Abstract
The structural and functional integrity of the gastric and duodenal mucosa represents equilibrium between aggressive factors and protective mechanisms. Mucus-buffers-phospholipid layer as pre-epithelial barrier, enhanced by prostaglandins and epidermal growth factor, remains a vanguard of mucosal protection. It maintains a neutral pH at the surface epithelial luminal interface, facing luminal pH dropping to 1.0, i.e., hydrogen ion concentration gradient equal 1,000,000. The surface epithelial cells, elaborating mucins, buffers, phospholipids, prostaglandins, trefoil peptides, peptide growth factor and their receptors, heat shock proteins, cathelicidins, and β-defensins form the second line of defense. Endothelium exerts mucosal protection through production of potent vasodilators like nitric oxide and prostacyclins and through release of angiogenic growth factors, securing adequate blood flow and representing the third and an ultimate line of mucosal protection. This microcirculation is instrumental for supply of oxygen, nitric oxide, hydrogen sulfide and removal of ad hoc generated toxic substances as well as for continuous mucosal cell renewal from progenitor cells, secured by growth factors accompanied by survivin preventing early apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The upper alimentary tract especially stomach and its two anatomical neighbors, duodenum and esophagus, are unique in terms of their physiological challenge that quite often turn into pathophysiology. This phenomenon takes place owing to a capacity of the gastric mucosa to secrete gastric acid resulting in hydrogen (H+) ion concentration gradient of one million between its lowest pH 1.0 within the lumen at peak secretion and pH 7.0 within the apical aspect of the gastric mucosal surface epithelium. This H+ ion concentration gradient represents the most potent bactericidal barrier in human body.
Gastric and duodenal mucosal integrity is maintained due to a balance between the so-called aggressive factors of endogenous or exogenous in origin and a number of protective mechanisms [1]. A unique property is its ability to withstand the corrosive action of not only endogenous hydrochloric acid together with pepsins within the luminal contents but also exogenous factors such as alcohol, smoking, and drugs [1]. Although the exact nature of the protective mechanisms remains elusive, it is multi-factorial [1–3].
Aggressive endogenous factors are acid, pepsin, and refluxed bile acids. The precursor of pepsins called pepsinogens or inactive polypeptide proenzymes are produced by the chief cells and mucous neck cells of gastric mucosa. Pepsinogens can be electrophoretically separated into 7 isoenzymes. Pepsinogens 1–5 are referred as group I and pepsinogens 6 and 7 as group II. Acetylcholine, cholecystokinin, gastrin, and gastrin-releasing peptide are the most important physiologic stimulants for their secretion. These pepsinogens are converted to pepsin by gastric acid in the gastric lumen, which is optimal at a pH of 1.6 to 3.5 or auto-catalytically by already active pepsins. At pH 5.0 they are inactivated reversibly but at pH 7.0 denaturated and inactivated irreversibly. If large amounts of acid and pepsins are secreted, mucus layer will be gradually proteolytically degraded by pepsins, and the presence of H+ ions creates low pH at the cell surface causing cellular damage [4]. Refluxed bile acids like hydrophobic deoxycholic acid (DCA) contribute to cytotoxic and epithelial tissue injury of gastric and especially esophageal mucosa in the setting of duodeno-gastroesophageal reflux [5, 6, 7•]. Aggressive factors which are exogenous in origin that play an important role in mucosal damage are non-steroidal anti-inflammatory drugs (NSAIDs), smoking, alcohol consumption, and emotional stress. NSAIDs inhibit prostaglandin synthesis, thereby reducing mucus production, bicarbonate secretion, and mucosal blood flow [4].
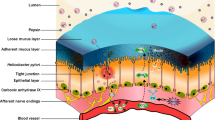
The gastric protective factors can act at various levels like mucus layer, mucosal surface epithelium, and mucosal vasculature. Despite the exposure to acid and pepsins under normal conditions, gastrointestinal mucosa maintains structural integrity and resists injury because of an entire array of defense mechanisms. They include a pre-epithelial component (mucus-buffers-phospholipid barrier) as the first line of defense [7•, 8••, 9, 10]. An epithelial component represents the second line of defense with continuous epithelial cell renewal accomplished by proliferation of progenitor cells, regulated by growth factors, prostaglandins, and survivin [7•, 8••]. Continuous blood flow through mucosal vasculature (provides oxygen and nutrients) and the endothelial microvascular barrier is recognized as the third line of defense [7•, 8••, 9]. This article aims at the review of various protective factors and their contribution towards the protection of gastric and duodenal mucosa against mucosal injury (summarized in Fig. 1 and Table 1) [11].
