Abstract
Purpose of Review
Although pervasive inequities in the health outcomes of youth and young adults with type 1 diabetes (T1D) exist, the role of provider bias in these inequities is not well-understood. The purpose of this review is to synthesize evidence from existing studies on the associations between patient characteristics, provider bias, and patient health.
Recent Findings
Fourteen articles were included. Determining the extent of the effects of provider bias on patient health is limited by a lack of consensus on its definition. Experiences of provider bias (e.g., shaming, criticism) negatively affects self-esteem, relationships with medical providers, and depressive symptoms. Provider bias also impacts diabetes technology recommendations, insulin regimen intensity, and risk for life-threatening T1D complications.
Summary
Future studies are needed to develop questionnaires and interviews that better account for diverse experiences and interpretations of bias in T1D healthcare. More research is also needed to investigate mitigating factors to reduce provider bias as a way to improve psychological and physical health in individuals with T1D.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In the USA, approximately 1.6 million people have type 1 diabetes (T1D) [1], which is increasing in prevalence, mostly in individuals from Hispanic and non-Hispanic Black backgrounds [2, 3]. Individuals with T1D from low socioeconomic backgrounds and/or underrepresented groups experience suboptimal glycemia, higher rates of hospitalization for diabetic ketoacidosis (DKA) and long-term complications, and increased mortality compared to individuals with higher socioeconomic backgrounds and/or who identify as White, non-Hispanic [4, 5]. Additionally, youth with T1D from families with incomes < $60,000 have higher HbA1c than those with higher incomes, an association that exists across ethnic groups [6]. Although socioeconomic background plays a significant role in T1D health inequities, Black children from high socioeconomic backgrounds have higher HbA1c than White children from low socioeconomic backgrounds [7]. Regardless of their socioeconomic background, Black children and adults with T1D have higher HbA1c and are significantly more likely to die from T1D complications compared to their White counterparts [6,7,8]. Hispanic youth with T1D also have increased risk for T1D complications and may need higher insulin doses compared to White youth with T1D [9]. These inequities represent problematic patterns in T1D medical care that place Black, Indigenous, and People of Color (BIPOC) and individuals from low socioeconomic backgrounds at-risk for significant health complications.
To mitigate T1D complications, the American Diabetes Association recommends that individuals with T1D attend quarterly appointments with a T1D provider to evaluate and problem-solve T1D management barriers to engaging in T1D care [10]. However, non-Hispanic Black and Hispanic youth have lower T1D-related appointment attendance than non-Hispanic White youth, suggesting that there are systematic barriers to appointment attendance including limited transportation or caregiver unemployment [11]. Area deprivation (e.g., using area-based geographic estimates of socioeconomic disadvantage based on social determinants of health [e.g., education, employment, housing, poverty] to determine the extent to which living in disadvantaged neighborhoods contributes to adverse health outcomes) also influences T1D medical appointment attendance, insulin pump and CGM use, and risk of severe hypoglycemia, HbA1c, and DKA-related hospitalizations [12, 13]. Provider bias is a potential explanation for lower T1D appointment attendance in BIPOC and/or individuals from low socioeconomic backgrounds.
Provider implicit (i.e., unconscious) and explicit (i.e., conscious) biases contribute to racial, ethnic, and socioeconomic inequities in healthcare delivery through stereotyping and prejudice [14, 15]. Provider implicit bias against Black individuals with myocardial infarctions results in decreased likelihood of prescribing thrombolytic medications compared to White individuals [16]. Provider bias is also linked with higher depression and poorer life satisfaction and social integration in adults with spinal cord injury [17]. Pediatric pain providers with greater implicit pro-White bias are more likely to prescribe narcotic medications for postoperative pain for White youth compared to Black youth [18]. However, little is known about provider bias as it relates to health outcomes in T1D.
Significant inequities in prescribed insulin regimens and diabetes technology prevent individuals with T1D who are BIPOC and/or from low-income backgrounds from achieving and maintaining optimal glycemia [19]. Black individuals and those from low socioeconomic backgrounds experience higher HbA1c across the lifespan, especially those who do not use diabetes devices [20]. Moreover, provider bias regarding insurance affects whether diabetes devices are prescribed [21••]; youth and adults who are prescribed CGMs often have private insurance, and higher household income and education [22]. Further, White children are 3.6 times more likely than Black children and 1.9 times more likely than Hispanic children to use an insulin pump [7]. These differences persist across socioeconomic backgrounds and parental education; 68% of White children whose parents earned college or graduate degrees are prescribed insulin pumps compared to 34% of Black children with parents of similar educational backgrounds [7]. Similarly, 71–72% of non-Hispanic White, but only 37–40% of Hispanic and 18–28% of non-Hispanic Black adolescents and young adults use diabetes devices [19]. Additionally, negative experiences of and/or discrimination against individuals with T1D are related to less technology use in non-Hispanic White individuals and those from low socioeconomic backgrounds [23]. Finally, medical mistrust stemming from experiences of racism in non-Hispanic White individuals may contribute to disparities in diabetes device usage between Black and non-Hispanic White youth [24••].
