Abstract
Purpose of Review
To provide insight into the role of urine biomarkers and electrolytes for the management of heart failure.
Recent Findings
The age-dependent decrease in glomerular filtration rate due to loss of functional nephrons occurs at a faster pace in heart failure, potentially exacerbated by episodes of acute kidney injury. Urine biomarkers have not convincingly demonstrated to improve detection of irreversible renal damage and predict long-term renal trajectories, compared with serial creatinine measurements. Recent data show that natriuresis and diuretic response track poorly with glomerular filtration, but strongly with prognosis. Urine sodium concentration > 50–70 mmol/L was recently put forward through expert consensus as an adequate diuretic response.
Summary
The value of urine biomarkers to detect structural renal damage in heart failure remains unsure and the latter is probably uncommon, especially over short-term follow-up. Urine electrolytes on the other hand predict diuretic response accurately and may allow better diuretic titration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The heart and kidneys are closely intertwined in the pathophysiology of heart failure. By ensuring an adequate cardiac output at low cardiac filling pressures, the heart can propel the whole body’s blood content (i.e., 5–6 L) through the renal vasculature every 4 min in normal circumstances. In heart failure, both low cardiac output and systemic venous congestion may contribute to impaired renal perfusion and cause acute kidney injury [1, 2]. Repetitive or long-standing events eventually accelerate nephron loss, enhancing the progression of chronic kidney disease (CKD) [3, 4•]. The kidneys on the other hand are responsible for sodium homeostasis, which plays a central role in the occurrence of congestion in heart failure [5].
In routine clinical practice, renal function is almost exclusively assessed by serum creatinine (Cr) or Cr-based estimations of the glomerular filtration rate (GFR). However, this approach has well-known limitations such as Cr analytic assay variability, Cr secretion by the proximal renal tubules (rendering it an imperfect biomarker for glomerular filtration), as well as dependency of serum Cr levels on muscle mass, diet, and physical activity [6,7,8,9]. Moreover, serum Cr levels may transiently increase in heart failure, not necessarily reflecting nephron loss or structural renal damage [10,11,12]. Alternatively, increased serum Cr may represent hemoconcentration associated with proper decongestion and favorable clinical outcome [13, 14]. Finally, neither serum Cr nor GFR estimates track reliably with natriuresis or response to diuretic therapy [15]. Importantly, both the latter are impaired from the beginning in heart failure and have emerged as powerful prognostic markers independently from the underlying GFR [16, 17, 18•, 19, 20••].
Urine biomarkers and electrolytes may help to answer 2 fundamental questions. First, is structural (and hence irreversible) renal damage implied when confronted with an increased serum Cr? And second, how to assess the response to diuretic therapy and tailor decongestive treatment? This review aims to offer comprehensive answers on both these questions.
Evolution of Renal Function in Heart Failure
Glomerular Filtration Rate and Number of Functional Nephrons
GFR, assessed through serum Cr measurements, is a powerful predictor of prognosis in both acute and chronic heart failure, outperforming cardio-specific parameters such as left ventricular ejection fraction [21, 22]. Importantly, total GFR constitutes the product of the number of functional nephrons with their average individual filtration rate (i.e., single-nephron GFR) [23]. While the latter is quite dynamic and strongly influenced by intra-glomerular and systemic hemodynamics as well as neurohumoral activation, the number of functional nephrons is rather fixed and slowly decreases with normal aging. Starting from approximately 1 million nephrons per kidney at birth, 5000–10,000 are lost every year with healthy aging [24]. This corresponds to an age-related decline in GFR of ≤ 1 mL/min/1.73 m2 per year with the single-nephron GFR remaining stable around 80 nL/min [24]. The robust relationship between lower GFR and higher risk for all-cause mortality in stable circumstances may thus intuitively be explained as the number of functional nephrons being a good surrogate for biological age of the kidneys [25].
Increased Nephron Loss in Chronic Kidney Disease
In its purest sense, CKD is defined as a greater loss of functional nephrons than would be anticipated through healthy aging. Obviously, it is inconceivable to use this definition in clinical practice as the number of functional nephrons can only be evaluated through biopsy. A GFR < 60 mL/min/1.73 m2 is therefore used as a reasonable surrogate, with this cutoff corresponding to a > 50% loss of functional nephrons at a normal single-nephron GFR [26]. Importantly, an increased single-nephron GFR may compensate for a loss of functional nephrons, reducing the impact on the total GFR assessed by serum Cr. Therefore, (micro-)albuminuria is an integral part of the CKD definition in patients with a GFR ≥ 60 mL/min/1.73 m2 [26]. The assumption is that in such patients, an increased urinary albumin (≥ 30 mg/g Cr) reflects an increased single-nephron GFR (i.e., glomerular hyper-filtration) and consequently a lower number of functional nephrons than reflected by the total GFR. Moreover, glomerular hyper-filtration is a key pathophysiological mechanism underlying further CKD progression, as it results in accelerated podocyte loss and a dysfunctional glomerular basement membrane [27]. Indeed, albuminuria is consistently associated with the risk of CKD progression and development of end-stage renal disease [28, 29].
