Abstract
Within the last decade, liver transplantation from DCD donors has gained interest and outcomes have improved, mainly due to an increased awareness of the overall risk and subsequent selection policy. Although most countries have steadily pushed their donor risk factor boundaries, the overall utilization rate of DCD livers remains suboptimal. The repeat occurrences of DCD-specific complications, difficult to reduce further, are highlighted as one reason behind such advancement standstill, despite the continuous technical, medical, and anesthesiologic innovation.
This chapter highlights most outcome measures after DCD liver transplantation including graft and patient survival with a specific focus on biliary, vascular, and renal complications. Overall graft and donor risk is discussed in context of liver reperfusion injury and graft function with the development of specific complications. Finally, suggestions on how to assess and report outcomes in a more standardized and transparent way are provided to further improve our results in the future.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
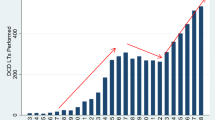
Liver transplantation has progressed from an experimental status to a standard treatment for end-stage liver disease and malignant liver lesions [10]. In addition to the ongoing improvement of surgical techniques, anesthesiologic and medical management, as well as donor and graft assessment, more livers from extended criteria donors (ECD) are frequently accepted. Liver transplantation from donation after circulatory death donors (DCD) was recently shown to be more beneficial compared to prolonged waiting for a presumably better DBD liver in the United Kingdom (UK) [111]. In the past decade, many countries have implemented a DCD liver transplant program (Fig. 10.1), which led to an increasing number of retrospective single-center or cohort studies, based on pooled national data (Table 10.1). Despite this success story, the utilization rate of DCD livers remain quite poor in many countries [69, 74, 83, 107, 114]. In order to better understand the overall donor and recipient risk, new tools were defined to suggest thresholds when to decline a certain donor-recipient combination in context of a predicted impaired outcome [39, 55, 103, 104]. However, which survival and complication rates to accept depends also on the number of available organs and the risk a center or country is willing to accept [18, 69].
Donors after circulatory death registered worldwide in 2018. (a) DCD donors in 2018 (PMP: per million population per country). (b) Percent DCD donors in relation to all deceased donors, which underwent procurement surgery. PMP per million population, DCD donation after circulatory death, UK United Kingdom, USA United States of America. (Based on data from annual report 2018: www.irodat.org)
In this chapter, we describe current outcomes reported after liver transplantation from controlled DCD donors with a specific focus on graft and patient survival, liver function, and biliary complications. In addition, we highlight the impact of DCD liver transplantation on other organ systems, including the kidneys, and we describe the rate of acute and chronic rejections. Finally, new tools to transparently quantify overall complications are presented including suggestions on how to improve outcomes after DCD liver transplantation further in the future.
Graft and Patient Survival
The proportion of DCD donors has grown in recent years and ranges presently between 5% and 50% of the total deceased donors (Fig. 10.1). Leading countries are, for example, Spain, the UK, Belgium, and the Netherlands, where DCD donors represented more than 50% of all deceased donors in 2018 (Fig. 10.1) [42, 52]. The higher overall number of DCD transplantations was mainly found due to an increased number of available donors, while the utilization rate remained largely stable in the last few years. Although experienced centers have improved their outcomes in DCD liver transplantation with modified techniques and a strict selection policy, the overall results have however plateaued within the last years, without further reduction of DCD-specific complications in context of standard cold storage liver preservation (Table 10.1) [74, 85].
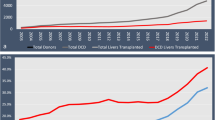
Most studies are of retrospective, single-center design and report 3-year outcomes (Table 10.1) [85]. The reported 5-year overall graft and recipient survival fluctuated between 54.4–79.5% and 68–88%, respectively (Table 10.1, Figs. 10.2, 10.3, and 10.4). Although most studies, which used pooled registry data, showed a generally inferior survival with DCD livers compared to DBD transplants, single-center analyses have also demonstrated comparable outcomes (Table 10.1) [22, 29, 34, 39, 40, 62, 111]. Such different results are largely based on the heterogeneity of risk among centers “pooled together” in large databases and the individual donor and graft risk accepted in each center and country. Specialized, large volume centers, for example, achieved excellent outcomes with a 5-year graft survival of almost 80% already in earlier years [22, 75]. The limitation of donor risk factors and a standardized organ retrieval practice with, for example, a short donor hepatectomy time and cold storage have contributed to such good outcomes [9, 22, 26, 55, 59]. The introduction of national guidelines has led to an increased utilization of livers from “good” DCD donors and subsequent excellent graft and patient survival rates, for example, in the UK or the United States of America (USA) (Figs. 10.2 and 10.3) [2, 8, 18, 26, 33, 83, 111].
Reported frequency of complications and survival rates after adult liver transplantation from controlled DCD donors. AKI acute kidney injury (overall mix of all severities), AS anastomotic stricture, CKD chronic kidney disease (overall rate reported, majority within 5 years), CR chronic rejection, DCD donation after circulatory death, HAT hepatic artery thrombosis, IC ischemic cholangiopathy (includes also nonanastomotic strictures, excluding HAT-related features), PNF primary nonfunction, 5 y five years. The frequency is reported as range and based on the most recent literature from the past 10 years
In context of the rather inhomogeneous follow-up in most studies together with the gradual loss of liver recipients at risk after transplant surgery, the literature information on 10-year survival rates are limited (Table 10.1). Only two retrospective, cohort studies reported on long-term graft and patient survivals of 43–44% and 54–56% after 10 years, respectively [5, 7]. Despite the difficulties to generally interpret various outcomes found in multiple studies, the higher adjusted odds ratio (OR) consistently reported for graft loss following DCD liver transplantation remains, considering a well-mixed donor and recipient risks combination as summarized in a recent meta-analysis [72, 85, 116].
In order to identify unfavorable donor-recipient risk combinations, the UCLA group was the first to suggest a prognostic scoring system with the aim to define cutoff values for risk factors to enable clinicians to decide whether to accept a certain donor and recipient combination [38]. Further scores were developed in the UK, for example, based on the King’s College DCD transplant cohort or the national DCD liver transplant cohort [55, 104]. Such models identified low-risk or “good quality” DCD livers, which led to excellent graft survival rates of more than 80% after 5 years, when respecting a balance between donor and recipient risk factors [10, 81, 82].
