Abstract
Purpose of Review
The gastroduodenal mucosal layer is a complex and dynamic system that functions in an interdependent manner to resist injury. We review and summarize the most updated knowledge about gastroduodenal defense mechanisms and specifically address (a) the mucous barrier, (b) membrane and cellular properties, and vascular, hormonal, and (c) gaseous mediators.
Recent Findings
Trefoil factor family peptides play a crucial role in cellular restitution by increasing cellular permeability and expression of aquaporin channels, aiding cellular migration and tissue repair. Additionally, evidence suggests that the symptoms of functional dyspepsia may be attributed to alterations in the duodenum, including low-grade inflammation and increased mucosal permeability.
Summary
The interaction of the various mucosal protective components helps maintain structural and functional homeostasis. There is increasing evidence suggesting that the upper GI microbiota plays a crucial role in the defense mechanisms. However, this warrants further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability of the gastroduodenal mucosa to resist injury in the setting of high acid concentration, pepsin, and refluxed bile to which it is exposed is a unique property and function of the mucosa. Daily, the adult stomach secretes about 1.5 L of gastric acid, and during periods of peak acid output, a pH of 1.0 is commonly achieved [1]. In the proximal duodenum, a pH of 2 is often reached after gastric emptying of acid, yet defense mechanisms can maintain homeostasis within these harsh environments. The integrity of the mucosal barrier is a dynamic, complex, and multi-factorial process, which involves interplay between physiological and anatomical components. Resistance to autodigestion was initially described approximately 250 years ago [2]. Since then, significant advances in understanding the physiology of gastroduodenal defense mechanisms have taken place. In this article, we review and summarize the most updated knowledge about gastroduodenal defense mechanisms and specifically address (a) mucous barrier, (b) membrane and cellular properties, and (c) vascular, hormonal, and gaseous mediators.
Protective Factors
Mucous Barrier
The first line of defense is the mucous layer secreted by the gastroduodenal epithelial cells. Mucous is an adherent, viscous, and elastic layer that protects underlying tissues from caustic damage. It has a mean thickness of 190–275 μm in the stomach and 170 μm in the duodenum. Its properties vary depending on its location on the gastrointestinal (GI) tract, but it is generally composed of 95% water and 0.2–5.0% mucins, ions, salts, lipids, cell debris, and DNA. Water acts as the solvent or medium to the other components, while mucins give it its viscoelastic and electrochemical properties [3,4,5,6]. Other key components of the mucous barrier include proteins (lysozymes, lactoferrin) and immunoglobulins (IgA) that contribute to its defensive properties [7]. The mucus layer also contains electrolytes that vary depending on the epithelial surface. The electrolytes that are usually found in the mucous layer are Na, K, Cl, HCO3, PO4, Mg, and Ca [4].
Mucins are an integral part of the mucus layer and are categorized as membrane-bound and secreted. Membrane-bound mucins are anchored on the cell membrane, and secreted mucins are packed into excretory vesicles and released. The membrane-bound mucins function for cellular adhesion, pathogen binding, and signal transduction. The secreted mucins are mainly responsible for the viscoelastic properties of the gastric mucus layer [5]. There are 21 different genes that code for mucin in the human body. The predominant mucin that is found in the human stomach is MUC5a, a secreted mucin, and it is predominantly located within the inner layer of the gastric mucus [5, 6].
Mucin Synthesis
Mucins are synthesized by the goblet cells and the submucosal epithelial glands. Synthesis begins in the rough endoplasmic reticulum where the apolipoproteins are created. They undergo further modifications such as the addition of carbohydrates, sialic acid, and sulfates. They are then packed in the Golgi apparatus and stored in secretory granules. These granules are released and fused with the cellular membrane through Ca-mediated exocytosis. Once the mucins are exposed to the extracellular milieu, they undergo rapid osmotic hydration. This is accompanied by diffusion of the complexed cations (calcium, sodium, and hydrogen) to the surrounding medium, freeing the negatively charged sulfates and sialic acid [4]. This process results in a rapid expansion of the mucin up to a 500-fold increase of packed size [5].
