Abstract
Purpose of Review
Great strides have recently been made in elucidating the role of genetic sequence variation in diabetes pathogenesis. Increasingly, studies are focusing on other factors that may contribute to the pathogenesis of diabetes, such as epigenetics, a term “traditionally” encompassing changes to the DNA that do not alter sequence and are heritable (primary methylation and histone modification) but often expanded to include microRNAs. This review summarizes latest findings on the role of epigenetics in diabetes pathogenesis.
Recent Findings
Recent studies illustrate roles for methylation changes, histone modification, imprinting, and microRNAs across several diabetes types and complications. Notably, methylation changes in the human leukocyte antigen (HLA) region have been found to precede the development of type 1 diabetes. In type 2 diabetes, lifestyle factors appear to interact with epigenetic mechanisms in pathogenesis.
Summary
Emerging technologies have allowed increasingly comprehensive descriptive analysis of the role of epigenetic mechanisms in diabetes pathogenesis which have yielded meaningful insights into effects on expression of relevant genes. These findings have the potential to inform future development of predictive testing to enable primary prevention and further work to uncover the complex pathogenesis of diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes has become a major health problem in the USA. Recent studies report that 9.5% of the US population has diabetes and 23.8% of people with diabetes are undiagnosed according to the National Diabetes Statistics Reports, 2017. It is predicted that there will be a 54% increase in diabetes prevalence in USA by 2030 [1]. Diabetes is a major cause of complications such as renal failure and stroke. It also brings huge economic burdens to families and the society. There is an urgent need for better understanding of prevention and treatment of diabetes.
The two major types of diabetes are type 1 diabetes and type 2 diabetes. Type 1 diabetes (T1D) is an autoimmune disease, in which β cells are destroyed. The human leukocyte antigen (HLA) genotypes and more than 50 non-HLA loci have been estimated to explain more than 75% of the heritability [2]. From the appearance of the first β cell autoantibody to the onset of T1D, there is often an environmental trigger [3]. Type 2 diabetes (T2D) is a complex disease, resulting from a combination of increased insulin resistance and reduced insulin secretion. Until now, more than 100 loci have been found to be associated with T2D, accounting for less than 20% of the heritability [4]. Only recently have epigenetic mechanisms begun to be explored.
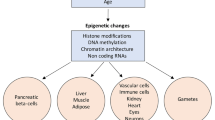
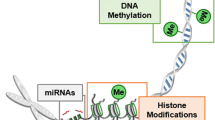
Epigenetics is a group of mechanisms of gene expression regulation, consisting of heritable changes in DNA that do not alter the DNA sequence. Epigenetic changes are often specific to developmental stages, which contributes to the regulation of cell differentiation [5]. Similarly, they are often tissue specific, for example regulating expression of gene loci in specific cell types by modifying the histone methylation status [6]. Since its discovery, epigenetics has attracted attention from many research areas, including diabetes. An epigenome-wide association study has found T1D-specific methylations that occur before the first autoantibody appears [7]. The maintenance of β cell function also depends on epigenetic regulations [8]. There are also methods developed to detect the methylation level of circulating DNA from mouse peripheral blood for monitoring β cell death. Epigenetic changes are also influenced by environmental factors including the metabolic state of the organism [9]. Epigenetic mechanisms discussed here include DNA methylation, histone modification, and microRNA (miRNA) regulation. The latter does not create heritable changes but is often grouped with epigenetic mechanisms because of its important regulatory role involving non-protein coding regions of the genome.
Diabetes and DNA Methylation
DNA methylation is the process of adding a methyl group to cytosine, and this process mostly happens in CpG islands, in which a cytosine is followed by a guanine. DNA methylation usually results in gene silencing. Many diabetes-related genes have been found to be dysregulated by methylation in patients with diabetes or model organisms, as reviewed by Wang et al. in T1D [10] and Drong et al. in T2D [11]. Comparing methylomes in case-control cohorts or monozygotic twin pairs is a powerful method for searching for epigenetic variations. Recent research has also focused on the interaction between DNA methylation and other factors such as lifestyle or environment.
