Abstract
Cholangiocarcinoma (CCA) is a rare tumor arising from the epithelium of the intrahepatic or the extrahepatic bile ducts. It is rarely diagnosed before 40 years of age except in patients with primary sclerosing cholangitis. CCA is usually clinically silent until the tumor obstructs the bile ducts. Carbohydrate antigen 19-9 is the most commonly used tumor marker, and magnetic resonance cholangiopancreatography is the best available imaging modality for CCA. Endoscopic retrograde cholangiopancreatography and cholangioscopy allow tissue acquisition. Positron emission tomography may play a role in identifying occult metastases. Tissue diagnosis is obtained by brush cytology or bile duct biopsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cholangiocarcinoma (CCA) is a tumor arising from the epithelial cells of the intrahepatic or the extrahepatic bile ducts. It was originally reported by Durand-Fardel in 1840 [1] and is usually an adenocarcinoma with variable desmoplastic reaction. CCAs are classified according to their anatomic location as intrahepatic (ICCA), perihilar, or distal extrahepatic (ECCA). Perihilar CCAs, first described as a separate entity by Klatskin in 1965, represent 60–70% of all CCAs, whereas intrahepatic tumors represent 5–10% and distal extrahepatic tumors 20–30% [2]. CCAs are slow-growing tumors that metastasize late in their progression. They usually present with cholestasis as the tumor obstructs the bile duct [3]. The prognosis of CCA is poor because most tumors are advanced at the time of diagnosis. In the recent years, several improved diagnostic and therapeutic modalities have emerged and new targeted therapies are being developed, thus offering the potential for earlier detection and improved survival for patients with CCA.
Epidemiology
CCA accounts for about 3% of all gastrointestinal malignancies; however, it represents the second most common hepatic malignancy after hepatocellular carcinoma (HCC) [4]. The incidence of CCA varies widely in different geographic regions, with the highest in Southeast Asia and the lowest in Australia [4]. However, interpreting the available epidemiologic data has been complicated because some studies grouped ICCA with HCC, and other studies classified Klatskin tumors as ICCA.
The ICCA incidence rates vary from as high as 96 per 100,000 in men and 38 per 100,000 in women in northeast Thailand, to as low as 0.2 per 100,000 among Australian men and 0.1 per 100,000 among Australian women [4]. Epidemiologic studies suggest the incidence of ICCA has been increasing around the world [5]. In the United States, the age-adjusted incidence rates of ICCA increased from 0.32 per 100,000 between 1975 and 1979 to 0.85 per 100,000 between 1995 and 1999 [4, 5]. Mortality from ICCA has been increasing as well [6]. Khan et al. [6] noted a trend of increasing ICCA mortality between 1979 and 1997 in several countries, including the United States, Japan, Australia, England and Wales, Scotland, France, and Italy. In the United States, the age-adjusted mortality rates for ICCA increased from 0.07 per 100,000 in 1973 to 0.69 per 100,000 in 1997 [5]. Shaib and El-Serag [4] examined the temporal trends in survival for patients diagnosed with ICCA between 1975 and 1999. Although the 1-year relative survival increased from 16.4% in 1975–1979 to 27.6% in 1995–1999, the 5-year survival did not show any significant change and has remained below 5% [4].
Strom et al. [7] reported incidence rates of ECCA that varied between 0.53 per 100,000 in the United Kingdom and 1.14 per 100,000 in Manitoba, Canada. In the United States, the age-adjusted incidence of ECCA was 1.2 per 100,000 for men and 0.8 per 100,000 for women as reported by the Surveillance, Epidemiology, and End Results Program (SEER) registry between 1973 and 1987 [8]. In contrast to ICCA, however, there is evidence that the incidence of ECCA has been decreasing over time. The age-adjusted incidence of ECCA decreased from 1.08 per 100,000 in 1979 to 0.82 per 100,000 in 1998 [4]. The mortality related to ECCA has been decreasing in many areas around the world [6]. In the United States, the age-adjusted mortality rates decreased from 0.6 per 100,000 in 1979 to 0.3 per 100,000 in 1998 [5]. Carriaga and Henson [8] reported that the 5-year survival of ECCA patients increased from 11.7% in 1973–1977 to 15.1% in 1983–1987.