Mucin Structure
Mucins are high molecular weight molecules that have a protein foundation rich in serine, proline, and threonine repeats and have amino and carboxy terminals rich in Cysteine. This infrastructure is responsible for the carbohydrate-heavy density of the mucin, which is composed of 70% carbohydrates [4, 5]. The cysteine-rich regions of the mucin consist of oligomers that are responsible for the adhesive properties of the mucus layer [5]. These cysteine-rich regions form disulfide bonds with other oligomers, allowing dimerization and further multimerization [4].
Mucous Barrier Properties
The mucous barrier is divided into two layers: a layer adherent to the epithelial surface and a loose layer on the luminal side. The consistency is affected by the gastric pH. The loose mucous on the luminal side of the gastric mucus layer is formed by contact with the acidic pH, causing the random coil conformation of the mucin to create an extended conformation, which results in a gel-like consistency [5]. The pepsin in the gastric lumen also contributes to the degradation of the mucin in the luminal side, producing mucin subunits which constitute the viscous liquid state of the mucus gel on the luminal side [6].
The mucin component of the mucous barrier provides a pH gradient throughout the luminal and epithelial layers. A pH of 1–2 is present in the luminal side while a pH of 6 or near neutral is found in the epithelial side [5]. The HCO3 secretion in the epithelial surface is enough to neutralize the acidic pH, preventing caustic damage [8]. The luminal acid is neutralized even before reaching the epithelial side [6].
Additionally, the mucous layer is stabilized by covalent and non-covalent interactions such as electrostatic bonds, hydrogen bonds, and hydrophobic bonds that create a network that acts as a filter which decreases the diffusion of specific molecules and particles [4, 9].
All these various interactions between the components of the mucous layer interact with each other forming a convoluted surface, with a mesh-like layer that has varying pore sizes from 10 to 500 nm [9]. This, in turn, acts as a semipermeable membrane that limits the diffusion of large molecules such as microbes and permits diffusion of low molecular weight solutes such as ions [6].
Mucosal Surface Epithelium
Epithelium
The gastric mucosa is lined by simple secretory columnar epithelial cells with a basal membrane [10]. This epithelial layer continuously secretes bicarbonate, contributing to the pH gradient along the gastric mucus layer, therefore, preventing caustic damage to the gastric epithelium. The gastric lining acts as the second line of defense against the H+ in the stomach. The gastric epithelial surface is known to be hydrophobic which repels acid and water-soluble agents [11].
Epithelium Components (Tight Junctions, Claudins, Cadherins, and Occludins)
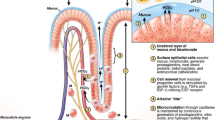
In between the gastric columnar cells are tight junctions. Tight junctions connect the cells of the gastric mucosa, thus maintaining the integrity and preventing backflow of H+ ions in between the cells. They also play a role in the process of cellular differentiation, polarization, and epithelial proliferation signaling [12, 13]. Different protein complexes constitute the tight junction architecture such as claudins, cadherins, occludins, actin, ZO-1, and junctional adhesion molecule-As (Fig. 1).
Gastric surface and the different elements that act in concert to maintain the integrity of the gastric mucosa. The mucus layer is composed of mucin, water, and bicarbonate, and it is continuously replenished by the gastric surface epithelial cells. Tight junctions form a tight-knit barrier between epithelial cells. H. pylori targets the tight junctions and disrupts the integrity of the epithelial barrier facilitating proton backflow, leading to mucosal injury. Neurovascular factors, gaseous mediators, and hormones stimulate vasodilation, mucin and bicarbonate secretion, and the antioxidant system to maintain the integrity of the gastroduodenal barrier
Claudins play a major role in tight junction permeability, cell signaling, cell cycle regulation, maintenance of cell polarity, and vesicle trafficking. They are primarily responsible for the regulation of paracellular permeability. Preclinical studies have identified 27 different claudins, each having unique characteristics which maintain the mucosal selectivity and integrity. Claudin 18 has been identified as the most abundant and is responsible for reducing the paracellular permeability of Na+ and H+ ions, working as a cation exclusion pore. The underexpression of this claudin 18 has been linked to atrophic gastritis, and early stages of gastric carcinoma development attributed to H+ ion leakage [12].
Due to the presence of tight junctions and these specific claudins, the gastric epithelium is considered electrically tight, preventing caustic damage from H+ ions. In contrast, the small intestinal epithelium’s tight junction is considered leaky, which aids the absorption of nutrients [12].
