Abstract
Type 2 diabetes (T2D) and obesity are the major public health problems. Substantial efforts have been made to define loci and variants contributing to the individual risk of these disorders. However, the overall risk explained by genetic variation is very modest. Epigenetics is one of the fastest growing research areas in biomedicine as changes in the epigenome are involved in many biological processes, impact on the risk for several complex diseases including diabetes and may explain susceptibility. In this review, we focus on the role of DNA methylation in contributing to the risk of T2D and obesity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Epigenetics: current status of knowledge
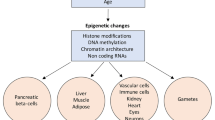
It is now well recognized that environmental factors including diet, physical activity, drugs and smoking, affect the phenotype and provide a major contribution to susceptibility to most chronic non communicable diseases [1]. Epigenetics acts at the interface between the genome and environmental factors, and might be broadly defined as the sum of all the mechanisms necessary to unfold the genetic program into development [2]. In the early 1940 s, Conrad Waddington linked genetics and developmental biology coining the term epigenetics. He defined epigenetics as “the branch of biology which studies the causal interactions between genes and their products which bring the phenotype into being” [3]. However, the meaning of the word has gradually changed over the following years, and epigenetics is known today as “the study of changes in gene function that are mitotically and/or meiotically heritable and that do not entail a change in DNA sequence” [4]. Differently from traditional genetics, based on cell lineages and clonal inheritance, epigenetic changes often occur in groups of cells while some epigenetic events are clonal. In addition, genetic changes are, almost by definition, stable, whereas epigenetic changes are plastic events [2]. An example of the latter concept is provided by genomic imprinting, where DNA methylation may be lost during development, or when persisting, it is erased and re-setted during gametogenesis [5]. Epigenetic mechanisms are plastic genomic processes that change genome function under endogenous and exogenous influences [6, 7], and may propagate modifications of gene activity from one cell generation to the next [8]. These mechanisms imply chemical modification of DNA, such as DNA methylation, post-traslational changes in histone proteins altering chromatin conformation, and transcriptional gene silencing mediated by non-coding RNAs (ncRNAs) [9] (Fig. 1). Abnormalities in one or more of these mechanisms can lead to inappropriate expression or silencing of genes, resulting in imbalance of the epigenetic network and may result in metabolic disorders such as T2D and obesity [10, 11].
Schematic representation of epigenetic modifications. Epigenetic modifications include DNA methylation, histone modifications and miRNAs. DNA methylation preferentially occurs at CpG di-nucleotides and is generally recognized as an epigenetic silencing mechanism. Histone modifications, instead, influence gene expression by directly altering the chromatin conformation through the passage from a condensed transcriptionally silent heterochromatin to transcriptionally active euchromatin and vice versa. Different types of modifications are known and include acetylation (Ac), methylation (Me), and phosphorylation (P) of histones tails. Finally, miRNAs act at post-transcriptional level by suppressing target gene expression or, through a non-perfect complementarity between miRNA and target mRNA that causes translation inhibition of the target or through near-perfect complementarity which results in the degradation of the target mRNA
Epigenetic mechanisms and gene function
DNA methylation
DNA methylation is a covalent modification of DNA that occurs at position 5 of the cytosine pyrimidine ring [12]. Nearly four decades ago, DNA methylation was identified as hotspot for spontaneous base substitutions [13]. Indeed, while spontaneous deamination of cytosine produces uracil, a nitrogenous base that does not belong to DNA and that is immediately recognized and corrected by the system of DNA repair; deamination of 5-methyl cytosine produces thymine, causes C:G to T:A transitions, and creates a mismatch that the system of DNA repair does not always preserve [10, 14]. DNA methylation is the better characterized epigenetic mark. In mammals, it is essential during development and is involved in a variety of biological processes, including genomic imprinting and X chromosome inactivation [15]. DNA methylation is established during embryogenesis by de-methylation and de novo methylation events that can be inherited and maintained clonally by the action of specific enzymes termed DNA methyltransferases (DNMTs) [15, 16]. DNMT1 faithfully and symmetrically propagates cytosine methylation through recognition of methylated cytosines from an existing DNA strand to its novel partner upon replication and is primarily responsible for the maintenance of DNA methylation in cells. DNMT3A and DNMT3B are mainly involved in de novo methylation and establish new methylation patterns [4]. DNA methylation has long been recognized as an epigenetic silencing mechanism [17] which preferentially occurs at CpG di-nucleotides that are usually clustered in the CpG islands (CGIs) [18]. Quite often, un-methylated CpG sites at gene promoters create a transcriptionally permissive chromatin state by destabilizing nucleosomes and facilitating the recruitment of transcription factors [19]. On the other hand, dense DNA methylation of CpGs mediates stable long-term gene silencing by direct inhibition of transcription factors binding or by a combination of events mediated by methyl-CpG binding domain proteins (MBDs) which recruit methylated DNA mediators of chromatin remodeling, such as histone deacetylases (HDACs), or other repressors of gene expression (Table 1) [17, 20, 21].