Pediatric DCD Recipients
Utilization of DCD liver grafts in the pediatric recipient population remains controversial, with a limited number of outcome studies available [3]. However, in context of a strict selection policy, current data support the use of pediatric DCD grafts in children. Experience with DCD donors appears crucial to achieve good results in this cohort, as demonstrated by excellent results from single centers [95]. The team from UCLA has demonstrated equivalent long-term results comparing pediatric DCD grafts and other variants in children, including partial grafts (Segment II and III) from living or deceased donors in 2009 [37]. Such earlier results were recently supported by a UNOS database analysis, where 57 pediatric DCD liver recipients achieved comparable survival rates as with DBD grafts [41].
Liver Function
Through risk minimization, the primary nonfunction (PNF) rate has significantly reduced in recent years and ranges between 0% and 6.5% following controlled DCD liver transplants (Table 10.1, Fig. 10.4) [7, 23, 24, 27, 30, 73, 92]. Although no clear cutoff when to decline a certain donor-recipient risk combination is available, there is a general consensus to limit the donor warm ischemia and the cold storage for DCD liver grafts [18, 63, 74]. Please see also Chap. 7.
In contrast to the clear PNF definition, the occurrence of any sort of impaired liver function or dysfunction is more difficult to capture and frequently found in DCD liver recipients. Olthoff et al. have therefore developed the formula for early allograft dysfunction (EAD) – which includes parameters for graft injury (quantified by liver enzyme release: alanine or aspartate aminotransferase of >2000 U/L) and elevated liver function tests assessed on day 7 after transplantation, including the coagulation parameter INR (≥1.6) and bilirubin (≥10 mg/dl) [64, 88, 98]. EAD following DCD liver transplantation is covered in Chap. 11.
Biliary Complications
Despite the improved medical treatment and surgical technique with a better awareness of risk transmitted with a DCD liver, one main cause of graft loss and subsequent patient death remains with biliary complications. The reported rate of 18–51% overall biliary complications depends on the follow-up duration and includes anastomotic strictures (AS), ischemic cholangiopathy (IC), bile leaks, and other types, such as biliary casts and stones found in the biliary tree [4, 12, 45, 53, 122]. IC is covered in detail in Chap. 12. While the majority of anastomotic strictures can be addressed through endoscopic ballooning and stent placement by an expert endoscopist, hilar strictures and intrahepatic abscesses appear more difficult to treat successfully with a conservative approach [1, 54]. Enormous variations have been reported regarding the type, location, and clinical impact of ICs (Table 10.1) [12, 31, 89]. Additionally, the clinical picture of IC appears very different and may range individually from episodes of elevated parameters of cholestasis to repeat diagnostic procedures including endoscopic retrograde cholangiopancreatography (ERCP) and percutaneous transhepatic cholangiography (PTC) with stent or drain placement to rotating antibiotics and retransplantation. The clinical consideration and reporting of ICs differ significantly in the literature with the lack of a uniform clinical classification, which could provide an overview of clinically relevant ischemic strictures and link different levels of donor, graft, and recipient risk profiles [85]. The IC rate following controlled DCD liver transplantation has been reported between 2.6% and 34% in the past decade (Table 10.1) [21, 22, 30, 33, 46, 62, 99].
Multiple factors were nominated to contribute to the development of biliary complications and include the entire spectrum of donor and graft parameters, procurement surgery, preservation, and implantation [52, 85, 104]. The majority of risk factors are simply given by the donor situation, which has led to a selective policy on how to allocate DCD livers best to a certain recipient, which is one main policy clinically applied to reduce biliary complications in context of standard cold storage preservation (Table 10.1) [22, 32, 59]. An increasing body of literature is available to understand the multifactorial pathogenesis of such ischemic strictures [53]. Several factors, including vessel patency, cumulative donor risk with subsequent level of ischemia reperfusion injury, potential cytomegaly virus infection, chronic rejection, ABO incompatibility, and other toxic factors, have been discussed as contributors [74]. The level of reperfusion injury in the large compound of hepatocytes in the liver triggers a toxic composition of the early bile, initially produced after reperfusion, which injures the vulnerable biliary epithelium further, enhancing the reperfusion injury in such sensitive cells [12, 84, 106, 122]. In this context, any additional episode of warm hepatic ischemia throughout the liver pathway from the donor to implantation will contribute to harmful damage [74]. The duration of donor hepatectomy time and implantation time has therefore been assessed with impact on complication rates following DCD liver transplantation [11, 26, 44, 55, 94]. Another “wheel to adjust” is the liver preservation, and novel perfusion concepts are currently evaluated with their potential impact on the occurrence of biliary complication and subsequent graft loss [36, 61, 79, 105, 120].
Vascular Complications
Posttransplant issues with vascular structures are frequently underreported in the field of DCD liver transplantation, because most analyses focus on biliary complications and graft or patient survival (Table 10.1) [8, 19, 21, 25, 27, 62, 102]. In context of advanced reperfusion injury with subsequent higher inotrope requirements, DCD liver grafts may have a higher degree of stiffness which promotes the development of arterial complications further, including hepatic artery thrombosis (HAT) [19, 20, 109]. Data on such additional issues appear scarce, and the reported HAT rate is found between 0% and 7.7% (Table 10.1) [8, 21, 24, 25, 28, 33, 59, 62, 110].
Despite the general lack of information in the literature, recipients with an unfavorable underlying disease may be exposed to an even higher risk of vascular complications, for example, primary sclerosing cholangitis (PSC) or autoimmune hepatitis, where the additional pro-coagulative status and higher immune system activation contribute further to arterial complications [113]. The rate of later hepatic artery stenosis (HAS) appears with 2.7–10% similarity when compared to the HAT rate in the currently available literature (Table 10.1) [19, 62, 115].
Relevant portal or hepatic vein occlusions are very rare and therefore often not reported. For example, only two retrospective studies showed the rate of venous complications, including portal vein thrombosis or hepatic vein obstruction, which ranged between 1.1% and 2.6% (Table 10.1) [25, 27, 115].
Acute and Chronic Kidney Injury
Acute kidney injury (AKI) following liver transplantation is inconsistently reported as all other outcome measures. The increased use of riskier DCD livers is paralleled by a relatively high overall AKI rate between 12% and 81% [24, 48, 49, 62, 64, 67, 112]. Of particular impact on the reported rate of renal complications are, for example, various criteria applied to define AKI, the severity, and the indication to implement renal replacement therapy (RRT) comparing different transplant centers [57, 66, 86]. The occurrence of AKI was recently linked with a higher risk of mortality after liver transplantation in a meta-analysis [112].
