Abstract
Purpose of Review
Heart failure is characterized by episodes of congestion with need for hospitalization. The current metrics lack the accuracy to predict and prevent episodes of congestion and to guide diuretic titration to reach euvolemia in case of decompensation. This article aims to provide answers to the role of urinary sodium measurements in acute and chronic heart failure.
Recent Findings
In acute heart failure, urinary sodium concentrations at the moment of admission and after diuretic administration are correlated with short- and long-term outcome. As this is a reflection of the degree of sodium retention, it can be used as a guide in the diuretic titration. In chronic heart failure, it might be used to predict and consequently prevent episodes of decompensation.
Summary
Urinary sodium measurements hold great promises to be a novel diagnostic and therapeutic parameter in patients with acute and chronic heart failure. However, more research is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Signs and symptoms of congestion remain the main reason for hospitalization in patients with heart failure. The novel quadruple guideline-directed medical therapy has proven to reduce morbidity and mortality in heart failure with reduced ejection fraction (HFrEF) patients [1]. However, the need for hospitalization remains high with rehospitalization rates up to 15% within 1 to 2 years in the latest heart failure (HF) trials, which runs up to 27% within a year in patients with more advanced heart failure [2,3,4,5]. After an acute heart failure event, the risk for rehospitalization is even higher, counting up to 25% within 3 months [6, 7]. Diuretic resistance and discharge with residual signs of congestion are a major risk factor for adverse events [8]. Neurohumoral upregulation in HF increases renal sodium avidity, compromising natriuresis and resulting in a positive sodium balance [9, 10]. There is growing evidence that spot urinary sodium measurements may help to guide treatment and follow-up of patients with both acute and chronic heart failure [1, 9, 11, 12].
Renal Alterations and Neurohumoral Upregulation in Heart Failure
In normal physiological circumstances, the human body regulates the urinary sodium excretion in balance to the dietary sodium intake. The ingested salt is almost completely reabsorbed by the gastro-intestinal tract, which leads to an increase in plasma osmolality and consequently an increase in the release of arginine-vasopressin in order to reduce diuresis. The baroreceptors in the large and small vasculatures are triggered by this increase in total body water, causing an increase in sodium and water excretion in the kidney. Sodium is freely filtered in the renal glomerulus; however, almost 99% is reabsorbed in the renal tubules with the largest part being reabsorbed by the proximal tubule (Fig. 1A). While the body aims to balance the sodium excretion with the sodium intake, recent insights suggest that sodium is not distributed in the body solely as a free cation, but that it is also bound to large interstitial glycosaminoglycan networks in different tissues, which have an important regulatory function [13, 14].
In HF, hemodynamic alterations and neurohumoral upregulation give rise to an increased renal sodium avidity with a net positive sodium balance (Fig. 1B). A decreased cardiac output, increased filling pressures, and elevated abdominal pressure lead to a diminished renal blood flow (RBF) [15]. Increased filling pressures together with renal venous congestion result in a decreased RBF and an increase in hydrostatic capillary pressures, which are further aggravated by the neurohumoral activation. The increased glomerular pressures lead to an acceleration of nephron loss in patients with heart failure [16]. In order to maintain the glomerular filtration rate, the kidney increases the filtration fraction in case of a drop in RBF (autoregulation), which results in an increase in sodium and water reabsorption in the proximal tubule (glomerulotubular balance). As a result, the amount of sodium reaching the loop of Henle and the distal parts of the tubules is dramatically reduced. In addition, the neurohumoral upregulation in HF increases the sodium reabsorption along the ascending loop of Henle. Further down in the nephron, an increase in aldosterone levels triggers sodium reabsorption by the sodium-chloride co-symporter and by the insertion of the epithelial natrium channels (ENaC) in the distal convoluted tubules and in the collection ducts. Despite the general volume overload in patients with HF, the tubular flow in the distal part of the nephron is low due to the increased proximal sodium and water reabsorption. The decreased blood flow in the vasa recta in combination with the increased sodium reabsorption renders the interstitium of the renal medulla hypertonic. This osmotic gradient, together with the increase in arginine-vasopressin release due to angiotensin II activation, promotes free water retention in the collection ducts and impairs the diluting capacity of the kidney. The increased sodium avidity and in parallel chloride reabsorption, in the proximal parts of the nephron, result in a reduced chloride concentration presented at the macula densa, which activates the release of renin with an ongoing activation of the renin–angiotensin–aldosterone axis (tubuloglomerular feedback) [9, 17].
As impaired natriuresis is one of the key pathophysiological components leading to HF with an increase in plasma and extravascular volume, it seems rational that measuring the urinary sodium content may hold important information.