Histone modifications
In eukaryotic cells, nucleosomes are the repeating and functional structural units of chromatin. They are composed of DNA wrapped around eight histone proteins, two homo-dimers histones H3 and H4, and two hetero-dimers histones H2A/H2B. Nucleosomes are mutually connected among themselves by the stretches of variable length of DNA linker conveyed by histone H1 [22, 23]. Histone modifications, such as acetylation, methylation, phosphorylation, ubiquitination, sumoylation and ADP ribosylation, are reversible epigenetic modifications, occurring at the histone tails. These modifications regulate gene expression by dynamically altering chromatin conformation causing electrostatic change and/or modulating binding proteins to chromatin [22, 24]. Even more extensively than other types of modifications, acetylation and methylation mediate formation of the condensed transcriptionally silent heterochromatin and of the transcriptionally active euchromatin. In mammalian cells, heterochromatin prevails and is generally characterized by high levels of DNA methylation and histone de-acetylation, and is enriched in tri-methylation of H3-Lys9, H3-Lys27, and H4-Lys20 [25, 26]. On the other hand, euchromatin exhibits lower levels of DNA methylation, and is typically enriched in acetylation of lysine residues at histones H3 and H4 and in mono- and tri-methylation of H3-Lys4 (Table 1) [27, 28].
NcRNAs
Findings over the past ten years have progressively revealed the relevance of ncRNAs in most epigenetically controlled events including modulation of gene transcription, transposon activity and silencing, X-chromosome inactivation and paramutation [29]. NcRNAs include multiple classes of RNA transcripts that do not encode proteins but rather regulate gene expression at the post-transcriptional level [30]. The most extensively studied ncRNAs are the miRNAs, small RNA molecules (21–25 nucleotides in length) often implicated in cell- and tissue-specific differentiation and development and associated to different disorders [31]. In the human genome, the exact number of reported sequences coding for these regulatory molecules continues to rise. In 2015, an analysis of 13 human cell types has revealed the existence of 3707 novel miRNA sequences, in addition to the 1900 sequences previously described [32]. miRNAs in the human genome are transcribed from both introns and exons of non-coding genes and from introns of protein coding genes as well [33]. In addition, some mammalian miRNAs derived from various transposons and processed pseudogenes [34]. miRNAs are critical regulators of post-transcriptional gene expression. In particular, suppression of the target gene expression mediated by miRNAs occurs based on the degree of complementarity of the miRNA with the 3’ Untranslated Region (3’UTR) of the target mRNA. Non-perfect complementarity between miRNA and target RNA, generally due to a pairing of only six to eight nucleotides, causes translation inhibition of the target mRNA, while near-perfect complementarity results in the degradation of the target mRNA by the RNA-induced silencing complex (Table 1) [35]. Interestingly, miRNAs are also susceptible to epigenetic modulation. Aberrant DNA methylation of miRNA gene promoters frequently occurs in human cancer and results in miRNAs expression down-regulation [36]. On the other hand, miRNAs are able to regulate both DNA methylation and histone modifications. Indeed, miRNAs may control the expression of important epigenetic regulators including DNMTs and HDACs thereby impacting on the entire gene expression profile [37].
Epigenetics in T2D and obesity
T2D and obesity are common metabolic disorders, which have reached epidemic proportions globally [38, 39]. Population and family (including twins) studies have extensively documented the familial aggregation of these diseases [40–45] with more than 175 genetic loci conclusively associated [46, 47]. Nevertheless, the impact of these loci, even in combination, on risk is very modest (5–10 % for T2D and ~2% for body mass index, BMI), leaving the heritability issue unsolved [48]. Technical limitations might, in part, account for this situation [49]. More likely, inheritance may be explained by epigenetics. Indeed, familial aggregation may reflect not only genetic influences, but also represent the effects of a shared family environment and thus of common environmentally induced epigenetic modifications [48]. In addition, while not been proved in humans yet, in rodents, certain environmentally induced epigenetic modifications can be trans-generationally transmitted to the offspring [50–52]. Environmentally induced epigenetic modifications may further explain the global epidemics of T2D and obesity, whose exponential rise in the past decades have been related to rapid cultural and social changes, such as socio-economic status, dietary changes, physical inactivity and unhealthy behaviors, all of which tend to cluster in family groups [38, 53]. Finally, epigenetics may help to understand the identical twin discordance for obesity and T2D [54, 55]. For example, the concordance rates for T2D among monozygotic twins are only ~ 70 % [56]. In these metabolic disorders, the incomplete concordance may be in part due to stochastic or environmentally determined epigenetic modifications that change over the lifetime and is responsible for the phenotypic differences and susceptibility to disease. Epigenetic processes may, therefore, contribute to the development of T2D and obesity and mediate the effects of environmental exposure on risk [9, 57]. In the following paragraphs, examples from our own as well as other investigators will be presented supporting the evidence linking epigenetic modifications, in particular DNA methylation, to T2D and obesity in both humans and rodents.