This illustration depicts the major protective factors of gastric and duodenal mucosa (from reference 9 and 32). (1) Mucus layer constitutes mucin, phospholipids, bicarbonate and non-bicarbonate buffers, forming mucus-buffers-phospholipids layer, a pre-epithelial barrier. (2) Surface epithelial cells are interconnected by tight junctions forming a selectively permeable barrier preventing back diffusion of acid and pepsin. They form second line of defense. (3) Continuous epithelial cell renewal from mucosal progenitor cells. (4) Alkaline tide is created by the concurrent secretion of bicarbonate by gastric parietal cells from the basolateral membrane, during acid secretion. (5) Mucosal vasculature provides oxygen and nutrition, forms endothelial barrier, and generates potent vasodilators such as nitric oxide and prostacyclin, which protect from mucosal injury. (6) Sensory nerves sense the mucosal acid and activate the secretion of bicarbonate secretion. Sensory nerve stimulation leads to the release of neurotransmitter calcitonin gene related peptide (CGRP), which causes vasodilation and enhances mucosal blood flow. (7) Prostaglandins enhance all mucosal defensive mechanisms and maintain mucosal integrity
Protective Factors of the Mucus Layer
The mucus-buffers-phospholipid layer is the first line of protection that tenaciously adheres to the epithelial surfaces [2, 12, 13]. The surface mucus maintains a dynamic equilibrium with the preformed intracellular mucus within the secretory granules of the surface epithelial and crypt cells, forming the “mucous barrier” [2, 14, 15]. The major protective components of this layer is constituted by mucus glycoprotein so-called mucin, surfactant phospholipids, prostaglandins, bicarbonate-forming mucus-buffers-phospholipids trio accompanied by non-bicarbonate phosphate buffers and peptide growth factor (EGF) [9].
Mucus Gel
Mucus gel is generated by mucin granules through their apical expulsion from surface epithelial cells and it contains 95 % water, 5 % mucin as products of mucin genes (MUC2, MUC5AC, MUC5B, and MUC6). The gel-forming mucin units polymerize into large mucin multimers essential for gel formation [9, 10, 16–18]. The efficiency of the mucus depends on its gel structure and thickness of the adherent mucus layer [19]. MUC5AC and MUC6 are gastric mucin gene products in alternate layers of the mucus layer [19, 20]. This gel layer provides a structural protection by creating a stable, unstirred layer with imbedded buffers to support surface neutralization of acid and also prevents the potential diffusion of luminal pepsin molecules reaching the underlying epithelium [19]. In addition, gastric mucin molecules are structurally bound to fatty acids making them more hydrophobic thus slowing the H+ ion back diffusion.
Phospholipids
Lipids account for up to 25 % of the dry weight of the gastric mucus and they comprise of neutral lipids, glycolipids, and phospholipids [2]. The mucus is coated with a film of surfactant phospholipids on the luminal surface which creates a potent hydrophobic nature that slows hydrogen ion diffusion [9, 10, 21]. Among the lipid components of mucus, the greatest effect on the retardation capacity of hydrogen ion diffusion is exhibited by phospholipids followed by glycolipids and neutral lipids [2, 15]. Phospholipids significantly enhance the viscosity and permeability of the mucus layer, determining the protective quality of gastric mucin [22, 23]. Ulcerogenic substances such as aspirin and bile salts disrupt the mucus gel and phospholipid layer, promoting mucosal injury [9, 24]. Furthermore, Helicobacter pylori proteolyses and lipolyses the mucin-lipid network, thus impairing defense mechanism establishing a strong causal relationship to gastritis, duodenitis, gastric ulcer, and duodenal ulcer [12, 17, 22, 25, 26].
Trefoil Factor Family (TFF) Peptides
TFF peptides are present in mucin secretory vesicles and involve in intracellular assembly and packaging of mucins [9]. They play an important role in epithelial defenses through their promotion of mucosal epithelial restitution and re-epithelialization [27•]. Three trefoil factor family peptides TFF1, TFF2, and TFF3 are expressed throughout the gastric mucosa [27•]. TFF1 is co-secreted with MUC5AC mucin in gastric epithelial cells, TFF2 with MUC6 mucin in glands of stomach and duodenum and TFF3 with MUC2 mucin in goblet cells [19]. TFF2 increases the viscosity of the mucosal layer and stabilizes the gel network [28]. Recently, Castro-Combs et al. demonstrated that impaired viscosity of gastric secretion and its mucin content might diminish lubrication within the gastrointestinal tract setting the stage for the development of symptoms related to chronic constipation [29].