Increased Nephron Loss in Heart Failure
In the effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (GISSI-HF) trial, the mean decrease in estimated GFR was 2.57 mL/min/1.73 m2 per year over a 3-year follow-up period [4•]. Assuming a normal single-nephron GFR, this would translate into a loss of approximately 25,000 functional nephrons per year, or 2.5–5 times the number expected through healthy aging only. Because many of these patients have glomerular hyper-filtration and hence an increased single-nephron GFR, this is likely an underestimation. Importantly, even after adjustments for risk factors of CKD progression, heart failure patients in the GISSI-HF trial still demonstrated an abnormally rapid decline of the GFR over time [4•]. Additionally, in a matched cohort of 3.4 million individuals without heart failure and 156,743 heart failure patients, the latter group was 3 times more likely to manifest with a very rapid GFR decline (> 5 mL/min/1.73 m2 per year), which occurred in 22% [3]. Conclusively, heart failure seems to be associated with an accelerated aging of the kidneys. The pace of this process, evaluated by the slope of serial GFR assessments over mid- to long-term follow-up, might be an attractive surrogate end-point for clinical trials [30]. Yet, more data are needed to confirm this, especially in the context of heart failure.
Urine Biomarkers to Assess Kidney Injury
Urine biomarkers have the potential to be more specific for kidney injury and/or irreversible structural damage, when compared with serum Cr or Cr-based GFR estimates. This premise holds 2 interesting prospects. First, by a more accurate assessment of ongoing injury and existing damage, the future GFR trajectory might be anticipated better. Secondly, in the context of dynamic changes in the single-nephron GFR, as would be expected in acute heart failure (AHF) or during decongestive treatment with rapidly changing systemic and/or intra-glomerular hemodynamics, urine biomarkers may better reflect the impact of an insult on the number of functional nephrons, when compared with GFR estimates. Several candidate biomarkers have been proposed (Table 1), most of whom are not readily available in clinical practice, but rather the topic of research.
Candidate Urine Biomarkers for Kidney Injury
Urinary Albumin
One urine biomarker with widespread availability is urinary albumin. Albuminuria is common in heart failure and linked to worse prognosis [31,32,33,34]. Microalbuminuria (30–299 mg/g Cr) is present in approximately 30% of heart failure patients and associated with a 40–60% increased risk of all-cause mortality [31]. Macroalbuminuria (≥ 300 mg/g Cr) carries an even higher relative risk increase of approximately 75% and is found in roughly 1 in 10 heart failure patients [31]. The absence of albuminuria is generally interpreted as a sign of preserved glomerular integrity. However, the link between albuminuria, glomerular hyper-filtration, and increased intra-glomerular pressure is mainly established for diabetic kidney disease, while it remains unclear to what extent findings can be extrapolated to the heart failure context.
Urinary Neutrophil Gelatinase-Associated Lipocalin
The most extensively studied biomarker for tubular injury in heart failure is gelatinase-associated lipocalin (NGAL). NGAL is a 25-kDa protein secreted by renal tubular cells, leucocytes, and several other types of epithelial cells in response to ischemic or toxic injury [35]. Because NGAL is normally reabsorbed by the proximal renal tubules after glomerular filtration, increased urine levels reflect dysfunctional proximal reabsorption or increased production in distal nephron segments because of injury. After multivariate adjustments, elevated urinary NGAL levels were associated with a borderline significantly increased risk of 10% for all-cause mortality or heart failure readmission in the GISSI-HF trial [33].
Kidney Injury Molecule-1
Another urine biomarker for tubular injury that has been investigated in heart failure is kidney injury molecule-1 (KIM-1). KIM-1 is a transmembrane glycoprotein belonging to the immunoglobulin gene superfamily [35]. It is expressed on the apical membrane cilia of proximal tubular cells in case of injury, but is absent in the normal kidney. In the few studies that have compared urinary NGAL with urinary KIM-1, the latter showed a stronger association with clinical outcome [33, 36, 37].
Other Urine Biomarkers for Tubular Injury
Among the many other potential urine biomarkers to assess tubular injury (Table 1) are interleukin-18 (IL-18), N-acetyl-β-d-glucosaminidase (NAG), and liver fatty acid–binding protein (L-FABP). Currently, they are used almost exclusively in research settings.
Urine Biomarkers to Predict Renal Trajectories in Chronic Heart Failure
If urine biomarkers allow a more accurate assessment of ongoing renal injury and irreversible structural damage, they might help to predict renal trajectories in chronic heart failure [38]. A head-to-head comparison of the slope of serial GFR assessments with instantaneous urine biomarker assessment is currently not available. However, in an analysis from the GISSI-HF trial, urinary albumin, urinary NGAL, urinary KIM-1, and urinary NAG were compared to predict worsening renal function, defined as a ≥ 0.3 mg/dL increase in serum Cr over a follow-up period of approximately 3 years [39]. All 4 urine biomarkers were associated with incident worsening renal function, with a relative risk increase of approximately 20% for albuminuria or urinary NGAL versus 40% for urinary KIM-1 or NAG. After multivariate adjustments, only the association with urinary KIM-1 proved to be robust. A smaller study (n = 138) that defined progression of CKD as a ≥ 25% drop in estimated GFR with a minimal nominal decrease of 15 mL/min/1.73 m2 found that urinary KIM-1 and NAG, but not albuminuria or urinary NGAL levels, were significantly higher in patients with deteriorating renal function [40•]. Finally, another small study in chronic heart failure with reduced ejection fraction (n = 85) did demonstrate that patients with a deterioration of the estimated GFR ≥ 25% over the 16-month follow-up period had significantly higher urinary NGAL levels [41].
