Abstract
In the last two decades, morbidity and mortality of patients with chronic heart failure could be further reduced by improved pharmacological and cardiac device therapies. However, despite these advances, there is a substantial unmet need for novel therapies, ideally specifically addressing repair and regeneration of the damaged or lost myocardium and its vasculature, given the limited endogenous potential for renewal of cardiomyocytes in adults. In this respect, cardiac cell-based therapies have gained substantial attention and have entered clinical feasibility and safety studies a decade ago. Different cell-types have been used, including bone marrow–derived mononuclear cells, bone marrow–derived mesenchymal stem cells, mobilized CD34+ cells, and more recently cardiac-derived c-kit+ stem cells and cardiosphere-derived cells. Some of these studies have suggested a potential of cell-based therapies to reduce cardiac scar size and to improve cardiac function in patients with ischemic cardiomyopathy. While first clinical trials examining the impact of cardiac cell–based therapy on clinical outcome have now been initiated, improved understanding of underlying mechanisms of action of cell-based therapies may lead to strategies for optimization of the cardiac repair potential of the applied cells. In experimental studies, direct in vivo reprogramming of cardiac fibroblasts towards cardiomyocytes, and microRNA-based promotion of cardiomyocyte proliferation and cardiac repair have recently been reported that may represent novel therapeutic approaches for cardiac regeneration that would not need cell-administration but rather directly stimulate endogenous cardiac regeneration. This review will focus mainly on recently completed clinical trials (within the last 2 years) investigating cardiac cell-based therapies and the current status of experimental studies for cardiac cell-based repair and regeneration with a potential for later translation into clinical studies in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Loss of myocardium rapidly after myocardial infarction and the ongoing death of cardiomyocytes thereafter frequently terminates in heart failure, as endogenous regeneration pathways cannot replace damaged myocardium and vasculature. Unlike in zebrafish [1, 2], division of differentiated cardiomyocytes (CM) is a rare event in humans [3].
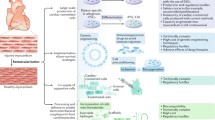
In the last decade, numerous different human cell populations, including bone marrow-derived mononuclear cells and CD34+ cells, have been suggested to enhance cardiac function and repair in experimental animal models. Several clinical studies largely examining feasibility and safety have been performed and have yielded mixed results with respect to effects on cardiac function. Cell isolation procedures, cell types, number of transplanted cells, and the functional cardiac repair capacity of the transplanted cells are likely determinants of their effects on cardiac function [4–6]. Here, we describe recent experiences of cardiac cell-based therapies using different cell populations (Fig. 1, Table 1
Clinically and experimentally emerging cells for heart regeneration therapies. Allo allogeneic; Auto autologous; BM-MNC bone marrow mononuclear cells; CDCs cardiosphere-derived cells; EOCs early outgrowth cells (also known as circulating angiogenic cells (CACs) or early endothelial progenitor cells (EPCs); iPSC induced pluripotent stem cells; LV-EF left ventricular ejection fraction; MSCs mesenchymal stem cells; NYHA new york heart association
).
Embryonic Stem Cells and Induced Pluripotent Stem Cells
Embryonic stem cells (ESCs) share the ability to differentiate in all three germ layers (pluripotency) and can be infinitely expanded (clonogenicity and self-renewal) [7]. Therefore, ESCs can be considered as an infinite source to generate the desired tissue, particularly as numerous studies have shown differentiation into cardiomyocyte-like cells and endothelial cells, and improvement of cardiac function after transplantation in experimental cardiac injury models [8–10]. However, allogeneic transplantation is required, which may cause immunologic reactions after transplantation and, as they are obtained from blastocytes, i.e., an early embryonic stage, ethical concerns set an additional barrier for wider clinical applications. Moreover, because of their ability to expand clonogenically, there is a substantial risk of teratogenic potential, at least for undifferentiated ESCs. These aspects limit their current use for potential human heart regeneration therapies.
Man-made dedifferentiated cells, which share similar properties with ESCs, are termed induced pluripotent stem cells (iPSCs). iPSCs have initially been reprogrammed from differentiated fibroblasts in 2006 [11, 12]. Since then, reprogramming protocols have been refined, thereby raising the efficiency and by transfection of recombinant proteins or RNA molecules, such as microRNAs, circumvented the initially required transfection procedures with stemness factors (i.e. transcription factors highly expressed in ESCs) via retroviruses [13–15]. As they can be directed to differentiate towards cardiomyocytes, iPSCs represent a potential resource of personalized heart tissue replacement and a valuable tool to further understand potential pathways towards cardiac regeneration. Using in vivo imaging, we have recently observed viability, tissue distribution and long-term engraftment of cellular iPSC-derived grafts in a large animal model of myocardial infarction [16].
However, iPSCs share the teratogenic potential with ESCs and recently the immuno-compatibility of undifferentiated autologous iPSCs has been questioned [17]. Moreover, as they are genetically modified and require prolonged cultivation times, iPSC-derived cells may have a risk for mutations. Hence, the use of iPSCs is challenging and not yet feasible for clinical applications. Direct reprogramming of cardiac fibroblasts into cardiomyocytes in vivo therefore represents a highly interesting perspective [18•, 19].
