Abstract
Purpose of Review
Over the past decade, donation after circulatory death (DCD) liver transplantation has expanded in the United States due to improved surgical experience and perioperative management. Despite these advances, there remains a reluctance towards broader utilization of DCD liver allografts due to lack of standardized donation process, concern for inferior graft survival, and risk of ischemic cholangiopathy associated with temporary lack of oxygenated perfusion during withdrawal of life-supporting treatment during procurement.
Recent Findings
New perfusion technologies offer potential therapeutic options to mitigate biliary complications and expand utilization of marginal DCD grafts. As these modalities enter routine clinical practice, DCD utilization will continue to increase, and liver allocation policies in turn will evolve to reflect this growing practice.
Summary
This review describes recent progress in DCD LT, current challenges with utilization of DCD liver allografts, and how novel technologies and policies could impact the future of the field.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Donation after circulatory death (DCD) is defined as the procurement of organs after confirmed irreversible cessation of cardiopulmonary function, pronounced by a non-procuring physician after withdrawal of life supporting treatment (WLST) [1]. The time spanning WLST until cessation of cardiopulmonary function has been termed the donor warm ischemia time (DWIT). While the initial experience of DCD liver transplantation (LT) was confined to a few centers and marked by inferior graft survival due to biliary complications [2,3,4], the field of transplantation has made great strides in DCD experience and utilization. Several studies have advocated for the increased utilization of DCD organs to mitigate the organ shortage [5].
In the previous decade, despite initial interest in expanding the donor pool through DCD LT, an important study by Mathur et al. reported significantly inferior graft survival with a probability of 3-year graft failure greater than 35% [6]. These findings, and the experiences reported by several single centers, dampened enthusiasm for expanding DCD LT in the United States, leading to nearly a decade of stagnation in the growth of DCD utilization. A critical finding in the report by Mathur et al. was that the key components for successful DCD LT included scrutinized DWIT, shortened cold ischemia time (CIT), and careful recipient selection. These lessons shaped the subsequent decade of DCD LT, which was associated with decreased DWIT (mean ≥ 25 min to < 23 min), shortened CIT (mean 10 h to < 6 h), and subsequent reduction in the rate of retransplant after DCD LT to just ≤ 2% [5]. An improved understanding of outcome-determining parameters lead to a resurgence in DCD LT utilization, with improved patient and graft survival and even shorter length of stay compared to their DBD counterparts [5].
Based on these trends, it can be projected that rates of DCD LT will continue to increase in the coming years. Due to improvements in neurocritical care, broader acceptance of organ donation, and the expanding definition of eligible donors, the number and percentage of DCD organ donors are continuing to rise [7]. However, despite similar 10-year patient survival between DCD (60.7%) and DBD (57.5%) LT recipients, rates of DCD LT graft survival, resource stewardship, and overall utilization remain inferior [8].
As the experience with DCD LT grows nationally and internationally, controversies surrounding procurement practices, perioperative management, recipient selection, and the utilization of perfusion technology remain. It is the goal of this paper to outline these controversies and review the current data supporting widespread adoption of DCD utilization.
Controversies
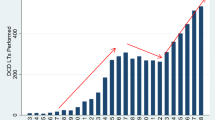
Since 2016 the number of organ donors has increased dramatically and DCD donors have increased even more significantly, with DCD donors now representing more than 30% of all organ donors (Fig. 1). However, rates of LT have risen at a more modest rate, largely due to the limited acceptance of DCD LT. In an important international comparison by Eden et al., the authors revealed that the United States represented the lowest DCD utilization percentage compared to 7 other nations, with a utilization rate of 27%. The authors described two critical assessment points at which a liver is utilized: the first assessment is prior to organ donation, at which time point nearly 70% of livers are declined, largely as a result of overall reluctance towards DCD, strict protocols (which limit donor age, height, weight, and DWIT), limited understanding regarding donor limitations, and logistical challenges (Fig. 2). Once accepted for donation, the second assessment results in another 29% of offers being declined, likely due to liver assessment, histology, quality of flush or issues associated with donation. Many of the controversies outlined here are thought to drive high organ decline rates at the first assessment, and thus pose a significant barrier to widespread adoption of DCD utilization.