Bicarbonate Buffers
The main role of bicarbonate secretion and its retention within the mucus gel layer is to neutralize the acid and pepsin [19, 30]. It forms a pH gradient from highly acidic luminal surface to neutral epithelial surface of stomach and duodenum forming the mucus-buffer barrier and acts as the first line of mucosal defense along with highly hydrophobic phospholipids [10, 19]. Mucus gel minimizes luminal loss of bicarbonate sufficiently to maintain a neutral pH at the apical surfaces [30]. Sodium-bicarbonate co-transport play an important role in importing bicarbonate at the basolateral membrane [9].
In the stomach, prostaglandin E synthase (PGES) catalyzes the conversion of prostaglandin H2 to prostaglandin E2 (PGE2) which is a key reaction [27•]. PGE2 increases intracellular calcium and cyclic adenosine monophosphate (AMP) to ultimately increase bicarbonate secretion [27•, 31]. Luminal acid, corticotrophin-releasing factor (CRF), melatonin, uroguanylin, and orexin A also stimulate bicarbonate secretion [9, 10]. Parietal cells simultaneously secrete bicarbonate along with hydrochloric acid into the mucosal interstitium and blood vessels which forms the alkaline tide [8••, 19]. Secreted bicarbonate is imported by the surface epithelial cells and significantly enhances the mucosal and surface alkalinity [8••, 19].
In duodenum, the epithelium secretes bicarbonate in addition to mucin at higher rates up to fivefold than stomach and distal small intestine, when exposed to acid and pepsin. Furthermore, melatonin released by the enterochromaffin cells of gastric mucosa enhances bicarbonate secretion through enterocyte membrane receptors [19]. Thus, mucus-buffers-phospholipid layer is an important pre-epithelial barrier with many integrated components, operating solely between lumen and epithelium. When it breaks down in any disease, the next series of protective mechanisms have to come into play, otherwise, severe injury to surface epithelium will follow [9, 19, 24].
Non-Bicarbonate Buffers
Although bicarbonate buffers represent the major neutralizing capacity of hydrogen ions and/or gastric acid, the non-bicarbonate buffers formed by various proteins/glycoproteins and mostly inorganic phosphates within the gastric and duodenal secretions play an important supportive role as well [2, 4, 17].
Protective Factors of the Mucosal Surface Epithelium
The second line of mucosal defense is formed by a continuously renewed layer of surface epithelial cells, which secrete mucus and bicarbonate, and synthesize prostaglandins, heat shock proteins, TFF peptides, antimicrobial cathelicidins, and β-defensins [8••, 9, 24]. Surface epithelial cells are interconnected by tight junctions and gap junctions forming a regulated selectively permeable or permselective “barrier” preventing back diffusion of acid and pepsin [7, 9, 10, 24, 32••]. The permeability of the surface epithelial cells is mainly regulated by apical intercellular junction referred as tight junction. Tight junction molecules contain filamentous F- actin that maintains mucosal integrity, and E-cadherin that regulates intestinal permeability forming strands between the cells [32••].
The epithelial cells are hydrophobic and repel acid and water soluble damaging agents because of presence of phospholipids on their surface [9, 10]. EGF may also regulate permeability and furthermore increases the time in which the epithelial layers resist acidification and maintains transmural epithelial electrical resistance (TEER) during the phase of acidification. The duodenal mucosa has only 5 % of TEER when compared to stomach [32••, 33].
Heat shock proteins (HSP) also contribute to the intestinal epithelial barrier [32••]. Increased temperature, stress, and cytotoxic agents stimulate the secretion of HSP by gastric epithelial cells [32••]. HSP prevents protein denaturation and promotes intestinal permeability during temperature elevation, thereby protecting cells from injury [9, 32••]. Activation of HSP is one of the major defense mechanisms of the antacid hydrotalcite [9, 24]. TFF peptides regulate re-epithelialization and play an integral role in the epithelial defenses through their promotion of mucosal restitution after injury to surface epithelium [9, 27•, 28]. Cathelicidins and β-defensins are cationic peptides that play roles in the innate defensive system at mucosal surfaces preventing bacterial colonization [24]. They have been previously demonstrated in gastric epithelial cells accelerating ulcer healing [9, 24].
Protective Factors Enhancing the Mucosal Vasculature
Continuous blood flow through the microvessels is very crucial for the function and maintenance of structural integrity of the gastrointestinal tract [7•]. The critical role of microcirculation is to deliver oxygen and nutrients to all tissues and cells and to remove ad hoc generated toxic metabolites [7•, 34, 35].
Endothelial Barrier
Endothelial cells of the mucosal vasculature are interconnected by adhesion junctions forming an endothelial “barrier” which prevents diffusion between cells [10]. A characteristic feature of gastric endothelial cells is the presence of fenestrations and pinocytotic vesicles involved in the transport of substances through the barrier [7•]. In addition, special vesicles termed Weibel-Palade bodies are present inside epithelium which stores von Williebrand factor (that helps in blood coagulation), P-selectin (that helps in the recruitment of leukocytes and migrate to the site of injury), chemokines, interleukin-8, endothelin-8, and angiopoietin-2 [7•, 36].
