Abstract
Imaging has not only an established role in screening and diagnosis of hepatocellular carcinoma (HCC) in patients with chronic liver inflammatory diseases, but also a crucial importance for patient stratification and treatment allocation, as well as for assessing treatment response. In the setting of increasing therapeutic options for HCC, the Barcelona Clinic Liver Cancer (BCLC) system still remains the most appropriate way to select candidate cohorts for best treatments. This classification takes into account the imaging information on tumor burden and extension, liver function, and cancer-related symptoms, stratifying patients in five risk categories (Stages 0, A, B, C and D) associated with different treatment options. Still now, there are no clear roles for biomarkers use in treatment allocation. The increasing use of locoregional non-surgical therapies in the different stages is highly dependent on reliable evaluation of treatment response, in particular when they are used with curative intention or for downstaging at liver transplantation re-assessment. Moreover, objective response (OR) has emerged as an important imaging biomarker, providing information on tumor biology, which can contribute for further prognostic assessment. Current guidelines for OR assessment recommend only the measurement of viable tumor according to mRECIST criteria, with further classification into complete response, partial response, stable disease or progressive disease. Either computed tomography (CT) or magnetic resonance (MR) imaging can be used for this purpose, and the Liver Imaging Reporting and Data System (LI-RADS) committee has recently provided some guidance for reporting after locoregional therapies. Nevertheless, imaging pitfalls resulting from treatment-related changes can impact with the correct evaluation of treatment response, especially after transarterial radioembolization (TARE). Volume criteria and emerging imaging techniques might also contribute for a better refinement in the assessment of treatment response and monitoring. As the role of imaging deeply expands in the multidisciplinary assessment of HCC, our main objective in this review is to discuss state-of-the-art decision-making aspects for treatment allocation and provide guidance for treatment response evaluation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Hepatocellular carcinoma staging and treatment allocation
Hepatocellular carcinoma (HCC) is frequently a complication in patients with chronic liver disease, and an important cause of death [1]. Imaging is paramount for its early detection and diagnosis, which is established through well-defined imaging criteria, obviating the need for biopsy in most cases [2].
However, as treatment modalities for HCC develop and improve, stratification and prognostic assessment are particularly important to select the best candidates for each therapy. Since most patients have underlying chronic liver inflammatory diseases, this stratification is also heavily dependent on the severity of liver changes [3]. Therefore, current European Association for the Study of the Liver (EASL) Clinical Practice Guidelines and the American Association for the Study of Liver Diseases (AASLD) still endorse the BCLC classification [2, 4]. First proposed in 1999, BCLC takes into account not only the estimated tumor burden (size and number of lesions) but also the liver function and cancer-related symptoms [5]. Moreover, the complexity of this stratification, the large spectrum of patients within each category, and the availability of multiple treatment options require decisions based on dedicated multidisciplinary team meetings with all medical specialties involved, including Hepatobiliary Surgery, Hepatology, Oncology, Pathology, Interventional and Diagnostic Radiology [6]. A flow chart based on BCLC categories, which can be used as a guide for multidisciplinary discussion on treatment allocation, is presented in this article (Fig. 1).
Very early stage (BCLC-0) and early stage (BCLC-A)
BCLC classification was initially proposed for effectively identifying patients with early disease and good prognosis, which could benefit from radical treatments with curative intent [5]. BCLC-A includes patients presenting with single tumors or 2–3 nodules < 3 cm in diameter, good health status and preserved liver function. A very early stage (BCLC-0) was subsequently incorporated as the first stage in this classification, addressing the increasing diagnostic capabilities that allowed the earlier detection of smaller lesions (< 2 cm) though effective ultrasound screening programs in patients with liver cirrhosis [7].
If a small single lesion is found in patients with preserved liver function, especially if single focality is confirmed with staging MRI, they will usually be good candidates for either surgical resection or percutaneous ablation with curative intent.
Surgical resection should take into account not only the resectability of the lesion but also the absence of significant portal hypertension and guarantee of an adequate hepatic reserve [2]. When feasible, pathology has the important advantage of offering prognostic biomarkers which can provide liver transplantation indication in patients with aggressive lesions and high risk of recurrence [8, 9]. If the patient does not meet the criteria for resection, image-guided ablation is a convenient and less invasive alternative. This technique is usually performed as radiofrequency ablation (RFA) or microwave ablation (MCA), which is increasingly considered in most centers. Although infrequently, ethanol injection might be also be considered in tumors ≤ 2 cm, when thermal ablation is not technically feasible. Ethanol injection in tumors > 2 cm is associated with incomplete necrosis and high local recurrence rate [10].