Worldwide, the average age at presentation of CCA is 50 years. However, there is a shift toward an older age at diagnosis, with most cases diagnosed in people more than 65 years old [4].
Ethnic variations in the prevalence of CCA was highlighted in a study by McLean and Patel [9] that examined racial and ethnic variations in the epidemiology of ICCA in the United States. The age-adjusted prevalence was highest for Hispanics (1.22 per 100,000) and lowest for African Americans (0.3 per 100,000) [9].
Risk Factors
In most cases, no etiology of CCA can be identified. However, the association between CCA and conditions leading to chronic biliary tract inflammation and cholestasis is well established. Several conditions have been clearly identified as risk factors for CCA; others have been shown to be associated with CCA in epidemiologic studies (Table 1).
Primary sclerosing cholangitis (PSC) is a major risk factor for CCA in the western world. The lifetime risk of CCA in patients with PSC ranges from 7% to 20%. The annual incidence rate for CCA in the setting of PSC is 0.6–1.5% [10]. The average age at diagnosis of CCA is generally lower in patients with PSC, and has been reported to be in the mid 40s [11]. CCA is usually diagnosed early after the diagnosis of PSC. In a long-term follow-up of 305 PSC patients, Broome et al. [12] reported 24 cases of CCA. The average time from PSC diagnosis to CCA diagnosis was 33 months. No difference was noted in the duration of PSC between patients who developed CCA and those who did not [12]. The risk of CCA in PSC is not related to the presence of concomitant inflammatory bowel disease [12].
Infection with liver flukes—particularly Opisthorchis viverrini and to a lesser extent Clonorchis sinensis, which are endemic in some areas of Southeast Asia—has been associated with the development of CCA [13]. Humans are infected by eating undercooked fish. Adult worms inhabit the biliary system and lay eggs there. Potential risk factors for CCA were investigated in a case-control study (103 cases) among inhabitants of northeast Thailand, which is endemic for O. viverrini and has the highest incidence rate of CCA in the world. In this study, a clear association with past or present infection with O. viverrini, as indicated by raised serum antibodies, was found (OR 5.0), and at least two thirds of cases can be attributed to this cause [13].
Hepatolithiasis is rare in the western world, but it is more common in several areas of Asia including China, Korea, and Japan. Hepatolithiasis seems to be associated particularly with peripheral ICCA. In a study from Taiwan, 69% of patients undergoing hepatic resection for CCA had hepatolithiasis [14]. It is believed that biliary stasis and recurrent infections in people with hepatolithiasis lead to chronic inflammation and carcinogenesis.
Choledochal cysts and Caroli’s disease have been associated with an increased risk of CCA. The incidence of malignancy increases with age. In a recent systematic review, the incidence of CCA in patients with biliary cysts ranged from 10% to 30%. The mean age at diagnosis was 32 years [15].
An increased risk of CCA has also been reported in patients with a history of biliary-enteric drainage procedures for benign disease [16].
Thorotrast, a radiologic contrast medium containing 232-Thorium dioxide, an α-particle emitter (used extensively before being banned in the 1950s), was associated with an increased incidence of CCA. Malignancy usually develops 30–35 years after exposure [17]. Several other toxins, including dioxin and polyvinyl chloride, have been correlated with an increased risk of CCA [18, 19].
Cirrhosis from any cause was found to be associated with a tenfold increase in the risk of CCA in a cohort study from Denmark [20]. Several studies have noted an association between hepatitis C and CCA [21]. A less compelling association has been suggested with hepatitis B [22].
Pathogenesis
In recent years, molecular and genetic studies provided better insight into the molecular pathogenesis of CCA. It is widely believed that cholestasis and chronic inflammation are important factors. They seem to provide an environment favorable for DNA damage and carcinogenesis [23]. Several mutations in oncogenes and tumor suppressor genes have been identified in CCA. Mutations of the oncogene K-ras have been found in 20–100% of histopathologically proven CCA [24, 25], and mutations in the tumor suppressor gene p-53 are present in 20–80% of ICCA [24, 26]. Recent studies have demonstrated that cholangiocytes release cytokines, such as interleukin 6 (IL-6), transforming growth factor-β (TGF-β), IL-8, tumor necrosis factor-α (TNF-α), and platelet-derived growth factor (PDGF). These factors can interact with cholangiocytes in an autocrine and paracrine fashion to regulate cholangiocyte intracellular signaling responses, which are thought to be altered during cholangiocarcinogenesis [27]. Cytokines, such as IL-6, appear to play an important role in cholangiocyte evasion of apoptosis. Cytokines also activate inducible nitric oxide synthase (iNOS) in cholangiocytes. This activation results in the generation of nitric oxide, which, along with other reactive oxygen species, may alter DNA bases, leading to direct DNA damage and triggering the downregulation of DNA repair mechanisms [28]. In addition, alterations of the expression of adhesion molecules and angiogenic factors have been described, further mediating tumor invasion and spread [27].