While research examining health inequities in individuals with T1D has grown significantly, associations between provider bias and health outcomes of individuals with T1D have not been synthesized. To inform clinical practice and future research, the current review elucidates the association between provider bias and the physical and psychological health of youth and young adults with T1D.
Materials and Methods
Review Design and Study Selection
The methodology for the current systematic review is in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [25]. PubMed, PsycINFO, CINAHL, Embase, and Web of Science databases were searched on 03/30/2022 and 04/05/2022. Search results were limited to English-language publications without restriction on study period or geographic location. Search terms relating to diabetes, provider bias, health outcomes, and children were used (see Table 1 in Supplemental Materials). Database-specific MeSH-equivalent terms were used when applicable to increase the breadth of search results. Studies were limited to peer-reviewed articles examining the impacts of provider bias on physical and/or psychological health outcomes in youth and young adults with a mean age between 12 and 35 years. Single case studies, measure validation studies, studies of individuals without T1D, systematic reviews, and meta-analyses were excluded. Search results were imported into Covidence, a record management tool for systematic reviews, wherein duplicate articles were automatically removed. Two independent reviewers (SWW, ACH) each screened 100% of the articles for eligibility based on titles and abstracts. All eligible articles were then full-text reviewed by SWW and ACH who met to resolve any discrepancies. Following the selection of articles, SWW conducted forward and backward reference searches.
Data Extraction and Quality Assessment
SWW and ACH extracted data from included studies using a standardized data extraction log embedded in Covidence: study location, design, aims, funding source(s), author-disclosed conflicts of interest, study sample characteristics, demographic information, assessed outcome information, study findings, covariates included in the study model, and study limitations. Qualitative articles were assessed for quality using the Critical Appraisal Skills Programme Qualitative Studies Checklist [26]. The quality of quantitative studies was assessed using the Study Quality Assessment Tools established by the National Heart, Lung, and Blood Institute [27]. Studies using mixed methods were evaluated for quality using both tools; scores were averaged to determine study quality. Every article was evaluated independently by both SWW and ACH using a standardized rating form (i.e., good = low risk of bias, fair = moderate risk of bias, or poor = high risk of bias) to assess study design-specific methodology and results reporting; rating disagreements were resolved through discussion. We used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework [28] to assess the quality of evidence in quantitative studies. We used ConQual [29] to rate confidence of the synthesized findings of qualitative studies. Study evidence begins as high but can be downgraded due to concerns about dependability and credibility of evidence.
Results
Included Articles
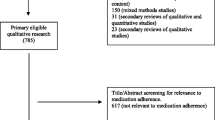
Database searches yielded 2547 nonduplicate records; following title and abstract review, 74 full-text articles were screened for eligibility. Three articles were identified in forward–backward literature searches: of these, one met inclusion criteria. In total, 14 articles met inclusion criteria and were included in the current review (see Fig. 1) [25].
Participant Characteristics
A total of 771 participants were included in this review (sample size range = 14–178). The majority of studies (n = 10, 71.4%) included data from only youth or young adults with T1D; 5 studies (35.7%) included caregivers’ data, and 4 studies (28.5%) included perspectives from healthcare providers. Race and ethnicity were inconsistently reported across studies (Table 1); 29.7% of participants were Black/African American (n = 229), 29% Hispanic/Latinx (n = 224), 24.4% White/Caucasian (n = 188), 1.1% Asian/Hawaiian/Pacific Islander (n = 8), and 0.4% identified as other race and ethnicity (n = 3). Notably, race and ethnicity were not reported in 15.4%, but these studies were conducted in other countries. Mean participant age was 25.1 years (range = 3–34 years). Average caregiver and medical provider ages were 41.2 years (range = 21–80 years) and 44.1 years old, respectively. See Table 1 for additional study and sample characteristics.
Study Characteristics and Design
All 14 articles used a cross-sectional design; 57.4% (n = 8) were qualitative with 21.4% (n = 3) using mixed methods and 21.4% (n = 3) including quantitative data. Half of the studies (n = 7) were conducted in the USA [21••, 24••, 30, 31••, 32, 40, 41]; the others were conducted in Puerto Rico [33], Tajikistan [34], Denmark [35], Ghana [38], the UK [37], and Italy [39••]. One study did not report study location [36]. Publication year ranged from 1997 to 2022; 71.4% (n = 10) were published in the previous 5 years.