Differently, patients with larger solitary lesions (especially if > 5 cm) are usually not considered for upfront resection, and alternatives should be defined. If the patient is otherwise a good surgical candidate, a strategy of lobar TARE plus resection might be the best choice. If not, ablation with or without combined TACE is another reasonable option.
LT should be reserved for patients in which other curable options are not adequate. LT has a good outcome in patients within the Milan criteria, with a 5-year survival exceeding 70% [11]. Therefore, it should be the best option for patients with solitary HCC with diameter ≥ 5 cm that are not good surgical candidates, or up to 3 nodules with diameter ≤ 3 cm, especially if they are bilobar [12]. Moreover, it is understandably the best option in patients with early disease and clinical signs of portal hypertension or decompensated cirrhosis. In this scenario, LT should be prioritized given that it treats both the tumor and the underlying liver disease.
Exceptionally, patients in the BCLC-A category that are not eligible for any curative treatment can still be considered for transarterial chemoembolization (TACE), according to a “stage migration” strategy in which the next suitable option from the next prognostic stage is offered [2].
Intermediate stage (BCLC-B) and advanced stage (BCLC-C)
BCLC-B stage includes multinodular and unresectable disease, with good health status and preserved liver function. BCLC-C stage is reserved for patients with portal invasion, extra-hepatic spread, or with worse performance status. These patients were usually excluded from curative treatments, but they include a heterogeneous group of patients, with an extended range of reported survival. As a result, treatment allocation is usually less straightforward and new strategies or treatment modalities have been introduced over the years.
For BCLC-B patients, TACE has been the most popular treatment modality for most patients with multinodular HCC which are deemed unresectable and poor candidates for LT. The indications for TACE are growing [13] as the superselective nature of this technique minimizes the risk of ischemic liver injury and drug-related systemic toxicity. In addition, retreatment is frequently possible to obtain higher response. The selection of patients that might benefit from this strategy remains a challenge.
In this sense, the heterogeneity of BCLC-B patients has been previously recognized in attempts to further subclassify them [14]. Even though no subclassification system has been widely accepted, many patients in this category are increasingly considered for curative treatment. In particular, despite being outside Milan criteria, similar survival rates after LT have been reported for selected patients in this group [12, 15]. These results have led to the increasing consideration for LT patients with higher disease stage, either using extended criteria for their selection (including the up-to-seven criteria) [15] or re-evaluation after successful downstaging with locoregional therapies, leading to inclusion in Milan criteria [12].
Despite ongoing controversy regarding this issue [16], indication for LT is consensually removed when facing larger tumors with portal vein invasion, extra-hepatic spread, or progressive liver disease post-TACE, as they usually show poor results after LT.
In patients with advanced stage (BCLC-C), systemic therapy is usually recommended, and Sorafenib is currently considered the first line agent. Other systemic therapies and clinical trials are reserved for patients with radiological progression under Sorafenib. Nevertheless, alternative therapies such as transarterial radioembolization (TARE, also known as SIRT systemic internal radiation therapy) and new systemic agents including immunotherapy agents are gaining wider acceptance.
In particular, TARE has emerged as an alternative not only to Sorafenib in patients with advanced disease but also as an extremely interesting alternative to TACE in patients with intermediate stage [17]. Current indications include larger tumors (> 5–8 cm), multinodular disease with more than 4 nodules, presence of portal thrombosis (lobar or segmental) and in patients considered non-responders to TACE. In contrast to TACE, TARE is microembolic and its therapeutic action is predominately attributable to the radiation effect of yttrium 90, which explains significant differences not only in treatment response but also in the expected imaging appearance after treatment. Commonly, tumors treated with TARE show a delayed response with a median time to response of 30–120 days [18]. An interesting feature of TARE is the presence of heterogeneous enhancement in treated area due to fibrosis which can retract and cause atrophy over time. Compensative hypertrophy of the remaining segments may be seen, which can even result in a significant increase of the liver volume and, consequently, liver reserve [19]. This has increased the attention for this technique as an interesting option to improve the chances of a surgical resection in patients that are otherwise good surgical candidates for this curative treatment [4].
Terminal stage (BCLC-D)
In patients with non-transplantable disease, end-stage liver function and bad performance status, only the best available supportive care can be offered.