The other protein complexes that comprise the tight junction architecture are located in the apical (occludins) and basolateral surface (E-cadherins). Beside the E-cadherins located on the basolateral surface, we also find carbonic anhydrase IX which converts protons that backflow to CO2 and H2O, contributing to the tight junction defense mechanism [14].
Tight junctions are composed of five to six strands with a honeycomb architecture. The passage of substances through the tight junctions is usually via paracellular transport and only charged differentiated molecules of less than four angstroms may cross freely. Temporary breaks of this architecture may result in bigger molecules being able to cross. H. pylori is said to target these tight junctions and gastric epithelial cells directly compromising the integrity and continuity of the epithelial barrier. The CagA protein of H. pylori targets claudins, leading to tight junction dysfunction and proton backflow [12].
Epithelial Restitution
There is a cell migration-dependent process that in the event of cell death or mucosal integrity disruption, the intact neighboring epithelium migrates to the exposed basal lamina, closing the gap and restoring the epithelial integrity [15].
The process of epithelial restitution begins within minutes after an injury, in contrast to cellular differentiation which can occur over days [16]. Actin and myosin II play a major role in epithelial restitution by guiding the intact epithelium to the denuded areas. Actin polymerizes on the lateral surface of intact epithelial cells towards the direction of the denuded zone, forming a network that guides cell migration to the empty basal lamina. The process of cellular migration requires changes in the cellular shape with the formation of lateral protrusions called lamellipodium. The process of cell migration also leads to the exfoliation of the dead cells to the gastric lumen [15].
Role of Trefoil Factors
Trefoil factors are a group of secreted peptides that can be found on the mammalian GI tract [17]. Trefoil factor 2 expression is increased on injured sites, and they play a role in cellular restitution. TFF2 increases AQP3 expression on the migrating cells which mediate water influx to the cell which is essential in the formation of the lamellipodium [18•].
Vascular and Hormonal Mediators
Vascular
Maintenance of constant blood flow is essential for the integrity of the mucosal surface. Decreased blood flow can cause damage by depriving the cells vital oxygen and nutrients and also by reducing mucosal barrier thickness [19]. Various mediators maintain adequate blood flow to the gastric lining. These include prostaglandins, gaseous mediators (NO, H2S, and CO) and afferent nerves which stimulate vasodilation [20].
Prostacyclin or PGI2 and PGE2 contribute to the mucosal defense by increasing mucus and HCO3 secretion, and by promoting vasodilation, resulting in increased blood flow [20]. PGE2 has four known receptors named EP1, EP2, EP3, and EP4. Activation of EP1 and EP4 increases mucus and bicarbonate secretion of surface mucosal cells. EP3 activation directly inhibits parietal cell H+ secretion, and indirectly via enterochromaffin cells by inhibiting histamine release [16]. PGE2 stimulates KATP channels which result in vasodilation. NSAIDs are known to impair the gastric blood flow by inhibiting the COX system [21].
The two components of the autonomic nervous system (parasympathetic and sympathetic) have an essential role in visceral blood flow. The parasympathetic system promotes blood flow while the sympathetic system decreases blood flow. Stress activates the sympathetic system may lead to ischemia and reperfusion injury resulting in inflammation and increased microvascular permeability [19, 22]. The afferent C neuronal fibers found in the gastric submucosa also play an important role in gastroprotection. Proton ions that back diffuse activate TRPV-1 receptors on the said nerves which releases calcitonin gene-related peptide (CGRP) resulting in nitric oxide-mediated vasodilation. The increased blood flow facilitates increased delivery of bicarbonate resulting in buffering of the diffused proton [23, 24•].
Gaseous Mediators (NO, CO, and H2S)
Nitric oxide (NO) is produced by nitric oxide synthase, which has three subtypes: neuronal, endothelial, and inducible. eNOS is found on endothelial cells. Nitric oxide produced by endothelial cells is known to improve ulcer healing and plays a role in maintaining the integrity of the epithelial barrier by stimulating mucus secretion via the epithelial cGMP messenger system. It has direct vasodilator effects facilitating the delivery of bicarbonate, nutrients, and oxygen. It also plays a role in the regulation of gastric acid secretion and inhibits neutrophil aggregation and adherence, thus reducing damage [23, 25, 26].