Type 1 Diabetes and DNA Methylation
Comparing methylation profiling of peripheral blood within monozygotic (MZ) twin pairs discordant for T1D enables the elimination of the influence of DNA sequence heterogeneity between individuals and enables the evaluation of the impact of other factors such as epigenetic changes. Rakyan et al. performed genome-wide DNA methylation profiling on CD14+ monocytes from 15 T1D-discordant MZ twin pairs and identified 132 T1D-methylation variable positions (T1D-MVPs). These positions include the HLA class II gene HLA-DQB1, the strongest known genetic risk factor for T1D, and GAD2, which encodes the GAD65 autoantigen, which has well-established involvement in T1D etiology. They also confirmed most of the T1D-MVPs in singletons prior to T1D diagnosis and after the appearance of the autoantibody as well as just after diagnosis but before treatment initiation, providing evidence that the methylation changes precede diagnosis and are not caused by treatment [7]. Stefan et al. also found 88 MVPs in three MZ discordant twin pairs. Through a combination of methylation data and genome-wide association study (GWAS) data, they found that the differentially methylated sites include HLA-E, HLA-DOB, HLA-DQ2A, INS, IL-2RB, and CD226. These results also support the hypothesis that DNA methylation of relevant genes contributes to T1D pathogenesis [12].
The INS VNTR polymorphism is an established risk factor for T1D heritability [13], and methylation changes in INS of islet cells have also been implicated. In the non-obese diabetic (NOD) mouse model, with the progression of diabetes, increased expression of cytokines induced changes in the methylation level and expression level of Ins1 and Ins2 [14]. Although the mechanism of INS methylation on the pathogenesis of T1D has not been fully established, a correlation between DNA methylation and T1D has been observed in several studies and may prove useful in disease detection. Several studies looked at the association of DNA methylation on the INS gene and the progression of T1D or diabetic complications. Because of the β cell destruction in T1D, the DNA specifically released by the destroyed β cells shows detectable methylation differences in the insulin genes responsible for transcriptional regulation. Several groups have utilized these methylation differences as a biomarker for T1D diagnosis. Akirav et al. found an increased number of demethylated copies of the insulin gene (Ins1) circulating in mice before the development of hyperglycemia and confirmed this finding in patients with T1D [15]. Husseiny et al. developed a quantitative method for monitoring demethylated genes as a way to monitor disease progress and confirmed this observation in the mouse Ins2 gene [16]. Fisher et al. found that there were elevations of both unmethylated and methylated INS genes in individuals with T1D at disease onset. However, the unmethylated INS fell to control levels by 8 weeks after onset, and the methylated INS levels normalized by 1 year after onset [17]. Fradin et al. found that four out of seven CpGs located within the proximal INS promoter were differentially methylated between individuals with T1D and controls [18]. These findings not only could inform promising methods for early T1D diagnosis, but also highlight the importance of INS gene methylation in T1D etiology, onset, and progression.
Type 2 Diabetes and DNA Methylation
With the availability of methylation arrays and large-scale bisulfite sequencing, the whole-genome DNA methylation landscape can now be studied. Recently, the first comprehensive DNA methylation analysis of a T2D case-control cohort in pancreatic islets was completed using whole-genome bisulfite sequencing (WGBS) [19••]. Among the 25,820 differentially methylated regions (DMRs) discovered in islets from patients with T2D compared to controls, some are confirmed to have known islet functions, such as PDX1, TCF7L2, and ADCY5. There are also novel loci mapped to islet-specific transcription factor binding sites and enhancer regions. When combining DMRs with RNA-seq data, NR4A3, PARK2, PID1, SLC2A2, and SOCS2 were found to have both methylation and expression changes. Functional studies of NR4A3, PID1, SOCS2, and PARK2 in rat β cells confirmed that the altered expression impaired glucose-stimulated insulin secretion. Jeon et al. performed WGBS on blood samples from a population-based cohort and found another gene, MSI2, associated with insulin secretion and β cell proliferation [20]. This finding was replicated in T2D case-control studies in blood and pancreatic islet cells [21]. An epigenome-wide association study (EWAS) on obesity traits also found MSI2 differential methylation to be associated with body mass index (BMI) and waist circumference (WC) [22•].
DNA methyltransferases are responsible for establishing and maintaining DNA methylation patterns. One of the DNA methyltransferases, encoded by DNMT3A, was found to mediate insulin resistance in adipose tissue. Dnmt3a was shown to negatively regulate FGF21, and elevated FGF21 methylation was shown to correlate with decreased expression [23].