Predictive imaging biomarkers
Besides currently accepted BCLC classification, in the last decade there has been a large interest in developing imaging biomarkers to better allocate treatments and estimate patient prognosis, mainly in the intermediate and advanced HCC stages. This has been done either by assessing the prognostic value of specific imaging findings [20] or more advanced quantitative biomarkers such as tumor texture and tissue perfusion metrics [21,22,23,24,25]. Radiomics has emerged as a promising field for the extraction of quantitative imaging biomarkers which can give important information on tumor biology. Quantitative features are not currently used in daily practice, as they require post-processing tools for its extraction, as well as further high-scale clinical validation [26]. However, if proven accurate, this “virtual biopsy” approach could provide important predictive biomarkers and might even have important advantages to histologic analysis after biopsy, given its non-invasive nature and capability to evaluate the entire lesion after 3D segmentation on the different time points. Furthermore, peri-tumoral region [27] and remaining liver parenchyma might also be assessed by imaging radiomics. However, this emerging field faces a lot of technical challenges for wider implementation, specially regarding the lack of homogeneity between imaging studies between different vendors or protocols and the continuous improvement of technology. Nevertheless, the use of an artificial intelligence (AI)-based approach might be very promising in this field, which might change the current approach for treatment allocation.
Evaluation of treatment response in hepatocellular carcinoma
Rationale for treatment response assessment
As locoregional treatments are increasingly popular options for HCC treatment in different stages, either with curative intent or for the reduction of tumor burden, their success relies on the assessment of radiologic objective response (OR) to assess full technical accomplishment.
When tumor ablation is used with curative intent in early stage disease, the presence of radiologic OR predicts survival benefit [28]. However, significant risk of recurrence has been reported after tumor ablation [29]. Therefore, and given that no current indication for adjuvant therapy exists, all patients should be carefully monitored, and imaging evaluation performed at least every 3–6 months [4]. The same is true for patients after tumor resection, in which retreatment or salvage LT might be considered when recurrent disease is detected [30, 31].
For patients with more advanced disease in which locoregional treatments are used for the reduction of tumor burden, the possibility of downstaging relies on the assessment of a successful treatment response. Particularly in patients outside Milan criteria, re-evaluation of transplantation eligibility should be done in successful cases [32].
Interestingly, locoregional treatments have also gained popularity in LT candidates within Milan criteria. In this setting, they are mainly used as bridging modalities while patients are in the waiting list for transplantation to avoid HCC progression if the estimating waiting time is longer than 6 months and also to minimize HCC recurrence after LT [33].
Treatment response also provides important information on tumor biology [34], given that a good response to treatment has shown correlation with histologic markers of good prognosis. Moreover, non-responders have shown higher HCC recurrence after LT. Importantly, OR has also shown to be a surrogate biomarker for patient survival [35, 36], highlighting its role as an important imaging biomarker with potential as a selection tool for treatment allocation during multidisciplinary discussion [16].
Despite the known value of OR as a prognostic biomarker in patients submitted to locoregional treatments such as TACE or ablation, its value in patients treated with systemic therapy is not clearly established [3]. The same is true for new treatments such as TARE in which treatment response evaluation is also more challenging and defined standardized protocols are still lacking [37].
Current guidelines for objective response (OR) assessment
After locoregional treatments, imaging assessment is done with multiphasic CT or MR [2]. Treatment evaluation highly depends on a good quality dynamic contrast-enhanced exam, either with CT or MR. Like pre-treatment assessment, the presence of hyperenhancement and/or washout appearance indicates viable tumor. If CT is used as the method of choice in the early assessment, a pre-contrast acquisition is suggested to correctly identify blood products or embolic material and for easier evaluating the presence of enhancement. On MR studies, image subtraction is recommended, as it is quite useful for the detection of post-treatment coagulative necrosis and hemorrhage changes [38, 39].
Traditionally, response has been evaluating measuring the whole lesions through the Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Nevertheless, more recent EASL and modified RECIST (mRECIST) criteria focus on the measurement of viable tumor, ignoring the treatment induced necrosis [40]. After ablation with curative treatment, visualization of complete necrosis is straightforward. However, partial necrosis is frequently observed, especially after TACE. In both cases, mRECIST has been favored for treatment evaluation after locoregional therapies as this approach has higher reproducibility and prognostic value when compared to the standard oncologic criteria (RECIST 1.1).
The mRECIST criteria classifies patients according to four categories: complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD). CR corresponds to the disappearance of any intra-tumoral arterial enhancement in all target lesions, PR is applied when there is at least 30% decrease in the sum of diameters of viable target lesions, SD for any cases that do not classify for partial response or progressive disease, and PD when there is at least 20% increase in the sum of diameters of viable target lesions. For outcome prediction (Figs. 2, 3), patients can be further classified into responders (CR or PR) or non-responders (SD or PD), as the latter group shows significant lower overall survival [41].