Angiogenesis
Angiogenesis is an excellent mechanism of repair and restoration of microvascular network through the formation of new capillaries at the site of injury. Angiogenesis is triggered by basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), and angioproteins, which are called as angiogenic growth factors [8••].
Endothelial Mediators
Endothelial cells generate potent vasodilators such as nitric oxide (NO) and prostacyclin (PGI2), which protect the gastric mucosa against injury and oppose the mucosal damaging action of vasoconstrictors such as leukotriene C4, thromboxane A2, and endothelin [3, 7•, 35]. The protective action of endothelial cells is also mediated by PGE2, carbon monoxide, tissue plasminogen activator (t-PA), VEGF, and bFGF. These protective endothelial mediators reduce adhesion of platelets and leukocytes, prevent thrombi formation, promote thrombolysis, maintain tissue perfusion, and protect the microvascular wall from acute damage [7•]. NO is responsible for maintaining gastric epithelium integrity and mucus barrier. NO inhibits hydrochloric acid secretion from parietal cells, thereby protecting the mucus barrier and gastric epithelial integrity [3, 37]. Hydrogen sulfide is another endogenously generated vasodilator that exerts strong mucosal protective action. It decreases leukocyte adherence to endothelium, reduces tumor necrosis factor α (TNF α) expression, and inhibits NSAID-induced gastric mucosal injury [3, 9, 38].
In the stomach, the presence of luminal acid increases bicarbonate secretion onto the overlying mucus layer by the mucosal microcirculation, thereby neutralizing hydrogen ions invading from the lumen [39, 40]. This protective hyperemia is mediated by stimulation of capsaicin-sensitive extrinsic sensory nerve fibers, release of calcitonin gene-related peptide (CGRP), and subsequent formation of NO, which is termed as the capsaicin pathway [32••, 39, 40].
Protective Factors Involved in Continuous Cell Renewal and Regeneration
Continuous cell renewal is one of the most important defense mechanism maintaining mucosal integrity. The epithelium is renewed every 2–4 days by a well-coordinated and controlled proliferation of the progenitor cells that replaces damaged or aged surface epithelial cells. Complete replacement of the gastric epithelium usually takes 3–7 days [3, 9, 24]. Growth factors promote mesenchymal to epithelial cell signaling to secure progenitor cell survival and thus regulate the cell proliferation of progenitor cells [41]. Re-epithelialization and gland reconstruction occur within minutes after injury by migration of preserved epithelial cells in the neck area of gastric glands to the site of injury [3, 8••, 9, 10, 42].
Growth Factors
Epidermal growth factor receptor (EGF-R) is the most important growth factor receptor expressed in progenitor cells of gastric epithelium [43]. The major mitogenic growth factors that activate this receptor are transforming growth factor-α (TGF-α) and insulin-like growth factor-1 (IGF-1) [9, 41]. PGE2 and gastrin activate EGF-R and trigger the mitogen-activated protein kinase pathway stimulating cell renewal and mucosal repair preventing the progression of the chronic atrophic gastritis to metaplasia and ultimately adenocarcinoma of the stomach [41, 44]. It has been demonstrated that gastric PGE2, EGF, and TGF-α can be tested in patients with already proven chronic atrophic gastritis, as these potential markers may be helpful to identify the disease progression to gastric adenocarcinoma during follow-up visits [45]. Furthermore, gastric EGF and its salivary component are powerful protective factors in patients with Zollinger-Ellison syndrome facing abnormally very high rate of gastric acid secretion [13].
Stress Proteins Induced by Mucosal Injury
When the mucosa is stressed by NSAIDs, inflammation, ethanol, acid, pepsin or other factors, stress proteins like matrix metalloproteinase, survivin, leptin, annexin, and heme oxygenase are induced that repair mucosal injury by a variety of mechanisms (like surface re-epithelialization) since expression of these proteins correlates with mucosal protection. Furthermore, all of these stress-induced proteins mitigate the oxidative stress imposed on the mucosa by injurious agents such as indomethacin [32••].
Survivin
Survivin is a 16.5-kDa anti-apoptosis protein from gastric progenitor cells, broad spectrum suppressor of cell death, and a protein-promoting mitosis [46]. It inhibits apoptosis by binding to caspase-3 and 7, and also inhibits caspase-independent cell death [46]. Thus, it plays an important role in protecting gastric mucosa against cell death [7•, 9]. It has been previously demonstrated that survivin expression is decreased by a non-selective NSAID like indomethacin predisposing gastric mucosal cells to greater severity of injury [46].