Hydrogen sulfide (H2S) is another gaseous mediator that has gastroprotective properties. It inhibits leukocyte adhesion, lipid peroxidation, and limit gastric damage. It also increases SOD-2 and glutathione peroxidase 1 activity, producing antioxidants and increasing resistance against oxidative ischemia-reperfusion-induced gastric mucosal damage [27]. H2S additionally increases blood flow by increasing the activity of TRPV-1 receptors on afferent nerve fibers and by enhancing the expression of COX-1 and COX-2 which leads to an increase in prostaglandins [27, 28].
Carbon monoxide (CO) is the third gaseous mediator that plays a role in gastroduodenal protection. CO is a byproduct of the metabolism of heme which is catalyzed by the enzyme heme oxygenase. CO activates K-channels which utilizing the cGMP second messenger system resulting in increased gastric blood flow. It decreases lipid peroxidation, increases antioxidants such as SOD and GSH. Donors of H2S also increase HCO3 secretion in the duodenum [29]. It exhibits antiapoptotic effects by utilizing various pathways such as MAPK8 and NFκB pathway.
Preclinical studies demonstrated that CO was protective against ethanol-induced gastric ulcers by increasing the gastric blood flow by stimulation of vanilloid receptors found in the afferent sensory nerves. It also decreases the expression of pro-inflammatory factors such as COX-2, TNF-α, IL-1β, and HIF-1α [30].
There are significant overlaps between the mechanisms of action of the gaseous mediators. All the three mediators cause vasodilation, therefore, increasing mucosal blood flow, thus potentially affecting the mucus and bicarbonate excretion of epithelial cells. It also is shown that the three mediators decrease leukocyte adhesion in the vascular wall resulting in reduced infiltration. They also support ulcer healing by promoting cell migration, proliferation, and collagen deposition [23].
Growth Factors and Hormones
Growth factors such as EGFr and VEGF are important factors that stimulate re-epithelialization and injury repair [31]. They are located on the edge of healing ulcers and were noted to have increased expression in rats who had received daily H2S donors [30].
Nerve growth factor also plays a role in angiogenesis and epithelial cell proliferation [32]. Various hormones contribute to gastroprotection by acting with the above mechanisms.
Angiotensin (1–7) is a product of the renin-angiotensin system which also exhibits gastroprotective properties. It binds to the Mas receptor in the vascular bed which results in increased blood flow and tissue oxygenation. It also increases the expression of superoxide dismutase 2, decreases levels of inflammatory markers such as TNF-α and IL-1β, and demonstrates antiapoptotic activity by decreasing gastric caspases 3. The prostaglandin and nitric oxide system play a role in the gastroprotective activity of angiotensin (1–7), but this is still poorly understood [33, 34•].
Acetylcholine and serotonin are known to promote NO production by endothelial cells [28]. Hormones such as acetylcholine, gastrin, and histamine are known to stimulate MUC5a production, as well as paracrine mediators such as NO, epidermal growth factors, and hepatocyte growth factors [16].
Thyroid hormones also stimulate mucin production by increasing the number of goblet cells and improving NSAID-induced gastric healing by increasing gastric blood flow and hastening the clearance of inflammatory cells [35].
Recent Development and Future Considerations
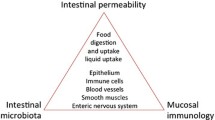
The stomach was traditionally believed to be sterile due to its caustic environment. However, in 1982, this belief was reformed after the discovery of H. pylori. A meta-analysis study by Hooi et al. determined that approximately 4.4 billion individuals were infected with H. pylori in 2015, accounting for more than half of the world’s population [36]. Yet, only a small proportion of infected individuals are symptomatic. Factors that can explain the discrepancy are strain virulence, varying immune responses, and host-microbe interactions [37]. Since the discovery of H. pylori, multiple studies have established the presence of other organisms in the stomach. Pereira et al. suggested that the microbiota in the upper GI tract play a role in the development of symptoms attributed in the past only to H. pylori [38]. The use of proton pump inhibitors can potentially influence the microbiota by altering the stomach’s caustic environment. However, this warrants further investigation.