a: Total number of organ donor and liver transplants performed over time from 2003–2022. LT, Liver Transplantation; DCD, donation after circulatory death b: Percentage of total organ donors that are DCD compared with the percentage of total liver transplants that are DCD, and the overall percentage of total donors that do not donate a liver for transplant. LT, Liver Transplantation; DCD, donation after circulatory death
Pathway of DCD liver donation and acceptance. The factors and stages influencing the acceptance of DCD-III liver offers are visualized. The majority of offers, e.g., two-thirds, are currently discarded before any retrieval. COR, controlled oxygenated rewarming; DCD, donation after circulatory death; DHOPE, dual HOPE; HOPE, hypothermic oxygenated perfusion; NMP, normothermic machine perfusion; NRP, normothermic regional perfusion. ([2023] Eden et al. Reproduced with permission from authors [40])
The Definition of Donor Warm Ischemia Time (DWIT)
While there is general consensus that prolonged DWIT leads to biliary complications and graft failure [9], there is a lack of standardization as to how DWIT is actually defined. Functional donor WIT (fDWIT) arose from the concept that individual events prior to procurement (hypotension, hypoxia, mandatory wait time, time to cross clamp) all had different effects on the outcome of the liver graft. Thus, some studies defined the start of fDWIT based on hemodynamic parameters (systolic blood pressure, mean arterial pressure) or oxygen saturation. The issue remains that there is no consensus as to what these exact parameters are [10]. The ASTS defines the start of fDWIT as MAP < 60 mmHg [11], the British Transplantation Society as systolic blood pressure < 50 mmHg [12], Eurotransplant as SpO2 < 80% or MAP < 50 mmHg, [13] and the Spanish National Transplant Organization as systolic blood pressure < 60 mmHg [10]. In 2019, the ILTS created a multidisciplinary working group to propose a universal definition of fDWIT based on hemodynamic parameters (time starting at when SpO2 < 80% or MAP < 60 mmHg), and a threshold of 30 min beyond which to carefully weigh risk of graft loss against benefits of transplantation [14]. Schlegel et al.’s 2022 benchmarking study to determine parameters predicting best outcomes with DCD LT found that DWIT < 30 min, asystolic WIT < 15 min, recipient MELD < 20 were the best predictors of patient and graft survival [15]. This lack of consensus definitions will remain a barrier to improved standardization and study of best practices.
Biliary Complications
Ischemic cholangiopathy (IC) is the feared complication of DCD LT, occurring in 2.6—30% of DCD liver recipients, and impacting graft survival compared to DCD LT recipients without IC [16, 17]. Although once thought to result in inevitable graft failure, IC does not always lead to a graft loss for the recipient. In a retrospective study of 770 DCD LTs performed within the Mayo Clinic hospital system [18•], Croome et al. identified four IC sub-types with distinct radiologic characteristics: diffuse necrosis, multifocal progressive, confluence dominant, and minor form. Both diffuse necrosis, characterized by early post-LT strictures throughout the intrahepatic biliary system, and multifocal processive IC, characterized by worsening stenosis of peripheral bile ducts over time, are associated with long-term need for biliary stenting and high rates of graft failure requiring re-transplant. Confluence dominant disease, characterized by strictures of the hilar confluence with preservation of the peripheral biliary system, typically requires stenting but eventually improves over time. Finally, minor form IC is characterized primarily by radiologic abnormalities of the biliary tree and normal graft function without need for repeat procedures.
IC and other biliary complications do translate to decreased patient and graft survival for DCD LT recipients compared to their DBD counterparts. Reported long-term survival outcomes show that DCD liver recipients have 3.2% lower patient survival at 10 years post-transplant; the difference increases to 4.7% lower patient survival when including patients requiring re-transplantation [8]. However, the major difference between DCD and DBD liver transplant outcomes is graft survival, with DCD recipients having 11% lower graft survival at 10 years [8]. This difference in graft survival due to biliary complications remains a barrier to utilization of DCD allografts nationwide, leading the majority of DCD LT to be confined to a few centers in the United States.
Management of Biliary Complications
Endoscopic Therapy
Endoscopic management options for IC include endoscopic retrograde cholangiopancreatography (ERCP), percutaneous transhepatic cholangiogram (PTC), and surgical placement of a biliary stents, but the disease process is ultimately often irreversible. ERCP and PTC are useful in removing biliary sludge and casts from bile ducts, followed by balloon dilation of ischemic strictures and placement of plastic stents. However, IC raises challenges for balloon dilation as IC strictures are diffuse, bilobar, and tend to be intrahepatic [19]. Due to these treatment challenges, endoscopic therapy for IC remains first line management, although historically considered a bridge to inevitable retransplantation [20]. However, with advanced experience and understanding of DCD and IC, some centers have demonstrated that not all patients will require retransplantation [18•].