Quantitative Studies: Measures of Provider Bias
Measures used to assess provider bias varied across the 3 quantitative studies [21••, 32, 41]: (1) Diabetes Provider Implicit Bias Tool includes a case vignette and providers rank-order factors influencing their recommendations about diabetes technology [21••]; (2) Perception of Racism questions from the Survey Interview Schedule [32] assess caregivers’ perceptions of racism from health care providers [42]; and (3) a 7-item questionnaire was developed specifically for one study to determine physician perceptions of family and child T1D management [41].
Quantitative Studies: Measures of Psychological and Physical Health Outcomes
Two questionnaires were used to assess psychological health outcomes in the context of provider bias: the Family Inventory of Life Events and Changes [43] and a 71-item questionnaire specifically developed for the study to determine family stress [32].
A variety of methods were used to assess physical health: (1) provider recommendations about diabetes technology [21••] in which provider bias was defined as either recommending more technology for those with private versus public insurance or ranking insurance in the top 2 of 7 factors considered when offering diabetes devices; (2) Patient Satisfaction Questionnaire [44] assessed maternal satisfaction with T1D care through two subscales, Total Access to Care, Doctor Manner and Competence, and a Total Satisfaction with Medical Care score; (3) Adherence and Insulin Dependent Diabetes Mellitus Questionnaire-R [45] was completed by mothers and youth and assessed adherence to prescribed medical regimens; (4) HbA1c during the previous 6–8 weeks [32]; and (5) physician report of prescribed diabetes regimen and its intensity based on insulin type and dosage frequency [41].
Quantitative Studies: Quality and Evidence
For all 3 quantitative studies [21••, 32, 41], quality was rated fair (moderate risk of bias). Limitations that compromised quality and increased risk of bias included use of inadequately defined or unvalidated measures [41], insufficient description of study population [21••, 32, 41], lack of power analyses to justify sample size [21••, 32, 41], and failure to assess and statistically adjust for confounding variables [21••]. See Table 1 for overall quality assessment scores.
The quality of evidence presented in all 3 quantitative studies was low; all were observational studies. No study gave cause for concern regarding limitations, inconsistency, indirectness, imprecision, or publication bias. See Supplemental Table 2 for a summary of GRADE evidence profile for quantitative studies.
Quantitative Studies: Findings
None of the quantitative studies found significant associations between demographic variables (e.g., race, ethnicity, age, insurance status, income, sex) and provider bias. One study found that Black youth are less likely to be prescribed intensive insulin regimens and diabetes devices than White, non-Hispanic youth but physician perceptions of family competence were not associated with race or ethnicity [41]. Providers who had more practice-years [21••] and who cared for families with fewer resources [32] had biases in clinical decision-making. Families experiencing higher stress were less satisfied with doctor manner and competence, have greater perceptions of racism, and demonstrate lower engagement in dietary prescriptions [32]. See Table 2 for detailed study findings.
Qualitative and Mixed-Methods Studies: Measures of Provider Bias
There were 8 qualitative and 3 mixed-methods studies (total n = 11); of these 11, 9 used semi-structured interviews to evaluate perceptions about having T1D and experiences with T1D providers [24••, 30, 31••, 34,35,36,37,38, 39••]. Of the 9 studies using semi-structured interviews, none included quantitative measures to specifically assess bias; however, each of these 9 studies included unique semi-structured interviews that were developed specifically for that study. Negative or biased interactions with providers were identified using qualitative methods.
However, one mixed-methods study [40] included a questionnaire assessing families’ perceived barriers to T1D management associated with the 5 Healthy People Social Determinants of Health. The other mixed-methods study [33] used the Adolescent Diabetes-Related Experiences Worksheet, which included 5 open-ended questions regarding adolescents’ accounts of troubling situations or discussions with healthcare professionals. See Table 2 for measures assessing provider bias.
Qualitative and Mixed-Methods Studies: Measures of Psychological and Physical Health Outcomes
Nine studies (64.3%) used interviews to identify themes such as T1D self-care, T1D management, and diabetes device use to assess impacts of provider bias on psychological and physical health outcomes [24••, 30, 31••, 34,35,36,37,38, 39••]. One study [33] used the Children’s Depression Inventory and the Diagnostic Interview Schedule for Children-IV, whereas another study [40] measured families’ T1D management (e.g., HbA1c) as an extension of the questionnaire that assessed barriers associated with the 5 Healthy People Social Determinants of Health. See Table 2 for methods used to assess psychological and physical health outcomes.