The interest in a more specific analysis of AKI and underlying causes has recently evolved, and reports identified about 15–40% DCD recipients with severe AKI grade 2 and 3, where between 16% and 40% require RRT [24, 51, 62, 67, 92, 102]. The AKI rate is also significantly higher in DCD liver transplants when compared to good DBD liver grafts [24, 67]. However, implantation of extended DBD livers (ECD) with, for example, advanced donor age, donor BMI, cold storage, or steatosis was also shown to induce higher AKI rates [66, 101]. Such findings parallel other publications, in which more severe liver reperfusion injury has been shown to be a driver of development of AKI [65, 93]. A higher rate of post-reperfusion syndrome with lower mean arterial pressures (MAP) and higher cardiovascular support was also shown to be related to the severity of AKI [47]. Wadei et al. have finally demonstrated the link between reperfusion injury-related EAD development and the presence of AKI in context of DCD liver transplantation [117, 118]. And human kidneys significantly contribute to the clearance of reperfusion injury-related circulating cytokines, following liver transplantation as shown by many [80, 93]. The higher AKI frequency in DCD transplantation with the link to an impaired outcome has further supported the selective policy with regard to donor and recipient risk factors and the early introduction of medical preventive treatment in the early posttransplant phase [43, 50, 66]. Centers ideally aim to limit the duration of donor warm ischemia time and allocate DCD grafts to rather fit recipients without hepatorenal syndrome and able to cope with potential reperfusion injury [49, 50]. Moreover, renal-sparing immunosuppression is the preferred regimen in many centers and includes, for example, induction therapy with basiliximab in combination with a delayed introduction of calcineurin inhibitors (CNI) to protect kidneys following liver transplantation [13, 49].
In context of an overall longer recipient follow-up today with improved survival, chronic and long-term complications are more in focus. The cumulative incidence of severe CKD with end-stage renal failure (ESRF) was shown to increase up to almost 25% within 10 years after liver transplantation. This was, however, in earlier days when traditional immunosuppressive regiments with higher through levels were used [87].
The severity of AKI was recently found to predict the later development of chronic kidney disease (CKD) [50]. Five years after liver transplantation, more than one-third of recipients present with signs of CKD (25–54%), while severe CKD with ESRF remains rare with only 1–2% [50, 66, 67].
In addition to the initial development of severe AKI, which was shown to predict later CKD (1.8-fold increased risk), other factors have impact on impaired kidney function 5–10 years after LT, including immunosuppression and cardiovascular or renal diseases. This was further underlined by the fact that most liver recipients with AKI recover from the initial renal hit, and Kalisvaart et al. did not find any differences in the development of CKD comparing different grafts types, such as good or marginal DBD and DCD livers [50]. Very high plasma through levels of calcineurin inhibitors were shown to impact on the early development of CKD [97]. Please see further information regarding renal complications after DCD liver transplantation in Chap. 11.
Acute and Chronic Rejection
Immunosuppression (IS) regimens have changed enormously within the last 20 years, not only based on renal complications but also cancer development and infections. The higher awareness of such drug-related long-term complications has led to an overall decrease in through levels and the introduction of new combinations of different drugs. Despite such modifications, the overall incidence of acute cellular rejection (ACR) following liver transplantation has steadily decreased and is currently reported with 10% [13, 58].
Pronounced reperfusion injury has been previously linked to a higher rejection rate in solid organ transplantation. Results comparing DCD and DBD liver transplants remain therefore controversial, and some studies reported higher ACR rates when DCD livers are utilized [29, 105]. The overall rate of ACR episodes is currently reported between 0% and 61% in the setting of DCD liver transplantation (Table 10.1, Fig. 10.4) [13, 24, 59, 75, 91, 102]. However, as seen with any other complications, such a wide range of frequencies is based on multiple contributing factors, including donor risk, level of reperfusion injury, type of immunosuppression, and other parameters related to center practice and the time window of observation after transplantation.
Some authors, for example, highlight exclusively the number of treated rejections, where the type and dosage of medical treatment appear difficult to identify [102] and true rates of ACR remain underreported. Younger recipients with an active immune system or transplant candidates with autoimmune liver disease are more prone to experience ACR episodes. Such increased immune response seems to be even more evident in DCD transplants and further increased through an elevated reperfusion injury [6, 13, 100]. The majority of DCD liver recipients are effectively treated with a dual or triple combination today [13, 15]. By far, not all experienced transplant centers add an induction therapy routinely [13, 62]. Halldorson et al. have assessed the impact of basiliximab compared to ATG induction and found similar acute rejection rates of 21% and 22% in a small DCD liver cohort [35].
Compared to other solid organs, ACR in liver transplantation is of less importance, because some studies showed a protective effect of ACR episodes with regard to graft survival [96]. Future research will identify more tailored immunosuppressive regimen with the aim for a significant drug reduction to achieve operational tolerance and complete withdrawal.
Chronic rejections with subsequent graft loss were reported with an equally low rate of 0.8–3.1% following DCD liver transplants when compared to DBD grafts [8, 24, 26, 34, 73, 75, 91]. And with today’s immunosuppressive regimen, very limited chronic rejection rates are seen in children receiving DCD liver transplants, as shown by a recent report from the Netherlands [95].