BCLC-C staged HCC in a patient with cirrhosis, classified as non-responder after TACE. Annotated pre-treatment CT images (a late arterial phase, b portal venous phase) showing a large HCC with extra-hepatic nodal disease (*). After treatment, late arterial phase image c shows an increase in size (over 20% increase in diameter of viable tumor), consistent with progressive disease (PD) according to mRECIST criteria
BCLC-B staged HCC in a patient with alcoholic liver disease classified as responder after TACE. On pre-treatment late arterial phase CT image (a) there is a 5 cm lesion with rim hyperenhancement, classified as LR-M and histologically proven as HCC. After treatment, late arterial phase CT image (b) shows nodular arterial phase hyperenhancement on the periphery of the lesion (arrow), compatible with the presence of viable tumor with more than 30% decrease in the diameters of viable tumor and consistent with partial response (PR) according to mRECIST criteria
Importantly, mRECIST follows strict rules for the definition of target lesions, which should only include typical HCC lesions, and for calculation of the final categories [41]. Furthermore, it also includes guidelines regarding the evaluation of vascular invasion, lymph nodes, effusions and new lesions. Important limitations exist in the assessment of infiltrative lesions, which cannot be considered target lesions, and in the setting of systemic therapies, as EASL recognizes that the current methods are still suboptimal for the evaluation of treatment response in these patients [2]. For the time being, the use of both mRECIST and RECIST 1.1 is recommended after systemic therapy. The application of immunotherapy will also require the adoption of new criteria, such as iRECIST.
For the evaluation of objective response and the application of mRECIST criteria, an important knowledge regarding imaging appearance of necrotic and viable treated HCC is needed. Moreover, changes in the surrounding liver parenchyma related with the effect of the local treatment are usually present. These surrounding abnormalities may be due to perfusion modifications, parenchymal inflammation, necrosis, fibrosis, or a combination of them [39]. Therefore, assessment of treatment response should take in consideration the timing of imaging after treatment, as well as the type of treatment that was performed.
Even though ideal timing for treatment assessment is not specified on current guidelines, early evaluation starting 1 month after treatment is common practice. Nevertheless, and especially after TARE, treatment-related findings can only resolve after 6 months follow-up. Multiple phase MR or CT is recommended in 3–4 months intervals for these cases [42].
LI-RADS criteria application and expected problems
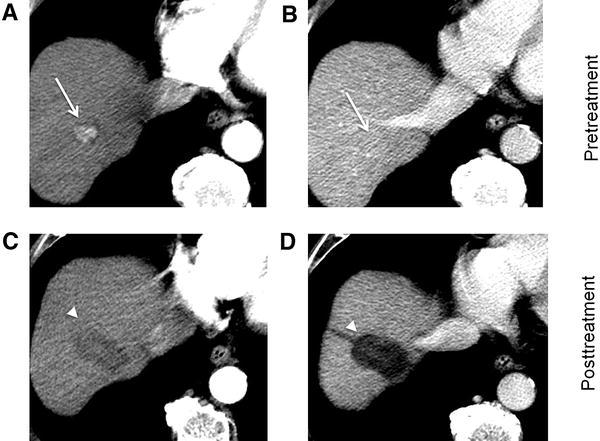
The Liver Imaging Reporting and Data System (LI-RADS) Committee currently provides some guidance for interpretation and reporting lesions after locoregional therapies, which can be also be applied to patients submitted to surgical resection [32, 43]. LR-TR (LI-RADS Treatment Response) algorithm allows categorization according to three categories: LR-TR Non-viable, LR-TR Equivocal and LR-TR Viable. The first is quite straightforward and related to lesions with no tumor enhancement or showing treatment-specific expected enhancement pattern. The LR-TR Equivocal category is applied when there is atypical lesion enhancement for treatment-specific expected pattern but not meeting the criteria for probable or definite tumor viability. LR-TR Viable category should be used when there is nodular, mass-like, or thick irregular enhancement suspicious for viable tumor (Fig. 4). Moreover, it should also be used when there is washout appearance and enhancement similar to that before treatment.