DNA methylation has been evaluated as a biomarker for T2D risk prediction. The ABCG1 locus cg06500161 methylation in blood was found to be associated with increased T2D risk, while the PHOSPHO1 locus cg02650017 was associated with decreased T2D risk. These findings may inform useful tools for diabetes prevention and targets to study T2D mechanisms [24].
Several researchers have investigated the association between non-genetic factors such as diet and exercise with DNA methylation. When exposed to high glucose levels, pancreatic islets exhibited changes in expression and methylation level in GLRA1, RASD1, VAC14, SLCO5A1, and CHRNA5. These genes are involved with TGF-beta signaling, Notch signaling and SNARE (Soluble NSF Attachment protein Receptors) interactions in vesicular transport, relevant pathways to islet function. Also, CpG sites in islet PDX1 showed increased methylation after glucose exposure [25]. Gillberg et al. fed young men a high-fat diet for 5 days. Differential methylation was observed in 53 sites in adipose tissue in low birth weight men as compared to normal birth weight men. However, no changes in gene expression were observed. In the whole group, high-fat feeding altered the expression of 3276 genes and altered DNA methylation at 652 CpG sites; this pattern did not differ between normal and low birth weight men [26]. PGC1α, encoded by the gene PPARGC1A, is a major regulator of energy metabolism [27]. Hypermethylation of PPARGC1A at the nucleotide 260 nucleotides upstream of the transcription start site (− 260 nt) was observed in skeletal muscle of patients with T2D [28], and it was the result of epigenetic changes due to exercise [28, 29]. Based on the level of hypermethylation at − 260 nt, participants were divided into high responders and low responders. There is a significant association between − 1 nucleosome repositioning and the PPARGC1A mRNA expression level in the high responders but not in the low responders [30]. The − 1 nucleosome repositioned itself away from − 260 nt and toward the transcription start site. This result may help explain the different responses to exercise among different people.
Diabetes and Imprinting
Imprinting is a form of transcriptional regulation in which only one parental allele of a specific gene is expressed, sometimes only in certain tissues and/or at certain developmental stages. Establishing and maintaining gene imprinting rely on DNA methylation and histone modification in the germline. There are more than 100 imprinted genes and another 100 predicted to be imprinted in human in the gene imprint database (www.geneimprint.com). Expression of imprinted genes is controlled by imprinting control regions (ICRs), regions that are contained within DMRs. Loss of imprinting (LOI) is implicated in several disorders, including as transient neonatal diabetes (TNDM), Silver-Russel syndrome, and Prader-Willi syndrome. LOI results from four major causes: deletion of the expressed copy, mutation of the expressed copy, uniparental disomy (UPD; inheritance of both copies of the imprinted gene or region from the same parent), and epimutation, i.e., changes imprinting control regions or disruption of histone modifications [31].
Imprinting has been implicated in the pathogenesis of many types of diabetes. Two thirds of TNDM cases are caused by abnormalities at an imprinted locus on chromosome 6q24. The imprinted genes PLAGL1 and HYMAI are located in this region, and normally, only the paternal allele of both genes is expressed. Duplication of the paternal allele, pUPD, and hypomethylation of the maternal allele are the major causes of overexpression of PLAGL1 and HYMAI [32]. HYMAI encodes untranslated RNA and the function is still unknown. PLAGL1 regulates cell cycle arrest and apoptosis and presumably its overexpression causes apoptosis, loss of β cell mass, and consequently reduced insulin secretion. In addition, overexpression of PLAGL1 may also influence SOCS3 and thus lead to dysregulation of insulin signaling [33].
Imprinted regions are also involved in T1D and T2D pathogenesis. The imprinted region on chromosome 14q32.2, containing the genes DLK1 and MEG3, was found to be associated with paternally inherited T1D in a GWAS [34]. Later, the methylation level of the MEG3 DMR in placenta was shown to be negatively associated with gains in weight and length in babies in the first postnatal year [35]. The genomic domain DLK1-DIO3, containing MEG3, also contains the largest miRNA cluster [36]. High-throughput sequencing of small RNAs from patients with T2D and healthy donors demonstrated that miRNAs from the DLK1-MEG3 domain are specifically expressed in β cells. However, individuals with T2D exhibited significant downregulation of DLK1-MEG3 miRNAs [37]. These down-regulated miRNAs target T2D pathogenesis-related genes such as IAPP and TP53INP1. In T2D, several genes in imprinted regions show evidence of parent-of-origin-specific effects on disease susceptibility, including KLF14, a transcription factor regulating multiple genes involved in metabolic syndrome-related phenotypes [38].