Cardiac-Derived Progenitor/Stem Cells
Amongst somatic progenitor cells, cardiac progenitor cells (CPCs) have been postulated to have the highest capacity to promote cardiac regeneration. The identification of c-kit+ cells [20] residing in stem cell niches [21] in the murine heart that can give rise to the main cellular components of the heart, namely cardiomyocytes, endothelial cells and smooth muscle cells have rendered the heart an organ with potential regenerative capacity. In experimental studies, transplantation of c-kit+ cells reconstituted the heart and improved cardiac function [20]. Next to c-kit+ cells, other populations as defined by surface markers or culture conditions have been suggested as an endogenous source of heart regeneration. Isl-1+ cells [22] derive from the second heart field, but can be rarely found in postnatal development stages (reviewed in [23]). Sca-1+ (stem cell antigen-1) cells [24] are restricted to murine hearts (no orthologue in human), but have also been suggested for heart regeneration [25]. Cardiosphere-derived cells (CDCs) are cultured from heart biopsies and are so named because of their ability to form spheroids in cell suspension [26, 27]. CDCs are multicellular clusters containing a mixed cell population, which comprise, next to cardiac progenitor cells with c-kit and CD105 surface marker expression, other cell types also, such as mesenchymal stem cells [26, 28]. Experimental data suggest that heart regeneration of CDCs depends on the release of paracrine factors, induction of endogenous regenerative capacity, and to a lesser extent on the differentiation into cardiomyocytes and endothelial cells in vivo (which has also been controversial) [26, 28–30].
All these adult cardiac-derived stem cells are suggested to have self-renewal capacities and the ability of multilineage differentiation. Importantly, adult cardiac stem cells have the potential to reconstitute damaged myocardium and improve cardiac function after heart injury [20, 25, 28, 29, 31]. Moreover, CPCs can be obtained by endomyocardial biopsies and sorted according to their surface markers and/or expanded in cell culture.
To date, two published clinical phase I trials have conducted transplantation of cardiac-derived cell products in patients with ischemic cardiomyopathy. In the randomized SCIPIO-trial, 1x106 c-kit+ cardiac stem cells were delivered intracoronary to patients with coronary artery bypass surgery (CABG) and left ventricular ejection fraction (LVEF) <40%. The control group received no cell therapy. After 4 months, infarct size as assessed by cMRI decreased (with, however, a lack of a control group for comparison) and LVEF as assessed by echocardiography significantly increased in patients receiving cell therapy. In a subset of patients, cMRI and echocardiography measurements were performed after 12 months, which showed an even more-reduced scar size and improved LVEF. Moreover, although a low number of cells were injected, adverse events were comparable with standard care treated patients [32••, 33].
Another, recently published trial, the Cardiosphere-Derived Autologous Stem Cells To Reverse Ventricular Dysfunction (CADUCEUS) trial [34••], used intracoronary infusion of cardiosphere-derived cells (CDCs) in patients with ventricular dysfunction 2–3 months after myocardial infarction.
The CADUCEUS trial [34••] suggested a reduction in scar mass and an enhanced viable heart mass at 6 and 12 months after transplantation. However, despite these beneficial effects, no significant change in LV-function could be observed.
The pilot data of these two clinical trials indicate that intracoronary delivery of heart-derived cell products is feasible and safe and may improve cardiac function in patients with ischemic cardiomyopathy. Although these trials, using either selected c-kit+ cells or CDCs (a mixed cell population), are not directly comparable because of different patient population and study designs, both suggest a reduction in scar size, which renders cardiac stem/progenitor cells to an interesting candidate for cell-based therapies. However as only a few patients were enrolled in the treatment arm (17 patients in the CADUCEUS trial and 16 patients in the SCIPIO trial), and control groups received only standard care, safety and efficacy has to be proven in a randomized-blinded, placebo-controlled study design.
Moreover, the finding that CDCs lack MHC II antigens, and therefore cause only a mild immune reaction after transplantation in the rat infarcted heart [35], initiated the ongoing randomized, double-blind, placebo-controlled ALLSTAR (NCT01458405) trial in patients with myocardial infarction and left ventricular dysfunction. With allogeneic cell transplantation, biopsies of patients would be needless; cells could be injected to a specific time point and circumvent the suggested impairment of adult progenitor cells [36, 37].
Bone-Marrow Derived Stem Cells
Mechanisms of Effects of Bone-Marrow Derived Cells on Cardiac Function
Initially, bone-marrow mononuclear cell (BM-MNC)-transplantation was thought to yield its effects on cardiac function by transdifferentiation into cardiomyocytes and endothelial cells [38]. Later, this concept has been challenged [39, 40]. Experimental studies have indicated that direct transdifferentiation of BM-MNCs into cardiomyocytes or endothelial cells is (if it ever occurs) a very rare event [39, 40], and could not explain the observed effects on cardiac function. Early studies may therefore have observed cell fusions, rather than true transdifferentiation of BM-MNCs into cardiomyocytes [40]. It is more conceivable that BM-MNCs enhance cardiac repair by paracrine effects [41•, 42]. Release of growth factors from transplanted BM-MNCs are suggested to promote migration of endothelial cells and CPCs, and can exert cytoprotective effects on resident cardiomyocytes [43, 44]. Particularly, BM-MNCs support cardiac angiogenesis and neovascularization in the infarcted heart [45]. However, a recent study has also suggested that bone marrow–derived c-kit+ cells promote augmentation of cardiomyocyte progenitor activity, which may lead to cardiomyocyte formation [46].