HCC dynamic contrast-enhanced CT images before (a late arterial, b portal phases) and after (c late arterial, d portal phases) TACE, with viable tumor (LR-TR Viable). On pre-treatment images there is a typical HCC (LR-5 observation) with arterial hyperenhancement, washout and pseudo-capsule appearance. Three months after treatment there is nodular arterial hyperenhancement on the periphery of the lesion (arrow) with washout on portal phase, due to viable tumor representing a partial response
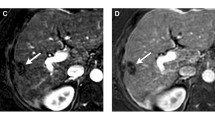
Some specifications should be considered regarding different treatment modalities when determining the presence of residual disease [43]. In patients after surgical resection, residual tumor should be evaluated at the resection margin. After ablation therapies (RFA or MWA), the treated area includes the peri-tumoral parenchyma, and the presence of recurrent or residual tumor usually appears as nodular arterial hyperenhancement nodule along this margin (Fig. 5).
Importantly, peri-tumoral parenchyma HCC enhancement should not be confused with a thin halo of necro-inflammatory enhancement, which is frequently seen in the early period after therapy and usually resolves after time [43]. Like the transient peripheral rim seen after tumor ablation, a similar phenomenon might persist after TACE (Fig. 6). It should be differentiated from nodular or mass-like enhancement within the tumoral lesion. Furthermore, peripheral arterioportal shunting may also mimic tumor hypervascularization, although hepatobiliary phase MR imaging is usually able to stablish the correct diagnosis.
The use of LI-RADS classification has shown both high positive predictive (86–96%) and high negative predictive (81% to 87%) values in the assessment of histopathologic viability of HCC treated with TACE [44], and can be extremely useful to guide the decision of further treatment or surveillance timing.
LI-RADS criteria apply to individual lesions, although they can be incorporated as patient level response assessment similar to mRECIST, even though the existence of an “equivocal viable tumor” category or the use of washout for the definition of viable tumor are not accounted in mRECIST [32]. The existence of a LR-TR equivocal category was introduced to allow some uncertainty in the assessment of treatment response, but most recent evidence suggests that these might correspond more frequently to incompletely necrotic lesions at histopathology [44]. The use of washout to define the presence of viable tumor might be particularly important in atypical lesions with no arterial hyperenhancement on pre-treatment CT (Fig. 7). Hypovascular HCC might be particularly difficult to assess after treatment given that mRECIST criteria cannot be applied [41], and the use of dual-energy CT, CEUS or MR perfusion studies are promising tools to improve imaging accuracy in these cases.
Furthermore, these criteria might not be applicable after TARE. Unlike other locoregional therapies, TARE does not cause immediate tumor size reduction or devascularization [37]. In fact, tumor size and arterial enhancement may transiently increase in the early (within 1 month) post-treatment period in a pseudo-progression phenomenon. But even after 1 month, patchy arterial enhancement can frequently be found either intra-tumoral or in the adjacent parenchyma from post-treatment inflammatory changes, simulating an infiltrative tumor appearance which is very difficult to differentiate real tumor infiltration. These changes often become less apparent in subsequent evaluations and usually resolve after 6 months, which is the reason why serial imaging with either CT or MR images are recommended in 3–4 months interval [42, 45] (Fig. 8).
HCC after TARE with partial response (PR). Serial imaging with CT (a late arterial, b portal phases) and MRI (c late arterial phase, d portal phase, e T2-weighted, f ADC map), were obtained for treatment response evaluation. MRI performed 6 months after treatment more efficiently detects the presence of viable tumor, with arterial hyperenhancement (black arrow) and washout with capsule appearance, moderate to high T2 signal and restricted diffusion (white arrow)
In particular, multiparametric MR imaging can be particularly important for clarification in difficult cases [45, 46]. Nevertheless, proper validation of treatment response criteria after TARE is still lacking.
Therefore, LI-RADS criteria are strongly encouraged for treatment evaluation, but further validation is still needed and will further clarify how to classify patient level response in problematic cases.
Future directions in treatment assessment
The assumption that tumor growth is isometric can be an extremely important limitation considering one-dimensional mRECIST, or even two-dimensional EASL measurement of the enhanced portion, given that liver tumors are frequently asymmetrical and show inhomogeneous patterns of enhancement, frequently the case after locoregional therapy. Particularly after TACE, this variability might be due to the presence of multiple tumor feeding vessels that are unequally treated. Three-dimensional (3D) evaluation of tumor burden after treatment has shown a good correlation with pathologic findings after TACE [47], and represents a promising imaging biomarker to better identify non-responders and predict patient survival [48]. This can be done using specific criteria, such as the approach called qEASL or vRECIST, in which a volumetric assessment of the enhancing tumor or the entire tumor is done, respectively. Moreover, these quantitative techniques have also been validated on patients treated with radioembolization, which could mean this could be an interesting option to traditionally problematic response evaluation after TARE [49].