Diabetes and Histone Modification
The most common histone modifications include histone methylation, acetylation, phosphorylation, and ubiquitination. Histone methylation and acetylation are the most-well studied types. Some work [39,40,41,42,43] has been done on genome-wide or targeted region histone modification profiling on patients with diabetes or controls, but limited to few modification types due to the complexity of histone modification.
T1D-associated genes are the major targets of histone modification research. The level of acetylation of the lysine 9 of the H3 histone protein (H3K9Ac) in the upstream regions of major T1D susceptibility genes HLA-DRB1 and HLA-DQB1 was increased in patients with T1D relative to controls. This increase was further confirmed in in vitro studies [39]. CTLA4 encodes a T cell receptor that regulates apoptosis and is a non-HLA T1D susceptibility gene [40]. The CTLA4 promoter was shown to have increased demethylation of the lysine 9 of the H3 histone protein (H3K9me2) in blood lymphocytes from patients with T1D compared to controls [41].
Recent research on histone modification has focused more on the manipulation of histone modification “writers”, such as histone acetyltranferases (HATs), histone deactylases (HDACs), and the histone methyltransferase SET7 in animal models or cell lines. Many investigators have tried to understand the involvement of histone modification in diabetic complications as part of a further search for therapeutic targets. For example, SET7 expression was found to be increased in patients with T2D. Methylation of lysine 4 of H3 (H3K4me1) of the transcription factor NF-κB promoter region was also increased in patients with T2D compared to controls. Knocking down of Set7 in human endothelial cells averted H3K4me1 in the NF-κB promoter region, reducing expression of NF-κB-related oxidant and inflammatory genes [42, 43].
Diabetes and microRNAs
microRNAs (miRNAs) are non-coding molecules ranging in size from of 18 to 22 nucleotides. Through binding to target mRNAs at 3′ untranslated regions (UTR), miRNAs cause mRNA degradation or inhibition of translation decided by the degree of complementarity [44]. While some would argue that miRNA effects do not fall under the epigenetics umbrella because the modifications they cause are not known to be heritable, we include them here because the heritable component of the epigenetics definition is debated [45], and they are proving to be an important emerging component of diabetes pathogenesis. Many studies have confirmed the involvement of miRNA changes in the diabetes pathogenesis, both from T1D and T2D. Altered miRNAs level could serve as marker for diagnosis or prognosis; miRNAs and their targets could be further developed for therapeutic targets.
Type 1 Diabetes and miRNAs
Recent studies implicate miRNAs in the formation of autoimmunity and β cell dysfunction in T1D. MiR-150, miR-146a, and miR-424 were found to be decreased in individuals with T1D compared to controls and individuals with T2D. When comparing them between GADA-autoantibody positive and GADA-autoantibody negative patients, they were significantly decreased in the positive group, indicating their participation in the autoimmune process [46]. A new perspective to look at miRNAs and T1D is through the interaction of miRNAs and genetic polymorphisms. The miR-146a rs2910164 C allele and miR-155 rs767649 A allele are less frequent in T1D [47]. MiR-375, the most abundant miRNA in pancreatic islets [48], was found to be a biomarker of β cell death: its increase in blood indicates decrease of β cell mass in mice [49]. Treatment of mice with streptozotocin (STZ)-induced diabetes with the β cell protective compound phenylpropenoic acid glucoside (PPAG) reduced the STZ-induced β cell mass loss induced by STZ and normalized circulating miR-375 levels [50].
Diabetes-related complications such as cardiovascular disease (CVD), retinopathy, nephropathy, microalbuminuria, and macroalbuminuria are of interest with respect to miRNA, given their possible role as a mediator in the pathological process [51]. MiR-126 was associated with multiple vascular complications in a case-control study [52]. MiR-320a and miR-27b were found to be associated with the onset of diabetic retinopathy and its progression [53]. Further studies are needed to determine whether the miRNAs play a causal role in diabetic pathogenesis or serve as a biomarker. In patients with T1D, plasma levels of four miRNAs, miR-125b-5p, miR-365a-3p, mir-5190, and miR-770-5p, were found to be highly significantly associated with HbA1C and hypothesized to potentially contribute to diabetes complications [54].