Diagnosis
Clinical Presentation
CCA usually remains clinically silent until reaching an advanced stage. The ECCAs usually present with biliary obstruction and jaundice, which occurs in more than 90% of patients. Common symptoms include pruritus, weight loss, abdominal pain, and fever. Cholangitis occurs in 10% of cases [1]. ICCAs usually present as an intrahepatic mass causing dull right upper quadrant pain and weight loss.
Ultrasonography
Transabdominal ultrasound is frequently the first radiologic study ordered for the evaluation of cholestasis. Ultrasound is accurate in the diagnosis of biliary obstruction; however, its findings are nonspecific and its value in the diagnosis of CCA is limited [29].
Computed Tomography
CT scanning is widely available and frequently obtained in patients with suspected hepatic and biliary malignancy. Contrast-enhanced CT is sensitive for detecting intrahepatic bile duct tumors, the level of biliary obstruction, and the presence of liver atrophy. In addition, CT may also permit visualization of the pertinent nodal basins. Performance of a triple-phase helical CT will detect essentially all CCAs greater than 1 cm [30]. However, CT is limited in its ability to determine intraductal tumor spread. It may only be able to establish resectability in about 60% of patients [31]. CT has a limited sensitivity for N2 nodal disease [32].
MRI and Magnetic Resonance Cholangiopancreatography
In recent years, MRI with magnetic resonance cholangiopancreatography (MRCP) has become the imaging method of choice for the diagnosis of CCA [29]. On MRI, CCAs appear hypointense on T1-weighted images, and hyperintense on T2-weighted images. Central hypointensity can be seen on T2-weighted images, and corresponds to fibrosis. On dynamic MRI, CCAs show moderate peripheral enhancement followed by progressive and concentric filling in the tumor with contrast material. Pooling of contrast within the tumor on delayed MRI images is suggestive of peripheral CCA [33]. Several studies, including a meta-analysis, have shown that MRI has a sensitivity and a specificity greater than 90% in diagnosing biliary obstruction [34]. The accuracy of MRI in diagnosing a malignant biliary stricture is lower and variable, according to the authors (sensitivity of 48–88% and specificity of 71–95%) [34]. Because of its intrinsic high tissue contrast and multiplanar capability, MRI and MRCP are able to detect and preoperatively assess patients with CCA, investigating all involved structures such as bile ducts, vessels, and hepatic parenchyma [33].
Endoscopic Retrograde Cholangiopancreatography and Percutaneous Transhepatic Cholangiography
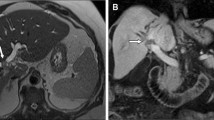
Although MRCP has emerged as a less invasive method for the evaluation of the biliary tree, endoscopic retrograde cholangiopancreatography (ERCP) (Fig. 1) and percutaneous transhepatic cholangiography (PTC) have the advantage of allowing therapeutic interventions, such as stenting, as well as tissue sampling for pathologic and cytologic evaluation. Brush cytology has a high specificity of nearly 100%, but sensitivity is much lower, ranging from 18% to 60% in most series [35, 36]. The sensitivity of brush cytology can be further improved by detecting chromosomal abnormalities with advanced cytologic methods, including digital image analysis and fluorescence in situ hybridization [37].
Cholangioscopy
Cholangioscopy uses visible light to directly visualize the biliary tract lumen, and may be used to determine whether a focal biliary stricture is benign or malignant. Typical signs for malignancy, including mucosal ulcerations, irregular mucosa, and asymmetric stricture, may be visible. The use of peroral cholangioscopy increases the diagnostic accuracy of ERCP for assessing malignancy in biliary tract strictures. In a study published by Shah et al. [38] in 2006, sensitivity was 89% and specificity was 96% for cholangioscopy to detect malignancy. Most recently, a single-operator peroral cholangiopancreatoscopy system known as SpyGlass (SpyGlass Direct Visualization System; Microvasive Endoscopy, Boston Scientific Corp., Natick, MA) has been developed [39•]. In a feasibility study, the preliminary sensitivity and specificity of Spyglass to detect malignancy were 71% and 100%, respectively [39•].