Recently Published (Within Last 2 Years) Clinical Studies of Cardiac Cell-Based Therapies Using BM-MNCs in Patients with Myocardial Infarction and Ischemic Cardiomyopathy
As BM-MNCs are an easily accessible cell source (via bone marrow aspiration), initial clinical studies have used transplantation of this heterogeneous cell population [47–50, 51••]. Clinical trials so far showed an excellent safety profile and feasibility. The effects observed in recent clinical studies on LV-function were more modest as expected [52–55], however, a meta-analysis of 1765 participants has suggested a significant improvement of LV-EF, both in short (3.26 %) and long term (3.91 %) follow-up [56].
In this regard, clinical trials were initiated by the Cardiovascular Cell Therapy Research Network (CCTRN) in patients with significant LV-dysfunction caused by ischemic cardiomyopathy and patients with ST-elevation myocardial infarction (STEMI) [57, 58•]. In the FOCUS-CCTRN trial, patients with ischemic cardiomyopathy were enrolled to receive BM-MNCs by transendocardial administration [57]. In this phase II randomized trial, at 6 months, LV end-systolic volume (as assessed by echocardiography) did not significantly differ between BM-MNCs administration and placebo group. However, exploratory analysis indicated a significant increase in LVEF (2.7 %) and stroke volume in the treatment group. Although this was the largest recent clinical trial conducted in patients with severe LV-dysfunction (LV-EF: 32.4 %) caused by ischemic cardiomyopathy, the sample size may have been too small.
Clinical data (and some later experimental observations) had suggested that timing of BM-MNC delivery after acute myocardial infarction may have an impact on its effects on cardiac function [51••, 59]. The TIME-trial [58•] focused on different time points of intracoronary BM-MNC delivery at day 3 and day 7 in patients with ST-elevation infarction treated with percutaneous primary intervention. However, no benefit on cMRI detected LV-performance could be observed 6 months after infusion of BM-MNCs in either group [58•].
Moreover, as assessed in the LateTIME [60•] and SWISS-AMI [61•] trial, BM-MNC administration 2 to 3 weeks after acute myocardial infarction did not significantly affect LV-function. However, although the SWISS-AMI trial was not optimized to evaluate this endpoint, subgroup analysis indicates a beneficial effect on LV-function after 4 months when revascularization therapy was performed less than 4.5 hours after symptom onset [61•]. In addition, these trials may not be geared to detect smaller changes in LV function.
Potential Impact of Cell Isolation Procedures and Impaired Functional Capacity of Adult Bone-Marrow Derived Cells
Whereas all clinical trials have supported the safety of delivery of BM-MNCs, the lack of a significant beneficial effect after BM-MNC delivery on LV-function in some of these studies raises the question of whether this patient-derived cell population will be efficient enough for long-term improvement of cardiac function. Importantly, however, the mode of bone marrow–derived cell preparation may play a critical role that likely has been underestimated.
For example, certain agents, such as buffer and medium composition during cell isolation, have been shown to crucially alter cellular function. Heparin has been observed recently to exert detrimental effects on the functionality of BM-MNCs by interacting with the CXCR4/SDF-1 axis [62, 63]. The CCTRN–trials have been performed using an automated cell-sorting system for the isolation of BM-MNCs [64, 65]. However, whether these cells are efficient in an experimental myocardial infarction model in vivo has not been reported. Therefore, as isolation procedure steps may have a crucial influence on cell functionality, the functional properties of these cells after automatic separation may be a determinant for the in vivo effects.
In addition, our group could show that the cardiac repair capacity of angiogenic early outgrowth cells [EOCs, also known as circulating angiogenic cells (CACs)] is impaired in patients with chronic heart failure caused by ischemic cardiomyopathy as compared to healthy subjects in an experimental myocardial infarction model [36]. Together with other studies, which show an impairment of migration and angiogenic capacity of adult bone marrow–derived mononuclear cells [66, 67], this might contribute to the limited capacity of BM-MNCs in clinical trials to effectively impact on cardiac function. In this respect, a phase I trial was recently published using allogeneic bone marrow–derived cells [68] from healthy donors as an off-the-shelf product, which may circumvent impairment of autologous cell function in patients with cardiovascular disease.
Bone-Marrow-Derived and Mobilized CD34+ Cells for Cell-Based Cardiac Therapy
Instead of unselected BM-MNCs, distinct cell populations with cardiac repair capacity can be isolated from the bone marrow. CD 34+ cells represent a rare subpopulation of BM-MNCs with an experimentally high potential of promoting angiogenesis and neovascularization in ischemic tissues [69]. In the ACT-34CMI trial, intramyocardial administration of low- or high-dose CD34+ cells was performed in patients with ischemic cardiomyopathy and refractory angina pectoris. Of interest, in the low-dose CD34+ cell group, a reduction in angina pectoris frequency and improvement in exercise tolerance was observed at 6 and 12 months after treatment [70•]. This study also delineates an example for trials, which not only takes functional endpoints into consideration, but focuses more on clinical endpoints in patients with chronic heart failure, and also questions whether higher numbers of intramyocardially applied cells are indeed more efficient.