Retransplantation
The primary surgical management of DCD graft failure remains retransplantation. When DCD LT emerged in the United States in the late 1990s, the percentage of DCD LT recipients requiring retransplantation was over 25%, compared to less than 10% for DBD LT. As clinical experience with DCD LT has improved over the past two decades, rates of DCD and DBD retransplantation have converged at less than 5% [5]. This improvement is largely attributed to advancements in post-operative management of biliary complications via interventional radiology and advanced endoscopy approaches, as well as better recipient selection following the 2009 ASTS recommendations [11]. While the use of DCD vs. DBD at initial transplant does not appear to have a significant effect on patient or graft survival in retransplanted patients, patients relisted for IC without MELD exception had far worse waitlist survival [21]. In 2017, Croome et al. demonstrated that patients awaiting retransplantation in the setting of IC had worse survival than patients with all other indications for MELD exception scores [22]. These data motivated a change in practice by the National Liver Review Board (NLRB) to grant a MELD exception based on median MELD at transplant (MMaT) for patients relisted with the complication of IC.
Solutions and Innovations
Tissue Plasminogen Activator (tPA)
The use of tissue plasminogen activator (tPA) has been suggested to mitigate biliary complications in DCD LT. Hashimoto et al. demonstrated that back-table injection of tPA into the hepatic artery could reduce the rate of ischemic type biliary strictures (ITBS) by lysing microthrombi in the peribiliary vascular plexus [23]. These findings were supported in a retrospective study by Seal et al.; in addition to showing that tPA livers had improved 1- and 3-year patient and graft survival, the authors also found no increased risk of bleeding [24]. However, critics of this work point out that tPA is not biochemically effective in cold temperatures used for static cold organ storage. A potential solution to this problem is the use of ex-vivo machine perfusion (EVMP) as a platform to administer plasminogen and tPA into the hepatic artery of livers on pump, mitigating the recipient bleeding risk associated with systemically-administered tPA. Haque et al. showed that by instilling plasminogen into the perfusate of a normothermic machine perfusion system and then administering tPA into the hepatic artery of warm DCD livers on pump, markers of bile duct injury were reduced at 12 h of perfusion compared to non-tPA livers [25]. Even with these promising early findings, the long-term benefits of machine perfusion-based interventions on ITBS and biliary complications in DCD livers remain to be fully realized and the role of tPA continues to be debated.
Induction with Depleting Agents
Another possible approach to mitigating early graft failure in DCD LT is with the choice of induction therapy. Halldorson et al. showed that induction therapy with anti-thymocyte globulin (ATG) reduced rates of graft failure and IC, suggesting a novel mechanism beyond DWIT that may drive inflammation and fibrosis contributing to biliary complications [26]. This strategy has been employed by a number of centers with significant DCD experiences, and a recent study using the UNOS Dataset demonstrated improved survival for recipients of DCD LT allografts who received antibody induction [27]. Because the physiologic mechanism underlying this biliary pathology is not well characterized, one can only speculate an important role for immunomodulation which has yet to be better studied.
Normothermic Regional Perfusion
The logistics of withdrawal of life-sustaining treatment (WLST) pose a unique challenge to the success of DCD LT and the donation process. New innovations have been adopted to help mitigate allograft damage associated with the ischemic injury at WLST, followed by cold storage and CIT (Fig. 3). As CIT has been demonstrated to be a critical outcome-determining variable, minimizing this injury following WLST has been the focus. Normothermic regional perfusion (NRP) has been the most widely implemented innovation in Europe; although it was utilized in the early donor experience with Starzl before brain death was established, adoption in the US has been slow [28]. This technique involves using oxygenated normothermic blood to perfuse organs in situ. In the early 2000s, studies from the University of Michigan demonstrated improved organ yield, but IC remained prevalent in LT recipients [29]. With recent renewed interest in NRP, protocols have been developed and standardized to greatly expand the utilization of donors with advanced age, the extended DWIT, and graft steatosis [30, 31•]. As standard practice in Europe, NRP is run for 1–4 h prior to organ retrieval to allow reconditioning of the organ while minimizing end organ injury.