Conclusively, urinary KIM-1 and to a lesser extent NAG may offer the best hope to predict renal trajectories in chronic heart failure. These urine biomarkers offer the advantage of an instantaneous measurement in comparison with the slope of GFR evolution over time that requires multiple Cr assessments and a long enough follow-up period to be reliable. Still, optimal cutoffs for these biomarkers should be determined from large, representative, real-world populations of patients with chronic heart failure before their use could be recommended in clinical practice. Even then, therapeutic implications remain unsure. Renin-angiotensin blockers that have been demonstrated to slow down the progression of CKD are indicated anyway in chronic heart failure with reduced ejection fraction and in many patients with preserved ejection fraction and arterial hypertension [42,43,44]. Novel drugs such as sodium-glucose transporter-2 inhibitors and/or sacubitril/valsartan may offer additional nephroprotection in heart failure and might be considered earlier in patients at high risk for deteriorating renal function [45, 46]. However, the hypothesis that urine biomarkers identify such population reliably should be tested formally in prospective studies.
Urine Biomarkers to Detect Structural Nephron Damage in Acute Heart Failure
Contrary to the long-term evolution of the GFR in chronic heart failure, short-term changes in AHF are less consistently associated with prognosis. Worsening renal function, defined as a ≥ 0.3 mg/dL increase of the serum Cr during a hospitalization for AHF, is associated with a 81% relative risk increase for subsequent mortality on a population level [47]. However, transient elevations in serum Cr are more frequently observed when thorough decongestion is achieved and this is nevertheless associated with better outcomes [13]. As explained, the highly dynamic single-nephron GFR in AHF because of changing hemodynamics, volume status, and neurohumoral activation, as well as the potential effect of hemoconcentration with decongestion, clouds the relationship between serum Cr and the number of functional nephrons that is prognostically relevant. Moreover, persistent (subclinical) congestion that is associated with hemodilution and a lower serum Cr is an important driver for deteriorating renal function on the long-term [48, 49].
Urine biomarkers have therefore been studied extensively in AHF, as they may be more sensitive to kidney injury and irreversible structural damage when compared with serum Cr. However, many of those studies have taken short-term changes in Cr or Cr-based GFR estimates as an end-point, which seems not entirely appropriate. One interesting observation is that tubular injury biomarkers such as NGAL are only slightly elevated in AHF, not to the extent observed in tubular necrosis with nephrotoxic medications [10, 50]. The lack of immediate, significant tubular injury in most cases of AHF may explain why urinary NGAL, KIM-1, and IL-18 are poor predictors of long-term renal impairment after 6 months [11]. In the Acute Kidney Injury NGAL Evaluation of Symptomatic Heart Failure Study (AKINESIS), peak or admission urine NGAL was not better than serum Cr levels to predict the need for renal replacement therapy, which was infrequent in 18/927 patients (1.9%), again suggesting that structural nephron damage is actually a rare event in AHF. This is notwithstanding that repetitive or long-standing events of acute kidney injury may accelerate nephron loss and progression of CKD. Indeed, in the infrequent cases where tubular injury biomarkers are markedly elevated in AHF, there seems to be a better relationship with progressive renal deterioration and clinical outcomes independently from the underlying GFR [51]. Notably in this respect, both urinary NGAL and KIM-1 were associated with true worsening renal function, defined as a ≥ 0.3 mg/dL increase in serum Cr in AHF patients without successful decongestion [52].
Conclusively, there is insufficient evidence to recommend the use of urine biomarkers to improve detection of acute kidney injury and irreversible renal damage during episodes of AHF. The main reason for this is that the latter is an uncommon event. Furthermore, persistent congestion is likely more harmful to the kidneys on the long-term than aggressive decongestive therapies on the short-term. This is further supported by an observation from the Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) that in cases with evolving worsening renal function, intensive volume removal resulted in a rise of urinary NGAL, KIM-1, and NAG indicating tubular injury, but superior decongestion and renal functional recovery after 60 days [53•]. This constitutes the best current evidence that at least part of the injury detected by urine biomarkers, especially at slightly to moderately elevated levels, is in fact reversible and probably not associated with a loss of functional nephrons.
Urine Electrolytes to Assess Diuretic Response and Guide Decongestive Treatment
Extracellular volume is governed by sodium homeostasis [5]. Renal sodium avidity is a hallmark feature of the heart failure syndrome, already early in its development [16]. The resulting positive sodium balance eventually leads to volume overload, which is the most common reason for hospital admission with AHF [54]. Typically, in such cases, diuretics are used to get rid of any excessive fluid accumulation and subsequently prevent recurrence.
A very consistent observation from multiple studies is that any residual congestion after treatment for AHF—whether evaluated clinically, through biomarkers, or with invasive hemodynamic measurements—portends worse prognosis [55,56,57]. Nevertheless, a substantial number of patients admitted with AHF leave the hospital with inadequate decongestion [58]. Remarkably, there is no consensus on how to define optimal decongestion. Intriguingly, in the Renal Optimization Strategies Evaluation in Acute Heart Failure (ROSE-AHF) trial, total sodium excretion was strongly associated with 6-month mortality, while traditional fluid-based metrics (i.e., urine output, net fluid balance, and weight change) were not [20••]. This is already a strong argument to reappraise the role of urine electrolyte assessment in heart failure and test prospectively whether natriuresis is a valid parameter to guide decongestive treatment. Moreover, it is now clearly established that urine electrolyte assessment helps to accurately predict response to diuretic treatment [59•, 60, 61••].
Urine Electrolyte Assessment in Heart Failure
Sodium Versus Chloride
Most studies on urine electrolyte assessment in heart failure have looked only at sodium and natriuresis, although chloride and chloruresis are potentially at least equally important. Renal autoregulation through tubulo-glomerular feedback is mediated by chloride rather than sodium [62]. Additionally, only sodium ingested under the form of sodium chloride but not sodium bicarbonate is osmotically active in the extracellular compartment [63]. However, because of the paucity of data regarding urine chloride assessment in heart failure, further focus will be on sodium. Three different metrics may be used to assess natriuresis and should be clearly specified to avoid confusion (Table 2).