Endoscopic Ultrasound and Fine Needle Aspiration
Endoscopic ultrasound (EUS) complements the role of ERCP in the evaluation of biliary strictures and may provide tissue diagnosis through fine needle aspiration (FNA). It proves to be a valuable tool in patients with ERCPs that are nondiagnostic for malignancy. It also provides visualization of the regional lymph nodes, and FNA of these nodes could serve both diagnostic and staging purposes. In patients with suspected hilar CCA and negative brush cytology, EUS-FNA had accuracy of 91%, sensitivity of 89%, and specificity of 100% [40]. In another study evaluating the yield of EUS-FNA and its impact on patient management for patients with suspected CCA, sensitivity was 86%, specificity was 100%, positive predictive value was 100%, negative predictive value was 57%, and accuracy was 88%. EUS-FNA had a positive impact on patient management in 84% of patients [41].
Positron Emission Tomography
Positron emission tomography (PET) using the radionucleotide tracer 18-fluorodeoxyglucose has evolved into a useful staging technique in many neoplastic disorders. Several studies have shown sensitivity and specificity greater than 90% for PET in the diagnosis of CCA [42]. PET has a reported specificity of 80% to 100% [42, 43]. A much lower specificity of 33% has been reported in ECCAs [44]. The sensitivity of PET in the detection of local lymph node metastasis is low (12–38%) [42, 44]. On the other hand, PET appears to be the best technique in detecting distant metastases, especially when using integrated PET and CT (PET/CT). PET/CT had a sensitivity of 94–100% in detecting distal metastasis [44].
Tumor Markers
The most studied and the most used tumor markers in clinical practice are the carbohydrate antigen 19-9 (CA 19-9) and the carcinoembryonic antigen (CEA). Both CEA and CA 19-9 can be elevated in CCA [45, 46]. However, CEA levels alone are neither sensitive nor specific for CCA [47]. In a retrospective study of 208 patients with sclerosing cholangitis, including 14 patients with CCA, CA 19-9 had a sensitivity of 79% and a specificity of 98% for diagnosing CCA, with a cut-off value of 129 U/mL [48]. CA 19-9 can also be elevated in other malignancies and in cholangitis [49].
Staging
Staging of CCA is according to the American Joint Committee on Cancer Staging (AJCCS) tumor, nodes, and metastases (TNM) system. This classification is a pathologic staging system, and it is not useful for preoperative staging. It predicts neither resectability nor survival [50].
Perihilar tumors can be classified according to the Bismuth classification, which stratifies the patients based on the location and extent of the cancer in the biliary tree.
Currently, no useful clinical staging system exists for ICCAs. The AJCCS TNM classification for primary liver cancer is applied to both HCC and ICCA, but is of little practical value.
Conclusions
CCA is a rare tumor with a poor prognosis. Despite advances in diagnostic techniques and new therapeutic options, 5-year survival for CCA continues to be less than 5%. This is mainly attributed to the delayed diagnosis, because the tumor remains silent until it is advanced and obstructs the bile duct. Diagnosis and accurate staging are improved with better imaging and advanced cytologic techniques. Cholangioscopy allowed visualization of this tumor, and made it more accessible for diagnostic and therapeutic inventions.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Olnes MJ, Erlich R: A review and update on cholangiocarcinoma. Oncology 2004, 66(3):167–79.
Nakeeb A, Pitt HA, Sohn TA, et al.: Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg 1996, 224(4):463–73; discussion 473–5.
Malhi H, Gores GJ: Cholangiocarcinoma: modern advances in understanding a deadly old disease. J Hepatol 2006, 45(6):856–67.
Shaib Y, El-Serag HB: The epidemiology of cholangiocarcinoma. Semin Liver Dis 2004, 24(2):115–25.
Patel T: Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001, 33(6):1353–7.
Khan SA, Taylor-Robinson SD, Toledano MB, et al.: Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol 2002, 37(6):806–13.