Qualitative and Mixed-Methods Studies: Quality and Evidence
Of the 8 qualitative studies, 7 were rated good (87.5%; low risk of bias [24••, 31••, 34,35,36,37, 39••]) and one was rated fair (12.5%; moderate risk of bias [38]). One study did not include a detailed description of recruitment strategies or ethical considerations, which affected the risk of bias [38]. All 3 mixed-methods studies were rated fair (moderate risk of bias [30, 33, 40]); use of unvalidated measures [30, 33, 40], insufficient description of the study population [30, 40], lack of power analyses to justify sample size [30, 33, 40], and failure to assess and statistically adjust for confounding variables [30, 33, 40] increased the risk of bias. See Table 1 for overall quality assessment scores. The quality of the synthesized findings from qualitative and mixed-methods studies was rated moderate; the majority of studies did not include a statement describing study location or an acknowledgment of their potential influence on the research. See Supplemental Table 3 for a summary of the ConQual evidence profile for qualitative and mixed-methods studies.
Qualitative and Mixed-Methods Studies: Findings
Results from the qualitative and mixed-methods studies provide inconclusive evidence of the association between demographics and provider bias in youth and young adults with T1D. Race and ethnicity, urban zone of residence, and greater family size were associated with more experiences of bias, while employment status, number of caregivers in the home, and school type (i.e., public vs. private) were not [33]. Group differences in health outcomes could not be assessed because 64.3% of studies used only interviews [24••, 30, 31••, 34,35,36,37,38, 39••].
Individuals with T1D who experience negative or biased interactions with providers (e.g., dissatisfaction with T1D care, shame about T1D management) reported feelings of self-doubt and incompetency to manage T1D care [30, 36, 37], as well as clinically significant depressive symptoms and disorders [33]. Additionally, age-related bias or misunderstandings about T1D responsibility led providers to discuss care with caregivers rather than youth, increasing self-doubt [35, 37]. Transition of care from pediatric to adult endocrinology was implicated as a time when bias occurs, with adult endocrinologists perceived as having less investment in, knowledge of, and time for young adults with T1D [39••]. Provider negative perceptions of an individual’s T1D management also significantly impacted whether recommendations for diabetes technology are made [30, 31••]; however, providers required different standards for glycemia when prescribing diabetes devices (e.g., one provider requires 7% HbA1c, whereas another provider requires 9% HbA1c). Further, provider misunderstanding or repudiation of an individual’s or family’s culture, background, and competing demands negatively affected trust with the provider and engagement with recommendations, thereby increasing glycemia [24••, 40]. Finally, limited knowledge of T1D and how its symptomatology differs from T2D emerged as an area of bias, particularly in countries with limited T1D educational resources and exposure to youth with T1D, which led to life-threatening complications [34, 38].
Discussion
Until recently, provider bias has not been considered as a contributing factor to T1D health inequities. We identified 14 articles for the current review that assessed the relationship between bias and psychological and physical health outcomes in youth and young adults with T1D. Provider bias and its impact on health is a burgeoning area of research; 10 of the 14 (71.4%) articles were published between 2017 and 2022.
Findings from this review reinforce that problematic inequities exist in the experiences and psychological and physical health outcomes of youth and young adults with T1D. Evidence was inconclusive regarding the roles of sex, age, race, and ethnicity on psychological and physical health outcomes in individuals with T1D who experienced provider bias. Some studies reported that demographic characteristics did not affect the psychological and physical health of individuals with T1D or provider bias [21••, 32], while another study reported that those of non-White race and ethnicity and older age were less likely to be prescribed diabetes technology or intensive insulin regimens [41]. More provider practice-years [21••], fewer family resources and increased neighborhood stressors [32], urban zone of residence [33], and larger family size [33] were associated with increased risk of provider bias as was caregivers’ dissatisfaction with providers’ demeanor and competence. Taken together, these mixed findings suggest that inequities in T1D medical care and psychological and physical health outcomes among youth and young adults with T1D are impacted by a variety of individual, interpersonal, systemic, and societal factors.
The majority of studies included investigated T1D physical health outcomes as they relate to provider bias. Provider bias regarding public insurance [21••] and provider perceptions of a family’s lower competence to engage in T1D self-management [31••, 41] impacts whether diabetes technology and intensive insulin regimens are prescribed, which are impacted by providers’ misunderstanding of barriers associated with a family’s ability to engage in T1D management (e.g., competing demands, financial constraints) [40]. In turn, individuals with T1D may not engage in T1D management if they perceive their provider to be biased [24••]. Additionally, medical mistrust from systemic racism in healthcare and lack of consideration for cultural foods or traditions in dietary recommendations facilitate skepticism about providers’ willingness to recommend diabetes devices [24••]. Because many studies examined similar aspects of T1D self-management (e.g., HbA1c), further research is necessary to explore other aspects of T1D management such as time in range, medical appointment attendance, hospitalizations, and episodes of DKA. Finally, misinformation and/or lack of education about T1D may cause provider bias about how T1D presents in youth and young adults, which can result in a misdiagnosis of T1D and life-threatening complications [34, 38]. It is possible that information about T1D is limited in low-resource countries due to the World Health Organization’s emphasis on T2D management [46]. In addition, individuals residing in low-resource countries are susceptible to infectious diseases which may be higher priorities [47].