Role in Acute Heart Failure
The main goal in the treatment of AHF is to achieve decongestion (Class I recommendation), as this is linked to improved survival and a reduction in rehospitalization rate [1, 18]. Recently, the Acetazolamide in Acute Decompensated Heart Failure with Volume OveRload (ADVOR) trial demonstrated that it is necessary to target congestion early during hospitalization with combination diuretic therapy of acetazolamide and loop diuretics as a delay in decongestive therapy cannot fully be corrected during the further hospital stay [19]. This is also reflected in the fact that an early initiation of diuretic therapy in patients with AHF is associated with a lower in-hospital mortality [20]. Additionally, achieving euvolemia is associated with better long-term result with an improvement in survival and a lower risk for rehospitalization [8, 21]. However, there is no “one-size fits all” model in treating patients with AHF and optimization of diuretic therapy requires an individualized approach. In current daily practice, diuretic titration is based upon clinical, hemodynamic, biochemical, and echocardiographic parameters. However, most of these parameters are rather inaccurate in evaluating adequate decongestion and do not allow an early adaptation of diuretic therapy as these metrics require some time to change after loop diuretic administration [22]. Urinary sodium measurement may be a valid and more importantly a fast and easily accessible tool in guiding diuretic therapy in patients with AHF [10, 12].
Loop diuretics are the cornerstone of the treatment of patients with acute heart failure, promoting sodium excretion in the ascending part of the loop of Henle by blocking the sodium/potassium/2chloride (NKCC2) cotransporter. Renal adaptations increase sodium reabsorption in proximal and distal parts of the nephron, which is defined as loop diuretic resistance [23]. Loop diuretics not only block the NKCC2-cotransporter at the ascending part of the loop of Henle, but also the apical NKCC2 in the macula densa. By inhibiting the NKCC2 cotransporter, the chloride concentration delivered at the macula densa is decreased, which leads to a release of prostaglandin E2 (PGE2) and nitric oxide (NO). The increased levels of PGE2 and NO cause vasodilatation of the afferent arteriole and trigger the juxtaglomerular cells to release renin, which further increases neurohormonal activation (Fig. 1B) [24]. Therefore, optimizing diuretic therapy in patients with volume overload requires an individualized approach. The dosage of loop diuretics and need for combination therapy varies between and within patients over time [25, 26]. The urinary sodium excretion is a marker of their efficacy as it directly reflects its mechanism of action. In order to make a judgement about the sodium balance, the total natriuresis should be interpreted in the light of the dietary sodium intake [27].
The level of sodium excretion can be measured in a 24-h urine collection, a 6-h urine collection, or a urinary sample spot. A spot sample has the advantage of being simpler and easier to obtain and to make an immediate assessment regarding diuretic response. However, bear in mind that this only gives the sodium concentration (mmol/L) and does not determine the daily total urinary sodium excretion (mmol).
To Guide Diuretic Therapy in AHF
Singh et al. were the first to demonstrate in 52 patients with AHF that a diminished urinary sodium excretion on spot samples measurements was associated with a low net 24-h volume output [28]. A poor diuretic response was later defined by Testani et al. as a cumulative urinary sodium output below 50 mmol in a 6-h urinary collection after diuretic administration [25]. This threshold was chosen based on the pharmacodynamic principle that loop diuretics have a half-life of 1.5–2 h and that their natriuretic effect is completed within 6-h [29]. A suboptimal diuretic efficiency was defined as a urinary sodium excretion below 100 mmol in the first 6-h after diuretic administration. Damman et al. confirmed the association between a 6-h sodium excretion and the total 24-h urine output [30]. However, this definition of poor diuretic response requires timed urinary collections in the hospital, which is known to be difficult and is associated with a high level of sampling errors. Therefore, spot urinary sodium samples are suggested as an alternative method. Testani et al. demonstrated that a highly accurate prediction of sodium content of a timed 6-h urine collection was achievable with a physiology-derived equation using the sodium concentration in a spot urine sample collected 2 h after diuretic administration (area under the curve (AUC) 0.90–0.92) [27, 31]. The natriuretic response prediction equation (NRPE) calculates the 6-h urinary sodium excretion by estimating the instantaneous rate of urine production, multiplied by the urinary sodium concentration on a spot sample 2-h after diuretic administration. The rate of urine production can be calculated from the estimated glomerular filtration rate and the serum to urine creatinine ratio as creatinine is limited reabsorbed or secreted in the tubules. This suggests that an early evaluation of the diuretic response with timely therapy alterations is possible in patients with AHF [25]. However, NRPE is still complex and requires not only urinary sodium content, but also the blood and urinary creatinine levels together with body weight and length.