Prostaglandins
Prostaglandins maintain mucosal integrity and enhance almost all mucosal defensive mechanisms [9]. They regulate gastric acid and mucus-bicarbonate secretion, mucosal blood flow and motility through E-type prostaglandin (EP) receptors 1–4 that may contribute to gastric cytoprotection [9, 32••, 47]. PGE2 inhibits hydrochloric acid secretion by parietal cells directly and histamine release by enterochromaffin-like cells indirectly through EP3 receptors [32••]. PGE2 increases mucus and bicarbonate secretion through EP4 and EP1 receptors. Mucosal blood flow is increased by EP2 and EP4 receptors. EP1 receptors affect gastric motility and provide mucosal protection against gastric lesions [32••, 47, 48]. Prostaglandins also increase surface active phospholipids, accelerate epithelial restitution and mucosal healing [10]. Furthermore, they inhibit mast cell activation, leukocyte and platelet adherence to the vascular endothelium and prevent free radical formation and microvascular ischemia [9, 49, 50].
Prostaglandins are produced from arachidonic acid metabolism produced by the stomach and duodenum through the cyclooxygenase (COX) enzymes [45, 49, 50]. Cyclooxygenase-1 (COX-1) is notably involved in the gastric functional responses such as increase in the blood flow and decrease in the acid secretion even after barrier disruption [32••, 50, 51]. These functional changes after barrier disruption are gastric adaptive responses that protect mucosa against acid by disposing hydrogen ions and maintaining a favorable microclimate for cellular restitution [32••, 47, 49, 51].
The protective action of various neuropeptides and hormones like gastrin, thyrotropin-releasing hormone, cholecystokinin, estrogen, CGRP, gastrin-releasing peptide, leptin, and ghrelin against damage induced by corrosive substances, has been attributed to the release of prostaglandins and activation of sensory nerves, promoting mucosal repair and ulcer healing [24, 52–54]. Prostaglandins play a very important role in maintaining gastric mucosal integrity especially when neuronal defense mechanisms are impaired [54]. Furthermore, PGE2 inhibits gastric motility through EP1 receptors, the exact mechanism needs to be explored [32••]. PGE2 relaxes circular smooth muscle but contracts the longitudinal muscle of the stomach mediated by EP1 receptors [32••, 55].
Other Protective Factors Within Enteric Nervous System (ENS), Central Nervous System (CNS), and Endocrine System
Sensory Nerve Innervation
Gastric mucosa and submucosal vessels are innervated by primary afferent sensory neurons and nerves forming a dense plexus at the mucosal base [24]. These nerve endings can sense the luminal content and entry of acid into mucosa via acid-sensing channels [9, 32••]. Activation of these nerves directly affects the tone of the submucosal vessels, regulating the blood flow [9]. Stimulation of sensory nerves leads to the release of CGRP and substance P, which causes mucosal protection by NO-mediated vasodilation [9, 10, 39].
Neurohormonal Regulation
Nervous system and hormonal factors have a vital role in regulating gastric mucosal defense mechanisms. Central corticotropin releasing factor (CRF) signaling pathways are involved in visceral responses to stress and peripheral CRF receptors play a protective role by inhibiting apoptosis [9]. Various hormones such as gastrin, cholecystokinin, thyrotropin-releasing hormone, bombesin, and intragastric peptone exert gastroprotection through NO synthase, CGRP receptors, and afferent nerve pathways [54]. Ghrelin is a peptide hormone of the gastric mucosa that enhances mucosal expression after exposure to ethanol and exhibits a strong gastroprotection, mediated by prostaglandins [56]. It also increases gastric mucosal blood flow through stimulation of NO production and CGRP release from sensory afferent nerves [9, 57, 10].
Conclusions
The structural integrity of the gastroduodenal mucosa is constantly challenged by harmful agents like acid, pepsin, bile acids, alcohol, and drugs. Many multi-componential protective mechanisms are activated for mucosal protection. Mucus layer acts as a first line of defense against luminal pepsin by forming a pre-epithelial mucus-buffers barrier along with phospholipids. It provides a stable unstirred layer that supports surface neutralization of acid by bicarbonate buffers. PGE2 increases intracellular calcium and cyclic AMP, and ultimately stimulate bicarbonate secretion. Increased interstitial bicarbonate forms the alkaline tide enhancing the mucosal and surface alkalinity which provides further protection against acid.
Phospholipids coat the luminal surface of the mucus and retard hydrogen ion diffusion with strong hydrophobic properties. Helicobacter pylori damages this mucin-phospholipid layer through proteolysis and lipolysis, thus impairs mucosal protection. Surface epithelial cells secrete mucus, bicarbonate, prostaglandins, HSP, TFF peptides and form a second line of defense. Surface epithelial cells are interconnected by tight junctions and gap junctions forming a regulated selectively permeable “barrier” preventing back diffusion of acid and pepsin. Endothelial cell lining microvessels constitute not only an important endothelial barrier, but also facilitate the exchange of oxygen and nutrients and generation of many vasoactive substances like PGE2, VEGF, and bFGF.