The recent Rome IV defined functional dyspepsia as the presence of at least one of the following symptoms for more than 3 months with the onset of symptoms of greater than 6 months with no evidence of organic disease: bothersome postprandial fullness, early satiety, epigastric pain, and epigastric burning [39]. The exact pathophysiology of functional dyspepsia is still unknown. A recent review article by Miwa et al. proposed that the duodenum is the probable explanation for generating the symptoms termed “functional dyspepsia.” They suggested that increased duodenal permeability, which allows passage of various stimuli such as acid, bile acids, nutrients, allergens, and microorganisms through the duodenal lining resulting to an inflammatory response and subsequent activation of submucosal afferent nerves leading to gastric dysmotility and gastric hypersensitivity resulting to dyspeptic symptoms [40•]. There are numerous hypotheses regarding the exact mechanism of the increased duodenal mucosal permeability. According to Wallace, et al., the distal part of the duodenum is in a chronic state of inflammation due to trans-epithelial migration of the mucosa-associated-microbiota and interaction of their byproducts with the mucosal immune system [23]. This, however, does not explain why only a certain number of people are diagnosed with functional dyspepsia. One possible explanation is a difference in the duodenal content, specifically the microbiota. Alterations in the mucosa-associated microbiota, including reduced diversity and increased density of bacteria, could lead to mucosal barrier disruption with subsequent passage of various agents. This in turn, can elicit an inflammatory response leading to a vicious cycle of increased barrier permeability and afferent nerve activation, which could explain the chronicity of functional dyspepsia. This field of gastric and duodenal microbiota is new and evolving. However, there are clear implications that duodenal microbiota can be perturbated and become “out of balance,” by exposure to gastroenteritis, antibiotics, changes in pH by use of proton pump inhibitors which decrease acidity or by “stress” which induces hyperacidity.
Conclusion
The gastroduodenal mucous layer is a complex and dynamic environment exposed continuously to different harmful agents. The interaction of different gastroduodenal protective factors helps to maintain structural and functional homeostasis. The mucous layer serves as a semipermeable membrane that restricts the passage of various molecules to the underlying epithelium. When the gastroduodenal epithelium is injured, an efficient process of restitution enables the environment to rapidly recover. The mediators of this process include different hormones and growth factors which signal and coordinate different molecules to re-establish the continuity of the epithelium. Several drugs and bacteria injure the gastroduodenal environment by disrupting the epithelium and preventing the restitution process; both of these, affect particular components that maintain structural integrity. A direct injury is sustained by acting against the mucous layer or secretory epithelium. An indirect injury usually occurs when hormonal or vasoactive factors are targeted and impair secretion of mucus, bicarbonate, and other molecules. The mechanism of Helicobacter pylori injury is well understood and has allowed for the development of effective treatments. However, a still unknown component of the gastroduodenal environment is the upper GI microbiota. There is increasing evidence suggesting that the upper GI microbiota plays a crucial role in the defense mechanisms of the gastroduodenal epithelium. Further research in this field is needed to fully understand the role of upper GI microbiota and what role abnormalities in this microbiota can contribute to different pathologies.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lewin MJ. Cell physiology and pharmacology of gastric acid secretion. Therapie. 1992;47(2):93–6.
Hunter J. On the digestion of the stomach after death, by John Hunter, F.R.S. and surgeon to St. George’s Hospital. Phil Trans R Soc A. 1772;62:447–54.
Pelaseyed T, Bergström JH, Gustaffson JK, et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev. 2014;260(1):8–20.
Bansil R, Turner BS. The biology of mucus: composition, synthesis and organization. Adv Drug Deliv Rev. 2018;124:3–15.
Leal J, Smyth HDC, Ghosh D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int J Pharm. 2017;532(1):555–72.
Mall AS, Habte H, Mthembu Y, Peacocke J, de Beer C. Mucus and mucins: do they have a role in the inhibition of the human immunodeficiency virus? Virol J. 2017;14(1):192.
Cárdenas-Mondragón MG, Torres J, Flores-Luna L, et al. Epstein-Barr virus association with peptic ulcer disease. Anal Cell Pathol (Amst). 2015;2015:164840.
Lewis OL, Keener JP, Fogelson AL. A physics-based model for maintenance of the pH gradient in the gastric mucus layer. Am J Physiol Gastrointest Liver Physiol. 2017;313(6):G599–612.
Lock JY, Carlson TL, Carrier RL. Mucus models to evaluate the diffusion of drugs and particles. Adv Drug Deliv Rev. 2018;124:34–49.