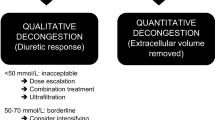
A urine spot sample sodium concentration is a simplified strategy and still holds an AUC of 0.89 to predict a poor loop diuretic response [31]. In order to simplify the recommendations, a post hoc analysis showed that a spot urinary sodium concentration below 50–70 mmol/L identifies patients with a poor loop diuretic response and patients who may benefit from early diuretic up-titration. A protocol for decongestive therapy based upon urinary sodium concentration measurement and volume output has been suggested by the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) position paper, which was more recently incorporated in the latest ESC heart failure guidelines [1, 10]. The usefulness of the natriuretic concentration threshold was confirmed in a monocentric observational trial in which patients with a urinary sodium concentration below 50 mEq/L had a higher risk for developing diuretic resistance during hospital stay (risk ratio 5.0, 95% confidence interval 2.4–10). Neither weight change nor initial diuresis was able to predict diuretic resistance [11]. Brinkley et al. conducted a prospective trial in 176 patients with AHF receiving intravenous loop diuretics in an outpatient clinic. They demonstrated that a spot urinary sodium measurement on the first void after diuretic administration correlated well with the urinary output after 3-h and higher sodium concentrations were associated with a lower risk for hospitalization or emergency visits within 30 days [32].
Importantly, the urinary sodium composition during decongestive therapy changes significantly over time when using loop diuretic mono therapy. Although urinary volume output remains stable (diuresis), a drop in natriuresis is expected during the consecutive days of loop diuretic therapy [30]. The diagnostic and prognostic value of a urinary sodium measurement after the first administration of loop diuretics has been demonstrated in several studies; however, its diagnostic value during the consecutive days remains unanswered.
Importantly, as suggested by the ESC heart failure guidelines, urinary sodium concentration early during decongestive therapy may help identify patients who are in need of diuretic intensification in order to maximize diuretic efficacy. Observational studies demonstrated that up to 40% of patients presenting with AHF met criteria for diuretic resistance, which highlights the importance of this strategy [25, 33,34,35]. Patients with diuretic resistance are more likely to have a worse glomerular filtration rate, a higher dose of home maintenance therapy with loop diuretics, a higher NYHA class, a lower blood pressure, higher natriuretic peptides, and lower hemoglobin levels at presentation [11, 30, 36,37,38]. Urinary spot sodium measurement may be the new kid in the block, leading to early optimization of diuretic treatment in patients with AHF. Beside the evaluation of diuretic response, urinary sodium concentration measurements also have a role as a prognostic marker in AHF (Table 1).
Prognostic Marker in AHF
As mentioned above, the urinary sodium concentration after diuretic administration is useful in predicting the diuretic response and may guide the decongestive therapy. However, a measurement of the urinary sodium concentration prior to loop diuretic administration may also hold information regarding the intrinsic renal sodium avidity. A large retrospective analysis of a Japanese registry divided a total of 669 patients into tertiles based upon the urinary sodium result at the moment of hospital admission before diuretic therapy was administered. Patients with a lower baseline urinary sodium concentration tended to have higher plasma renin, aldosterone, cortisol, and dopamine levels, indicating that these patients had a higher neurohumoral upregulation which resulted in an increased renal sodium avidity with lower renal sodium excretion. These patients required higher diuretic dosages during hospital stay and in the long-term had an increased risk for all-cause mortality and worsening renal failure [44]. Cox et al. demonstrated that in patients with AHF and volume overload, compensatory post-diuretic sodium reabsorption is not a major driver of diuretic resistance. On the contrary, patients with a higher natriuretic response after loop diuretics also tended to have a higher post-diuretic spontaneous natriuresis. The diuretic response itself was largely determined by the basal sodium avidity, enhancing the prognostic importance of a baseline urinary sodium measurement [49]. A post hoc analysis of the IMPROVE trial demonstrated that for every 20 mmol/L increase in baseline urine sodium concentration, there was a 25% decrease in all-cause mortality and an 8% decrease in all-cause readmissions. The inability to increase the urinary sodium excretion after 24-h was associated with an increase in mortality rates. However, the ability to increase natriuresis after diuretic administration seemed only of importance in patients with a baseline low urinary sodium excretion as there was no effect found in the group with a urinary sodium excretion above 50 mmol/L [50]. It should be noted that this was a retrospective analysis of a relatively small sample size (n = 160) and patients could have received intravenous loop diuretics prior to randomization, i.e., prior to urine sample collection. A prospective, observational trial performing serial urinary sodium measurements in patients with AHF was not able to confirm a correlation between the baseline urinary sodium concentration and clinical outcome. However, a low urinary sodium at 6-h or the inability to increase the urinary sodium concentration after diuretic administration was associated with a higher 1-year mortality risk compared to patients with an increase in sodium concentration. There was no difference in baseline glomerular filtration rate between the two groups, highlighting the different role and the disconnection of the tubular and glomerular function of the kidney [46]. Other small observational studies confirmed these results, stating that a stronger natriuretic response was associated with a better long-term outcome and a lower risk for all-cause mortality and heart failure rehospitalizations, independently from baseline glomerular filtration rate [30, 35, 39, 40, 42]. A retrospective analysis of the ROSE-AHF demonstrated in almost 30% of the patients a poor natriuretic response after 24-h, which was associated with an increased risk for all-cause mortality at 6 months, even in the context of a negative fluid balance [47]. Damman et al. performed a prospective, single-center, open-label study and were able to demonstrate in a cohort of 175 AHF patients that a 6-h sodium output below 89 mmol was a strong predictor of a higher all-cause mortality with a median follow-up of 257 days (hazard ratio of 3.81 (CI 1.92–7.57)) [30].