Total Natriuresis
Total natriuresis, or the total amount of sodium excreted through the urine, equals sodium intake during the steady state. A sodium intake that is higher than the excreted amount results in an increased extracellular volume. Conversely, a lower intake than excretion contracts the extracellular compartment, which is generally the aim in AHF. Assessing total natriuresis requires a timed urine collection because both urine sodium concentration and the amount of urine production are needed for its calculation. After diuretic administration, total natriuresis reflects the reduction in extracellular volume that will eventually be achieved.
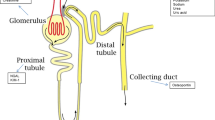
Urine Sodium Concentration
Urine sodium concentration, which may be evaluated on a spot urine sample as well as a timed collection, is not so much an indicator of sodium intake or extracellular volume, but rather a reflection of renal tubular sodium handling. A low urine sodium concentration (< 50 mmol/L) indicates intensive tubular sodium reabsorption, which requires a structurally intact nephron and an activated renin-angiotensin-aldosterone system [64]. Interestingly, it was recently shown that heart failure patients with a lower urine sodium concentration on a morning spot sample before administration of any diuretics had a higher risk of hospital admission for AHF [65••]. Moreover, with serial measurements, there was a clear temporal relationship between a drop in urine sodium concentration (generally < 50 mmol/L) and subsequent decompensation. This strongly suggests that increased renal sodium avidity was the cause of a positive sodium balance, resulting in extracellular volume expansion and decompensation. Based on the results of this small observational study (n = 80) that requires further confirmation, a urine sodium concentration > 70 mmol/L (outside the window of diuretic administration) indicates acceptable natriuresis without risk of impending decompensation.
Fractional Sodium Excretion
Fractional sodium excretion (FENa) on a spot urine sample is theoretically the most accurate reflection of renal tubular sodium handling. However, its reliance on 4 measurements (urine sodium and serum Cr concentration as well as serum sodium and urine Cr concentration), each with inherent measurement error, makes it a much less precise parameter compared with the urine sodium concentration alone. As the inflated measurement error offsets any gains in accuracy, the added value of this more complex metric of natriuresis is questionable in clinical practice [15].
Urine Sodium Concentration to Assess Diuretic Response
As natriuresis is the primary goal of diuretic therapy, urine sodium assessment offers a unique insight into the diuretic response. In an experiment with 52 AHF patients, presenting with clear volume overload, a urine spot sample was obtained under continuous furosemide infusion for > 3 h to allow a steady state [15]. Both the urine sodium concentration and FENa showed a linear correlation with 24-h urine output and net fluid balance. Patients with a urine sodium concentration < 50 mmol/L had a very poor diuretic response, precluding any meaningful decongestion. In another experiment, it was demonstrated that early total sodium excretion in AHF, assessed within 1–2 h after diuretic administration, did accurately predict total natriuresis after 6 h [59•]. The authors were able to validate a formula that estimates total natriuresis after diuretic therapy based on the estimated GFR, serum and urine Cr levels, and the urine sodium concentration on a spot urine sample taken within 1–2 h after diuretic administration.
Diuretic Response as Natriuresis Predicts Prognosis in Acute Heart Failure
An accurate assessment of diuretic response is gaining increased attention because of its important association with prognosis in AHF, irrespectively of the underlying GFR [17, 19]. Different metrics that have been used are typically expressed as an effect (i.e., urine output, net fluid balance, weight loss, or natriuresis) per dose of loop diuretics administered [19]. Among these metrics, natriuresis seems to have the most robust association with clinical outcome [20••, 66]. Both higher total natriuresis and a higher urine sodium concentration under diuretic treatment are associated with a lower risk for all-cause mortality or heart failure readmissions [15, 18•, 20••, 67•, 68•]. The exact mechanistic underpinning of this robust association is less obvious than at first sight. A logical assumption might be that patients who present with poor diuretic response have a lower chance of achieving appropriate decongestion [18•, 69]. Yet, even with similar congestion signs at discharge, patients with good diuretic response still have favorable clinical outcomes [17, 70]. Alternatively, diuretic response might be interpreted as the result of a renal stress test, indicating the functional reserve of the kidneys to excrete sodium and water (in analogy to the maximal aerobic capacity being reflective of the cardiac reserve during exercise). Finally, poor diuretic response might indicate as well that volume overload is not present and sodium levels are possibly depleted [60, 71]. In the latter scenario, diuretic therapy is unlikely to target the underlying pathophysiological culprit of AHF and may be harmful instead.
Clinical Implications of Urine Electrolyte Assessment in Heart Failure
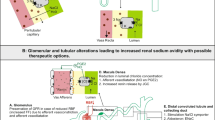
Urine electrolyte assessment in heart failure might have important implications for clinical practice (Fig. 1). In a recently published position statement from the Heart Failure Association study group on cardio-renal dysfunction, the importance of a systematic and early evaluation of diuretic response is highlighted [61••]. Both urine output 6 h after diuretic administration and urine sodium concentration on a spot sample after 2 h are proposed to achieve this goal. The consensus between experts was that a urine sodium concentration > 50 mmol/L and preferably > 70 mmol/L should be achieved to consider diuretic therapy effective. Urine sodium concentration < 50–70 mmol/L would indicate the need for loop diuretic dose escalation or consideration of combinational diuretic therapy or in selected cases ultrafiltration. It should be emphasized though that this recommendation is based on limited evidence, mostly from observational, non-randomized studies. Notably, the 50–70 mmol/L cutoff for an acceptable diuretic response is probably a conservative estimate in patients with clear signs of volume overload, most of whom achieve a urine sodium concentration > 100 mmol/L with adequately dosed diuretic therapy.