Tumor Recurrence in Context of DCD Liver Transplantation
With regard to recurrence rates of underlying recipient diseases or hepatocellular carcinoma (HCC), the available literature remains limited. The overall HCC recurrence rate was found between 10% and 15% [16, 17, 56]. Such results from experienced centers and the variation in recurrence rates seen point to other factors with impact, including the cumulative donor and graft risk, the tumor load and activity, and the vascular invasion. The recurrence risk after DCD liver transplantation has been presented based on subgroup analyses, where the initial tumor load in the recipient was inside Milan criteria [17, 70]. Many centers are currently extending their acceptance criteria for HCC. The reported impact of DCD livers on outcomes in this cohort appears therefore inconsistent and may require new analysis in the future. Studies on the HCC recurrence following DCD liver transplantation with similar donor and recipient risk have demonstrated different results. For example, in 2013, Croome et al. have demonstrated inferior survival rates found in DCD liver recipients with an HCC in the large UNOS database until 2011. This report was followed by another study 2 years later from Mayo Clinic , Florida, with opposite findings and similar recurrence rates found in DCD compared to DBD liver recipients [16, 17]. Such results were however paralleled by a paper from King’s College, London, where authors showed similar survivals in HCC candidates transplanted with DBD or DCD livers [56]. Both studies included a rather low cumulative donor risk with an overall good recipient survival. Another recent assessment of the impact of graft quality on recurrence rate in the UK did not support earlier results, where, for example, Nagai et al. showed a higher recurrence rate in liver transplantations with prolonged cold ischemia times [78, 119]. Others reported a link between reperfusion injury and higher recurrence rates also triggered by an inflammatory milieu in the gut [60, 90]. The Hongkong group has provided a summary on underlying mechanisms leading to the perfect environment for cancer cells to migrate and regrow in the newly implanted liver, which include all features of reperfusion injury [68]. Such limitation of donor risk and reperfusion injury through new preservation technology may mitigate the HCC recurrence, where future studies are urgently required.
Assessment of Cumulative Complications
Reported frequencies of single complications appear somewhat difficult to interpret and should always be seen in context of the overall donor and recipient risk. The majority of complications as summarized in Table 10.1 and Fig. 10.4 are routinely presented in percent and with several confounding factors. Slankamenac et al. have therefore developed a new metric system to better quantify complications. Authors present this new tool, the comprehensive complication index (CCI), which serves as novelty to assess the median of complications following any type of surgical procedure. Such model was recently applied in DBD and DCD liver transplants and demonstrated an overall median CCI during hospital stay and at 6 months of 38.2 and 53.4 points, respectively, on an overall scale between 0 and 100, where 100 points represent recipient death [46, 108]. During hospital stay, the CCI was comparable to DBD liver transplantations, while through further follow-up, the DCD cohort experienced more complications, summarized by a higher CCI at 6 months [46]. Other reports from Canada demonstrated similar in-hospital complication rates with a mean CCI of 28.2 points, which was slightly higher compared to transplants from living donors or other DBD grafts [59]. This new tool has been recently used in multiple surgical disciplines to assess outcomes and enables comparative analyses between surgical procedures, centers, national cohorts, and even single surgeons [14].
How to Report and Improve Outcomes Further?
The majority of outcome reports rely on retrospective analyses from single center or national cohort studies, with either specific risk profiles or large volumes of missing data in pooled cohorts. In this context, future analyses should aim for international data collection with inclusion of most relevant outcomes and the CCI. A benchmarking-type analysis with DCD liver transplants is therefore currently performed, where results from most cases transplanted in all Western countries are included. Such benchmarking concept appears not new but has previously defined valid reference values for most outcome measures in DBD liver transplantation, where the impact of new technology and the results from large randomized controlled trials can be compared with [76].
The overall donor and recipient risk a specific country, center, or surgeon is willing to accept depends also on national regulations and the internal and external support a center receives. A more uniform donor and recipient risk factor application with subsequent development of general thresholds would be of importance to compare results, and the consensus conference planned for 2020 will possibly develop some guidelines.
Novel machine perfusion technology is currently improved and tested in the clinical setting of liver transplantation and in other solid organs. Results expected from various randomized controlled trials are awaited and will possibly impact on future applications. Importantly, viability criteria are currently developed for various types of cold and warm in situ and ex situ perfusion strategies to increase the generally poor utilization rate and safety of extended DBD and DCD donor liver transplants [71, 77, 121]. Future prediction models will therefore retain not only donor and recipient risk factors but also capture the metabolic liver assessment to more accurately predict outcomes and the risk for certain complications prior to decision-making whether to utilize a graft or not.
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- AUC:
-
Area under the receiver operating characteristic curve statistic
- BAR score:
-
Balance of risk score
- BMI:
-
Body mass index
- CIT:
-
Cold ischemia time
- CVA:
-
Cerebrovascular accident
- DAA:
-
Direct-acting antiviral medications
- DBD:
-
Donation after brain death
- DCD:
-
Donation after circulatory death
- DCD-RI:
-
DCD-Risk Index
- DM:
-
Diabetes mellitus
- DRI:
-
Donor Risk Index
- EAD:
-
Early allograft dysfunction
- ECMO:
-
Extracorporeal membrane oxygenation
- ERCP:
-
Endoscopic retrograde cholangiopancreatography
- fDWIT:
-
functional donor warm ischemia time
- GDA:
-
Gastroduodenal artery
- GGT:
-
Gamma-glutamyl-transferase
- HAS:
-
Hepatic artery stenosis
- HAT:
-
Hepatic artery thrombosis
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- HMP:
-
Hypothermic machine perfusion
- HOPE:
-
Hypothermic oxygenated perfusion
- IC:
-
Ischemic cholangiopathy
- ICU:
-
Intensive care unit
- IT:
-
Implantation time
- KCH:
-
King’s College Hospital
- MELD:
-
Model of End Liver Disease
- NHS:
-
National Health Service
- NHSBT:
-
National Health Service Blood and Transplant
- NMP:
-
Normothermic machine perfusion
- NRP:
-
Normothermic regional perfusion
- OLT:
-
Orthotopic liver transplantation
- PNF:
-
Primary nonfunction
- PTC:
-
Percutaneous transhepatic cholangiography
- ROS:
-
Reactive oxygen species
- UHB:
-
University Hospitals Birmingham
- UK:
-
United Kingdom
- UK-DCD-Risk Score:
-
United Kingdom Donation After Circulatory Death Risk Score
- UKELD:
-
United Kingdom Model of End-Stage Liver Disease
- UNOS:
-
United Network of Organ Sharing
- USA:
-
United States of America
References
Abbass AA, et al. Biliary complications after orthotopic liver transplantation from donors after cardiac death: broad spectrum of disease. Transplant Proc. 2010;42(9):3392–8. https://doi.org/10.1016/j.transproceed.2010.07.099.
Andrews PA, Burnapp L, Manas D. Summary of the british transplantation society guidelines for transplantation from donors after deceased circulatory death. Transplantation. 2014; https://doi.org/10.1097/01.TP.0000438630.13967.c0.
Angelico R, et al. Donation after circulatory death in paediatric liver transplantation: current status and future perspectives in the machine perfusion era. Biomed Res Int. 2018; https://doi.org/10.1155/2018/1756069.