This review provides evidence that provider bias negatively affects the psychological health of individuals with T1D. Only one study examined depressive symptoms [33], but providers who engaged in shaming and criticism had adversarial relationships and self-doubt and blame occurred in individuals with T1D about its management [35, 37]. Additionally, caregiver stress was associated with greater dissatisfaction with provider demeanor and competence, which was in turn associated with greater caregiver perceptions of racism [32]. One study [39••] found that providers paid little attention to the psychological health of youth during routine T1D appointments, which is exacerbated by the transition from pediatric to adult care, where providers are perceived to have less time and interest in psychological health. Across all studies, mood was not directly assessed as being related to provider bias, despite evidence that mood affects physical health [48, 49]. Thus, there is an urgent need to investigate how provider bias impacts mood specifically, but also other aspects of psychological health (e.g., anxiety, suicidality) in individuals with T1D.
No studies investigated moderating or mediating variables that may impact the relationship between provider bias and psychological and physical health outcomes in T1D. For example, prolonged exposure to discrimination in the general population may result in heightened stress reactivity, which can increase risk for negative psychological and physical health outcomes across time [50, 51]. Characteristics of healthcare settings (e.g., hospital clinics, primary care offices), intersectionality of identities, and patient-provider interactions may also moderate or mediate associations between provider bias and psychological or physical health outcomes [52, 53]. Future studies should account for histories of discrimination and other potential confounding variables such as an individual’s race and ethnicity, gender identity, age, and income to fully understand the experiences of provider bias and subsequent impacts on health. In addition, future studies should explore providers’ clinical decision-making to better understand factors that affect providers’ treatment recommendations. Further, healthcare organizations should incorporate trainings on implicit bias and cultural competence into provider continuing education, allowing them to better support individuals with T1D.
A significant limitation of the studies included in this review is the lack of consistency in how provider bias is conceptualized. Because different approaches to examining bias were used, it was difficult to determine which methods are the most useful in future research. Therefore, a questionnaire demonstrating high reliability and validity for assessing bias and a standardized, comprehensive structured interview specific to T1D are needed. Until these are developed, the Diagnostic and Statistical Manual of Mental Disorders—Fifth Edition (DSM-V) Cultural Formulation Interview provides a framework for asking culturally relevant questions (e.g., “Are there any aspects of your background or identity that make a difference to your [T1D];” “Sometimes doctors and patients misunderstand each other because they come from different backgrounds or have different expectations. Have you been concerned with this [during T1D appointments]?”) [54]. Moreover, individuals vary widely in their backgrounds, cultures, and experiences, as do their definitions of bias. For this reason, future development of bias measures should first incorporate qualitative information from individuals with T1D and strive for adaptation and validation to account for their diverse experiences.
Individual studies had methodological weaknesses including small sample sizes, lack of inclusion of study participants’ demographic characteristics, study location, confounding or intervening variables and power analyses, and cross-sectional designs hindering the ability to make inferences across studies. Future studies with robust methodology should assess group differences in experiences of provider bias and physical and psychological health outcomes to understand how these mechanisms affect individuals with T1D.
Finally, race and ethnicity are conceptualized differently in the USA, which uses specific, but flawed racial and ethnic categories compared to other countries [55]. For example, individuals from Middle Eastern countries are categorized as White, but individuals from the Middle East often do not self-identify as White [56]. In addition, because T1D disproportionately affects individuals of White European descent [57], representation of individuals from other backgrounds (e.g., Native American or Indigenous, Asian) in studies of bias is lacking. Future research in the USA should recruit diverse samples of participants in accordance with National Institutes of Health guidelines [58] for the inclusion of underrepresented groups.
Strengths of the current systematic review include use of a systematic search strategy and gold standard methods of assessing the quality of studies and evidence [28]; inclusion of quantitative and qualitative studies which provided rich data on the experiences of youth and young adults with T1D and their families; and a review sample that was heterogeneous, with the majority of participants being Black, non-Hispanic or Hispanic/Latinx. Spanning 7 countries and 3 continents, this diverse study sample allows for representation of varied accounts of provider bias.
Conclusions
The results of this review highlight the significant opportunities for future research to expand our understanding of the experiences of individuals with T1D who encounter racial and ethnic bias in their T1D medical care. Highly reliable and valid questionnaires and comprehensive semi-structured interviews to assess bias are needed as are interventions to decrease provider bias. National and international clinical practice guidelines can then be established to improve the lives of all individuals with T1D.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mulvey A. More people being diagnosed with type 1 diabetes. 2020. https://www.jdrf.org/blog/2020/02/18/more-people-being-diagnosed-type-1-diabetes/. Accessed February 27 2023.