The time line from withdrawal of life sustaining treatment (WLST) to transplantation with the various technologies illustrated. Static cold storage compared to Normothermic Regional Perfusion compared to Normothermic Regional Pefusion plus Hypothermic oxygenated machine perfusion compared to direct Normothermic Machine Perfusion compared to back-to-base Normothermic Machine Perfusion compared to back-to-base Hypothermic Oxygenated machine Perfusion. WLST, Withdrawal of Life-Sustaining Treatment; SCS, Static Cold Storage; DWIT, Donor Warm Ischemia Time; NMP, normothermic machine perfusion; NRP, normothermic regional perfusion; HOPE, hypothermic oxygenated perfusion
The benefits of NRP in DCD LT outcomes are well-demonstrated. A recent Spanish study showed that using NRP in controlled DCD donor liver procurement reduced rates of biliary complications by 23% at a median follow-up of 20 months post-transplant [32]. A similar study from the UK showed a 20% reduction in anastomotic biliary strictures in DCD livers procured with the addition of post-mortem NRP [33]. Together, these data present a strong case for standard incorporation of NRP into DCD procurement practices in the United States. Because of the similarity of NRP to cardiopulmonary bypass or extracorporeal membrane oxygenation (ECMO), a number of groups have raised ethical concerns regarding NRP as a form of resuscitation. However, many US transplant centers and OPOs are gaining experience with this technology, and broader implementation will require multidisciplinary agreement between transplant surgeons, ethicists, donor families, and policy makers.
Ex Vivo Machine Perfusion
Machine perfusion is a highly dynamic modality that has the potential to redefine the practice of LT. Several studies have now shown the benefits of normothermic machine perfusion (NMP) on the liver allograft, specifically with respect to the repletion of glycogen stores [34], the upregulation of genes involved in repair and mitigating inflammation [35], and improvement in transplant outcomes [36•]. Furthermore, NMP allows real-time assessment of liver allograft function, as well as the use of pharmacologic interventions (eg. tPA, defatting cocktails) prior to reimplantation. The first randomized trial investigating the role of NMP demonstrated the benefits of machine perfusion in reducing injury associated with static cold storage (SCS). In this study, the outcomes for 34 DCD recipients with NMP were compared to 21 DCD recipients with SCS and demonstrated a reduction in IC from 26 to 11%; however this was not statistically significant. The recently published Organ Care System (OCS) PROTECT randomized clinical trial showed that the use of NMP in DCD LT significantly increased DCD liver donor utilization. The authors also showed OCS-perfused livers had lower rates of both ischemia reperfusion injury and ischemic biliary complications [37•].
Another perfusion modality, hypothermic (4-6C) oxygenated machine perfusion (HOPE), has been shown to reduce rates of IC [38•]. The studied mechanism of this effect is centered around mitochondrial reprogramming under hypothermic aerobic conditions not achieved in NMP, which prevents oxidative injury while still replenishing ATP. The mitigation of mitochondrial oxidative stress and downstream inflammation is postulated to protect against ischemia reperfusion injury (IRI), reducing rates of biliary injury [39]. The main disadvantage of HOPE compared to NMP is the limited ability to assess organ function and viability under hypothermic conditions.
Importantly, the use of perfusion technology is associated with reduced organ discard rates and increased use of DCD livers outside consensus-recommended parameters. Eden et al. evaluated DCD utilization practices and outcomes across the US, UK and 6 European countries, and found that in Italy and Switzerland, where perfusion protocols are well-established, discard rates were lower and grafts with WIT > 30 min were utilized frequently [40••]. In Spain, the majority of DCD livers are recovered with NRP, and willingness to utilize grafts outside the ILTS criteria is reflected in the consensus statement from Spanish Liver Transplant Society [41]. Taken together, use of NRP at time of procurement, followed by EVMP to assess viability and allow for reconditioning of DCD liver allografts, would not only increase DCD liver utilization but could drastically improve graft survival. Over time, increasing familiarity with these modalities will hopefully lessen the trepidation of using DCD livers.