Another approach is measuring the urinary sodium concentration on the first void after diuretic administration. A trial conducted in 103 patients with AHF compared clinical outcome between patients with a urinary sodium concentration below and above 60 mmol/L. The time to first void did not differ between the two groups. Patients with a urinary sodium concentration below the predefined cut-off point were more than twice as likely to need mechanical circulatory or inotropic support during admission or to die during the next 90 days [43]. Despite the limitations of the small sample size and the arbitrary cut-off, the main message remains that an early urinary sodium measurement may identify patients at risk for poor outcome. The median time to first void was 157 min (86–244 min) and could be compared to a urine sample after 2-h as was proposed by Testani. The same cut-off value was used in a post hoc analysis of the Renal Optimization Strategies Evaluation in Acute Heart Failure (ROSE-AHF) trial, which showed that patients with a urinary sodium concentration below 60 mmol/L had a longer length of hospital stay and a lower weight loss after 72-h [48]. Collins et al. demonstrated that an early assessment of urinary sodium excretion after loop diuretic therapy was not only useful to determine diuretic response, but was also a predictor of worsening heart failure (WHF). Patients who developed WHF during hospitalization had a diminished urinary sodium concentration 1 h after diuretic administration, with a 100% sensitivity for predicting WHF in patients with a urinary sodium content below 35.4 mmol [45]. A poor natriuretic response had a stronger association with short-term and long-term outcome than historically used parameters such as weight change, urinary output, or fluid balance. Perez et al. confirmed these results and a low natriuresis on a spot sample 2-h after diuretic administration was associated with a higher risk for diuretic resistance, longer hospital stay, and worse clinical outcome (rehospitalization and death) at 6 months [52].
Biegus et al. investigated whether the prognostic information of the urinary sodium concentration depended on the time course of the AHF hospitalization. The urine sodium concentration after diuretic intake at the moment of admission, at 24-h and 48-h, all entailed significant prognostic information regarding a composite end point of 1-year all-cause mortality or heart failure hospitalization. However, the urinary sodium concentration at discharge was not able to predict clinical outcome [51]. This emphasizes the importance of knowing the timing and volume status at the moment of sodium measurement in order to make an adequate assumption regarding prognosis.
Ongoing Trials Assessing the Value of Spot Urinary Sodium in AHF
It should be noted that all these data are collected from retrospective or observational studies with a relatively small sample size (Table 1). Currently, three prospective trials are investigating the importance of a urinary sodium guided diuretic approach. The Pragmatic Urinary Sodium‐based treatment algoritHm in Acute Heart Failure (PUSH-AHF, NCT04606927) is a single-center, randomized, open-label trial in the Netherlands. It aims to randomize 310 patients towards an intervention group or a standard of care group. In the intervention arm, diuretic therapy is titrated based upon the urinary sodium concentration with a stepwise approach of increasing dose and starting combination therapy. The co-primary end point is the total natriuresis after 24-h and the combined end point of mortality or first heart failure rehospitalization within 6 months [54]. The Efficacy of a Standardized Diuretic Protocol in Acute Heart Failure (ENACT-HF) trial is an international, non-randomized, open-label, pragmatic study, aiming to enroll 500 patients. In order to be eligible for inclusion, patients should be treated with a maintenance dose of loop diuretics of at least 40 mg of furosemide or equivalents. Participating study centers will first include patients in phase 1 of the study, i.e., standard of care, and afterwards in the phase 2 of the study. In phase 2, patients will receive diuretic up-titration based upon the protocol suggested by the HFA algorithm and the result of the urinary sodium measurement. The primary end point is the total urinary sodium excretion after 24-h [55]. A large, randomized, triple-blinded study aims to include 484 AHF patients in the emergency department, with a stratification in a 1:1 ratio towards standard of care or intervention group. In the intervention group, results of urine and serum creatinine will be entered in a diuretic calculator three times daily after which a diuretic dose will be chosen based on daily urine output goals. The primary endpoint is the days of clinical benefit from randomization through day 14, which includes global clinical status and hospital days (NCT04481919). These trials will provide important insights whether an improvement in natriuresis will also lead to changes in morbidity and mortality, or whether it is just a reflection of a more advanced disease stage without any influence on outcome (Fig. 2).
Role of Urinary Sodium Assessment in Patients with Chronic Heart Failure
In contrast to AHF where the goal is to achieve decongestion with a net negative sodium and fluid balance, the goal in patients with chronic heart failure (CHF) is to maintain euvolemia with a neutral sodium and fluid balance. The assessment of urinary sodium concentrations might be helpful to monitor changes in volemic state (Table 2).