Mayer-Davis EJ, Beyer J, Bell RA, Dabelea D, D’Agostino R Jr, Imperatore G, et al. Diabetes in African American youth: prevalence, incidence, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32 Suppl 2(Suppl 2):S112-22. https://doi.org/10.2337/dc09-S203.
Bell RA, Mayer-Davis EJ, Beyer JW, D’Agostino RB Jr, Lawrence JM, Linder B, et al. Diabetes in non-Hispanic white youth: prevalence, incidence, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32 Suppl 2(Suppl 2):S102-11. https://doi.org/10.2337/dc09-S202.
Delamater AM, Shaw KH, Applegate EB, Pratt IA, Eidson M, Lancelotta GX, et al. Risk for metabolic control problems in minority youth with diabetes. Diabetes Care. 1999;22(5):700–5. https://doi.org/10.2337/diacare.22.5.700.
Gallegos-Macias AR, Macias SR, Kaufman E, Skipper B, Kalishman N. Relationship between glycemic control, ethnicity and socioeconomic status in Hispanic and white non-Hispanic youths with type 1 diabetes mellitus. Pediatr Diabetes. 2003;4(1):19–23. https://doi.org/10.1034/j.1399-5448.2003.00020.x.
Springer D, Dziura J, Tamborlane WV, Steffen AT, Ahern JH, Vincent M, et al. Optimal control of type 1 diabetes mellitus in youth receiving intensive treatment. J Pediatr. 2006;149(2):227–32. https://doi.org/10.1016/j.jpeds.2006.03.052.
Willi SM, Miller KM, DiMeglio LA, Klingensmith GJ, Simmons JH, Tamborlane WV, et al. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135(3):424–34. https://doi.org/10.1542/peds.2014-1774.
Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–40. https://doi.org/10.2337/dc17-1600.
Redondo MJ, Libman I, Cheng P, Kollman C, Tosur M, Gal RL, et al. Racial/ethnic minority youth with recent-onset type 1 diabetes have poor prognostic factors. Diabetes Care. 2018;41(5):1017–24. https://doi.org/10.2337/dc17-2335.
Standards of care in diabetes-2023 abridged for primary care providers. Clin Diabetes. 2022;41(1):4–31. https://doi.org/10.2337/cd23-as01.
Lipman TH, Smith JA, Patil O, Willi SM, Hawkes CP. Racial disparities in treatment and outcomes of children with type 1 diabetes. Pediatr Diabetes. 2021;22(2):241–8. https://doi.org/10.1111/pedi.13139.
Auzanneau M, Lanzinger S, Bohn B, Kroschwald P, Kuhnle-Krahl U, Holterhus PM, et al. Area deprivation and regional disparities in treatment and outcome quality of 29,284 pediatric patients with type 1 diabetes in Germany: a cross-sectional multicenter DPV analysis. Diabetes Care. 2018;41(12):2517–25. https://doi.org/10.2337/dc18-0724.
Govan L, Maietti E, Torsney B, Wu O, Briggs A, Colhoun HM, et al. The effect of deprivation and HbA1c on admission to hospital for diabetic ketoacidosis in type 1 diabetes. Diabetologia. 2012;55(9):2356–60. https://doi.org/10.1007/s00125-012-2601-6.
Nosek BA, Smyth FL, Hansen JJ, Devos T, Lindner NM, Ranganath KA, et al. Pervasiveness and correlates of implicit attitudes and stereotypes. Eur Rev Soc Psychol. 2007;18(1):36–88.
Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc. 2002;94(8):666–8.
Green AR, Carney DR, Pallin DJ, Ngo LH, Raymond KL, Iezzoni LI, et al. Implicit bias among physicians and its prediction of thrombolysis decisions for black and white patients. J Gen Intern Med. 2007;22(9):1231–8. https://doi.org/10.1007/s11606-007-0258-5.
Hausmann LR, Myaskovsky L, Niyonkuru C, Oyster ML, Switzer GE, Burkitt KH, et al. Examining implicit bias of physicians who care for individuals with spinal cord injury: a pilot study and future directions. J Spinal Cord Med. 2015;38(1):102–10. https://doi.org/10.1179/2045772313y.0000000184.
Sabin JA, Greenwald AG. The influence of implicit bias on treatment recommendations for 4 common pediatric conditions: pain, urinary tract infection, attention deficit hyperactivity disorder, and asthma. Am J Public Health. 2012;102(5):988–95. https://doi.org/10.2105/ajph.2011.300621.
Agarwal S, Schechter C, Gonzalez J, Long JA. Racial-ethnic disparities in diabetes technology use among young adults with type 1 diabetes. Diabetes Technol Ther. 2021;23(4):306–13. https://doi.org/10.1089/dia.2020.0338.