Axelrod DA, et al. National assessment of early biliary complications following liver transplantation: incidence and outcomes. Liver Transpl. 2014; https://doi.org/10.1002/lt.23829.
Bellingham JM, et al. Donation after cardiac death: a 29-year experience. Surgery. 2011; https://doi.org/10.1016/j.surg.2011.07.057.
Berger M, et al. Immunologic challenges in small bowel transplantation. Am J Transplant. 2012; https://doi.org/10.1111/j.1600-6143.2012.04332.x.
Blok JJ, et al. Longterm results of liver transplantation from donation after circulatory death. Liver Transpl. 2016; https://doi.org/10.1002/lt.24449.
Bohorquez H, et al. Safety and outcomes in 100 consecutive donation after circulatory death liver transplants using a protocol that includes thrombolytic therapy. Am J Transplant. 2017; https://doi.org/10.1111/ajt.14261.
Boteon A, et al. Retrieval practice or overall donor and recipient risk – what impacts on outcomes after DCD liver transplantation in the United Kingdom? Liver Transpl. 2019;25:545.
Briceño J, Ciria R, De La Mata M. Donor-recipient matching: myths and realities. J Hepatol. 2013;58(4):811–20. https://doi.org/10.1016/j.jhep.2012.10.020.
Buchholz BM, et al. Revascularization time in liver transplantation: independent prediction of inferior short- and long-term outcomes by prolonged graft implantation. Transplantation. 2018; https://doi.org/10.1097/TP.0000000000002263.
Buis CI, et al. Altered bile composition after liver transplantation is associated with the development of nonanastomotic biliary strictures. J Hepatol. 2009; https://doi.org/10.1016/j.jhep.2008.07.032.
Cillo U, et al. Identifying risk profiles in liver transplant candidates and implications for induction immunosuppression. Transplant Rev. 2018; https://doi.org/10.1016/j.trre.2018.04.001.
Clavien PA, et al. The comprehensive complication index (CCI ®): added value and clinical perspectives 3 years “down the line”. Ann Surg. 2017; https://doi.org/10.1097/SLA.0000000000002132.
Colaneri J. An overview of transplant immunosuppression–history, principles, and current practices in kidney transplantation. Nephrology nursing journal. 2014;41(6):549–60; quiz 561. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26287052.
Croome KP, et al. Inferior survival in liver transplant recipients with hepatocellular carcinoma receiving donation after cardiac death liver allografts. Liver Transpl. 2013; https://doi.org/10.1002/lt.23715.
Croome KP, et al. The use of donation after cardiac death allografts does not increase recurrence of hepatocellular carcinoma. Am J Transplant. 2015; https://doi.org/10.1111/ajt.13306.
Croome KP, et al. Improving national results in liver transplantation using grafts from donation after cardiac death donors. Transplantation. 2016; https://doi.org/10.1097/TP.0000000000001483.
Croome KP, et al. Comparison of longterm outcomes and quality of life in recipients of donation after cardiac death liver grafts with a propensity-matched cohort. Liver Transpl. 2017; https://doi.org/10.1002/lt.24713.
Croome KP, et al. Waitlist outcomes for patients relisted following failed donation after cardiac death liver transplant: implications for awarding model for end-stage liver disease exception scores. Am J Transplant. 2017;17(9):2420–7. https://doi.org/10.1111/ajt.14383.
Croome KP, et al. Outcomes of donation after circulatory death liver grafts from donors 50 years or older: a multicenter analysis. Transplantation. 2018; https://doi.org/10.1097/TP.0000000000002120.
DeOliveira ML, et al. Biliary complications after liver transplantation using grafts from donors after cardiac death: results from a matched control study in a single large volume center. Ann Surg. 2011;254(5), pp. 716–722; discussion 722–3. doi: https://doi.org/10.1097/SLA.0b013e318235c572.
Detry O, et al. Donor age as a risk factor in donation after circulatory death liver transplantation in a controlled withdrawal protocol programme. Br J Surg. 2014;101(7):784–92. https://doi.org/10.1002/bjs.9488.
Doyle MB, et al. Outcomes using grafts from donors after cardiac death. J Am Coll Surg. 2015;221(1):142–52. https://doi.org/10.1016/j.jamcollsurg.2015.03.053. Epub 2015 Apr 8.
Dubbeld J, et al. Similar liver transplantation survival with selected cardiac death donors and brain death donors. Br J Surg. 2010;97(5):744–53. https://doi.org/10.1002/bjs.7043.
Farid SG, et al. Impact of donor hepatectomy time during organ procurement in donation after circulatory death liver transplantation. Transplantation. 2018; https://doi.org/10.1097/TP.0000000000002518.
Firl DJ, et al. Impact of donor age in liver transplantation from donation after circulatory death donors: a decade of experience at Cleveland Clinic. Liver Transpl. 2015;21(12):1494–503. https://doi.org/10.1002/lt.24316.
Firl DJ, et al. Role of donor hemodynamic trajectory in determining graft survival in liver transplantation from donation after circulatory death donors. Liver Transpl. 2016; https://doi.org/10.1002/lt.24633.
Foley DP, et al. Donation after cardiac death: the University of Wisconsin experience with liver transplantation. Ann Surg. 2005;242(5):724–31. https://doi.org/10.1097/01.sla.0000186178.07110.92.
Foley DP, et al. Biliary complications after liver transplantation from donation after cardiac death donors: an analysis of risk factors and long-term outcomes from a single center. Ann Surg. 2011;253(4):817–25. https://doi.org/10.1097/SLA.0b013e3182104784.
Giesbrandt K, et al. Atlas of ischemic cholangiopathy in donation after-cardiac-death liver transplants. J Vasc Interv Radiol. 2015;26(2):S216–7. Available at: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L71806031.
Giorgakis E, et al. Minimization of ischemic cholangiopathy in donation after cardiac death liver transplantation: is it thrombolytic therapy or warm ischemic time stringency and donor bile duct flush? Am J Transplant. 2017; https://doi.org/10.1111/ajt.14429.
Goldberg DS, et al. Interpreting outcomes in DCDD liver transplantation: first report of the multicenter IDOL consortium. Transplantation. 2017;101(5):1. https://doi.org/10.1097/TP.0000000000001656.