When serial assessments of natriuresis are performed during successful decongestive treatment for AHF, it is clear that sodium excretion diminishes, even when urine output is still preserved [60]. Low urine sodium or chloride concentration in this specific context may predict whether repeating a diuretic dose would still be effective. A high urine sodium (> 85 mmol/L) or chloride (> 50 mmol/L) in this respect would indicate a continued response to the diuretic regimen and the likely presence of congestion, even when not clinically obvious [72•]. In such way, urine electrolyte assessment might be a promising approach to aid clinical decisions as when to make the switch from intravenous to oral diuretic administration or plan discharge from the hospital. However, more mechanistical studies are needed to confirm or refute these hypotheses.
Conclusions
Heart failure is associated with an accelerated aging of the kidneys and a GFR decrease > 1 mL/min/1.73 m2 per year. Total GFR assessment through serum Cr or Cr-based GFR estimates incompletely captures the irreversible loss of functional nephrons over time, as the individual filtration rate of the remaining nephrons may be compensatory increased. In addition, rapidly changing volume status, hemodynamics, and neurohumoral activation in AHF or during decongestive therapies may temporarily impact on the GFR without causing structural nephron damage. Urine biomarkers have the potential to be more specific for ongoing kidney injury and irreversible structural damage when compared with serum Cr assessment. Although there might be a role for urine biomarkers to predict renal trajectories in chronic heart failure, especially for urinary KIM-1 and NAG at moderately elevated levels, this has limited therapeutic implications. In addition, urine biomarker assessments in AHF have yielded overall disappointing results. In contrast, urine electrolyte (mainly sodium) assessment has gathered increased attention lately. Evaluating natriuresis is an accurate method to assess diuretic efficacy and is a powerful predictor of prognosis in AHF. According to expert consensus, a urine sodium concentration < 50–70 mmol/L on a spot urine sample obtained 2 h after diuretic administration should be considered diuretic resistance and trigger intensification of decongestive treatments when volume overload is still present.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ljungman S, Laragh JH, Cody RJ. Role of the kidney in congestive heart failure. Relationship of cardiac index to kidney function. Drugs. 1990;39(Suppl 4):10–21 discussion 2-4.
Dupont M, Mullens W, Tang WH. Impact of systemic venous congestion in heart failure. Curr Heart Fail Rep. 2011;8(4):233–41. https://doi.org/10.1007/s11897-011-0071-7.
George LK, SKG K, Molnar MZ, Thomas F, Lu JL, Kalantar-Zadeh K. et al, Heart failure increases the risk of adverse renal outcomes in patients with normal kidney function. Circ Heart Fail. 2017;10(8). https://doi.org/10.1161/CIRCHEARTFAILURE.116.003825.
• Damman K, Masson S, Lucci D, Gorini M, Urso R, Maggioni AP, et al. Progression of renal impairment and chronic kidney disease in chronic heart failure: an analysis from GISSI-HF. J Card Fail. 2017;23(1):2–9. https://doi.org/10.1016/j.cardfail.2016.09.006One of the few studies that describe the long-term evolution of renal function over time in chronic heart failure.
Verbrugge FH, Dupont M, Steels P, Grieten L, Swennen Q, Tang WH, et al. The kidney in congestive heart failure: ‘are natriuresis, sodium, and diuretics really the good, the bad and the ugly?’. Eur J Heart Fail. 2014;16(2):133–42. https://doi.org/10.1002/ejhf.35.
Walser M. Assessing renal function from creatinine measurements in adults with chronic renal failure. Am J Kidney Dis. 1998;32(1):23–31.
Miller WG, Myers GL, Ashwood ER, Killeen AA, Wang E, Thienpont LM, et al. Creatinine measurement: state of the art in accuracy and interlaboratory harmonization. Arch Pathol Lab Med. 2005;129(3):297–304.
Stevens LA, Levey AS. Measurement of kidney function. Med Clin North Am. 2005;89(3):457–73.
Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. Lower serum albumin level is associated with higher fractional excretion of creatinine. Clin Exp Nephrol. 2013. https://doi.org/10.1007/s10157-013-0841-5.
Dupont M, Shrestha K, Singh D, Awad A, Kovach C, Scarcipino M, et al. Lack of significant renal tubular injury despite acute kidney injury in acute decompensated heart failure. Eur J Heart Fail. 2012;14(6):597–604. https://doi.org/10.1093/eurjhf/hfs039.
Verbrugge FH, Dupont M, Shao Z, Shrestha K, Singh D, Finucan M, et al. Novel urinary biomarkers in detecting acute kidney injury, persistent renal impairment, and all-cause mortality following decongestive therapy in acute decompensated heart failure. J Card Fail. 2013;19(9):621–8. https://doi.org/10.1016/j.cardfail.2013.07.004.
Coca SG, Zabetian A, Ferket BS, Zhou J, Testani JM, Garg AX, et al. Evaluation of short-term changes in serum creatinine level as a meaningful end point in randomized clinical trials. J Am Soc Nephrol. 2016;27(8):2529–42. https://doi.org/10.1681/ASN.2015060642.
Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122(3):265–72. https://doi.org/10.1161/CIRCULATIONAHA.109.933275.