Policy Changes
As the landscape of DCD LT evolves to become a more integral part of clinical practice, policies that govern the allocation of these organs will also be forced to evolve. The number of DCD LTs performed in the United States is on the rise, which will lead to a potentially higher number of patients relisted for LT secondary to IC. Early relisting for DCD graft failure caused by primary nonfunction (PNF) or hepatic artery thrombosis (HAT) is defined as relisting less than 2 weeks after transplant, while late relisting is defined as 2 weeks to 3 years after transplant. With the new guidance regarding relisting for patients with IC, the NLRB may consider more standardized and expedited access for patients who suffer from this complication.
Another future challenge lies in incorporating the benefits of organ-reconditioning technologies into risk models and outcome predictions. Currently, DCD status, donor age, and cold ischemia time are among the most heavily weighted coefficients in current modeling of transplant outcomes. With the increased utilization of DCD, increasing use of NRP and NMP, and the subsequent use of more marginal allografts from older donors with continuing improvement in outcomes, these factors will likey require adjustment. CIT measurements are currently not tracked separately, and procurement utilizing NMP often results in inaccurate documentation of prolonged CIT. Importantly, while these adjustments to predictive modeling are needed, they may inadvertently disadvantage programs without the resources to provide these technologies, thereby increasing regional disparities that already exist in transplantation.
Conclusions
In summary, the practice of DCD LT is rapidly changing as it has become increasingly established worldwide. With greater utilization and broader acceptance, the transplant community continues to lack standardization in practice, and even common terminology – despite numerous efforts to achieve consensus. NRP has yet to be adopted in the United States to the extent that it has been in Europe, representing an untapped opportunity to improve DCD outcomes. HOPE and NMP are also modalities that can be used to recondition DCD livers, and in the case of NMP, allow for viability testing prior to transplantation. These technologies are likely to provide significant improvements in utilization of DCD livers, reducing waitlist mortality and improving post-transplant patient and graft survival. With these advances, policy changes will have to be enacted to protect higher risk DCD allograft recipients, balanced with reassessment of quality metrics and predictive modeling algorithms. Ultimately, despite the risk of biliary complications, DCD liver allografts in today's age represent a great opportunity to expand the donor pool of transplantable organs and offer hope to patients awaiting LT.
Abbreviations
- CIT:
-
Cold ischemia time
- DBD:
-
Donation after brain death
- DCD:
-
Donation after circulatory death
- ERCP:
-
Endoscopic retrograde cholangiopancreatography
- EVMP:
-
Ex vivo machine perfusion
- HOPE:
-
Hypothermic oxygenated machine perfusion
- ITBS:
-
Ischemic type biliary strictures
- IC:
-
Ischemic cholangiopathy
- LT:
-
Liver transplantation
- MMaT:
-
Median MELD at transplant
- NRP:
-
Normothermic regional perfusion
- NMP:
-
Normothermic machine perfusion
- PTC:
-
Percutaneous transhepatic cholangiogram
- SCS:
-
Static cold storage
- Tpa:
-
Tissue plasminogen activator
- fWIT:
-
Functional warm ischemia time
- WIT:
-
Warm ischemia time
- WLST:
-
Withdrawal of life supporting treatment
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Thuong M, Ruiz A, Evrard P, et al. New classification of donation after circulatory death donors definitions and terminology. Transpl Int Off J Eur Soc Organ Transplant. 2016;29(7):749–59.
Foley DP, Fernandez LA, Leverson G, et al. Donation after cardiac death: the University of Wisconsin experience with liver transplantation. Ann Surg. 2005;242(5):724–31.
Skaro AI, Jay CL, Baker TB, et al. The impact of ischemic cholangiopathy in liver transplantation using donors after cardiac death: the untold story. Surg. 2009;146(4):543–52 (discussion 552–553).
Jay C, Ladner D, Wang E, et al. A comprehensive risk assessment of mortality following donation after cardiac death liver transplant - an analysis of the national registry. J Hepatol. 2011;55(4):808–13.
Haque O, Yuan Q, Uygun K, Markmann JF. Evolving utilization of donation after circulatory death livers in liver transplantation: The day of DCD has come. Clin Transplant. 2021;35(3): e14211.
Mathur AK, Heimbach J, Steffick DE, Sonnenday CJ, Goodrich NP, Merion RM. Donation after cardiac death liver transplantation: predictors of outcome. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2010;10(11):2512–9.