Grewal HP, et al. Liver transplantation using controlled donation after cardiac death donors: an analysis of a large single-center experience. Liver Transpl. 2009; https://doi.org/10.1002/lt.21811.
Halldorson JB, et al. Differential rates of ischemic cholangiopathy and graft survival associated with induction therapy in DCD liver transplantation. Am J Transplant. 2015; https://doi.org/10.1111/ajt.12962.
Hessheimer A, et al. Normothermic regional perfusion versus super rapid recovery in controlled donation after circulatory death liver transplantation. J Hepatol. 2018; https://doi.org/10.1016/j.jhep.2018.12.013.
Hong JC, et al. Longterm outcomes for whole and segmental liver grafts in adult and pediatric liver transplant recipients: a 10-year comparative analysis of 2,988 cases. J Am Coll Surg. 2009; https://doi.org/10.1016/j.jamcollsurg.2009.01.023.
Hong JC, et al. Liver transplantation using organ donation after cardiac death: a clinical predictive index for graft failure-free survival. Arch Surg (Chicago, Ill.: 1960). 2011;146(9):1017–23. https://doi.org/10.1001/archsurg.2011.240.
Hong JC. Liver transplantation using organ donation after cardiac death. Arch Surg. 2011; https://doi.org/10.1001/archsurg.2011.240.
Hong JC, et al. Liver transplantation in children using organ donation after circulatory death: a case-control outcomes analysis of a 20-year experience in a single center. JAMA Surg. 2014; https://doi.org/10.1001/jamasurg.2013.3195.
Hwang CS, et al. Should more donation after cardiac death livers be used in pediatric transplantation? Pediatr Transplant. 2019; https://doi.org/10.1111/petr.13323.
Jochmans I, Van Rosmalen M, et al. Adult liver allocation in eurotransplant. Transplantation. 2017;101:1542–50. https://doi.org/10.1097/TP.0000000000001631.
Jochmans I, Meurisse N, et al. Hepatic ischemia/reperfusion injury associates with acute kidney injury in liver transplantation: prospective cohort study. Liver Transpl. 2017; https://doi.org/10.1002/lt.24728.
Jochmans I, Fieuws S, et al. The impact of hepatectomy time of the liver graft on post-transplant outcome. Ann Surg. 2017;269:712. https://doi.org/10.1097/SLA.0000000000002593.
de Jong IEM, et al. Peribiliary glands are key in regeneration of the human biliary epithelium after severe bile duct injury. Hepatology. 2019; https://doi.org/10.1002/hep.30365.
Kalisvaart M, De Haan JE, et al. Comparison of postoperative outcomes between donation after circulatory death and donation after brain death liver transplantation using the comprehensive complication index. Ann Surg. 2017; https://doi.org/10.1097/SLA.0000000000002419.
Kalisvaart M, de Haan JE, et al. The postreperfusion syndrome is associated with acute kidney injury following donation after brain death liver transplantation. Transpl Int. 2017; https://doi.org/10.1111/tri.12891.
Kalisvaart M, de Haan JE, et al. Onset of donor warm ischemia time in donation after circulatory death liver transplantation: hypotension or hypoxia? Liver Transpl. 2018; https://doi.org/10.1002/lt.25287.
Kalisvaart M, Schlegel A, et al. The impact of combined warm ischemia time on development of acute kidney injury in donation after circulatory death liver transplantation: stay within the golden hour. Transplantation. 2018; https://doi.org/10.1097/TP.0000000000002085.
Kalisvaart M, Schlegel A, Trivedi PJ, et al. Chronic kidney disease after liver transplantation: impact of extended criteria grafts. Liver Transpl. 2019; https://doi.org/10.1002/lt.25468.
Kalisvaart M, Schlegel A, Umbro I, et al. The AKI Prediction Score: a new prediction model for acute kidney injury after liver transplantation. HPB. 2019; https://doi.org/10.1016/j.hpb.2019.04.008.
Kalisvaart M, Muiesan P, Schlegel A. The UK-DCD-Risk-Score – practical and new guidance for allocation of a specific organ to a recipient? Expert Rev Gastroenterol Hepatol. 2019;13(8):771–83. https://doi.org/10.1080/17474124.2019.1629286.
Karimian N, Op Den Dries S, Porte RJ. The origin of biliary strictures after liver transplantation: Is it the amount of epithelial injury or insufficient regeneration that counts? J Hepatol. 2013; https://doi.org/10.1016/j.jhep.2013.02.023.
Karimian N, Westerkamp AC, Porte RJ. Biliary complications after orthotopic liver transplantation. Curr Opin Organ Transplant. 2014; https://doi.org/10.1097/MOT.0000000000000082.
Khorsandi S, et al. Developing a donation after cardiac death risk index for adult and pediatric liver transplantation. World J Transplant. 2017;7(3):203–12. https://doi.org/10.5500/wjt.v7.i3.203.
Khorsandi SE, et al. Does donation after cardiac death utilization adversely affect hepatocellular cancer survival? Transplantation. 2016; https://doi.org/10.1097/TP.0000000000001150.
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1–138. https://doi.org/10.1038/kisup.2012.6.
Kim WR, et al. OPTN/SRTR 2015 annual data report: liver. Am J Transplant. 2017; https://doi.org/10.1111/ajt.14126.
Kollmann D, et al. Expanding the donor pool: donation after circulatory death and living liver donation do not compromise the results of liver transplantation. Liver Transpl. 2018; https://doi.org/10.1002/lt.25068.
Kornberg A, et al. Treating ischaemia-reperfusion injury with prostaglandin E1 reduces the risk of early hepatocellular carcinoma recurrence following liver transplantation. Aliment Pharmacol Ther. 2015; https://doi.org/10.1111/apt.13380.
Kron P, et al. Hypothermic oxygenated perfusion (HOPE) for fatty liver grafts in rats and humans. J Hepatol. 2018;68(1):82–91. https://doi.org/10.1016/j.jhep.2017.08.028.
Laing RW, et al. Liver transplantation using grafts from donors after circulatory death: a propensity-matched study from a single centre. Am J Transplant. 2016;16:1795. https://doi.org/10.1111/ajt.13699.
Lan C, et al. Pediatric donor to adult recipients in donation after cardiac death liver transplantation: a single-center experience. Transplant Proc. 2017; https://doi.org/10.1016/j.transproceed.2017.01.088.