Strom BL, Hibberd PL, Soper KA, et al.: International variations in epidemiology of cancers of the extrahepatic biliary tract. Cancer Res 1985, 45(10):5165–8.
Carriaga MT, Henson DE: Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer 1995, 75(1 Suppl):171–90.
McLean L, Patel T: Racial and ethnic variations in the epidemiology of intrahepatic cholangiocarcinoma in the United States. Liver Int 2006, 26(9):1047–53.
Burak K, Angulo P, Pasha TM, et al.: Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol 2004, 99(3):523–6.
Chalasani N, Baluyut A, Ismail A, et al.: Cholangiocarcinoma in patients with primary sclerosing cholangitis: a multicenter case-control study. Hepatology 2000, 31(1):7–11.
Broome U, Olsson R, Loof L, et al.: Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut 1996, 38(4):610–5.
Parkin DM, Srivatanakul P, Khlat M, et al.: Liver cancer in Thailand. I. A case-control study of cholangiocarcinoma. Int J Cancer 1991, 48(3):323–8.
Chen MF: Peripheral cholangiocarcinoma (cholangiocellular carcinoma): clinical features, diagnosis and treatment. J Gastroenterol Hepatol 1999, 14(12):1144–9.
Soreide K, Soreide JA: Bile duct cyst as precursor to biliary tract cancer. Ann Surg Oncol 2007, 14(3):1200–11.
Tocchi A, Mazzoni G, Liotta G, et al.: Late development of bile duct cancer in patients who had biliary-enteric drainage for benign disease: a follow-up study of more than 1,000 patients. Ann Surg 2001, 234(2):210–4.
Sahani D, Prasad SR, Tannabe KK, et al.: Thorotrast-induced cholangiocarcinoma: case report. Abdom Imaging 2003, 28(1):72–4.
Bond GG, McLaren EA, Sabel FL, et al.: Liver and biliary tract cancer among chemical workers. Am J Ind Med 1990, 18(1):19–24.
Walker NJ, Crockett PW, Nyska A, et al.: Dose-additive carcinogenicity of a defined mixture of “dioxin-like compounds”. Environ Health Perspect 2005, 113(1):43–8.
Sorensen HT, Friis S, Olsen JH, et al.: Risk of liver and other types of cancer in patients with cirrhosis: a nationwide cohort study in Denmark. Hepatology 1998, 28(4):921–5.
Okuda K, Nakanuma Y, Miyazaki M: Cholangiocarcinoma: recent progress. Part 1: epidemiology and etiology. J Gastroenterol Hepatol 2002, 17(10):1049–55.
Donato F, Gelatti U, Tagger A, et al.: Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case-control study in Italy. Cancer Causes Control 2001, 12(10):959–64.
Jaiswal M, LaRusso NF, Gores GJ: Nitric oxide in gastrointestinal epithelial cell carcinogenesis: linking inflammation to oncogenesis. Am J Physiol Gastrointest Liver Physiol 2001, 281(3):G626–34.
Tannapfel A, Benicke M, Katalinic A, et al.: Frequency of p16(INK4A) alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut 2000, 47(5):721–7.
Su WC, Shiesh SC, Liu HS, et al.: Expression of oncogene products HER2/Neu and Ras and fibrosis-related growth factors bFGF, TGF-beta, and PDGF in bile from biliary malignancies and inflammatory disorders. Dig Dis Sci 2001, 46(7):1387–92.
Furubo S, Harada K, Shimonishi T, et al.: Protein expression and genetic alterations of p53 and ras in intrahepatic cholangiocarcinoma. Histopathology 1999, 35(3):230–40.
Berthiaume EP, Wands J: The molecular pathogenesis of cholangiocarcinoma. Semin Liver Dis 2004, 24(2):127–37.
Jaiswal M, LaRusso NF, Burgart LJ, et al.: Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res 2000, 60(1):184–90.
Khan SA, Davidson BR, Goldin R, et al.: Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut 2002, 51 Suppl 6:VI1–9.
Valls C, Guma A, Puig I, et al.: Intrahepatic peripheral cholangiocarcinoma: CT evaluation. Abdom Imaging 2000, 25(5):490–6.