Cappucci SP, Smith WS, Schwartzstein R, White DB, Mitchell SL, Fehnel CR. End-Of-Life Care in the Potential Donor after Circulatory Death: A Systematic Review. The Neurohospitalist. 2023;13(1):61–8.
Haque OJ, Roth EM, Fleishman A, Eckhoff DE, Khwaja K. Long-Term Outcomes of Early Experience in Donation After Circulatory Death Liver Transplantation: Outcomes at 10 Years. Ann Transplant. 2021;26: e930243.
Mateo R, Cho Y, Singh G, et al. Risk factors for graft survival after liver transplantation from donation after cardiac death donors: an analysis of OPTN/UNOS data. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2006;6(4):791–6.
Croome KP, Taner CB. The Changing Landscapes in DCD Liver Transplantation. Curr Transplant Rep. 2020;7(3):194–204.
Reich DJ, Mulligan DC, Abt PL, et al. ASTS recommended practice guidelines for controlled donation after cardiac death organ procurement and transplantation. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2009;9(9):2004–11.
Andrews PA, Burnapp L, Manas D. British Transplantation Society. Summary of the British Transplantation Society guidelines for transplantation from donors after deceased circulatory death. Transplant. 2014;97(3):265–70.
Braat AE, Blok JJ, Putter H, et al. The Eurotransplant donor risk index in liver transplantation: ET-DRI. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2012;12(10):2789–96.
Kalisvaart M, Croome KP, Hernandez-Alejandro R, et al. Donor Warm Ischemia Time in DCD Liver Transplantation-Working Group Report From the ILTS DCD, Liver Preservation, and Machine Perfusion Consensus Conference. Transplant. 2021;105(6):1156–64.
Schlegel A, van Reeven M, Croome K, et al. A multicentre outcome analysis to define global benchmarks for donation after circulatory death liver transplantation. J Hepatol. 2022;76(2):371–82.
Croome KP, Lee DD, Perry DK, et al. Comparison of longterm outcomes and quality of life in recipients of donation after cardiac death liver grafts with a propensity-matched cohort. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2017;23(3):342–51.
Laing RW, Scalera I, Isaac J, et al. Liver Transplantation Using Grafts From Donors After Circulatory Death: A Propensity Score-Matched Study From a Single Center. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2016;16(6):1795–804.
Croome KP, Mathur AK, Aqel B, et al. Classification of Distinct Patterns of Ischemic Cholangiopathy Following DCD Liver Transplantation: Distinct Clinical Courses and Long-term Outcomes From a Multicenter Cohort. Transplantation. 2022;106(6):1206–1214. This article describes the spectrum of ischemic cholangiopathy patterns and clarifies that not all instances of cholangiopathy result in graft failure.
Tung BY, Kimmey MB. Biliary complications of orthotopic liver transplantation. Dig Dis Basel Switz. 1999;17(3):133–44.
Mourad MM, Algarni A, Liossis C, Bramhall SR. Aetiology and risk factors of ischaemic cholangiopathy after liver transplantation. World J Gastroenterol. 2014;20(20):6159–69.
Allen AM, Kim WR, Xiong H, et al. Survival of recipients of livers from donation after circulatory death who are relisted and undergo retransplant for graft failure. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2014;14(5):1120–8.
Croome KP, Lee DD, Nguyen JH, Keaveny AP, Taner CB. Waitlist Outcomes for Patients Relisted Following Failed Donation After Cardiac Death Liver Transplant: Implications for Awarding Model for End-Stage Liver Disease Exception Scores. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2017;17(9):2420–7.
Hashimoto K, Eghtesad B, Gunasekaran G, et al. Use of tissue plasminogen activator in liver transplantation from donation after cardiac death donors. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2010;10(12):2665–72.
Seal JB, Bohorquez H, Reichman T, et al. Thrombolytic protocol minimizes ischemic-type biliary complications in liver transplantation from donation after circulatory death donors. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2015;21(3):321–8.
Haque O, Raigani S, Rosales I, et al. Thrombolytic Therapy During ex-vivo Normothermic Machine Perfusion of Human Livers Reduces Peribiliary Vascular Plexus Injury. Front Surg. 2021;8: 644859.
Halldorson JB, Bakthavatsalam R, Montenovo M, et al. Differential rates of ischemic cholangiopathy and graft survival associated with induction therapy in DCD liver transplantation. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2015;15(1):251–8.