Urinary Sodium and Prognosis
Multiple trials have demonstrated a strong correlation between the sodium concentration on a morning spot urinary sample prior to diuretic intake and the total 24-h urinary sodium excretion [38, 57]. A retrospective cohort study examined the long-term outcome in ambulatory chronic heart failure patients in relationship to the morning spot urinary sodium concentration. Patients with a furosemide dosage to urinary sodium concentration ratio above 0.8 (i.e., higher maintenance loop diuretic or lower baseline urinary sodium excretion) would have a three times higher risk of dying within the next 5 years. Patients with a baseline high urinary sodium excretion (> 80 mmol/L) and concomitantly a low dosage of maintenance loop diuretic therapy (furosemide < 80 mg per day) had the best survival rate, suggesting that patients with the least neurohormonal activation and consequently lowest diuretic resistance had the best long-term outcome. In line with this cohort, Elias et al. demonstrated that patients with more advanced disease reflected by a low baseline urinary sodium concentration (< 80 mmol/L) despite high maintenance diuretic therapy (furosemide > 80 mg per day) had a five times higher mortality risk in the next 5 years [38]. The prognostic role of urinary sodium excretion in stable, chronic heart failure patients is confirmed in a prospective cohort study with a median follow-up time of 10–14 years, including 180 men from the Kuopio Ischaemic Heart Disease Risk Factor Study (KIHD). The study lacked to demonstrate an association with major adverse coronary events (MACE), but was able to demonstrate an inverse association between the total 24-h urinary sodium excretion and all-cause mortality risk [59].
Martens et al. anticipated a role of home urinary sodium measurements in telemonitoring patients with CHF. This single-center, observational trial prospectively followed 80 patients with chronic, stable heart failure. Patients were asked to collect a first morning void once-weekly for 30 consecutive weeks. During a mean follow-up of 587 ± 54 days, 21 patients developed an AHF event and 16 of these events were during active measurements of the urinary sodium. The longitudinal urinary sodium profile of patients with an AHF event was in general lower than the profile of patients with stable CHF. Moreover, there was a clear drop in urinary sodium concentration in the week preceding an AHF event and the urinary sodium concentration returned to baseline after decongestion [57]. Despite the study limitations (small sample size, single-center, observational study), these results hold great promise for novel telemonitoring possibilities in the future (Fig. 2). Albeit more data with multicentric, randomized trials are necessary for urinary sodium measurements in patients with chronic heart failure, there is already a trend that this may pay an important role in the prediction of AHF events, maintenance loop diuretic titration, and prognostication (Table 2).
Urinary Sodium to Guide Loop Diuretic Withdrawal
Cox et al. demonstrated in patients with AHF that compensatory post-diuretic sodium retention is not an important driver of diuretic resistance, as patients with a higher natriuretic response on diuretic therapy also have a larger spontaneous natriuresis afterwards. Chronic loop diuretic therapy is associated with an increase in morbidity and mortality and may hamper up-titration of guideline-directed medical therapy. Therefore, Dauw et al. investigated whether cessation of maintenance loop diuretic therapy was possible in patients with stable chronic heart failure in whom the investigators felt there was a need to continue loop diuretic therapy. A 24-h urine collection was collected after diuretic intake and repeated the next day after cessation of diuretic therapy. On the day of diuretic omission, there was a tremendous drop in natriuresis (50 ± 23%) and urine output (30 ± 20%) in comparison with the 24-h urine collection the day before on diuretic therapy. Despite the small study sample, the study demonstrated that stable, CHF patients necessitating chronic maintenance loop diuretic therapy still have a clear loop diuretic response. The natriuresis and urine output on the day of diuretic intake was comparable to patients without the need for maintenance therapy, however with a distinct time pattern. After diuretic omission, a drop in urinary sodium excretion and volume production remained during the day and night. As demonstrated in AHF, the drop in natriuresis and urine output after diuretic cessation is most likely the result of an increased intrinsic renal sodium avidity reflecting the need for diuretic therapy in this patient cohort rather than a pure effect of diuretic omission [58].
In contrast, the ReBIC1 trial was a randomized, double blind, placebo-controlled trial investigating whether diuretic cessation was achievable in CHF patients (n = 188) on stable diuretic maintenance dose, without signs and symptoms of volume overload, without a heart failure hospitalization during the last 6 months, and with optimized and stable HF treatment. After diuretic withdrawal, there was no significant difference in patient-reported dyspnea score after 90 days with an excellent short-term prognosis [60]. An observational study examined down-titration of loop diuretic therapy in 50 patients with stable, chronic heart failure. After 30 and 180 days, down-titration was feasible in 62% of the patients and down-titration failure was always notable within 30 days [61]. Therefore, it should be feasible to down-titrate diuretic maintenance therapy in a pre-selected subgroup of CHF patients. A correct identification of these patients may still hamper down-titration efforts in clinical practice.
A small, observational study demonstrated a tendency towards easier down-titration in patients with low urinary chloride concentration post-diuretic therapy. Lower urinary chloride (and sodium) concentrations were reflective of better decongested state without the need for loop diuretics. The urinary chloride concentration correlated strongly with the urinary sodium concentration [56].