Lee DD, et al. Early allograft dysfunction in liver transplantation with donation after cardiac death donors results in inferior survival. Liver Transpl. 2014;20(12):1447–53. https://doi.org/10.1002/lt.23985.
Leithead JA, et al. Hepatic ischemia reperfusion injury is associated with acute kidney injury following donation after brain death liver transplantation. Transpl Int. 2013; https://doi.org/10.1111/tri.12175.
Leithead JA, et al. The evolving use of higher risk grafts is associated with an increased incidence of acute kidney injury after liver transplantation. J Hepatol. 2014; https://doi.org/10.1016/j.jhep.2014.02.019.
Leithead JA, et al. Donation after cardiac death liver transplant recipients have an increased frequency of acute kidney injury. Am J Transplant. 2012; https://doi.org/10.1111/j.1600-6143.2011.03894.x.
Li CX, Man K, Lo CM. The impact of liver graft injury on cancer recurrence posttransplantation. Transplantation. 2017; https://doi.org/10.1097/TP.0000000000001844.
Marcon F, et al. Utilisation of declined liver grafts yields comparable transplant outcomes and previous decline should not be a deterrent to graft use. Transplantation. 2018; https://doi.org/10.1097/TP.0000000000002127.
Mazzaferro V, et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology. 2018; https://doi.org/10.1053/j.gastro.2017.09.025.
Mergental H, et al. Transplantation of declined liver allografts following normothermic ex-situ evaluation. Am J Transplant. 2016;16(11):3235–45. https://doi.org/10.1111/ajt.13875.
Merion RM, et al. Donation after cardiac death as a strategy to increase deceased donor liver availability. Ann Surg. 2006;244(4):555–62. https://doi.org/10.1097/01.sla.0000239006.33633.39.
Meurisse N, et al. Outcomes of liver transplantations using donations after circulatory death: a single-center experience. Transplant Proc. 2012; https://doi.org/10.1016/j.transproceed.2012.09.077.
Monbaliu D, Pirenne J, Talbot D. Liver transplantation using donation after cardiac death donors. J Hepatol. 2012;56(2):474–85. https://doi.org/10.1016/j.jhep.2011.07.004.
Muiesan P, et al. Single-center experience with liver transplantation from controlled non-heartbeating donors: a viable source of grafts. Ann Surg. 2005; https://doi.org/10.1097/01.sla.0000186177.26112.d2.
Muller X, et al. Defining benchmarks in liver transplantation: a multicenter outcome analysis determining best achievable results. Ann Surg. 2017; https://doi.org/10.1097/SLA.0000000000002477.
Muller X, et al. Novel real time prediction of liver graft function during hypothermic oxygenated machine perfusion prior to liver transplantation. Ann Surg. 2019;270(5):783–90. https://doi.org/10.1097/SLA.0000000000003513.
Nagai S, et al. Ischemia time impacts recurrence of hepatocellular carcinoma after liver transplantation. Hepatology. 2015; https://doi.org/10.1002/hep.27358.
Nasralla D, et al. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018; https://doi.org/10.1038/s41586-018-0047-9.
Nastos C, et al. Global consequences of liver ischemia/reperfusion injury. Oxidative Med Cell Longev. 2014; https://doi.org/10.1155/2014/906965.
Nemes B, et al. Extended-criteria donors in liver transplantation Part II: reviewing the impact of extended-criteria donors on the complications and outcomes of liver transplantation. Expert Rev Gastroenterol Hepatol. 2016;10(7):841–59. https://doi.org/10.1586/17474124.2016.1149061.
Nemes B, et al. Extended criteria donors in liver transplantation Part I: reviewing the impact of determining factors. Expert Rev Gastroenterol Hepatol. 2016;10:827–39. https://doi.org/10.1586/17474124.2016.1149061.
NHSBT. NHS Blood and Transplant annual report and accounts 2017 to 2018’. 2018. https://www.gov.uk/government/publications/nhs-blood-and-transplant-annual-report-and-accounts-2018-to-2019. Accessed 24 Apr 2020.
Noack K, et al. The greater vulnerability of bile duct cells to reoxygenation injury than to anoxia. Implications for the pathogenesis of biliary strictures after liver transplantation. Transplantation. 1993; https://doi.org/10.1097/00007890-199309000-00001.
O’Neill S, et al. A meta-analysis and meta-regression of outcomes including biliary complications in donation after cardiac death liver transplantation. Transpl Int. 2014;27(11):1159–74. https://doi.org/10.1111/tri.12403.
O’Riordan A, et al. Acute renal disease, as defined by the RIFLE criteria, post-liver transplantation. Am J Transplant. 2007; https://doi.org/10.1111/j.1600-6143.2006.01602.x.
Ojo AO, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003; https://doi.org/10.1056/NEJMoa021744.
Olthoff KM, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16(8):943–9. https://doi.org/10.1002/lt.22091.
Op Den Dries S, et al. Injury to peribiliary glands and vascular plexus before liver transplantation predicts formation of non-anastomotic biliary strictures. J Hepatol. 2014;60(6):1172–9. https://doi.org/10.1016/j.jhep.2014.02.010.
Orci LA, et al. Effects of the gut–liver axis on ischaemia-mediated hepatocellular carcinoma recurrence in the mouse liver. J Hepatol. 2018; https://doi.org/10.1016/j.jhep.2017.12.025.
Pine JK, et al. Liver transplantation following donation after cardiac death: an analysis using matched pairs. Liver Transplant. 2009;15(9):1072–82. https://doi.org/10.1002/lt.21853.
Pitarch Martínez M, et al. Donation after cardiac death in liver transplantation: an additional source of organs with similar results to donation after brain death. Transplant Proc. 2019; https://doi.org/10.1016/j.transproceed.2018.02.208.
Pulitano C, et al. Molecular profiling of postreperfusion milieu determines acute kidney injury after liver transplantation: A prospective study. Liver Transpl. 2018; https://doi.org/10.1002/lt.25178.
van Leeuwen OB, et al. Donor hepatectomy time in donation after circulatory death donors is an independent risk factor for development of biliary strictures and early graft loss after transplantation Surgery. 2020 Mar 26:S0039-6060(20)30071-4. https://doi.org/10.1016/j.surg.2020.02.005. Online ahead of print. PMID: 32223984.
Van Rijn R, et al. Long-term results after transplantation of pediatric liver grafts from donation after circulatory death donors. PLoS One. 2017; https://doi.org/10.1371/journal.pone.0175097.