Other explored techniques include the use of diffusion weighted derived metrics, as responding HCC lesions exhibit increased ADC value in acellular and necrotic areas [50]. Moreover, perfusion studies have been explored for the detection of perfusion changes in HCC lesions, in particular after chemoembolization. DCE-MR (dynamic contrast-enhanced MR) imaging has been shown to assess these changes effectively even in a semi-quantitative fashion [51], and CEUS (contrast-enhanced ultrasound) is also being used to confirm the technical success in the early setting to confirm technical success, or as an alternative method after TACE with lipiodol [52].
Abbreviations
- AASLD:
-
American Association for the Study of Liver Diseases
- BCLC:
-
Barcelona Clinic Liver Cancer
- CEUS:
-
Contrast-enhanced ultrasound
- CR:
-
Complete response
- CT:
-
Computed tomography
- DCE-MR:
-
Dynamic contrast-enhanced magnetic resonance
- EASL:
-
European Association for the Study of the Liver
- HCC:
-
Hepatocellular carcinoma
- LI-RADS:
-
Liver Imaging Reporting and Data System
- LR-TR:
-
LI-RADS treatment response
- LT:
-
Liver transplantation
- MCA:
-
Microwave ablation
- MR:
-
Magnetic resonance
- mRECIST:
-
Modified Response Evaluation Criteria in Solid Tumors
- OR:
-
Objective response
- PD:
-
Progressive disease
- PR:
-
Partial response
- qEASL:
-
Quantitative European Association for the Study of the Liver
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- RFA:
-
Radiofrequency ablation
- SD:
-
Stable disease
- SIRT:
-
Systemic internal radiation therapy
- TACE:
-
Transarterial chemoembolization
- TARE:
-
Transarterial radioembolization
References
Fitzmaurice C, Akinyemiju T, Abera S, et al (2017). The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level results from the global burden of disease study 2015. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2017.3055
Galle PR, Forner A, Llovet JM, et al (2018). EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. https://doi.org/10.1016/j.jhep.2018.03.019
Forner A, Reig M, Bruix J (2018). Hepatocellular carcinoma. Lancet. https://doi.org/10.1016/s0140-6736(18)30010-2
Marrero JA, Kulik LM, Sirlin CB, et al (2018). Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. https://doi.org/10.1002/hep.29913
Llovet JM, Brú C, Bruix J (1999). Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin Liver Dis. https://doi.org/10.1055/s-2007-1007122
Yopp AC, Mansour JC, Beg MS, et al (2014). Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol. https://doi.org/10.1245/s10434-013-3413-8
Darnell A, Forner A, Rimola J, et al (2015). Liver imaging reporting and data system with MR imaging: Evaluation in nodules 20 mm or smaller detected in cirrhosis at screening US. Radiology. https://doi.org/10.1148/radiol.15141132
Sala M, Fuster J, Llovet JM, et al (2004). High pathological risk of recurrence after surgical resection for hepatocellular carcinoma: An indication for salvage liver transplantation. Liver Transplant. https://doi.org/10.1002/lt.20202
Ferrer-Fàbrega J, Forner A, Liccioni A, et al (2016). Prospective validation of ab initio liver transplantation in hepatocellular carcinoma upon detection of risk factors for recurrence after resection. Hepatology. https://doi.org/10.1002/hep.28339
Germani G, Pleguezuelo M, Gurusamy K et al (2010). Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocellular carcinoma: A meta-analysis. J Hepatol. https://doi.org/10.1016/j.jhep.2009.12.004
Mazzaferro V, Regalia E, Doci R, et al (2002). Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N Engl J Med. https://doi.org/10.1056/nejm199603143341104
Burra P, Burroughs A, Graziadei I, et al (2016). EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol. https://doi.org/10.1016/j.jhep.2015.10.006
Raoul JL, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T (2019). Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev. https://doi.org/10.1016/j.ctrv.2018.11.002
Bolondi L, Burroughs A, Dufour JF, et al (2012). Heterogeneity of patients with intermediate (BCLC B) hepatocellular carcinoma: Proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. https://doi.org/10.1055/s-0032-1329906
Mazzaferro V, Llovet JM, Miceli R, et al (2009). Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. https://doi.org/10.1016/s1470-2045(08)70284-5
Mazzaferro V (2016). Squaring the circle of selection and allocation in liver transplantation for HCC: An adaptive approach. Hepatology. https://doi.org/10.1002/hep.28420
Gramenzi A, Golfieri R, Mosconi C, et al (2015). Yttrium-90 radioembolization vs sorafenib for intermediate-locally advanced hepatocellular carcinoma: A cohort study with propensity score analysis. Liver Int. https://doi.org/10.1111/liv.12574
Keppke AL, Salem R, Reddy D, Huang J, Jin J, Larson AC, Miller FH (2007). Imaging of hepatocellular carcinoma after treatment with yttrium-90 microspheres. AJR Am J Roentgenol. 188(3):768.