Boccellato F, Woelffling S, Imai-Matsushima A, et al. Polarised epithelial monolayers of the gastric mucosa reveal insights into mucosal homeostasis and defence against infection. Gut. 2018.
Yandrapu H, Sarosiek J. Protective factors of the gastric and duodenal mucosa: an overview. Curr Gastroenterol Rep. 2015;17(6):24.
Caron TJ, Scott KE, Fox JG, et al. Tight junction disruption: helicobacter pylori and dysregulation of the gastric mucosal barrier. World J Gastroenterol. 2015;21(40):11411–27.
Balda MS, Matter K. Tight junctions as regulators of tissue remodelling. Curr Opin Cell Biol. 2016;42:94–101.
Li T, Liu X, Riederer B, Nikolovska K, Singh AK, Mäkelä KA, et al. Genetic ablation of carbonic anhydrase IX disrupts gastric barrier function via claudin-18 downregulation and acid backflux. Acta Physiol (Oxf). 2018;222(4):e12923.
Aihara E, Medina-Candelaria NM, Hanyu H, et al. Cell injury triggers actin polymerization to initiate epithelial restitution. J Cell Sci. 2018;131(16):jcs216317.
Sáenz JB, Mills JC. Acid and the basis for cellular plasticity and reprogramming in gastric repair and cancer. Nat Rev Gastroenterol Hepatol. 2018;15(5):257–73.
Chen X, Hu Y, Xie Y, Wang Y. High salt diet can down-regulate TFF2 expression level in gastric mucosa of MGs after H. pylori infection. Microb Pathog. 2018;118:316–21.
• Marchbank T, Playford RJ. Trefoil factor family peptides enhance cell migration by increasing cellular osmotic permeability and aquaporin 3 levels. FASEB J. 2018;32(2):1017–24 Trefoil factor family peptides play a crucial role in cellular restitution by increasing cellular permeability and expression of aquaporin channels, aiding in the formation of the lamellipodium, cellular migration, and tissue repair .
Saxena B, Singh S. Comparison of three acute stress models for simulating the pathophysiology of stress-related mucosal disease. Drug Discov Ther. 2017;11(2):98–103.
Farrugia G, Szurszewski JH. Carbon monoxide, hydrogen sulfide, and nitric oxide as signaling molecules in the gastrointestinal tract. Gastroenterology. 2014;147(2):303–13.
Shore R, Björne H, Omoto Y, Siemiatkowska A, Gustafsson JÅ, Lindblad M, et al. Sex differences and effects of oestrogen in rat gastric mucosal defence. World J Gastroenterol. 2017;23(3):426–36.
Yoon G, Kim HS. Gastric acid response to acute exposure to hypergravity. Oncotarget. 2017;8(1):64–9.
Wallace JL, Ianaro A, de Nucci G. Gaseous mediators in gastrointestinal mucosal defense and injury. Dig Dis Sci. 2017;62(9):2223–30.
• Magierowska K, Wojcik D, Chmura A, Bakalarz D, Wierdak M, Kwiecien S, et al. Alterations in gastric mucosal expression of calcitonin gene-related peptides, vanilloid receptors, and heme oxygenase-1 mediate gastroprotective action of carbon monoxide against ethanol-induced gastric mucosal lesions. Int J Mol Sci. 2018;19(10):E2960 Carbon monoxide protects against ethanol-associated mucosal injury by increasing gastric microcirculation through the activation of transient receptor potential vanilloid receptor type 1 (located on afferent sensory fiber endings) and calcitonin gene-related peptide.
Ribeiro AR, Diniz PB, Pinheiro MS, et al. Gastroprotective effects of thymol on acute and chronic ulcers in rats: the role of prostaglandins, ATP-sensitive K(+) channels, and gastric mucus secretion. Chem Biol Interact. 2016;244:121–8.
Magierowski M, Magierowska K, Kwiecien S, Brzozowski T. Gaseous mediators nitric oxide and hydrogen sulfide in the mechanism of gastrointestinal integrity, protection and ulcer healing. Molecules. 2015;20(5):9099–123.
Magierowski M, Magierowska K, Hubalewska-Mazgaj M, et al. Exogenous and endogenous hydrogen sulfide protects gastric mucosa against the formation and time-dependent development of ischemia/reperfusion-induced acute lesions progressing into deeper ulcerations. Molecules. 2017;22(2):E295.