Testani JM, Brisco MA, Chen J, McCauley BD, Parikh CR, Tang WH. Timing of hemoconcentration during treatment of acute decompensated heart failure and subsequent survival: importance of sustained decongestion. J Am Coll Cardiol. 2013;62(6):516–24. https://doi.org/10.1016/j.jacc.2013.05.027.
Singh D, Shrestha K, Testani JM, Verbrugge FH, Dupont M, Mullens W, et al. Insufficient natriuretic response to continuous intravenous furosemide is associated with poor long-term outcomes in acute decompensated heart failure. J Card Fail. 2014;20(6):392–9. https://doi.org/10.1016/j.cardfail.2014.03.006.
McKie PM, Schirger JA, Costello-Boerrigter LC, Benike SL, Harstad LK, Bailey KR, et al. Impaired natriuretic and renal endocrine response to acute volume expansion in pre-clinical systolic and diastolic dysfunction. J Am Coll Cardiol. 2011;58(20):2095–103. https://doi.org/10.1016/j.jacc.2011.07.042.
Testani JM, Brisco MA, Turner JM, Spatz ES, Bellumkonda L, Parikh CR, et al. Loop diuretic efficiency: a metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail. 2014;7(2):261–70. https://doi.org/10.1161/CIRCHEARTFAILURE.113.000895.
• Verbrugge FH, Dupont M, Bertrand PB, Nijst P, Penders J, Dens J, et al. Determinants and impact of the natriuretic response to diuretic therapy in heart failure with reduced ejection fraction and volume overload. Acta Cardiol. 2015;70(3):265–73. https://doi.org/10.2143/AC.70.3.3080630One of the first studies to explore natriuresis instead of traditional fluid-based metrics for diuretic response in acute heart failure, focussing on its determinants.
Verbrugge FH. Editor’s choice-diuretic resistance in acute heart failure. Eur Heart J Acute Cardiovasc Care. 2018;7(4):379–89. https://doi.org/10.1177/2048872618768488.
•• Hodson DZ, Griffin M, Mahoney D, Raghavendra P, Ahmad T, Turner J, et al. Natriuretic response is highly variable and associated with 6-month survival: insights from the ROSE-AHF trial. JACC Heart Fail. 2019;7(5):383–91. https://doi.org/10.1016/j.jchf.2019.01.007Pivotal study to demonstrate the strong prognostic impact of natriuresis in acute heart failure and its superiority over traditional fluid-based metrics for diuretic response.
Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, et al. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102(2):203–10.
Fonarow GC, Adams KF Jr, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293(5):572–80.
Verbrugge FH, Grieten L, Mullens W. Management of the cardiorenal syndrome in decompensated heart failure. Cardiorenal Med. 2014;4(3-4):176–88. https://doi.org/10.1159/000366168.
Denic A, Mathew J, Lerman LO, Lieske JC, Larson JJ, Alexander MP, et al. Single-nephron glomerular filtration rate in healthy adults. N Engl J Med. 2017;376(24):2349–57. https://doi.org/10.1056/NEJMoa1614329.
Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307(18):1941–51. https://doi.org/10.1001/jama.2012.3954.
Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17–28. https://doi.org/10.1038/ki.2010.483.
Kriz W, Lemley KV. A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J Am Soc Nephrol. 2015;26(2):258–69. https://doi.org/10.1681/ASN.2014030278.
Coresh J, Heerspink HJL, Sang Y, Matsushita K, Arnlov J, Astor BC, et al. Change in albuminuria and subsequent risk of end-stage kidney disease: an individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol. 2019;7(2):115–27. https://doi.org/10.1016/S2213-8587(18)30313-9.
Heerspink HJL, Greene T, Tighiouart H, Gansevoort RT, Coresh J, Simon AL, et al. Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol. 2019;7(2):128–39. https://doi.org/10.1016/S2213-8587(18)30314-0.
Inker LA, Heerspink HJL, Tighiouart H, Levey AS, Coresh J, Gansevoort RT, et al. GFR slope as a surrogate end point for kidney disease progression in clinical trials: a meta-analysis of treatment effects of randomized controlled trials. J Am Soc Nephrol. 2019. https://doi.org/10.1681/ASN.2019010007.
Jackson CE, Solomon SD, Gerstein HC, Zetterstrand S, Olofsson B, Michelson EL, et al. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet. 2009;374(9689):543–50. https://doi.org/10.1016/S0140-6736(09)61378-7.
Masson S, Latini R, Milani V, Moretti L, Rossi MG, Carbonieri E, et al. Prevalence and prognostic value of elevated urinary albumin excretion in patients with chronic heart failure: data from the GISSI-Heart Failure trial. Circ Heart Fail. 2010;3(1):65–72. https://doi.org/10.1161/CIRCHEARTFAILURE.109.881805.
Damman K, Masson S, Hillege HL, Maggioni AP, Voors AA, Opasich C, et al. Clinical outcome of renal tubular damage in chronic heart failure. Eur Heart J. 2011;32(21):2705–12. https://doi.org/10.1093/eurheartj/ehr190.
Katz DH, Burns JA, Aguilar FG, Beussink L, Shah SJ. Albuminuria is independently associated with cardiac remodeling, abnormal right and left ventricular function, and worse outcomes in heart failure with preserved ejection fraction. JACC Heart Fail. 2014;2(6):586–96. https://doi.org/10.1016/j.jchf.2014.05.016.
Cruz DN, Goh CY, Haase-Fielitz A, Ronco C, Haase M. Early biomarkers of renal injury. Congest Heart Fail. 2010;16(Suppl 1):S25–31.