In conclusion, it is noteworthy that no adverse events occurred in trials using BM-MNCs. In addition, a reduction of major adverse cardiovascular events was observed in the REPAIR-AMI trial, which maintained for 2 years after acute myocardial infarction [71]. This finding is underlined by a recently published meta-analysis [72•]. In this respect, large scaled phase 3 trials are on the way to identify the effects of BM-MNCs on clinical outcome and mortality in patients with acute myocardial infarction or ischemic cardiomyopathy (BAMI, NCT01569178; REPEAT, NCT01693042).
Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) are a subpopulation of bone-marrow mononuclear cells and can be cultured by repeated passaging on plastic surfaces [73]. Typically, MSCs are able to differentiate in cartilage, bone, or adipose tissue [73], but differentiation into cardiomyocyte-like cells has also been suggested [74], which has rendered them attractive for cardiac regeneration therapies. In addition, MSCs release growth factors, indicating a therapeutically important paracrine function and direct cell-cell interactions, which may additionally activate endogenous repair mechanisms [75–79]. Moreover, at least initially, MSCs prevent anti–donor T-cell responses and create an immunosuppressive milieu, thereby generating an immune-privileged state [80]. In this regard, experimental studies have demonstrated an improved LV-function after allogeneic transplantation of MSCs [81].
Both autologous and allogeneic MSC- administration was tested in clinical trials.
Chen et al [82]. recruited 69 patients after acute myocardial infarction (AMI) for a placebo-controlled trial using intracoronary delivery of autologous MSCs. Three months after administration, an improved LV-function and decreased left ventricular volumes were detected. In addition, a decrease in perfusion defect could be observed, indicating reverse remodeling and cardiac regeneration after autologous MSC administration. As an ‘off-the-shelf’ product, MSCs from healthy volunteers were also transplanted allogeneically in patients with AMI [83]. Importantly, in this placebo-controlled trial using intravenous injection, safety outcomes did not differ in the treatment arm and furthermore, a decrease in ventricular arrhythmias was observed [83]. Notably, LVEF, as assessed with echocardiography, increased significantly in patients treated with allogeneic MSCs [83].
In the recently published POSEIDON trial [84••], based on pilot data [85], a head-to-head comparison between autologous and allogeneic transplantation of MSCs in a dose-escalating manner in patients with LV-dysfunction caused by ischemic cardiomyopathy was performed. Cell-based treatment associated adverse events were low, though a placebo-treated group was missing, and adverse events did not differ between autologous and allogeneic cell transplantation. Thirteen month after transplantation of MSCs, reverse remodeling (as assessed by LV sphericity index) could be observed, along with a reduction of myocardial infarction size. However, a significant change in LV-EF was not observed. Interestingly, it appeared that low doses of MSCs resulted in the greatest reductions in LV volumes.
New insights using an intramyocardial delivery approach in patients with ischemic cardiomyopathy are under way: TAC-HFT (NCT00768066), PROMETHEUS (NCT00587990), and pilot data from the TAC-HFT study (a placebo-controlled trial) suggest potential beneficial effects of MSCs in this patient population [85].
Priming/Preconditioning of Stem Cells
As it was reported that adult progenitor/stem cells are impaired in their functional cardiac repair capacity [36, 37, 66], next to advances in allogeneic cell transplantation described above, strategies to enhance functional capacity of autologous progenitor/stem cells emerge as promising applications. Preconditioning of progenitor cells by ischemic, pharmacological, or genetic manipulation to render them resistant to the hostile environment in ischemic tissues may enhance their functional properties that is currently intensely investigated [86, 87].
eNOS-Overexpression in EOCs
Recruitment of angiogenic EOCs (also known as CACs) and dysfunction of endothelial cells is critically dependent on endothelial nitric oxide synthase (eNOS) [88–90]. In addition, eNOS-expression crucially alters cardiac repair capacity of bone marrow-derived progenitor cells in an experimental model of ischemic injury [91]. Based on these results, a randomized trial (ENACT-AMI (NTC00936819)) is currently under way to assess potential improvement after transplantation of EOCs transfected with human eNOS in patients with acute myocardial infarction [92].
Growth-Factor Treatment as a Strategy to Facilitate and Enhance Repair Capacity of Progenitor/Stem Cells
Retention and engraftment of transplanted progenitor/stem cells is still an important issue, which is not resolved yet, as only few cells injected reside in the designated location [93]. Instead, they are flushed away or die because of a hostile milieu in the ischemic heart region. Therefore, in order to equip injected cells with a friendlier milieu, Takehara et al. [94] transplanted CDCs with a hydrogel controlling the release of bFGF (basic fibroblast growth factor), a compound that is known to facilitate differentiation, proliferation, and survival. CDCs injected with hydrogels releasing bFGF showed a superior engraftment and facilitate effects of CDCs in pigs with heart failure caused by myocardial infarction [94]. These results led to the initiation of the ongoing ALCADIA-trial (NCT00981006) in CABG-patients. Another strategy is to pretreat progenitor/stem cells to enhance their efficacy after transplantation. In this regard, Behfar et al. pretreated human MSCs with a growth-factor cocktail [95]. Thereby, differentiation of human MSCs towards a cardiopoietic lineage commitment has been achieved, leading to an improved cardiac function and structural benefits in infarcted murine hearts after cell transplantation [95]. These cardiopoietic MSCs were subsequently used in a clinical trial with patients with ischemic cardiomyopathy. Transplantation of cardiopoietic MSCs was safe and at 6 months, an increase of LVEF could be observed as compared to the control group with standard care [96].