Feydy A, Vilgrain V, Denys A, et al.: Helical CT assessment in hilar cholangiocarcinoma: correlation with surgical and pathologic findings. AJR Am J Roentgenol 1999, 172(1):73–7.
Lee HY, Kim SH, Lee JM, et al.: Preoperative assessment of resectability of hepatic hilar cholangiocarcinoma: combined CT and cholangiography with revised criteria. Radiology 2006, 239(1):113–21.
Manfredi R, Barbaro B, Masselli G, et al.: Magnetic resonance imaging of cholangiocarcinoma. Semin Liver Dis 2004, 24(2):155–64.
Romagnuolo J, Bardou M, Rahme E, et al.: Magnetic resonance cholangiopancreatography: a meta-analysis of test performance in suspected biliary disease. Ann Intern Med 2003, 139(7):547–57.
Fogel EL, de Bellis M, McHenry L, et al.: Effectiveness of a new long cytology brush in the evaluation of malignant biliary obstruction: a prospective study. Gastrointest Endosc 2006, 63(1):71–7.
de Bellis M, Sherman S, Fogel EL, et al.: Tissue sampling at ERCP in suspected malignant biliary strictures (Part 2). Gastrointest Endosc 2002, 56(5):720–30.
Moreno Luna LE, Kipp B, Halling KC, et al.: Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology 2006, 131(4):1064–72.
Shah RJ, Langer DA, Antillon MR, et al.: Cholangioscopy and cholangioscopic forceps biopsy in patients with indeterminate pancreaticobiliary pathology. Clin Gastroenterol Hepatol 2006, 4(2):219–25.
• Chen YK, Pleskow DK: SpyGlass single-operator peroral cholangiopancreatoscopy system for the diagnosis and therapy of bile-duct disorders: a clinical feasibility study (with video). Gastrointest Endosc 2007, 65(6):832–41. This study proved the clinical feasibility of the SpyGlass Direct Visualization System (Boston Scientific) procedure. The procedure provided adequate tissue samples with a good sensitivity to diagnose malignancy.
Fritscher-Ravens A, Broering DC, Knoefel WT, et al.: EUS-guided fine-needle aspiration of suspected hilar cholangiocarcinoma in potentially operable patients with negative brush cytology. Am J Gastroenterol 2004, 99(1):45–51.
Eloubeidi MA, Chen VK, Jhala NC, et al.: Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin Gastroenterol Hepatol 2004, 2(3):209–13.
Kluge R, Schmidt F, Caca K, et al.: Positron emission tomography with [(18)F]fluoro-2-deoxy-D-glucose for diagnosis and staging of bile duct cancer. Hepatology 2001, 33(5):1029–35.
Anderson CD, Rice MH, Pinson CW, et al.: Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg 2004, 8(1):90–7.
Petrowsky H, Wildbrett P, Husarik DB, et al.: Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. J Hepatol 2006, 45(1):43–50.
Nakeeb A, Lipsett PA, Lillemoe KD, et al.: Biliary carcinoembryonic antigen levels are a marker for cholangiocarcinoma. Am J Surg 1996, 171(1):147–52; discussion 152–3.
Patel AH, Harnois DM, Klee GG, et al.: The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol 2000, 95(1):204–7.
Siqueira E, Schoen RE, Silverman W, et al.: Detecting cholangiocarcinoma in patients with primary sclerosing cholangitis. Gastrointest Endosc 2002, 56(1):40–7.
Levy C, Lymp J, Angulo P, et al.: The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci 2005, 50(9):1734–40.
Akdogan M, Sasmaz N, Kayhan B, et al.: Extraordinarily elevated CA19-9 in benign conditions: a case report and review of the literature. Tumori 2001, 87(5):337–9.
Jarnagin WR, Shoup M: Surgical management of cholangiocarcinoma. Semin Liver Dis 2004, 24(2):189–99.
Disclosure
Conflicts of interest: H. Charbel—none; F.H. Al-Kawas—consultancies, GlaxoSmithKline and Boston Scientific, honoraria, Cook, and travel expense reimbursements, Pentax and Mauna Kea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Charbel, H., Al-Kawas, F.H. Cholangiocarcinoma: Epidemiology, Risk Factors, Pathogenesis, and Diagnosis. Curr Gastroenterol Rep 13, 182–187 (2011). https://doi.org/10.1007/s11894-011-0178-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11894-011-0178-8