Continuous cell renewal from mucosal progenitor cells is regulated by growth factors along with survivin, which promotes re-epithelialization and reconstruction. Prostaglandins maintain mucosal integrity and enhance all mucosal defense mechanisms. Elucidating and understanding these protective factors may help identify potential therapeutic targets, leading to effective prevention and treatment options for mucosal injury in the future.
The interrelationship between the first, second, and third lines of mucosal defense mechanisms in prevention as well as response to injury leading to restitution and repair of the afflicted mucosa remains to be explored setting the stage for the best health of the gastric and duodenal mucosa, thus enhancing the quality of life of our communities.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Abdel-Salam OM, Czimmer J, Debreceni A, Szolcsanyi J, Mozsik G. Gastric mucosal integrity: gastric mucosal blood flow and microcirculation. An overview. J Physiol Paris. 2001;95(1-6):105–27.
Slomiany BL, Sarosiek J, Slomiany A. Gastric mucus and the mucosal barrier. Dig Dis (Basel, Switz). 1987;5(3):125–45.
Tulassay Z, Herszenyi L. Gastric mucosal defense and cytoprotection. Best Pract Res Clin Gastroenterol. 2010;24(2):99–108.
Richardson CT. Pathogenetic factors in peptic ulcer disease. Am J Med. 1985;79(2c):1–7.
Chen X, Oshima T, Shan J, Fukui H, Watari J, Miwa H. Bile salts disrupt human esophageal squamous epithelial barrier function by modulating tight junction proteins. Am J Physiol Gastrointest Liver Physiol. 2012;303(2):G199–208.
Jurgens S, Meyer F, Spechler SJ, Souza R. The role of bile acids in the neoplastic progression of Barrett’s esophagus—a short representative overview. Z Gastroenterol. 2012;50(9):1028–34.
Tarnawski AS, Ahluwalia A, Jones MK. The mechanisms of gastric mucosal injury: focus on microvascular endothelium as a key target. Curr Med Chem. 2012;19(1):4–15. This article presents defense mechanisms of gastric mucosa concentrating on the microvascular endothelial barrier. It also demonstrates the generation of prostaglandins, nitric oxide and various other factors that protect from injury and prevent platelet and leukocyte aggregation.
Tarnawski A, Ahluwalia A, Jones MK. Gastric cytoprotection beyond prostaglandins: cellular and molecular mechanisms of gastroprotective and ulcer healing actions of antacids. Curr Pharm Des. 2013;19(1):126–32. This article highlights acute gastric mucosal injury and the concept of cytoprotection describing the mucosal protective actions of antacids, thereby causing ulcer healing.
Laine L, Takeuchi K, Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology. 2008;135(1):41–60.
Matteo F, Luca A, Rocchina C, Marco T, Corrado B. Pathophysiology of gastric ulcer development and healing: Molecular mechanisms and novel therapeutic options. In: Chai J Ed. Peptic Ulcer Disease. ISBN 978-953-307-976-9; 2011:113–42.
Konturek SJ. Mechanisms of gastroprotection. Scand J Gastroenterol Suppl. 1990;174:15–28.
Sarosiek J, Peura DA, Guerrant RL, Marshall BJ, Laszewicz W, Gabryelewicz A, et al. Mucolytic effects of Helicobacter pylori. Scand J Gastroenterol Suppl. 1991;187:47–55.
Sarosiek J, Jensen RT, Maton PN, Peura DA, Harlow D, Feng T, et al. Salivary and gastric epidermal growth factor in patients with Zollinger-Ellison syndrome: its protective potential. Am J Gastroenterol. 2000;95(5):1158–65.
Glass GB, Slomiany BL. Derangements of biosynthesis, production and secretion of mucus in gastrointestinal injury and disease. Adv Exp Med Biol. 1977;89:311–47.
Slomiany BL, Piasek A, Sarosiek J, Slomiany A. The role of surface and intracellular mucus in gastric mucosal protection against hydrogen ion. Compositional differences. Scand J Gastroenterol. 1985;20(10):1191–6.
Kang JM, Kim N, Kim B, Kim JH, Lee BY, Park JH, et al. Gastroprotective action of Cochinchina momordica seed extract is mediated by activation of CGRP and inhibition of cPLA(2)/5-LOX pathway. Dig Dis Sci. 2009;54(12):2549–60.
Allen A, Flemstrom G, Garner A, Kivilaakso E. Gastroduodenal mucosal protection. Physiol Rev. 1993;73(4):823–57.
