic yttrium-90 microsphere therapy. Phys Med Biol 45(4):1023–1033
Lewandowski RJ, et al. (2007) Radioembolization with 90Y microspheres: angiographic and technical considerations. Cardiovasc Intervent Radiol 30(4):571–592
Louie JD, et al. (2009) Incorporating cone-beam CT into the treatment planning for yttrium-90 radioembolization. J Vasc Interv Radiol 20(5):606–613
Becker C, et al. (2011) C-arm computed tomography compared with positron emission tomography/computed tomography for treatment planning before radioembolization. Cardiovasc Intervent Radiol 34(3):550–556
Virmani S, et al. (2007) Effect of C-arm angiographic CT on transcatheter arterial chemoembolization of liver tumors. J Vasc Interv Radiol 18(10):1305–1309
Wallace MJ, et al. (2007) Impact of C-arm CT on hepatic arterial interventions for hepatic malignancies. J Vasc Interv Radiol 18(12):1500–1507
Boas FE, et al. (2015) Optimal imaging surveillance schedules after liver-directed therapy for hepatocellular carcinoma. J Vasc Interv Radiol 26(1):69–73
Gaba RC, et al. (2015) Clinical and imaging follow-up practices after transarterial therapy for primary and secondary hepatic malignancies. Acad Radiol 22(12):1510–1515
Gaba RC (2012) Chemoembolization practice patterns and technical methods among interventional radiologists: results of an online survey. Am J Roentgenol 198(3):692–699
Bruix J, Sherman M (2011) Management of hepatocellular carcinoma: an update. Hepatology 53(3):1020–1022
European Association for Study of Liver; European Organisation for Research and Treatment of Cancer (2012) EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56(4):908–943
Bruix J, et al. (2001) Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 35(3):421–430
Organization, W.H. (1979) WHO Handbook for Reporting Results of Cancer Treatment. Geneva: WHO
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30(1):52–60
Memon K, et al. (2011) Radiographic response to locoregional therapy in hepatocellular carcinoma predicts patient survival times. Gastroenterology 141(2):526–35, 535.e1–2
Jung ES, et al. (2013) Comparison of the methods for tumor response assessment in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. J Hepatol 58(6):1181–1187
Gillmore R, et al. (2011) EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol 55(6):1309–1316
Kulik LM, et al. (2006) Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol 94(7):572–586
Sangro B, et al. (2006) Radioembolization using 90Y-resin microspheres for patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 66(3):792–800
Salem R, et al. (2005) Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. J Vasc Interv Radiol 16(12):1627–1639
Kulik LM, et al. (2008) Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology 47(1):71–81
Tirumani SH, et al. (2014) Update on the role of imaging in management of metastatic colorectal cancer. Radiographics 34(7):1908–1928
Keppke AL, et al. (2007) Imaging of hepatocellular carcinoma after treatment with yttrium-90 microspheres. AJR Am J Roentgenol 188(3):768–775
Camacho JC, et al. (2015) (90)Y radioembolization: multimodality imaging pattern approach with angiographic correlation for optimized target therapy delivery. Radiographics 35(5):1602–1618
Riaz A, et al. (2009) Radiologic-pathologic correlation of hepatocellular carcinoma treated with internal radiation using yttrium-90 microspheres. Hepatology 49(4):1185–1193
Bester L, et al. (2011) Imaging characteristics following 90yttrium microsphere treatment for unresectable liver cancer. J Med Imaging Radiat Oncol 55(2):111–118
Atassi B, et al. (2008) Multimodality imaging following 90Y radioembolization: a comprehensive review and pictorial essay. Radiographics 28(1):81–99
Kallini JR, et al. (2016) Hepatic imaging following intra-arterial embolotherapy. Abdom Radiol (NY) 41(4):600–616
Minocha J, Lewandowski RJ (2015) Assessing imaging response to therapy. Radiol Clin North Am 53(5):1077–1088
Ibrahim SM, et al. (2009) Radiologic findings following Y90 radioembolization for primary liver malignancies. Abdom Imaging 34(5):566–581
Memon K, et al. (2012) Alpha-fetoprotein response correlates with EASL response and survival in solitary hepatocellular carcinoma treated with transarterial therapies: a subgroup analysis. J Hepatol 56(5):1112–1120
Author information
Authors and Affiliations
Contributions
Study concept and design: Ronald A Mora, Rehan Ali, Ahmed Gabr, Nadine Abouchaleh, Ali Al Asadi, Joseph Ralph Kallini, Ahsun Riaz, Robert J Lewandowski, Riad Salem. Acquisition of data: Ronald A Mora, Rehan Ali, Ahmed Gabr, Nadine Abouchaleh, Ali Al Asadi, Ahsun Riaz, Robert J Lewandowski, Riad Salem. Analysis and interpretation of data: Ronald A Mora, Rehan Ali, Ahmed Gabr, Nadine Abouchaleh, Ali Al Asadi, Ahsun Riaz, Robert J Lewandowski, Riad Salem. Drafting of the manuscript: Ronald A Mora, Rehan Ali, Ahmed Gabr, Nadine Abouchaleh, Ali Al Asadi, Joseph Ralph Kallini, Ahsun Riaz, Robert J Lewandowski, Riad Salem. Critical revision of the manuscript for important intellectual content: All Authors. Statistical analysis: Ronald A Mora, Rehan Ali, Ahmed Gabr, Nadine Abouchaleh, Ali Al Asadi, Ahsun Riaz, Robert J Lewandowski, Riad Salem. Administrative, technical, or material support: Ronald A Mora, Rehan Ali, Ahmed Gabr, Nadine Abouchaleh, Ali Al Asadi, Ahsun Riaz, Robert J Lewandowski, Riad Salem. Study supervision: Ronald A Mora, Rehan Ali, Ahmed Gabr, Nadine Abouchaleh, Ali Al Asadi, Joseph Ralph Kallini, Ahsun Riaz, Robert J Lewandowski, Riad Salem.
Corresponding author
Ethics declarations
Conflict of interest
RJL and RS are advisors to BTG. None of the other co-authors report any conflict of interest.
Ethical Compliance
The study was Health Insurance Portability and Accountability Act compliant. All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Informed consent was obtained from all individual participants included in the study.
No funding was obtained for this study.
No studies were performed on animal subjects by any of the authors.
Rights and permissions
About this article
Cite this article
Mora, R.A., Ali, R., Gabr, A. et al. Pictorial essay: imaging findings following Y90 radiation segmentectomy for hepatocellular carcinoma. Abdom Radiol 43, 1723–1738 (2018). https://doi.org/10.1007/s00261-017-1391-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-017-1391-1