Type 2 Diabetes and miRNAs
A systematic literature review and in silico analysis integrating recent findings on dysregulated miRNAs of T2D provided evidence that these miRNAs are involved in relevant processes including carbohydrate and lipid metabolism as well as insulin and adipokine signaling [55]. Experiments applied to animal models of diabetes or cell lines support the important role of miRNAs on insulin resistance. One pathway involved in insulin resistance is stat-induced stat inhibitor family member SOCS3-mediated insulin signaling. MiR-185 was shown to inhibit β-cell dysfunction through binding to the SOCS3 3′-UTR [56]. Later, Li et al. also found that miR-19a-3p also protects β cells from dysfunction by inhibiting SOCS3 [57]. Although both experiments were done on cell lines, and further animal model verification is needed, their results supported the role of SOCS3 in insulin signaling.
In high-fat diet-induced obese mice, the expression of miR-206 was down-regulated compared to normal mice. This observation was also seen in human hepatocytes treated with oleic acids in comparison to untreated cells. MiR-206 binds to protein tyrosine phosphatase, non-receptor type 1 (PTPN) and thus promotes insulin signaling pathway by enhancing insulin receptor (INSR) phosphorylation. It also inhibited Srebp1c transcription and further lipogenesis in hepatocytes [58]. Similarly, miR-96 upregulation in dietary obese mice impaired hepatic insulin signaling and glycogen synthesis, but by directly suppressing INSR and insulin receptor substrate 1 (IRS-1) [59].
There is emerging understanding of how miRNA interacts with non-genetic factors to influence T2D susceptibility. MiR-29a and miR-29c showed increased expression in skeletal muscle cells from patients with T2D compared to controls. In healthy men and healthy female rats, endurance exercise reduced miR-29a and miR-29c expression in skeletal muscle cells. The significance of miR-29 family in skeletal metabolism is that they connect with multiple proteins in related pathways. Both miR-29a and miR-29c decreased expression of IRS1, PIK3R3, and AKT2, which encode members of the insulin signaling pathway. Consistent with the reduced glucose uptake in human skeletal muscle cells, Glut4 expression was also decreased after miR-29a and miR-29c were overexpressed [60]. These findings support the role of exercise in reducing insulin resistance in skeletal muscle and provide evidence for the role of miRNA in this relationship. Further investigation is needed to fully understand the mechanism for this relationship.
Conclusion
There is emerging evidence of epigenetic abnormalities in individuals with diabetes, often in genetic regions previously implicated in diabetes pathogenesis. The ultimate goals of diabetes research are to prevent diabetes before it starts, make a correct diagnosis, and treat it properly after the diagnosis. Toward this goal, epigenetics research has the potential to inform prediction tools and drug targets. Additional research, including longitudinal studies, is needed to determine the causal relationship between epigenetic changes and disease status, and the mechanistic details of the modifying effect of environmental exposure.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major Importance
Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S. Diabetes 2030: insights from yesterday, today, and future trends. Popul Health Manag. 2017;20:6–12.
Groop L, Pociot F. Genetics of diabetes--are we missing the genes or the disease? Mol Cell Endocrinol. 2014;382:726–39.
Pociot F, Lernmark A. Genetic risk factors for type 1 diabetes. Lancet. 2016;387:2331–9.
Fuchsberger C, Flannick J, Teslovich TM, Mahajan A, Agarwala V, Gaulton KJ, et al. The genetic architecture of type 2 diabetes. Nature. 2016;536:41–7.
Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–32.
Aziz A, Liu QC, Dilworth FJ. Regulating a master regulator: establishing tissue-specific gene expression in skeletal muscle. Epigenetics. 2010;5:691–5.
Rakyan VK, Beyan H, Down TA, Hawa MI, Maslau S, Aden D, et al. Identification of type 1 diabetes-associated DNA methylation variable positions that precede disease diagnosis. PLoS Genet. 2011;7:e1002300.
Stankov K, Benc D, Draskovic D. Genetic and epigenetic factors in etiology of diabetes mellitus type 1. Pediatrics. 2013;132:1112–22.