As such, urinary sodium (and chloride) measurement may play an important role in guiding diuretic needs in the outpatient setting, but it is clear that this depends on the timing of the sample in relation with the loop diuretic intake (and dosing).
Conclusion
Neurohumoral upregulation is the driving mechanism leading to excessive renal sodium avidity in patients with heart failure, resulting in extracellular volume overload with signs and symptoms of congestion. As natriuresis is a direct marker of neurohumoral activation, it seems logical that this may be a useful parameter in patients with HF. In AHF, the role of early urinary sodium measurements is well established with its recommendation in the novel ESC guidelines. It allows early identification of patients with diuretic resistance with prompt adaptation of diuretic therapy. As patients with diuretic resistance have a more advanced disease process, the urinary sodium measurement also correlates with long-term outcomes. Data in patients with CHF are less robust; however, there are some data that urinary sodium measurements also hold a prognostic and therapeutic role but more research is necessary.
Data Availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726.
Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382(20):1883–93.
Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–24.
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–61.
McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004.
Dharmarajan K, Wang Y, Lin Z, Normand ST, Ross JS, Horwitz LI, et al. Association of changing hospital readmission rates with mortality rates after hospital discharge. JAMA. 2017;318(3):270–8.
Krumholz HM, Hsieh A, Dreyer RP, Welsh J, Desai R, Dharmarajan K. Trajectories of risk for specific readmission diagnoses after hospitalization for heart failure, acute myocardial infarction, or pneumonia. PLoS ONE. 2016;11(10):1–14.
Metra M, Davison B, Bettari L, Sun H, Edwards C, Lazzarini V, et al. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Hear Fail. 2012;5(1):54–62.
Mullens W, Damman K, Harjola V, Mebazaa A, Rocca HB, Martens P, et al. The use of diuretics in heart failure with congestion — a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21:137–55.
Mullens W, Damman K, Testani JM, Martens P, Mueller C, Lassus J, et al. Evaluation of kidney function throughout the heart failure trajectory – a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22(4):584–603.
Cobo M, Zegri I, Paloma R, Garcia-Gomez S, Garcia-Rodriguez D, Dominguez-Rodriguez F, et al. Usefulness of natriuresis to predict in-hospital diuretic resistance. Am J Cardiovasc Dis. 2020;10(4):350–5.
Tersalvi G, Dauw J, Gasperetti A, Winterton D, Cioffi GM, Scopigni F, et al. The value of urinary sodium assessment in acute heart failure. Eur Hear Journal Acute Cardiovasc Care. 2021;10(2):216–23.
Mullens W, Verbrugge FH, Nijst P, Hong W. Renal sodium avidity in heart failure : from pathophysiology to treatment strategies. Eur Heart J. 2017;38:1872–82.
Nijst P, Verbrugge F, Grieten L, Dupont M, Steels P, Tang WHW, et al. The pathophysiological role of interstitial sodium in heart failure. J Am Coll Cardiol. 2015;65(4):378–88.
Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53(7):589–96.
Martens P, Tang WHW, Mullens W. Renal sodium avidity, the prevailing renal target in heart failure. Eur Heart J. 2021;42(43):4478–81.
Verbrugge FH, Dupont M, Steels P, Grieten L, Swennen Q, Tang WHW, et al. The kidney in congestive heart failure : ‘ are natriuresis, sodium and diuretics really the good, the bad and the ugly?’ Eur J Heart Fail. 2014;16:133–42.
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Vol. 145. Circulation. 2022;2022:895–1032.
Mullens W, Dauw J, Martens P, Verbrugge FH, Nijst P, Meekers E, et al. Acetazolamide in acute decompensated heart failure with volume overload. N Engl J Med. 2022;387(13):1185–95.
Matsue Y, Damman K, Voors AA, Kagiyama N, Yamaguchi T, Kuroda S, Okumura T, et al. Time-to-furosemide treatment and mortality in patients hospitalized with acute heart failure. J Am Coll Cardiol. 2017;69(25):3042–51. https://doi.org/10.1016/j.jacc.2017.04.042.
Testani JM, Chen J, Mccauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122:265–72.
Testani JM, Brisco MA, Kociol RD, Jacoby D. Substantial discrepancy between fluid and weight loss during acute decompensated geart failure treatment. Am J Med. 2015;128(7):776–83.
Cox ZL, Rao VS, Testani JM. Classic and novel mechanisms of diuretic resistance in cardiorenal syndrome. Kidney360. 2022;3. https://doi.org/10.34067/KID.0006372021.
Neuwirt H, Burtscher A, Cherney D, Mayer G, Ebenbichler C. Tubuloglomerular feedback in renal glucosuria: mimicking long-term SGLT-2 inhibitor therapy. Kidney Med. 2020;2(1):76–9.
Testani JM, Hanberg JS, Cheng S, Rao V, Onyebeke C, Laur O, et al. Rapid and highly accurate prediction of poor loop diuretic natriuretic response in patients with heart failure. Circ Hear Fail Hear Fail. 2015;9(1):1–8.