microRNA-Based Pre-treatment to Optimize Cell-Based Cardiovascular Repair Capacity
Key regulators, which are already therapeutically used in patients with hepatitis C in a clinical trial (NCT01200420), but have not been translated yet in clinical applications for cell-based cardiac therapies, are microRNAs. These small RNAs [97], which regulate gene expression at the post-transcriptional level mostly by degradation of mRNAs, have a highly attractive potential to regenerate damaged myocardium in experimental studies after viral delivery [98]. Interestingly, dysregulation of microRNAs has been observed in bone marrow-derived cells from patients with cardiovascular diseases [36, 66, 99]. Overexpression of the proangiogenic microRNA-126 [36] or blocking of microRNA-21 or microRNA-34a [66, 100] may enhance functional capacity of impaired adult circulating or bone-marrow derived mononuclear cells. Moreover, several microRNAs have been transfected into progenitor cells and improved their biological functions [101].
These applications may potentiate and/or restore the functional capacities of applied progenitor/stem cells. Thus, preconditioning and priming of cells used for cell-based therapies may have not only an important impact on their own functional ability to improve cardiac function, but also to enhance the activation of endogenous repair mechanisms by paracrine signaling.
Conclusions and Future Directions
A decade ago, the first in-man administration of BM-MNCs was performed in a patient with myocardial infarction [93]. Since then, thousands of patients have been enrolled in clinical trials examining cardiac cell-based therapies. Safety and feasibility of bone marrow–derived cells have so far been excellent, and beneficial effects on cardiac function, reverse remodeling, and scar size have been observed in some studies. The main focus is still to unravel the ideal approach to regenerate the heart in different cardiovascular disease conditions.
However, reconstitution of the myocardium and sufficient neovascularization after cardiac injury may require more than a single injection and/or a combination of progenitor/stem cells. In this regard, recently, synergistic effects of simultaneously injected MSCs and c-kit+ cells on cardiac function have been observed after myocardial infarction in a swine model [102] and a clinical trial with repeated injections of BM-MNCs is planned (REPEAT (NCT01693042)).
iPSCs have a clear potential for cardiac regeneration, but substantial safety and practical hurdles are an important limitation. Direct reprogramming of cardiac fibroblasts into cardiomyocytes, thereby skipping the induction of pluripotent stem cells with the associated risks, represents a highly interesting direction of research [18•, 19]. Recently, systemic application of a microRNA-cocktail [19] or 3 cardiac transcription factors (Gata4, Mef2c and Tbx5 (GMT)) [18•] in a murine model of myocardial infarction has been reported to directly reprogram cardiac fibroblasts into cardiomyocyte-like cells in vivo, leading to an improved cardiac function [18•].
Recently published clinical trials with cardiac-derived stem cells and the non-inferiority of allogeneic versus autologous MSCs-transplantation represent interesting avenues worth to pursue in the future. Furthermore, phase III clinical trials are under way to examine the effects of BM-MNCs administration on all-cause mortality in patients with ischemic LV-dysfunction (BAMI, REPEAT). In addition, ex vivo preconditioning to enhance the cardiac repair potential of autologous cells for cardiac cell-based therapies may improve their efficacy, in particular in heart failure patients.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298(5601):2188–90. doi:10.1126/science.1077857.
Jopling C, Sleep E, Raya M, Marti M, Raya A, Izpisua Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464(7288):606–9. doi:10.1038/nature08899.
Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98–102. doi:10.1126/science.1164680.
Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451(7181):937–42. doi:10.1038/nature06800.
Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473(7347):326–35. doi:10.1038/nature10147.
Tongers J, Losordo DW, Landmesser U. Stem and progenitor cell-based therapy in ischaemic heart disease: promise, uncertainties, and challenges. Eur Heart J. 2011;32(10):1197–206. doi:10.1093/eurheartj/ehr018.
Gepstein L. Derivation and potential applications of human embryonic stem cells. Circ Res. 2002;91(10):866–76.
Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25(9):1015–24. doi:10.1038/nbt1327.
Li Z, Wu JC, Sheikh AY, Kraft D, Cao F, Xie X, et al. Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation. 2007;116(11 Suppl):I46–54. doi:10.1161/CIRCULATIONAHA.106.680561.
Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408(6808):92–6. doi:10.1038/35040568.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. doi:10.1016/j.cell.2007.11.019.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi:10.1016/j.cell.2006.07.024.
Maherali N, Hochedlinger K. Guidelines and techniques for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;3(6):595–605. doi:10.1016/j.stem.2008.11.008.
Anokye-Danso F, Trivedi CM, Juhr D, Gupta M, Cui Z, Tian Y, et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8(4):376–88. doi:10.1016/j.stem.2011.03.001.
Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481(7381):295–305. doi:10.1038/nature10761.
Templin C, Zweigerdt R, Schwanke K, Olmer R, Ghadri JR, Emmert MY, et al. Transplantation and tracking of human-induced pluripotent stem cells in a pig model of myocardial infarction: assessment of cell survival, engraftment, and distribution by hybrid single photon emission computed tomography/computed tomography of sodium iodide symporter transgene expression. Circulation. 2012;126(4):430–9. doi:10.1161/CIRCULATIONAHA.111.087684.
Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474(7350):212–5. doi:10.1038/nature10135.
• Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485(7400):593–8. doi:10.1038/nature11044. This experimental study shows that systemic delivery of cardiac transcription factors can directly reprogram resident cardiac fibroblasts into cardiomyocyte–like cells.
Jayawardena TM, Egemnazarov B, Finch EA, Zhang L, Payne JA, Pandya K, et al. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res. 2012;110(11):1465–73. doi:10.1161/CIRCRESAHA.112.269035.
Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114(6):763–76.
Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, et al. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci U S A. 2006;103(24):9226–31. doi:10.1073/pnas.0600635103.
Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433(7026):647–53. doi:10.1038/nature03215.
Laugwitz KL, Moretti A, Caron L, Nakano A, Chien KR. Islet1 cardiovascular progenitors: a single source for heart lineages? Development. 2008;135(2):193–205. doi:10.1242/dev.001883.
Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100(21):12313–8. doi:10.1073/pnas.2132126100.
Matsuura K, Honda A, Nagai T, Fukushima N, Iwanaga K, Tokunaga M, et al. Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice. J Clin Invest. 2009;119(8):2204–17. doi:10.1172/JCI37456.
Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115(7):896–908. doi:10.1161/CIRCULATIONAHA.106.655209.
Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95(9):911–21. doi:10.1161/01.RES.0000147315.71699.51.
Li TS, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B, et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol. 2012;59(10):942–53. doi:10.1016/j.jacc.2011.11.029.
Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR, et al. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120(12):1075–83. doi:10.1161/CIRCULATIONAHA.108.816058. 7 p following 83.
Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106(5):971–80. doi:10.1161/CIRCRESAHA.109.210682.
Welt FG, Gallegos R, Connell J, Kajstura J, D'Amario D, Kwong RY, et al. Effect of cardiac stem cells on left ventricular remodeling in a canine model of chronic myocardial infarction. Circ Heart Fail. 2012. doi:10.1161/CIRCHEARTFAILURE.112.972273.
•• Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378(9806):1847–57. doi:10.1016/S0140-6736(11)61590-0. This is the first clinical study using c-kit+ cardiac stem cells in patients with ischemic cardiomyopathy.
Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126(11 Suppl 1):S54–64. doi:10.1161/CIRCULATIONAHA.112.092627.
•• Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379(9819):895–904. doi:10.1016/S0140-6736(12)60195-0. This is the first clinical trial using cardiosphere-derived cells (CDCs) in patients with ischemic cardiomyopathy.
Malliaras K, Li TS, Luthringer D, Terrovitis J, Cheng K, Chakravarty T, et al. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation. 2012;125(1):100–12. doi:10.1161/CIRCULATIONAHA.111.042598.
Jakob P, Doerries C, Briand S, Mocharla P, Krankel N, Besler C, et al. Loss of AngiomiR-126 and 130a in angiogenic early outgrowth cells from patients with chronic heart failure: role for impaired in vivo neovascularization and cardiac repair capacity. Circulation. 2012. doi:10.1161/CIRCULATIONAHA.112.093906.
Dimmeler S, Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circ Res. 2008;102(11):1319–30. doi:10.1161/CIRCRESAHA.108.175943.
Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–5. doi:10.1038/35070587.
Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428(6983):664–8. doi:10.1038/nature02446.
Nygren JM, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J, et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10(5):494–501. doi:10.1038/nm1040.
• Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103(11):1204–19. doi:10.1161/CIRCRESAHA.108.176826. This comprehensive review describes and discusses one of the most important mechanism - namely paracrine signaling - by which adult stem cells exert their effects.
Burchfield JS, Dimmeler S. Role of paracrine factors in stem and progenitor cell mediated cardiac repair and tissue fibrosis. Fibrogenesis Tissue Repair. 2008;1(1):4. doi:10.1186/1755-1536-1-4.
Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7(4):430–6. doi:10.1038/86498.
Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39(5):733–42. doi:10.1016/j.yjmcc.2005.07.003.
Yoon CH, Koyanagi M, Iekushi K, Seeger F, Urbich C, Zeiher AM, et al. Mechanism of improved cardiac function after bone marrow mononuclear cell therapy: role of cardiovascular lineage commitment. Circulation. 2010;121(18):2001–11. doi:10.1161/CIRCULATIONAHA.109.909291.
Loffredo FS, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8(4):389–98. doi:10.1016/j.stem.2011.02.002.
Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI). Circulation. 2002;106(24):3009–17.
Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364(9429):141–8. doi:10.1016/S0140-6736(04)16626-9.
Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367(9505):113–21. doi:10.1016/S0140-6736(05)67861-0.
Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1199–209. doi:10.1056/NEJMoa055706.
•• Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1210–21. doi:10.1056/NEJMoa060186. This landmark study demonstrates improvement of cardiac function after BM-MNCs transplantation in patients with acute myocardial infarction.
Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167(10):989–97. doi:10.1001/archinte.167.10.989.
Zimmet H, Porapakkham P, Sata Y, Haas SJ, Itescu S, Forbes A, et al. Short- and long-term outcomes of intracoronary and endogenously mobilized bone marrow stem cells in the treatment of ST-segment elevation myocardial infarction: a meta-analysis of randomized control trials. Eur J Heart Fail. 2012;14(1):91–105. doi:10.1093/eurjhf/hfr148.
Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50(18):1761–7. doi:10.1016/j.jacc.2007.07.041.
Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J. 2008;29(15):1807–18. doi:10.1093/eurheartj/ehn220.
Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Clarke MJ, et al. Long-term effects of autologous bone marrow stem cell treatment in acute myocardial infarction: factors that may influence outcomes. PLoS One. 2012;7(5):e37373. doi:10.1371/journal.pone.0037373.
Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. Jama. 2012;307(16):1717–26. doi:10.1001/jama.2012.418.
• Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG et al. effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. Jama. 2012:1–10. doi:10.1001/jama.2012.28726.
Wang X, Takagawa J, Lam VC, Haddad DJ, Tobler DL, Mok PY, et al. Donor myocardial infarction impairs the therapeutic potential of bone marrow cells by an interleukin-1-mediated inflammatory response. Sci Transl Med. 2011;3(100):100ra90. doi:10.1126/scitranslmed.3002814.
• Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. Jama. 2011;306(19):2110–9. doi:10.1001/jama.2011.1670.
• Surder D. Intracoronary infusion of BM-MNC early or late after AMI – 4 months results of the SWISS-AMI trial. Scientific Sessions of the AHA – late braking trials. 2012. References 58, 60, and 61 are all well-designed clinical trials that investigated BM-MNCs administration at different time points in patients with acute myocardial infarction and left ventricular dysfunction.
Seeger FH, Tonn T, Krzossok N, Zeiher AM, Dimmeler S. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 2007;28(6):766–72. doi:10.1093/eurheartj/ehl509.
Seeger FH, Rasper T, Fischer A, Muhly-Reinholz M, Hergenreider E, Leistner DM, et al. Heparin disrupts the CXCR4/SDF-1 axis and impairs the functional capacity of bone marrow-derived mononuclear cells used for cardiovascular repair. Circ Res. 2012;111(7):854–62. doi:10.1161/CIRCRESAHA.112.265678.
Aktas M, Radke TF, Strauer BE, Wernet P, Kogler G. Separation of adult bone marrow mononuclear cells using the automated closed separation system Sepax. Cytotherapy. 2008;10(2):203–11. doi:10.1080/14653240701851324.
Marban E, Malliaras K. Mixed results for bone marrow-derived cell therapy for ischemic heart disease. Jama. 2012:1–2. doi:10.1001/jama.2012.64751.
Fleissner F, Jazbutyte V, Fiedler J, Gupta SK, Yin X, Xu Q, et al. Short communication: asymmetric dimethylarginine impairs angiogenic progenitor cell function in patients with coronary artery disease through a microRNA-21-dependent mechanism. Circ Res. 2010;107(1):138–43. doi:10.1161/CIRCRESAHA.110.216770.
Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, et al. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109(13):1615–22. doi:10.1161/01.CIR.0000124476.32871.E3.
Penn MS, Ellis S, Gandhi S, Greenbaum A, Hodes Z, Mendelsohn FO, et al. Adventitial delivery of an allogeneic bone marrow-derived adherent stem cell in acute myocardial infarction: phase I clinical study. Circ Res. 2012;110(2):304–11. doi:10.1161/CIRCRESAHA.111.253427.
Wang J, Zhang S, Rabinovich B, Bidaut L, Soghomonyan S, Alauddin MM, et al. Human CD34+ cells in experimental myocardial infarction: long-term survival, sustained functional improvement, and mechanism of action. Circ Res. 2010;106(12):1904–11. doi:10.1161/CIRCRESAHA.110.221762.
• Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, Bass T, et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109(4):428–36. doi:10.1161/CIRCRESAHA.111.245993. This clinical study demonstrates a reduction of angina pectoris frequency in patients with Canadian Cardiovascular Society (CCS) class III–IV refractory angina after intramyocardial delivery of CD34+ cells and emphasizes that relief of symptoms may emerge as an important target of cell-based therapies.
Assmus B, Rolf A, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, et al. Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ Heart Fail. 2010;3(1):89–96. doi:10.1161/CIRCHEARTFAILURE.108.843243.
• Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 2012;126(5):551–68. doi:10.1161/CIRCULATIONAHA.111.086074. This up-to-date meta-analysis highlights beneficial short- and long-term effects after BM-MNCs administration in 2625 patients with ischemic heart disease.
Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–36. doi:10.1038/nri2395.
Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105(1):93–8.
Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11(4):367–8. doi:10.1038/nm0405-367.
Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–84. doi:10.1002/jcb.20886.
Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109(8):923–40. doi:10.1161/CIRCRESAHA.111.243147.
Suzuki G, Iyer V, Lee TC, Canty Jr JM. Autologous mesenchymal stem cells mobilize cKit+ and CD133+ bone marrow progenitor cells and improve regional function in hibernating myocardium. Circ Res. 2011;109(9):1044–54. doi:10.1161/CIRCRESAHA.111.245969.
Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107(7):913–22. doi:10.1161/CIRCRESAHA.110.222703.
Griffin MD, Ryan AE, Alagesan S, Lohan P, Treacy O, Ritter T. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: what have we learned so far? Immunol Cell Biol. 2012. doi:10.1038/icb.2012.67.
Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106(33):14022–7. doi:10.1073/pnas.0903201106.
Chen SL, Fang WW, Qian J, Ye F, Liu YH, Shan SJ, et al. Improvement of cardiac function after transplantation of autologous bone marrow mesenchymal stem cells in patients with acute myocardial infarction. Chin Med J (Engl). 2004;117(10):1443–8.
Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54(24):2277–86. doi:10.1016/j.jacc.2009.06.055.
•• Hare JM, Fishman JE, Gerstenblith G, Difede Velazquez DL, Zambrano JP, Suncion VY et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. Jama. 2012:1–11. doi:10.1001/jama.2012.25321. This clinical trial investigated transendocardial delivery of allogeneic and autologous MSCs head-to-head in patients with ischemic cardiomyopathy (ICM).
Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res. 2011;108(7):792–6. doi:10.1161/CIRCRESAHA.111.242610.
Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–36.
Haider H, Ashraf M. Preconditioning and stem cell survival. J Cardiovasc Transl Res. 2010;3(2):89–102. doi:10.1007/s12265-009-9161-2.
Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9(11):1370–6. doi:10.1038/nm948.
Landmesser U, Engberding N, Bahlmann FH, Schaefer A, Wiencke A, Heineke A, et al. Statin-induced improvement of endothelial progenitor cell mobilization, myocardial neovascularization, left ventricular function, and survival after experimental myocardial infarction requires endothelial nitric oxide synthase. Circulation. 2004;110(14):1933–9. doi:10.1161/01.CIR.0000143232.67642.7A.
Bauersachs J, Bouloumie A, Fraccarollo D, Hu K, Busse R, Ertl G. Endothelial dysfunction in chronic myocardial infarction despite increased vascular endothelial nitric oxide synthase and soluble guanylate cyclase expression: role of enhanced vascular superoxide production. Circulation. 1999;100(3):292–8.
Jujo K, Ii M, Sekiguchi H, Klyachko E, Misener S, Tanaka T, et al. CXCR4 Antagonist AMD3100 promotes cardiac functional recovery after ischemia-reperfusion injury via eNOS-dependent mechanism. Circulation. 2012. doi:10.1161/CIRCULATIONAHA.112.099242.
Taljaard M, Ward MR, Kutryk MJ, Courtman DW, Camack NJ, Goodman SG, et al. Rationale and design of enhanced angiogenic cell therapy in acute myocardial infarction (ENACT-AMI): the first randomized placebo-controlled trial of enhanced progenitor cell therapy for acute myocardial infarction. Am Heart J. 2010;159(3):354–60. doi:10.1016/j.ahj.2009.12.021.
Strauer BE, Steinhoff G. 10 years of intracoronary and intramyocardial bone marrow stem cell therapy of the heart: from the methodological origin to clinical practice. J Am Coll Cardiol. 2011;58(11):1095–104. doi:10.1016/j.jacc.2011.06.016.
Takehara N, Tsutsumi Y, Tateishi K, Ogata T, Tanaka H, Ueyama T, et al. Controlled delivery of basic fibroblast growth factor promotes human cardiosphere-derived cell engraftment to enhance cardiac repair for chronic myocardial infarction. J Am Coll Cardiol. 2008;52(23):1858–65. doi:10.1016/j.jacc.2008.06.052.
Behfar A, Yamada S, Crespo-Diaz R, Nesbitt JJ, Rowe LA, Perez-Terzic C, et al. Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. J Am Coll Cardiol. 2010;56(9):721–34. doi:10.1016/j.jacc.2010.03.066.
Bartunek J, Wijns W, Dolatabadi D, Vanderheyden M, Dens J, Ostojic M, et al. C-cure multicenter trial: lineage specified bone marrow derived cardiopoietic mesenchymal stem cells for treatment of ischemic cardiomyopathy. J Am Coll Cardiol. 2011;57:E200.
Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011;469(7330):336–42. doi:10.1038/nature09783.
Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, et al. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492(7429):376–81. doi:10.1038/nature11739.
Jakob P, Landmesser U. Role of microRNAs in stem/progenitor cells and cardiovascular repair. Cardiovasc Res. 2012;93(4):614–22. doi:10.1093/cvr/cvr311.
Xu Q, Seeger FH, Castillo J, Iekushi K, Boon RA, Farcas R, et al. Micro-RNA-34a contributes to the impaired function of bone marrow-derived mononuclear cells from patients with cardiovascular disease. J Am Coll Cardiol. 2012;59(23):2107–17. doi:10.1016/j.jacc.2012.02.033.
Hu S, Huang M, Nguyen PK, Gong Y, Li Z, Jia F, et al. Novel microRNA prosurvival cocktail for improving engraftment and function of cardiac progenitor cell transplantation. Circulation. 2011;124(11 Suppl):S27–34. doi:10.1161/CIRCULATIONAHA.111.017954.
Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, et al. Enhanced effect of human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and restore cardiac function after myocardial infarction. Circulation. 2012. doi:10.1161/CIRCULATIONAHA.112.131110 [Epub ahead of print].
Acknowledgments
This work was supported by Swiss National Research Foundation grants (310030–122339, 33CM30-124112/1), the German Research Foundation (DFG-LA-1432/3-1), the Swiss Heart Foundation, Uniscientia Foundation, the Zurich Center for Integrative Human Physiology and the Clinical Research Focus Program of the University of Zurich.
Conflict of Interest
Philipp Jakob declares he has no conflict of interest.
Ulf Landmesser declares he has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jakob, P., Landmesser, U. Current Status of Cell-Based Therapy for Heart Failure. Curr Heart Fail Rep 10, 165–176 (2013). https://doi.org/10.1007/s11897-013-0134-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-013-0134-z