Rodríguez-Perálvarez M, et al. Early tacrolimus exposure after liver transplantation: Relationship with moderate/severe acute rejection and long-term outcome. J Hepatol. 2013; https://doi.org/10.1016/j.jhep.2012.09.019.
Rodríguez-Perálvarez M, et al. Area under trough concentrations of tacrolimus as a predictor of progressive renal impairment after liver transplantation. Transplantation. 2019; https://doi.org/10.1097/tp.0000000000002760.
Salvalaggio PR, et al. Early allograft dysfunction and liver transplant outcomes: a single center retrospective study. Transplant Proc. 2012; https://doi.org/10.1016/j.transproceed.2012.08.002.
Scalea JR, Redfield RR, Foley DP. Liver transplant outcomes using ideal donation after circulatory death livers are superior to using older donation after brain death donor livers. Liver Transpl. 2016; https://doi.org/10.1002/lt.24494.
Schlegel A, et al. Hypothermic Oxygenated Perfusion (HOPE) downregulates the immune response in a rat model of liver transplantation. Ann Surg. 2014;260(5):931–7. https://doi.org/10.1097/SLA.0000000000000941; discussion 937–8.
Schlegel A, et al. Risk assessment in high and low MELD liver transplantation. Am J Transplant. 2016;10:1–14. https://doi.org/10.1111/ajt.14065.
Schlegel A, et al. Impact of donor age in donation after cardiac death liver transplantation: Is the cut-off “60” still of relevance? Liver Transplant. 2017; https://doi.org/10.1002/lt.24865. [Epub ahead of print].
Schlegel A, et al. Reply to: “DCD consensus and futility in liver transplantation”. J Hepatol. 2018;69(1):257–8. https://doi.org/10.1016/j.jhep.2018.04.001.
Schlegel A, et al. The UK DCD Risk Score: a new proposal to define futility in donation-after-circulatory-death liver transplantation. J Hepatol. 2018;68(3):456–64. https://doi.org/10.1016/j.jhep.2017.10.034.
Schlegel AA, et al. Outcomes of liver transplantations from donation after circulatory death (DCD) treated by hypothermic oxygenated perfusion (HOPE) before implantation. J Hepatol. 2019;70(1):50–7. https://doi.org/10.1016/j.jhep.2018.10.005.
Schlegel A, Dutkowski P. Impact of machine perfusion on biliary complications after liver transplantation. Int J Mol Sci. 2018; https://doi.org/10.3390/ijms19113567.
Sher L, et al. Attitudes and barriers to the use of donation after cardiac death livers: comparison of a United States transplant center survey to the united network for organ sharing data. Liver Transpl. 2017;23(11):1372–83. https://doi.org/10.1002/lt.24855.
Slankamenac K, et al. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258(1):1–7. https://doi.org/10.1097/SLA.0b013e318296c732.
Stewart ZA, et al. Increased risk of graft loss from hepatic artery thrombosis after liver transplantation with older donors. Liver Transplant. 2009;15(12):1688–95. https://doi.org/10.1002/lt.21946.
Taner CB, et al. Asystole to cross-clamp period predicts development of biliary complications in liver transplantation using donation after cardiac death donors. Transpl Int. 2012;25(8):838–46. https://doi.org/10.1111/j.1432-2277.2012.01508.x.
Taylor R, et al. Survival advantage for patients accepting the offer of a circulatory death liver transplant. J Hepatol. 2019; https://doi.org/10.1016/j.jhep.2018.12.033.
Thongprayoon C, et al. Incidence and impact of acute kidney injury after liver transplantation: a meta-analysis. J Clin Med. 2019; https://doi.org/10.3390/jcm8030372.
Trivedi PJ, et al. Clinical outcomes of donation after circulatory death liver transplantation in primary sclerosing cholangitis. J Hepatol. 2017; https://doi.org/10.1016/j.jhep.2017.06.027.
Tsui SSL, Oniscu GC. Extending normothermic regional perfusion to the thorax in donors after circulatory death. Curr Opin Organ Transplant. 2017;22:245–50. https://doi.org/10.1097/MOT.0000000000000413.
Vanatta JM, et al. Liver transplant using donors after cardiac death: a single-center approach providing outcomes comparable to donation after brain death. Exp Clin Transplant. 2013; https://doi.org/10.6002/ect.2012.0173.
De Vera ME, et al. Liver transplantation using donation after cardiac death donors: long-term follow-up from a single center. Am J Transplant. 2009;9(4):773–81. https://doi.org/10.1111/j.1600-6143.2009.02560.x.
Wadei HM, et al. Early allograft dysfunction after liver transplantation is associated with short- and long-term kidney function impairment. Am J Transplant. 2016; https://doi.org/10.1111/ajt.13527.
Wadei HM, et al. Early allograft dysfunction is associated with higher risk of renal nonrecovery after liver transplantation. Transplant Direct. 2018; https://doi.org/10.1097/TXD.0000000000000771.
Wallace D, et al. Assessing the impact of suboptimal donor characteristics on mortality after liver transplantation: a time-dependent analysis comparing HCC with non-HCC patients. Transplantation. 2019; https://doi.org/10.1097/TP.0000000000002559.
Watson C, Hunt F, et al. In situ normothermic perfusion of livers in controlled circulatory death donation may prevent ischemic cholangiopathy and improve graft survival. Am J Transplant. 2018; https://doi.org/10.1111/ajt.15241.
Watson C, Kosmoliaptsis V, et al. Observations on the ex situ perfusion of livers for transplantation. Am J Transplant. 2018; https://doi.org/10.1111/ajt.14687.
Yska MJ, et al. The role of bile salt toxicity in the pathogenesis of bile duct injury after non-heart-beating porcine liver transplantation. Transplantation. 2008;85(11):1625–31. https://doi.org/10.1097/TP.0b013e318170f5f7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Schlegel, A., Panconesi, R., Muiesan, P. (2020). Outcomes in DCD Liver Transplantation. In: Croome, K., Muiesan, P., Taner, C. (eds) Donation after Circulatory Death (DCD) Liver Transplantation. Springer, Cham. https://doi.org/10.1007/978-3-030-46470-7_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-46470-7_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-46469-1
Online ISBN: 978-3-030-46470-7
eBook Packages: MedicineMedicine (R0)