Katada S, Imhof A, Sassone-Corsi P. Connecting threads: epigenetics and metabolism. Cell. 2012;148:24–8.
Wang Z, Xie Z, Lu Q, Chang C, Zhou Z. Beyond genetics: What causes type 1 diabetes. Clin Rev Allergy Immunol. 2017;52:273–86.
Drong AW, Lindgren CM, McCarthy MI. The genetic and epigenetic basis of type 2 diabetes and obesity. Clin Pharmacol Ther. 2012;92:707–15.
Stefan M, Zhang W, Concepcion E, Yi Z, Tomer Y. DNA methylation profiles in type 1 diabetes twins point to strong epigenetic effects on etiology. J Autoimmun. 2014;50:33–7.
Bell GI, Horita S, Karam JH. A polymorphic locus near the human insulin gene is associated with insulin-dependent diabetes mellitus. Diabetes. 1984;33:176–83.
Rui J, Deng S, Lebastchi J, Clark PL, Usmani-Brown S, Herold KC. Methylation of insulin DNA in response to proinflammatory cytokines during the progression of autoimmune diabetes in NOD mice. Diabetologia. 2016;59:1021–9.
Akirav EM, Lebastchi J, Galvan EM, Henegariu O, Akirav M, Ablamunits V, et al. Detection of beta cell death in diabetes using differentially methylated circulating DNA. Proc Natl Acad Sci U S A. 2011;108:19018–23.
Husseiny MI, Kuroda A, Kaye AN, Nair I, Kandeel F, Ferreri K. Development of a quantitative methylation-specific polymerase chain reaction method for monitoring beta cell death in type 1 diabetes. PLoS One. 2012;7:e47942.
Fisher MM, Watkins RA, Blum J, Evans-Molina C, Chalasani N, DiMeglio LA, et al. Elevations in circulating methylated and unmethylated preproinsulin DNA in new-onset type 1 diabetes. Diabetes. 2015;64:3867–72.
Fradin D, Le Fur S, Mille C, Naoui N, Groves C, Zelenika D, et al. Association of the CpG methylation pattern of the proximal insulin gene promoter with type 1 diabetes. PLoS One. 2012;7:e36278.
•• Volkov P, Bacos K, Ofori JK, Esguerra JL, Eliasson L, Ronn T, et al. Whole-genome bisulfite sequencing of human pancreatic islets reveals novel differentially methylated regions in type 2 diabetes pathogenesis. Diabetes. 2017;66:1074–85. Comprehensive whole-genome bisulfite sequencing analysis of DNA methylation of human islet cells from a T2D case/control study.
Szabat M, Kalynyak TB, Lim GE, Chu KY, Yang YH, Asadi A, et al. Musashi expression in beta-cells coordinates insulin expression, apoptosis and proliferation in response to endoplasmic reticulum stress in diabetes. Cell Death Dis. 2011;2:e232.
Jeon JP, Koh IU, Choi NH, Kim BJ, Han BG, Lee S. Differential DNA methylation of MSI2 and its correlation with diabetic traits. PLoS One. 2017;12:e0177406.
• Dhana K, Braun KVE, Nano J, Voortman T, Demerath EW, Guan W, Fornage M, van Meurs JBJ, Uitterlinden AG, Hofman A, Franco OH, and Dehghan A. An epigenome-wide association study (EWAS) of obesity-related traits. Am J Epidemiol. 2018. This study replicates previous results from African American on obesity-related traits, and also found novel association between DNA methylation and increased BMI in older adults.
You D, Nilsson E, Tenen DE, Lyubetskaya A, Lo JC, Jiang R, Deng J, Dawes BA, Vaag A, Ling C, Rosen ED, and Kang S. Dnmt3a is an epigenetic mediator of adipose insulin resistance. Elife. 2017; 6. https://doi.org/10.7554/eLife.30766.
Dayeh T, Tuomi T, Almgren P, Perfilyev A, Jansson PA, de Mello VD, et al. DNA methylation of loci within ABCG1 and PHOSPHO1 in blood DNA is associated with future type 2 diabetes risk. Epigenetics. 2016;11:482–8.
Hall E, Dekker Nitert M, Volkov P, Malmgren S, Mulder H, Bacos K, et al. The effects of high glucose exposure on global gene expression and DNA methylation in human pancreatic islets. Mol Cell Endocrinol. 2018;472:57–67.