Porchet N, Aubert JP. [MUC genes: mucin or not mucin? That is the question. Med Sci M/S. 2004;20(5):569–74.
Allen A, Flemstrom G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol Cell Physiol. 2005;288(1):C1–19.
Ho SB, Takamura K, Anway R, Shekels LL, Toribara NW, Ota H. The adherent gastric mucous layer is composed of alternating layers of MUC5AC and MUC6 mucin proteins. Dig Dis Sci. 2004;49(10):1598–606.
Lichtenberger LM. Gastroduodenal mucosal defense. Curr Opin Gastroenterol. 1999;15(6):463–72.
Gindzienski A, Zwierz K, Sarosiek J. The role of mucus and its components in protection and repair within the alimentary tract mucosa: polish experience. J Physiol Pharmacol Off J Pol Physiol Soc. 2003;54 Suppl 3:127–44.
Namiot Z, Sarosiek J, Marcinkiewicz M, Edmunds MC, McCallum RW. Declined human esophageal mucin secretion in patients with severe reflux esophagitis. Dig Dis Sci. 1994;39(12):2523–9.
Silva G, Izabel M, Florenco de Sous FC. Gastric ulcer pathology. In: Chai J Ed. Peptic Ulcer Disease. ISBN 978-953-307-976-9; 2011:3–28.
Sarosiek J, Slomiany A, Slomiany BL. Evidence for weakening of gastric mucus integrity by Campylobacter pylori. Scand J Gastroenterol. 1988;23(5):585–90.
Sarosiek J, Marshall BJ, Peura DA, Hoffman S, Feng T, McCallum RW. Gastroduodenal mucus gel thickness in patients with Helicobacter pylori: a method for assessment of biopsy specimens. Am J Gastroenterol. 1991;86(6):729–34.
Palileo C, Kaunitz JD. Gastrointestinal defense mechanisms. Curr Opin Gastroenterol. 2011;27(6):543–8. This article highlights the gastroduodenal mucosal defenses that enhance mucosal healing, focusing on pathophysiological mechanisms. Furthermore, it also describes elements that contribute to the failure of defense systems.
Thim L, Madsen F, Poulsen SS. Effect of trefoil factors on the viscoelastic properties of mucus gels. Eur J Clin Investig. 2002;32(7):519–27.
Castro-Combs J, Garcia CJ, Majewski M, Wallner G, Sarosiek J. Impaired viscosity of gastric secretion and its mucin content as potential contributing factors to the development of chronic constipation. Dig Dis Sci. 2014;59(11):2730–4.
Nassini R, Andre E, Gazzieri D, De Siena G, Zanasi A, Geppetti P, et al. A bicarbonate-alkaline mineral water protects from ethanol-induced hemorrhagic gastric lesions in mice. Biol Pharm Bull. 2010;33(8):1319–23.
Takeuchi K, Kita K, Hayashi S, Aihara E. Regulatory mechanism of duodenal bicarbonate secretion: roles of endogenous prostaglandins and nitric oxide. Pharmacol Ther. 2011;130(1):59–70.
Ham M, Akiba Y, Takeuchi K, Montrose MH, Kaunitz JD. Gastroduodenal Mucosal Defense. 2012:1169–208. This chapter summarizes the components of various defense mechanisms in detail including the physicial compositions, pH variations and various regulatory mechanisms.
Werther JL. The gastric mucosal barrier. Mt Sinai J Med N Y. 2000;67(1):41–53.
Kawano S, Tsuji S. Role of mucosal blood flow: a conceptional review in gastric mucosal injury and protection. J Gastroenterol Hepatol. 2000;15(Suppl):D1–6.
Tarnawski AS, Chai J, Jones MK. Esophageal and gastrointestinal microcirculation: essential for mucosal protection, a target for injury, and a critical component of injury and ulcer healing. In: Ishii I, Suematsu M, Tanishita K, Suzuki H, editors. Organ microcirculation: a gateway to diagnostic and therapeutic interventions. Tokyo: Springer Publisher; 2005(13): 49–61.
Bonfanti R, Furie BC, Furie B, Wagner DD. PADGEM (GMP140) is a component of Weibel-Palade bodies of human endothelial cells. Blood. 1989;73(5):1109–12.
Wallace JL. Nitric oxide, aspirin-triggered lipoxins and NO-aspirin in gastric protection. Inflamm Allergy Drug Targets. 2006;5(2):133–7.
Fiorucci S, Distrutti E, Cirino G, Wallace JL. The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology. 2006;131(1):259–71.
Abdel-Salam OM, Debreceni A, Mozsik G, Szolcsanyi J. Capsaicin-sensitive afferent sensory nerves in modulating gastric mucosal defense against noxious agents. J Physiol Paris. 1999;93(5):443–54.