Theysohn JM, Ertle J, Müller S, et al (2014). Hepatic volume changes after lobar selective internal radiation therapy (SIRT) of hepatocellular carcinoma. Clin Radiol. https://doi.org/10.1016/j.crad.2013.09.009
An C. Kim DW, Park YN et al (2015). Single Hepatocellular Carcinoma: Preoperative MR Imaging to Predict Early Recurrence after Curative Resection. Radiology. 276(2):433-43.
Park HJ, Kim JH, Choi SY et al (2017). Prediction of Therapeutic Response of Hepatocellular Carcinoma to Transcatheter Arterial Chemoembolization Based on Pretherapeutic Dynamic CT and Textural Findings. AJR Am J Roentgenol. 209(4):W211-W220.
Zhou Y, He L, Huang Y, et al (2017). CT-based radiomics signature: a potential biomarker for preoperative prediction of early recurrence in hepatocellular carcinoma. Abdom Radiol (NY). 42(6):1695-1704.
Chen S, Zhu Y, Liu Z, Liang C (2017). Texture analysis of baseline multiphasic hepatic computed tomography images for the prognosis of single hepatocellular carcinoma after hepatectomy: A retrospective pilot study. Eur J Radiol. 90:198-204.
Wu M, Tan H, Gao F, et al (2018). Predicting the grade of hepatocellular carcinoma based on non-contrast-enhanced MRI radiomics signature. Eur Radiol. https://doi.org/10.1007/s00330-018-5787-2
Mulé S, Thiefin G, Costentin C et al (2018). Advanced Hepatocellular Carcinoma: Pretreatment Contrast-enhanced CT Texture Parameters as Predictive Biomarkers of Survival in Patients Treated with Sorafenib. Radiology. 288(2):445-455.
Martí-Bonmatí L, Alberich-Bayarri A, Garcí-Marí G et al (2012). Imaging biomarkers, quantitative imaging, and bioengineering. Radiologia. 54(3):269-78. https://doi.org/10.1016/j-rx-2010-12-013
Chen S, Feng S, Wei J et al (2019). Pretreatment prediction of immunoscore in hepatocellular cancer: a radiomics-based clinical model based on Gd-EOB-DTPA-enhanced MRI imaging. Eur Radiol. 29(8):4177-4187. https://doi.org/10.1007/s00330-018-5986-x
Sala M, Llovet JM, Vilana R, et al (2004). Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology. https://doi.org/10.1002/hep.20465
N’Kontchou G, Mahamoudi A, Aout M, et al (2009). Radiofrequency ablation of hepatocellular carcinoma: Long-term results and prognostic factors in 235 western patients with cirrhosis. Hepatology. https://doi.org/10.1002/hep.23181
Poon RTP, Sheung TF, Chung M Lo, Chi LL, Wong J (2002). Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: Implications for a strategy of salvage transplantation. Ann Surg. https://doi.org/10.1097/00000658-200203000-00009
Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S (2015). Recurrence of Hepatocellular Cancer after Resection: Patterns, Treatments, and Prognosis. Ann Surg. https://doi.org/10.1097/sla.0000000000000710
Kielar A, Fowler KJ, Lewis S, et al (2018). Locoregional therapies for hepatocellular carcinoma and the new LI-RADS treatment response algorithm. Abdom Radiol. https://doi.org/10.1007/s00261-017-1281-6
Llovet JM, Mas X, Aponte JJ, et al (2002). Cost effectiveness of adjuvant therapy for hepatocellular carcinoma during the waiting list for liver transplantation. Gut. https://doi.org/10.1136/gut50.1.123
Cescon M, Cucchetti A, Ravaioli M, Pinna AD (2013). Hepatocellular carcinoma locoregional therapies for patients in the waiting list. Impact on transplantability and recurrence rate. J Hepatol. https://doi.org/10.1016/j.jhep.2012.09.021
Vincenzi B, Di Maio M, Silletta M, et al (2015). Prognostic relevance of objective response according to EASL criteria and mRECIST criteria in hepatocellular carcinoma patients treated with loco-regional therapies: A literature-based meta-analysis. PLoS One. https://doi.org/10.1371/journal.pone.0133488
Lencioni R, Montal R, Torres F, et al (2017). Objective response by mRECIST as a predictor and potential surrogate end-point of overall survival in advanced HCC. J Hepatol. 66(6):1166–1172.