Ig-Izevbekhai K, Goldberg DS, Karp SJ, Foley DP, Abt PL. Immunosuppression in Donation After Circulatory Death Liver Transplantation: Can Induction Modify Graft Survival? Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2020;26(9):1154–66.
Starzl TE, Groth CG, Brettschneider L, et al. Orthotopic homotransplantation of the human liver. Ann Surg. 1968;168(3):392–415.
Magliocca JF, Magee JC, Rowe SA, et al. Extracorporeal support for organ donation after cardiac death effectively expands the donor pool. J Trauma. 2005;58(6):1095–101 (discussion 1101–1102).
Hunt F, Johnston CJC, Coutts L, et al. From Haphazard to a Sustainable Normothermic Regional Perfusion Service: A Blueprint for the Introduction of Novel Perfusion Technologies. Transpl Int Off J Eur Soc Organ Transplant. 2022;35:10493.
Hessheimer AJ, de la Rosa G, Gastaca M, et al. Abdominal normothermic regional perfusion in controlled donation after circulatory determination of death liver transplantation: Outcomes and risk factors for graft loss. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2022;22(4):1169–1181. This manuscript presents one of the largest experiences from the Spanish transplant registry, demonstrating superior outcomes.
Hessheimer AJ, Coll E, Torres F, et al. Normothermic regional perfusion vs. super-rapid recovery in controlled donation after circulatory death liver transplantation. J Hepatol. 2019;70(4):658–65.
Watson CJE, Hunt F, Messer S, et al. In situ normothermic perfusion of livers in controlled circulatory death donation may prevent ischemic cholangiopathy and improve graft survival. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2019;19(6):1745–58.
Mergental H, Perera MTPR, Laing RW, et al. Transplantation of Declined Liver Allografts Following Normothermic Ex-Situ Evaluation. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2016;16(11):3235–45.
Jassem W, Xystrakis E, Ghnewa YG, et al. Normothermic Machine Perfusion (NMP) Inhibits Proinflammatory Responses in the Liver and Promotes Regeneration. Hepatol Baltim Md. 2019;70(2):682–95.
Nasralla D, Coussios CC, Mergental H, et al. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557(7703):50–56. This paper reports the first randomized controlled trial using ex vivo normothermic perfusion in liver transplantation.
Markmann JF, Abouljoud MS, Ghobrial RM, et al. Impact of Portable Normothermic Blood-Based Machine Perfusion on Outcomes of Liver Transplant: The OCS Liver PROTECT Randomized Clinical Trial. JAMA Surg. 2022;157(3):189–198. This paper rerports the first american randomized trial using normothermic perfusion in liver transplantation.
Czigany Z, Schöning W, Ulmer TF, et al. Hypothermic oxygenated machine perfusion (HOPE) for orthotopic liver transplantation of human liver allografts from extended criteria donors (ECD) in donation after brain death (DBD): a prospective multicentre randomised controlled trial (HOPE ECD-DBD). BMJ Open. 2017;7(10):e017558. This paper reports the first randomized trial using hypothermic machine perfusion in liver transplantation.
Schlegel A, Porte R, Dutkowski P. Protective mechanisms and current clinical evidence of hypothermic oxygenated machine perfusion (HOPE) in preventing post-transplant cholangiopathy. J Hepatol. 2022;76(6):1330–47.
Eden J, Sousa Da Silva R, Cortes-Cerisuelo M, et al. Utilization of livers donated after circulatory death for transplantation - An international comparison. J Hepatol. 2023;78(5):1007–1016. This excellent international comparison outlines some of the challenges that impede broader utilization of DCD.
Hessheimer AJ, Gastaca M, Miñambres E, Colmenero J, Fondevila C. in representation of the SETH Working Group on DCD. Donation after circulatory death liver transplantation: consensus statements from the Spanish Liver Transplantation Society. Transpl Int Off J Eur Soc Organ Transplant. 2020;33(8):902–16.
Funding
The authors have nothing to disclose.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the review of the literature, writing of the manuscript and final review and approval of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Human and animals rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Disclosures
None of the authors have any relevant potential conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Haque, O.J., Roth, E.M. & Lee, D.D. Modern-Day Practice of DCD Liver Transplantation: Controversies, Innovations, and Future Directions. Curr Gastroenterol Rep 25, 413–420 (2023). https://doi.org/10.1007/s11894-023-00902-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11894-023-00902-8