Gillberg L, Perfilyev A, Brons C, Thomasen M, Grunnet LG, Volkov P, et al. Adipose tissue transcriptomics and epigenomics in low birthweight men and controls: role of high-fat overfeeding. Diabetologia. 2016;59:799–812.
Liang H, Ward WF. PGC-1alpha: a key regulator of energy metabolism. Adv Physiol Educ. 2006;30:145–51.
Barres R, Osler ME, Yan J, Rune A, Fritz T, Caidahl K, et al. Non-CpG methylation of the PGC-1alpha promoter through DNMT3B controls mitochondrial density. Cell Metab. 2009;10:189–98.
Henagan TM, Stefanska B, Fang Z, Navard AM, Ye J, Lenard NR, et al. Sodium butyrate epigenetically modulates high-fat diet-induced skeletal muscle mitochondrial adaptation, obesity and insulin resistance through nucleosome positioning. Br J Pharmacol. 2015;172:2782–98.
Bajpeyi S, Covington JD, Taylor EM, Stewart LK, Galgani JE, Henagan TM. Skeletal muscle PGC1alpha −1 nucleosome position and −260 nt DNA methylation determine exercise response and prevent ectopic lipid accumulation in men. Endocrinology. 2017;158:2190–9.
Elhamamsy AR. Role of DNA methylation in imprinting disorders: an updated review. J Assist Reprod Genet. 2017;34:549–62.
Docherty LE, Kabwama S, Lehmann A, Hawke E, Harrison L, Flanagan SE, et al. Clinical presentation of 6q24 transient neonatal diabetes mellitus (6q24 TNDM) and genotype-phenotype correlation in an international cohort of patients. Diabetologia. 2013;56:758–62.
Hoffmann A, Spengler D. Role of ZAC1 in transient neonatal diabetes mellitus and glucose metabolism. World J Biol Chem. 2015;6:95–109.
Wallace C, Smyth DJ, Maisuria-Armer M, Walker NM, Todd JA, Clayton DG. The imprinted DLK1-MEG3 gene region on chromosome 14q32.2 alters susceptibility to type 1 diabetes. Nat Genet. 2010;42:68–71.
Prats-Puig A, Carreras-Badosa G, Bassols J, Cavelier P, Magret A, Sabench C, et al. The placental imprinted DLK1-DIO3 domain: a new link to prenatal and postnatal growth in humans. Am J Obstet Gynecol. 2017;217:350.e1–350.e13.
Benetatos L, Hatzimichael E, Londin E, Vartholomatos G, Loher P, Rigoutsos I, et al. The microRNAs within the DLK1-DIO3 genomic region: involvement in disease pathogenesis. Cell Mol Life Sci. 2013;70:795–814.
Kameswaran V, Bramswig NC, McKenna LB, Penn M, Schug J, Hand NJ, et al. Epigenetic regulation of the DLK1-MEG3 microRNA cluster in human type 2 diabetic islets. Cell Metab. 2014;19:135–45.
Small KS, Hedman AK, Grundberg E, Nica AC, Thorleifsson G, Kong A, et al. Identification of an imprinted master trans regulator at the KLF14 locus related to multiple metabolic phenotypes. Nat Genet. 2011;43:561–4.
Miao F, Chen Z, Zhang L, Liu Z, Wu X, Yuan YC, et al. Profiles of epigenetic histone post-translational modifications at type 1 diabetes susceptible genes. J Biol Chem. 2012;287:16335–45.
Nistico L, Buzzetti R, Pritchard LE, Van der Auwera B, Giovannini C, Bosi E, et al. The CTLA-4 gene region of chromosome 2q33 is linked to, and associated with, type 1 diabetes. Belgian diabetes registry. Hum Mol Genet. 1996;5:1075–80.
Miao F, Smith DD, Zhang L, Min A, Feng W, Natarajan R. Lymphocytes from patients with type 1 diabetes display a distinct profile of chromatin histone H3 lysine 9 dimethylation: an epigenetic study in diabetes. Diabetes. 2008;57:3189–98.
Li Y, Reddy MA, Miao F, Shanmugam N, Yee JK, Hawkins D, et al. Role of the histone H3 lysine 4 methyltransferase, SET7/9, in the regulation of NF-kappaB-dependent inflammatory genes. Relevance to diabetes and inflammation. J Biol Chem. 2008;283:26771–81.