Adcock CS, Florez E, Zand KA, Patel A, Howard CM, Fatemi A (2018). Assessment of Treatment Response Following Yttrium-90 Transarterial Radioembolization of Liver Malignancies. Cureus. https://doi.org/10.7759/cureus.2895
Gordic S, Corcuera-Solano I, Stueck A, et al (2017). Evaluation of HCC response to locoregional therapy: Validation of MRI-based response criteria versus explant pathology. J Hepatol. https://doi.org/10.1016/j.jhep.2017.07.030
Hussein RS, Tantawy W, Abbas YA (2019). MRI assessment of hepatocellular carcinoma after locoregional therapy. Insights Imaging. https://doi.org/10.1186/s13244-019-0690-1
Bruix J, Reig M, Rimola J, et al (2011). Clinical decision making and research in hepatocellular carcinoma: Pivotal role of imaging techniques. Hepatology. https://doi.org/10.1002/hep.24670
Lencioni R, Llovet JM (2010). Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. https://doi.org/10.1055/s-0030-1247132
Vogel A, Cervantes A, Chau I, et al (2018). Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. https://doi.org/10.1093/annonc/mdy308
Voizard, N., Cerny, M., Assad, A. et al (2019). Assessment of hepatocellular carcinoma treatment response with LI-RADS: a pictorial review. Insights Imaging. 10, 121. https://doi.org/10.1186/s13244-019-0801-z
Erin L. Shropshire, Mohammad Chaudhry, Chad M. Miller, Brian C. Allen, Erol Bozdogan, Diana M. Cardona, Lindsay Y. King, Gemini L. Janas, Richard K. Do, Charles Y. Kim, James Ronald MRB (2019). LI-RADS Treatment Response Algorithm: Performance and Diagnostic Accuracy. Radiology. 00(0):1–9. https://doi.org/10.1148/radiol.2019182135
Semaan S, Makkar J, Lewis S, Chatterji M, Kim E, Taouli B (2017). Imaging of hepatocellular carcinoma response after 90y radioembolization. Am J Roentgenol. https://doi.org/10.2214/ajr.17.17993
Chaudhry M, McGinty KA, Mervak B, et al (2019). The LI-RADS version 2018 MRI treatment response algorithm: evaluation of ablated hepatocellular carcinoma. Radiology. https://doi.org/10.1148/radiol.2019191581.
Chapiro J, Wood LD, De Lin M, et al (2014). Radiologic-pathologic analysis of contrast-enhanced and diffusion-weighted MR imaging in patients with HCC after TACE: Diagnostic accuracy of 3D quantitative image analysis. Radiology. https://doi.org/10.1148/radiol.14140033
Tacher V, Lin M De, Duran R, et al (2016). Comparison of existing response criteria in patients with hepatocellular carcinoma treated with transarterial chemoembolization using a 3D quantitative approach. Radiology. https://doi.org/10.1148/radiol.2015142951
Chapiro J, Duran R, Lin M De, et al (2015). Early survival prediction after intra-arterial therapies: a 3D quantitative MRI assessment of tumour response after TACE or radioembolization of colorectal cancer metastases to the liver. Eur Radiol. https://doi.org/10.1007/s00330-015-3595-5
Sahin H, Harman M, Cinar C, Bozkaya H, Parildar M, Elmas N (2012). Evaluation of treatment response of chemoembolization in hepatocellular carcinoma with diffusion-weighted imaging on 3.0-T MR imaging. J Vasc Interv Radiol. https://doi.org/10.1016/j.jvir.2011.08.030
Taouli B, Johnson RS, Hajdu CH, et al (2013). Hepatocellular carcinoma: Perfusion quantification with dynamic contrast-enhanced MRI. Am J Roentgenol. https://doi.org/10.2214/ajr.12.9798
Spârchez Z, Mocan T, Radu P, et al (2016). Contrast enhanced ultrasonography in assessing the treatment response to transarterial chemoembolization in patients with hepatocellular carcinoma. Med Ultrason. https://doi.org/10.11152/mu.2013.2066.181.scz
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amorim, J., França, M., Perez-Girbes, A. et al. Critical review of HCC imaging in the multidisciplinary setting: treatment allocation and evaluation of response. Abdom Radiol 45, 3119–3128 (2020). https://doi.org/10.1007/s00261-020-02470-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02470-1