Damman K, Van Veldhuisen DJ, Navis G, Vaidya VS, Smilde TD, Westenbrink BD, et al. Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart. 2010;96(16):1297–302. https://doi.org/10.1136/hrt.2010.194878.
Jungbauer CG, Birner C, Jung B, Buchner S, Lubnow M, von Bary C, et al. Kidney injury molecule-1 and N-acetyl-beta-D-glucosaminidase in chronic heart failure: possible biomarkers of cardiorenal syndrome. Eur J Heart Fail. 2011;13(10):1104–10. https://doi.org/10.1093/eurjhf/hfr102.
Brankovic M, Akkerhuis KM, van Boven N, Anroedh S, Constantinescu A, Caliskan K, et al. Patient-specific evolution of renal function in chronic heart failure patients dynamically predicts clinical outcome in the Bio-SHiFT study. Kidney Int. 2018;93(4):952–60. https://doi.org/10.1016/j.kint.2017.09.013.
Damman K, Masson S, Hillege HL, Voors AA, van Veldhuisen DJ, Rossignol P, et al. Tubular damage and worsening renal function in chronic heart failure. JACC Heart Fail. 2013;1(5):417–24. https://doi.org/10.1016/j.jchf.2013.05.007.
• Jungbauer CG, Uecer E, Stadler S, Birner C, Buchner S, Maier LS, et al. N-acteyl-ss-D-glucosaminidase and kidney injury molecule-1: new predictors for long-term progression of chronic kidney disease in patients with heart failure. Nephrology (Carlton). 2016;21(6):490–8. https://doi.org/10.1111/nep.12632Interesting study to suggest that the urine biomarkers KIM-1 and NAG might improve prediction of long-term renal trajectories in chronic heart failure.
Argan O, Ural D, Kozdag G, Sahin T, Bozyel S, Aktas M, et al. Associations between neutrophil gelatinase associated lipocalin, neutrophil-to-lymphocyte ratio, atrial fibrillation and renal dysfunction in chronic heart failure. Med Sci Monit. 2016;22:4765–72. https://doi.org/10.12659/msm.898608.
Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011;80(3):282–7. https://doi.org/10.1038/ki.2011.79.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891–975. https://doi.org/10.1002/ejhf.592.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA Focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136(6):e137–e61. https://doi.org/10.1161/CIR.0000000000000509.
Verbrugge FH, Martens P, Mullens W. SGLT-2 Inhibitors in heart failure: implications for the kidneys. Curr Heart Fail Rep. 2017. https://doi.org/10.1007/s11897-017-0345-9.
Damman K, Gori M, Claggett B, Jhund PS, Senni M, Lefkowitz MP, et al. Renal effects and associated outcomes during angiotensin-neprilysin inhibition in heart failure. JACC Heart Fail. 2018;6(6):489–98. https://doi.org/10.1016/j.jchf.2018.02.004.
Damman K, Valente MA, Voors AA, O'Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014;35(7):455–69. https://doi.org/10.1093/eurheartj/eht386.
Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53(7):582–8. https://doi.org/10.1016/j.jacc.2008.08.080.
Damman K. Ng Kam Chuen MJ, MacFadyen RJ, Lip GY, Gaze D, Collinson PO et al. Volume status and diuretic therapy in systolic heart failure and the detection of early abnormalities in renal and tubular function. J Am Coll Cardiol. 2011;57(22):2233–41. https://doi.org/10.1016/j.jacc.2010.10.065.
Legrand M, De Berardinis B, Gaggin HK, Magrini L, Belcher A, Zancla B, et al. Evidence of uncoupling between renal dysfunction and injury in cardiorenal syndrome: insights from the BIONICS study. PLoS One. 2014;9(11):e112313. https://doi.org/10.1371/journal.pone.0112313.
Chen C, Yang X, Lei Y, Zha Y, Liu H, Ma C, et al. Urinary biomarkers at the time of AKI diagnosis as predictors of progression of AKI among patients with acute cardiorenal syndrome. Clin J Am Soc Nephrol. 2016;11(9):1536–44. https://doi.org/10.2215/CJN.00910116.
Sokolski M, Zymlinski R, Biegus J, Siwolowski P, Nawrocka-Millward S, Todd J, et al. Urinary levels of novel kidney biomarkers and risk of true worsening renal function and mortality in patients with acute heart failure. Eur J Heart Fail. 2017;19(6):760–7. https://doi.org/10.1002/ejhf.746.
• Rao VS, Ahmad T, Brisco-Bacik MA, Bonventre JV, Wilson FP, Siew ED, et al. Renal effects of intensive volume removal in heart failure patients with preexisting worsening renal function. Circ Heart Fail. 2019;12(6):e005552. https://doi.org/10.1161/CIRCHEARTFAILURE.118.005552 Important study to show that tubular injury assessed through urine biomarkers, caused by aggressive decongestion with either ultrafiltration or diuretic treatment, is largely reversible .
Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149(2):209–16.
Bettencourt P, Azevedo A, Pimenta J, Frioes F, Ferreira S, Ferreira A. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110(15):2168–74. https://doi.org/10.1161/01.CIR.0000144310.04433.BE.
Lala A, McNulty SE, Mentz RJ, Dunlay SM, Vader JM, AbouEzzeddine OF, et al. Relief and recurrence of congestion during and after hospitalization for acute heart failure: insights from Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF). Circ Heart Fail. 2015;8(4):741–8. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001957.
Cooper LB, Mentz RJ, Stevens SR, Felker GM, Lombardi C, Metra M, et al. Hemodynamic predictors of heart failure morbidity and mortality: fluid or flow? J Card Fail. 2016;22(3):182–9. https://doi.org/10.1016/j.cardfail.2015.11.012.