Verbrugge FH, Nijst P, Dupont M, Penders J, Tang WHW, Mullens W. Urinary composition during decongestive treatment in heart failure with reduced ejection fraction. Circ Hear Fail. 2014;7:766–72.
Rao VS, Ivey-Miranda JB, Cox ZL, Riello R, Griffin M, Fleming J, et al. Natriuretic equation to predict loop diuretic response in patients with heart failure. JACC. 2021;77(6):695–708.
Singh D, Shrestha K, Testani JM, Verbrugge FH, Dupont M, Mullens W, et al. Insufficient natriuretic response to continuous intravenous furosemide is associated with poor long-term outcomes in acute decompensated heart failure. J Card Fail. 2014;20(6):392–9.
Ward A, Heel R. Bumetanide: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic use. Drugs. 1984;28:426–64.
Damman K, Ter Maaten JM, Coster JE, Krikken JA, van Deursen VM, Krijnen HK, et al. Clinical importance of urinary sodium excretion in acute heart failure. Eur J Heart Fail. 2020;22(8):1438–47.
Gleason O, Meegan G, Fleming J, Griffin M, Ivey-Miranda J, Cox Z, et al. Validation of natriuretic response prediction equation in patients with acute decompensated heart failure. J Card Fail. 2020;26(10):S2.
Brinkley DM, Burpee LJ, Chaudhry SP, Smallwood JA, Lindenfeld JA, Lakdawala NK, et al. Spot urine sodium as triage for effective diuretic infusion in an ambulatory heart failure unit. J Card Fail. 2018;24(6):349–54.
Galluzzo A, Frea S, Boretto P, Pidello S, Volpe A, Canavosio FG, et al. Spot urinary sodium in acute decompensation of advanced heart failure and dilutional hyponatremia: insights from DRAIN trial. Clin Res Cardiol. 2020;109(10):1251–9.
Ravera A, ter Maaten JM, Metra M. Diuretic Resistance and Chronic Heart Failure. In Cardiorenal Syndrome in Heart Failure. 2019 Springer International Publishing; 2020. 121–135. https://doi.org/10.1007/978-3-030-21033-5_9
Biegus J, Zymliński R, Testani J, Marciniak D, Zdanowicz A, Jankowska EA, et al. Renal profiling based on estimated glomerular filtration rate and spot urine sodium identifies high-risk acute heart failure patients. Eur J Heart Fail. 2021;23(5):729–39.
García-Magallón B, Cobo-Marcos M, Martiarena AD, Hernández EM, Martín Jiménez ML, García AM, et al. Role of early assesment of diuresis and natriuresis in detecting in-hospital diuretic resistance in acute heart failure. Front Physiol. 2022;13(May):1–5.
Ter Maaten JM, Valente MAE, Damman K, Hillege HL, Navis G, Voors AA. Diuretic response in acute heart failure - pathophysiology, evaluation, and therapy. Nat Rev Cardiol. 2015;12(3):184–92.
Elias C, Oliveira D, Soares-Carreira M, Amorim M, Araújo JP, Bettencourt P, et al. The ratio of furosemide dosage to urinary sodium concentration predicts mortality in patients with chronic stable heart failure. Polish Arch Intern Med. 2021;131(10):1–8.
Verbrugge FH, Nijst P, Dupont M, Reynders C, Penders J, Tang WHW, et al. Prognostic value of glomerular filtration changes versus natriuretic response in decompensated heart failure with reduced ejection. J Card Fail. 2014;20(11):817–24.
Verbrugge FH, Dupont M, Bertrand PB, Nijst P, Penders J, Dens J, et al. Determinants and impact of the natriuretic response to diuretic therapy in heart failure with reduced ejection fraction and volume overload. Acta Cardiol. 2015;70(3):265–73.
Ferreira JP, Girerd N, Medeiros PB, Santos M, Carvalho HC, Bettencourt P, et al. Spot urine sodium excretion as prognostic marker in acutely decompensated heart failure: the spironolactone effect. Clin Res Cardiol. 2016;105(6):489–507.
Doering A, Jenkins CA, Storrow AB, Lindenfeld JA, Fermann GJ, Miller KF, et al. Markers of diuretic resistance in emergency department patients with acute heart failure. Int J Emerg Med. 2017;10(1):17. https://doi.org/10.1186/s12245-017-0143-x.
Luk A, Groarke JD, Desai AS, Mahmood SS, Gopal DM, Joyce E, et al. First spot urine sodium after initial diuretic identifies patients at high risk for adverse outcome after heart failure hospitalization. Am Heart J. 2018;203:95–100.
Honda S, Nagai T, Nishimura K, Nakai M, Honda Y, Nakano H, et al. Long-term prognostic significance of urinary sodium concentration in patients with acute heart failure. Int J Cardiol. 2018;254:189–94.