Magierowski M, Jasnos K, Kwiecien S, Drozdowicz D, Surmiak M, Strzalka M, et al. Endogenous prostaglandins and afferent sensory nerves in gastroprotective effect of hydrogen sulfide against stress-induced gastric lesions. PLoS One. 2015;10(3):e0118972.
Kwiecien S, Magierowska K, Magierowski M, Surmiak M, Hubalewska-Mazgaj M, Pajdo R, et al. Role of sensory afferent nerves, lipid peroxidation and antioxidative enzymes in the carbon monoxide-induced gastroprotection against stress ulcerogenesis. J Physiol Pharmacol. 2016;67(5):717–29.
Magierowski M, Magierowska K, Hubalewska-Mazgaj M, Surmiak M, Sliwowski Z, Wierdak M, et al. Cross-talk between hydrogen sulfide and carbon monoxide in the mechanism of experimental gastric ulcers healing, regulation of gastric blood flow and accompanying inflammation. Biochem Pharmacol. 2018;149:131–42.
Alese MO, Adewole SO, Akinwunmi KF, et al. Aspirin-induced gastric lesions alters EGFR and PECAM-1 immunoreactivity in Wistar rats: modulatory action of flavonoid fraction of Musa paradisiaca. Open Access Maced J Med Sci. 2017;5(5):569–77.
Tarnawski AS, Ahluwalia A, Jones MK, et al. Expression of nerve growth factor in rat stomach. Implications for interactions between endothelial, neural and epithelial cells. J Physiol Pharmacol. 2016;67(6):879–83.
Hassan MKA, Aziz NM, Shaaban MAE, et al. Possible contribution of nitric oxide and prostaglandin in the protective effect of angiotensin (1-7) against stress induced gastric ulceration in adult male albino rats. Bratisl Lek Listy. 2016;117(12):715–21.
• Pawlik MW, Kwiecien S, Ptak-Belowska A, Pajdo R, Olszanecki R, Suski M, et al. The renin-angiotensin system and its vasoactive metabolite angiotensin-(1-7) in the mechanism of the healing of preexisting gastric ulcers. The involvement of Mas receptors, nitric oxide, prostaglandins and proinflammatory cytokines. J Physiol Pharmacol. 2016;67(1):75–91 Inhibition of angiotensin-converting enzyme and the blockade of angiotensin AT-1 receptor protects against gastric mucosal injury. Whether the metabolite of the renin-angiotensin system, Ang (1-7), accelerates the healing of already existing gastric ulcers remains to be elucidated.
Namulema J, Nansunga M, Kato CD, Kalange M, Olaleye SB. Thyroid hormones increase stomach goblet cell numbers and mucin expression during indomethacin induced ulcer healing in Wistar rats. Thyroid Res. 2018;11:6.
Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153(2):420–9.
Wang B, Yao M, Longxian L, et al. The human microbiota in health and disease. Engineering. 2017;3(1):71–82.
Pereira V, Abraham P, Nallapeta S, Shetty A. Gastric bacterial flora in patients harbouring Helicobacter pylori with or without chronic dyspepsia: analysis with matrix-assisted laser desorption ionization time-of-flight mass spectroscopy. BMC Gastroenterol. 2018;18(1):20.
Drossman DA, Hasler WL. Rome IV-functional GI disorders: disorders of gut-brain interaction. Gastroenterology. 2016;150(6):1257–61.
• Miwa H, Oshima T, Tomita T, et al. Recent understanding of the pathophysiology of functional dyspepsia: role of the duodenum as the pathogenic center. J Gastroenterol. 2019. https://doi.org/10.1007/s00535-019-01550-4 The involvement of psychological factors, diet, and H. pylori has been debated as causative factors for the pathophysiology of functional dyspepsia. New evidence suggests that the symptoms of functional dyspepsia may be brought on by alterations in the duodenum, including low-grade inflammation and increased mucosal permeability.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Stomach and Duodenum
Rights and permissions
About this article
Cite this article
Galura, G.M., Chavez, L.O., Robles, A. et al. Gastroduodenal Injury: Role of Protective Factors. Curr Gastroenterol Rep 21, 34 (2019). https://doi.org/10.1007/s11894-019-0701-x
Published:
DOI: https://doi.org/10.1007/s11894-019-0701-x