Miller KM, Beck RW, Foster NC, Maahs DM. HbA1c levels in type 1 diabetes from early childhood to older adults: a deeper dive into the influence of technology and socioeconomic status on HbA1c in the T1D exchange clinic registry findings. Diabetes Technol Ther. 2020;22(9):645–50. https://doi.org/10.1089/dia.2019.0393.
•• Addala A, Hanes S, Naranjo D, Maahs DM, Hood KK. Provider implicit bias impacts pediatric type 1 diabetes technology recommendations in the United States: findings from the Gatekeeper Study. J Diabetes Sci Technol. 2021;15(5):1027–33. An investigation of factors impacting provider recommendations for diabetes technology showed that provider implicit bias significantly impacts T1D treatment recommendations.
Wong JC, Foster NC, Maahs DM, Raghinaru D, Bergenstal RM, Ahmann AJ, et al. Real-time continuous glucose monitoring among participants in the T1D exchange clinic registry. Diabetes Care. 2014;37(10):2702–9. https://doi.org/10.2337/dc14-0303.
Walker AF, Hood KK, Gurka MJ, Filipp SL, Anez-Zabala C, Cuttriss N, et al. Barriers to technology use and endocrinology care for underserved communities with type 1 diabetes. Diabetes Care. 2021;44(7):1480–90. https://doi.org/10.2337/dc20-2753.
•• Mencher SR, Weinzimer SA, Nally LM, Van Name M, Nunez-Smith M, Sadler LS. Technology utilization in black adolescents with type 1 diabetes: exploring the decision-making process. Diabetes Technol Ther. 2022;24(4):249–57. A qualitative study of factors impacting diabetes technology use in Black adolescents showed medical mistrust and provider misunderstanding of patient culture may impact use of diabetes devices.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71. https://doi.org/10.1136/bmj.n71.
(CASP) CASP. CASP qualitative studies checklist. n.d. https://casp-uk.net/casp-tools-checklists/. Accessed June 20 2022.
National Heart L, and Blood Institute (NHLBI). Study quality assessment tools. n.d. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed December 9 2019.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. https://doi.org/10.1136/bmj.39489.470347.AD.
Munn Z, Porritt K, Lockwood C, Aromataris E, Pearson A. Establishing confidence in the output of qualitative research synthesis: the ConQual approach. BMC Med Res Methodol. 2014;14:108. https://doi.org/10.1186/1471-2288-14-108.
Addala A, Igudesman D, Kahkoska AR, Muntis FR, Souris KJ, Whitaker KJ, et al. The interplay of type 1 diabetes and weight management: a qualitativestudy exploring thematic progression from adolescence to young adulthood. Pediatr Diabetes. 2019;20(7):974–85.
•• Agarwal S, Crespo-Ramos G, Long JA, Miller VA. “I didn’t really have a choice”: qualitative analysis of racial-ethnic disparities in diabetes technology use among young adults with type 1 diabetes. Diabetes Technol Ther. 2021;23(9):616–22. A qualitative analysis of patient perceptions of T1D technology use revealed exacerbating factors of disparities in pump and CGM use including perceived provider gatekeeping of information about diabetes devices and limited opportunities for shared decision making.
Auslander WF, Thompson SJ, Dreitzer D, Santiago JV. Mothers’ satisfaction with medical care: perceptions of racism, family stress, and medical outcomes in children with diabetes. Health Soc Work. 1997;22(3):190–9.
Crespo-Ramos G, Cumba-Avilés E, Quiles-Jiménez M. “They called me a terrorist”: social and internalized stigma in Latino youth with type 1 diabetes. Health Psychol Rep. 2018;6:307–20.
Haugvik S, Beran D, Klassen P, Hussain A, Haaland A. “My heart burns” – a qualitative study of perceptions and experiences of type 1 diabetes among children and youths in Tajikistan. Chronic Illn. 2017;13:128–39.
Ingersgaard MV, Hoeeg D, Willaing I, Grabowski D. An exploratory study of how young people experience and perceive living with type 1 diabetes during late adolescence and emerging adulthood. Chronic Illn. 2021;17(4):475–92.
Jeong YM, Quinn L, Kim N, Martyn-Nemeth P. Health-related stigma in young adults with type 1 diabetes mellitus. J Psychosoc Nurs Ment Health Serv. 2018;56(10):44–51.
King KM, King PJ, Nayar R, Wilkes S. Perceptions of adolescent patients of the “lived experience” of type 1 diabetes. Diabetes Spectrum. 2017;30(1):23–35.
Kratzer J. Structural barriers to coping with type 1 diabetes mellitus in Ghana: experiences of diabetic youth and their families. Ghana Med J. 2012;46(2):39–45.
•• Montali L, Zulato E, Cornara M, Ausili D, Luciani M. Barriers and facilitators of type 1 diabetes self-care in adolescents and young adults. J Pediatr Nurs. 2022;62:136–43. A qualitative evaluation of barriers and facilitators to T1D self-care revealed provider-level barriers including limited emphasis on psychological well-being and transition from pediatric to adult endocrinology.