Ambrosy AP, Cerbin LP, Armstrong PW, Butler J, Coles A, DeVore AD, et al. Body weight change during and after hospitalization for acute heart failure: patient characteristics, markers of congestion, and outcomes: findings from the ASCEND-HF trial. JACC Heart Fail. 2017;5(1):1–13. https://doi.org/10.1016/j.jchf.2016.09.012.
• Testani JM, Hanberg JS, Cheng S, Rao V, Onyebeke C, Laur O, et al. Rapid and highly accurate prediction of poor loop diuretic natriuretic response in patients with heart failure. Circ Heart Fail. 2016;9(1):e002370. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002370This study demonstrated that early natriuresis after diuretic administration accurately predicts the total natriuresis achieved. As a result, urine spot sampling might be used to estimate total natriuresis with diuretic treatment.
Verbrugge FH, Nijst P, Dupont M, Penders J, Tang WH, Mullens W. Urinary composition during decongestive treatment in heart failure with reduced ejection fraction. Circ Heart Fail. 2014;7(5):766–72. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001377.
•• Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner-La Rocca HP, Martens P, et al. The use of diuretics in heart failure with congestion - a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21(2):137–55. https://doi.org/10.1002/ejhf.1369Expert consensus paper on the use of diuretics in heart failure, incorporating the recommendation to assess urine sodium concentration as an early marker of diuretic response.
Schnermann J. Juxtaglomerular cell complex in the regulation of renal salt excretion. Am J Physiol. 1998;274(2 Pt 2):R263–79.
Nijst P, Verbrugge FH, Grieten L, Dupont M, Steels P, Tang WH, et al. The pathophysiological role of interstitial sodium in heart failure. J Am Coll Cardiol. 2015;65(4):378–88. https://doi.org/10.1016/j.jacc.2014.11.025.
Kamel KS, Ethier JH, Richardson RM, Bear RA, Halperin ML. Urine electrolytes and osmolality: when and how to use them. Am J Nephrol. 1990;10(2):89–102. https://doi.org/10.1159/000168062.
•• Martens P, Dupont M, Verbrugge FH, Damman K, Degryse N, Nijst P, et al. Urinary sodium profiling in chronic heart failure to detect development of acute decompensated heart failure. JACC Heart Fail. 2019;7(5):404–14. https://doi.org/10.1016/j.jchf.2019.02.011Innovative study that suggests a pivotal role for renal sodium avidity, assessed through the urine sodium concentration on a spot urine sample outside the window of diuretic administration, in causing episodes of decompensated heart failure.
Ferreira JP, Girerd N, Bettencourt Medeiros P, Bento Ricardo M, Almeida T, Rola A, et al. Lack of diuretic efficiency (but not low diuresis) early in an acutely decompensated heart failure episode is associated with increased 180-day mortality. Cardiorenal Med. 2017;7(2):137–49. https://doi.org/10.1159/000455903.
• Brinkley DM Jr, Burpee LJ, Chaudhry SP, Smallwood JA, Lindenfeld J, Lakdawala NK, et al. Spot urine sodium as triage for effective diuretic infusion in an ambulatory heart failure unit. J Card Fail. 2018;24(6):349–54. https://doi.org/10.1016/j.cardfail.2018.01.009Interesting study showing that the natriuretic response to diuretic treatment in heart failure is associated with prognosis.
• Honda S, Nagai T, Nishimura K, Nakai M, Honda Y, Nakano H, et al. Long-term prognostic significance of urinary sodium concentration in patients with acute heart failure. Int J Cardiol. 2018;254:189–94. https://doi.org/10.1016/j.ijcard.2017.08.053Interesting study showing that the natriuretic response to diuretic treatment in heart failure is associated with prognosis.
Collins SP, Jenkins CA, Baughman A, Miller KF, Storrow AB, Han JH, et al. Early urine electrolyte patterns in patients with acute heart failure. ESC Heart Fail. 2019;6(1):80–8. https://doi.org/10.1002/ehf2.12368.
Luk A, Groarke JD, Desai AS, Mahmood SS, Gopal DM, Joyce E, et al. First spot urine sodium after initial diuretic identifies patients at high risk for adverse outcome after heart failure hospitalization. Am Heart J. 2018;203:95–100. https://doi.org/10.1016/j.ahj.2018.01.013.
Verbrugge FH, Grodin JL, Mullens W, Taylor DO, Starling RC, Tang WH. Transient hyponatremia during hospitalization for acute heart failure. Am J Med. 2016;129(6):620–7. https://doi.org/10.1016/j.amjmed.2016.01.016.
• Verbrugge FH, Martens P, Boonen L, Nijst P, Verhaert D, Noyens P, et al. Loop diuretic down-titration in stable chronic heart failure is often achievable, especially when urinary chloride concentration is low. Acta Cardiol. 2018;73(4):335–41. https://doi.org/10.1080/00015385.2017.1385152First study to suggest that diuretic downtitration is more likely successful in stable chronic heart failure patients with a low urine chloride concentration after diuretic administration. Findings suggest that a high urine chloride concentration might indicate subclinical congestion and the need for continued diuretic treatment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Biomarkers of Heart Failure
Rights and permissions
About this article
Cite this article
Verbrugge, F.H. Utility of Urine Biomarkers and Electrolytes for the Management of Heart Failure. Curr Heart Fail Rep 16, 240–249 (2019). https://doi.org/10.1007/s11897-019-00444-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-019-00444-z