Collins SP, Jenkins CA, Baughman A, Miller KF, Storrow AB, Han JH, et al. Early urine electrolyte patterns in patients with acute heart failure. ESC Hear Fail. 2019;6(1):80–8.
Biegus J, Zymliński R, Sokolski M, Todd J, Cotter G, Metra M, et al. Serial assessment of spot urine sodium predicts effectiveness of decongestion and outcome in patients with acute heart failure. Eur J Heart Fail. 2019;21(5):624–33.
Hodson DZ, Griffin M, Mahoney D, Ahmad T, Turner J, Wilson FP, et al. Natriuretic response is highly variable and associated with six-month survival: insights from the ROSE-AHF trial. JACC Hear Fail. 2019;7(5):383–91.
Cunningham J, Sun J, Mc Causland F, Ly S, Anstrom K, Lindenfeld J, et al. Lower urine sodium predicts longer length of stay in acute heart failure patients: insights from the ROSE AHF trial. Clin Cardiol. 2020;43:43–9.
Cox ZL, Rao VS, Ivey-Miranda JB, Moreno-Villagomez J, Mahoney D, Ponikowski P, et al. Compensatory post-diuretic renal sodium reabsorption is not a dominant mechanism of diuretic resistance in acute heart failure. Eur Heart J. 2021;42(43):4468–77.
de la Espriella R, Núñez E, Llàcer P, García-Blas S, Ventura S, Núñez JM, et al. Early urinary sodium trajectory and risk of adverse outcomes in acute heart failure and renal dysfunction. Rev Española Cardiol (English Ed). 2021;74(7):616–23.
Biegus J, Zymliński R, Fudim M, Testani J, Sokolski M, Marciniak D, et al. Spot urine sodium in acute heart failure: differences in prognostic value on admission and discharge. ESC Hear Fail. 2021;8(4):2597–602.
Caravaca Perez P, Nuche J, Moran Fernandez L, Lora D, Blazquez-Bermejo Z, Lopez-Azor JC, et al. Potential role of natriuretic response to furosemide stress test during acute heart failure. Circ Hear Fail. 2021;14(6):E008166.
Martens P, Chen HH, Verbrugge FH, Testani JT, Mullens W, Tang WHW. Assessing intrinsic renal sodium avidity in acute heart failure: implications in predicting and guiding decongestion. Eur J Heart Fail. 2022;24(10):1978–87. https://doi.org/10.1002/ejhf.2662.
ter Maaten JM, Beldhuis IE, van der Meer P, Krikken JA, Coster JE, Nieuwland W, et al. Natriuresis-guided therapy in acute heart failure: rationale and design of the Pragmatic Urinary Sodium-based treatment algoritHm in Acute Heart Failure (PUSH-AHF) trial. Eur J Heart Fail. 2022;24:385–92.
Dauw J, Lelonek M, Zegri-Reiriz I, Paredes-Paucar CP, Zara C, George V, et al. Rationale and design of the efficacy of a standardized diuretic protocol in acute heart failure study. ESC Hear Fail. 2021;8:4685–92.
Verbrugge FH, Martens P, Boonen L, Nijst P, Verhaert D, Noyens P, et al. Loop diuretic down-titration in stable chronic heart failure is often achievable, especially when urinary chloride concentration is low. Acta Cardiol. 2017;73(4):1–7.
Martens P, Dupont M, Verbrugge H, Damman K, Degryse N, Nijst P, et al. Urinary sodium profiling in chronic heart failure to detect development of acute decompensated heart failure. JACC Hear Fail. 2019;7(5):404–14.
Dauw J, Martens P, Tersalvi G, Schouteden J, Deferm S, Gruwez H, et al. Diuretic response and effects of diuretic omission in ambulatory heart failure patients on chronic low-dose loop diuretic therapy. Eur J Heart Fail. 2021;23(7):1110–9.
Ganes A, Davis JA, Virtanen JK, Voutilainen A, Tuomainen TP, Atherton JJ, et al. Urinary sodium concentration predicts time to major adverse coronary events and all-cause mortality in men with heart failure over a 28–33-year period: a prospective cohort study. BMC Cardiovasc Disord. 2022;22(1):391.
Rohde LE, Rover MM, Figueiredo Neto JA, Danzmann LC, Bertoldi EG, Simoes MV, et al. Short-term diuretic withdrawal in stable outpatients with mild heart failure and no fluid retention receiving optimal therapy : a. Eur Heart J. 2019;40:3605–12.
Martens P, Verbrugge FH, Boonen L, Nijst P, Dupont M, Mullens W. Value of routine investigations to predict loop diuretic down-titration success in stable heart failure. Int J Cardiol. 2018;250:171–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meekers, E., Mullens, W. Spot Urinary Sodium Measurements: the Future Direction of the Treatment and Follow-up of Patients with Heart Failure. Curr Heart Fail Rep 20, 88–100 (2023). https://doi.org/10.1007/s11897-023-00591-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-023-00591-4