Morone JF, Teitelman AM, Cronholm PF, Hawkes CP, Lipman TH. Influence of social determinants of health barriers to family management of type 1 diabetes in Black single parent families: a mixed methods study. Pediatr Diabetes. 2021;22(8):1150–61.
Valenzuela JM, La Greca AM, Hsin O, Taylor C, Delamater AM. Prescribed regimen intensity in diverse youth with type 1 diabetes: role of family and provider perceptions. Pediatr Diabetes. 2011;12(8):696–703.
Dressler WW. Stress and adaptation in the context of culture. New York: State University of New York Press; 1991.
McCubbin HI, Thompson AIE. Family assessment inventories for research and practice. Madison: University of Wisconsin; 1987.
Ware JE Jr, Snyder MK, Wright WR, Davies AR. Defining and measuring patient satisfaction with medical care. Eval Program Plann. 1983;6(3–4):247–63. https://doi.org/10.1016/0149-7189(83)90005-8.
Hanson CL, Henggeler SW, Burghen GA. Model of associations between psychosocial variables and health-outcome measures of adolescents with IDDM. Diabetes Care. 1987;10(6):752–8. https://doi.org/10.2337/diacare.10.6.752.
World Health Organization. Reducing the burden of noncommunicable diseases through strengthening of prevention and control of diabetes. In: Seventy-Fourth World Health Assembly. 2021. https://apps.who.int/gb/ebwha/pdf_files/WHA74-REC1/A74_REC1-en.pdf.
World Health Organization. Mid-point evaluation of the implementation of the WHO global action plan for the prevention and control of noncommunicable diseases 2013–2020. In: Seventy-Fourth World Health Assembly. 2021. https://apps.who.int/gb/ebwha/pdf_files/WHA74-REC1/A74_REC1-en.pdf.
Nguyen LA, Pouwer F, Winterdijk P, Hartman E, Nuboer R, Sas T, et al. Prevalence and course of mood and anxiety disorders, and correlates of symptom severity in adolescents with type 1 diabetes: results from diabetes LEAP. Pediatr Diabetes. 2021;22(4):638–48. https://doi.org/10.1111/pedi.13174.
Van Tilburg MA, McCaskill CC, Lane JD, Edwards CL, Bethel A, Feinglos MN, et al. Depressed mood is a factor in glycemic control in type 1 diabetes. Psychosom Med. 2001;63(4):551–5. https://doi.org/10.1097/00006842-200107000-00005.
Berger M, Leicht A, Slatcher A, Kraeuter AK, Ketheesan S, Larkins S, et al. Cortisol awakening response and acute stress reactivity in First Nations people. Sci Rep. 2017;7:41760. https://doi.org/10.1038/srep41760.
Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133(1):25–45. https://doi.org/10.1037/0033-2909.133.1.25.
Ogungbe O, Mitra AK, Roberts JK. A systematic review of implicit bias in health care: a call for intersectionality. IMC J Med Sci. 2019;13(1):005.
Govender V, Penn-Kekana L. Gender biases and discrimination: a review of health care interpersonal interactions. Glob Public Health. 2008;3(Suppl 1):90–103. https://doi.org/10.1080/17441690801892208.
American Psychiatric Association. Cultural formulation interview. In: Diagnostic and statistical manual of mental disorders, 5th edn. Arlington, VA. 2013;745–759.
Yudell M, Roberts D, DeSalle R, Tishkoff S, 70 signatories. NIH must confront the use of race in science. Science. 2020;369(6509):1313–4. https://doi.org/10.1126/science.abd4842.
Maghbouleh N, Schachter A, Flores RD. Middle Eastern and North African Americans may not be perceived, nor perceive themselves, to be White. Proc Natl Acad Sci. 2022;119(7):e2117940119. https://doi.org/10.1073/pnas.2117940119.
Divers J, Mayer-Davis EJ, Lawrence JM, Isom S, Dabelea D, Dolan L, et al. Trends in incidence of type 1 and type 2 diabetes among youths - selected counties and Indian reservations, United States, 2002–2015. MMWR Morb Mortal Wkly Rep. 2020;69(6):161–5. https://doi.org/10.15585/mmwr.mm6906a3.
Health NIo. NIH guidelines on the inclusion of women and minorities as subjects in clinical research. Fed Regist. 1994;59:1408–13.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This review does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wetter-Wren, S.E., Himelhoch, A.C. & Driscoll, K.A. A Systematic Review of the Effects of Provider Bias on Health in Youth and Young Adults with Type 1 Diabetes. Curr Diab Rep 24, 45–60 (2024). https://doi.org/10.1007/s11892-023-01527-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11892-023-01527-x