Paneni F, Costantino S, Battista R, Castello L, Capretti G, Chiandotto S, et al. Adverse epigenetic signatures by histone methyltransferase Set7 contribute to vascular dysfunction in patients with type 2 diabetes mellitus. Circ Cardiovasc Genet. 2015;8:150–8.
Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97.
Tammen SA, Friso S, Choi SW. Epigenetics: the link between nature and nurture. Mol Asp Med. 2013;34:753–64.
Wang G, Gu Y, Xu N, Zhang M, Yang T. Decreased expression of miR-150, miR146a and miR424 in type 1 diabetic patients: Association with ongoing islet autoimmunity. Biochem Biophys Res Commun. 2018;498:382–7.
Assmann TS, Duarte GC, Brondani LA, de Freitas PH, Martins EM, Canani LH, et al. Polymorphisms in genes encoding miR-155 and miR-146a are associated with protection to type 1 diabetes mellitus. Acta Diabetol. 2017;54:433–41.
Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–14.
Erener S, Mojibian M, Fox JK, Denroche HC, Kieffer TJ. Circulating miR-375 as a biomarker of beta-cell death and diabetes in mice. Endocrinology. 2013;154:603–8.
Song I, Roels S, Martens GA, Bouwens L. Circulating microRNA-375 as biomarker of pancreatic beta cell death and protection of beta cell mass by cytoprotective compounds. PLoS One. 2017;12:e0186480.
Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–74.
Barutta F, Bruno G, Matullo G, Chaturvedi N, Grimaldi S, Schalkwijk C, et al. MicroRNA-126 and micro−/macrovascular complications of type 1 diabetes in the EURODIAB prospective complications study. Acta Diabetol. 2017;54:133–9.
Zampetaki A, Willeit P, Burr S, Yin X, Langley SR, Kiechl S, et al. Angiogenic microRNAs linked to incidence and progression of diabetic retinopathy in type 1 diabetes. Diabetes. 2016;65:216–27.
Satake E, Pezzolesi MG, Md Dom ZI, Smiles AM, Niewczas MA, Krolewski AS. Circulating miRNA profiles associated with hyperglycemia in patients with type 1 diabetes. Diabetes. 2018;67:1013–23.
He Y, Ding Y, Liang B, Lin J, Kim TK, Yu H, Hang H, and Wang K. A systematic study of dysregulated MicroRNA in type 2 diabetes mellitus. Int J Mol Sci. 2017;18. https://doi.org/10.3390/ijms18030456.
Bao L, Fu X, Si M, Wang Y, Ma R, Ren X, et al. MicroRNA-185 targets SOCS3 to inhibit beta-cell dysfunction in diabetes. PLoS One. 2015;10:e0116067.
Li Y, Luo T, Wang L, Wu J, Guo S. MicroRNA-19a-3p enhances the proliferation and insulin secretion, while it inhibits the apoptosis of pancreatic beta cells via the inhibition of SOCS3. Int J Mol Med. 2016;38:1515–24.
Wu H, Zhang T, Pan F, Steer CJ, Li Z, Chen X, et al. MicroRNA-206 prevents hepatosteatosis and hyperglycemia by facilitating insulin signaling and impairing lipogenesis. J Hepatol. 2017;66:816–24.
Yang WM, Min KH, Lee W. Induction of miR-96 by dietary saturated fatty acids exacerbates hepatic insulin resistance through the suppression of INSR and IRS-1. PLoS One. 2016;11:e0169039.
Massart J, Sjogren RJO, Lundell LS, Mudry JM, Franck N, O'Gorman DJ, et al. Altered miR-29 expression in type 2 diabetes influences glucose and lipid metabolism in skeletal muscle. Diabetes. 2017;66:1807–18.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Haichen Zhang has no conflicts to declare.
Toni I. Pollin reports grant support from Regeneron Genetics Center.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Genetics
Rights and permissions
About this article
Cite this article
Zhang, H., Pollin, T.I. Epigenetics Variation and Pathogenesis in Diabetes. Curr Diab Rep 18, 121 (2018). https://doi.org/10.1007/s11892-018-1091-4
Published:
DOI: https://doi.org/10.1007/s11892-018-1091-4