Bi LC, Kaunitz JD. Gastroduodenal mucosal defense: an integrated protective response. Curr Opin Gastroenterol. 2003;19(6):526–32.
Radi ZA. Gastrointestinal Tract. In: Comparative pathophysiology and toxicology of cyclooxygenases. New Jersey: John Wiley & Sons Publishers; ISBN 9780470577547: 2012.
Lacy ER, Ito S. Rapid epithelial restitution of the rat gastric mucosa after ethanol injury. Lab Invest J Tech Methods Pathol. 1984;51(5):573–83.
Tarnawski A, Stachura J, Durbin T, Sarfeh IJ, Gergely H. Increased expression of epidermal growth factor receptor during gastric ulcer healing in rats. Gastroenterology. 1992;102(2):695–8.
Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med. 2002;8(3):289–93.
Dias A, Garcia C, Majewski M, Wallner G, McCallum RW, Poplawski C, et al. Gastric juice prostaglandins and peptide growth factors as potential markers of chronic atrophic gastritis, intestinal metaplasia and gastric cancer: their potential clinical implications based on this pilot study. Dig Dis Sci. 2011;56(11):3220–5.
Chiou SK, Tanigawa T, Akahoshi T, Abdelkarim B, Jones MK, Tarnawski AS. Survivin: a novel target for indomethacin-induced gastric injury. Gastroenterology. 2005;128(1):63–73.
Montrose MH, Akiba Y, Takeuchi K, Kaunitz JD. Gastroduodenal mucosal defense. In: Johnson LR, Barrett KE, Ghishan FK, Merchant JL, Said HM, Wood JD, editors. Physiology of the Gastrointestinal Tract. 4 th Edition. New York: Elsevier Academic Press; 2006 (1): 1259–91.
Kato S, Aihara E, Yoshii K, Takeuchi K. Dual action of prostaglandin E2 on gastric acid secretion through different EP-receptor subtypes in the rat. Am J Physiol Gastrointest Liver Physiol. 2005;289(1):G64–9.
Cryer B. Mucosal defense and repair: role of prostaglandins in the stomach and duodenum. Gastroenterol Clin N Am. 2001;30(4):877–94.
Atay S, Tarnawski AS, Dubois A. Eicosanoids and the stomach. Prostaglandins Other Lipid Mediat. 2000;61(3-4):105–24.
Takeuchi K, Kato S, Amagase K. Prostaglandin EP receptors involved in modulating gastrointestinal mucosal integrity. J Pharmacol Sci. 2010;114(3):248–61.
Konturek SJ, Konturek PC, Brzozowski T. Prostaglandins and ulcer healing. J Physiol Pharmacol Off J Pol Physiol Soc. 2005;56 Suppl 5:5–31.
Brzozowski T, Konturek PC, Konturek SJ, Brzozowska I, Pawlik T. Role of prostaglandins in gastroprotection and gastric adaptation. J Physiol Pharmacol Off J Pol Physiol Soc. 2005;56 Suppl 5:33–55.
Peskar BM. Neural aspects of prostaglandin involvement in gastric mucosal defense. J Physiol Pharmacol Off J Pol Physiol Soc. 2001;52(4 Pt 1):555–68.
Araki H, Ukawa H, Sugawa Y, Yagi K, Suzuki K, Takeuchi K. The roles of prostaglandin E receptor subtypes in the cytoprotective action of prostaglandin E2 in rat stomach. Aliment Pharmacol Ther. 2000;14 Suppl 1:116–24.
Konturek PC, Brzozowski T, Pajdo R, Nikiforuk A, Kwiecien S, Harsch I, et al. Ghrelin-a new gastroprotective factor in gastric mucosa. J Physiol Pharmacol Off J Pol Physiol Soc. 2004;55(2):325–36.
Brzozowski T, Konturek PC, Drozdowicz D, Konturek SJ, Pawlik M, Sliwowski Z, et al. Role of central and peripheral ghrelin in the mechanism of gastric mucosal defence. Inflammopharmacology. 2005;13(1-3):45–62.
Compliance with Ethics Guidelines
Conflict of Interest
Harathi Yandrapu and Jerzy Sarosiek declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors. With regard to the author’s research cited in this paper, all procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000 and 2008.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Stomach and Duodenum
Rights and permissions
About this article
Cite this article
Yandrapu, H., Sarosiek, J. Protective Factors of the Gastric and Duodenal Mucosa: An Overview. Curr Gastroenterol Rep 17, 24 (2015). https://doi.org/10.1007/s11894-015-0452-2
Published:
DOI: https://doi.org/10.1007/s11894-015-0452-2