T2D and DNA methylation
T2D is a metabolic abnormality characterized by elevated plasma glucose levels. T2D typically occurs when insulin secretion fails to keep pace with reduced sensitivity to the action of circulating insulin [38]. There is now substantial evidence indicating that environmentally induced epigenetic changes contribute to diabetes prevalence (Table 2). Ling et al. have recently demonstrated that the promoter of the transcriptional co-activator Peroxisome proliferator activated receptor gamma coactivator-1 alpha (PGC1-α) gene, mainly involved in mitochondrial function, is highly methylated in pancreatic islets obtained from diabetic patients compared with non-diabetic controls [58]. Additionally, Barrès et al. have shown that the hyper-methylation of the PGC-1α promoter occurs even in the skeletal muscle from type 2 diabetic subjects compared with normal glucose-tolerant (NGT) individuals. Hyper-methylation negatively correlates with PGC-1α mRNA expression in these subjects [59]. In addition, the exposure of primary human skeletal muscle cells from NGT individuals to external factors, such as free fatty acids and tumor necrosis factor-alpha (TNF-α) directly and acutely alters the methylation status of PGC-1α promoter. These findings illustrate how alterations in the extracellular milieu may predispose to T2D by inducing DNA methylation changes [59]. Also, a genome-wide DNA methylation analysis of skeletal muscle from obese subjects before and after bariatric surgery provides evidence that the promoter methylation of PGC-1α is altered by obesity and restored after weight loss. DNA methylation inversely correlates to BMI, leptin, triglyceride and insulin levels in these subjects, which supports the role of DNA methylation in the physiological control of PGC-1α gene transcription [60]. The Pancreatic duodenal homeobox 1 (PDX-1) promoter is also methylated. PDX-1 is a homeodomain-containing transcription factor that plays a key role in pancreas development and function. In humans, mutations of PDX-1 cause maturity onset diabetes of the young 4 (MODY4) [61], while Pdx-1 silencing in pancreatic β-cells causes diabetes in mice [62]. In humans pancreatic islets, Yang et al. have shown that 10 CpG sites in the PDX-1 promoter and enhancer regions are hyper-methylated in type 2 diabetics compared with healthy individuals and that glycosylated hemoglobin (HbA1c) negatively correlates with mRNA expression of PDX-1 and positively correlates with DNA methylation, suggesting a role of chronic hyperglycemia in the modulation of PDX-1 expression through epigenetic events [63]. In support of this concept, these authors also found an increased DNA methylation of the Pdx-1 gene in clonal rat β-cells exposed to high levels of glucose associated to increased mRNA expression and binding of the Dnmt1 on Pdx-1 promoter [63]. More recently, a genome-wide analysis of differentially methylated sites in genomic regions associated to T2D has recently revealed that the Fat mass and Obesity-associated (FTO) gene is hypo-methylated in a CpG site within the first intron in type 2 diabetics compared with control subjects in human peripheral blood. The T2D predictive power of this mark is significantly greater than all genetic variants so far described [64]. In the same investigation Toperoff et al. have also prospectively established, that, in an independent cohort hypo-methylation at the FTO intron is observed in young subjects that later progress to T2D. This further finding provides evidence that methylation changes predispose to T2D and deserve to be considered further investigated as T2D markers.
Obesity and DNA methylation
Obesity is a complex disorder resulting in an abnormal accumulation of fat in the organism due to alterations in energy homeostasis in terms of balance among energy intake, expenditure and storage [65]. It has been extensively documented in both humans and animal models that a relationship exists between obesity and the epigenetic regulation of genes involved in the control of food intake (Table 2) [51, 66–70]. In this context, the epigenetic modulation of the Agouti gene is a paradigmatic example of this association in mice. The Agouti gene encodes the paracrine-signaling molecule “Agouti signalling peptide” (ASIP), which antagonizes the melanocortin 1 receptor (MC1R) and leads melanocytes to produce yellow rather than black coat pigments. Additionally, ASIP acts as antagonist of the hypothalamic MC4R, inhibiting the anorexigenic neuropeptide alpha-melanocyte-stimulating hormone (α-MSH) signaling, thereby promoting the activation of orexigenic pathways which make mice hyperphagic and prone to develop obesity and diabetes [67]. It is now known that the Agouti gene is sensitive to cytosine methylation [69]. When its promoter is un-methylated, the Agouti gene is in an “ON” state, ASIP protein is abundant and mice show the typical agouti yellow coat and a tendency to develop obesity and diabetes [70]. On the contrary, when the promoter is heavily methylated, the Agouti gene is in an “OFF” state, ASIP levels are low resulting in mice that are lean and exhibit black coat. Interestingly, the Agouti gene is sensitive to environmental stimuli [51, 68, 71]. Nutrients and environmental pollutants impact on Agouti gene expression altering disease susceptibility through epigenetic modifications. Agouti pregnant mice fed diets supplemented with the methyl donors folic acid, vitamin B12 or choline generate lean brown offspring which show increased DNA methylation on the Agouti gene promoter and decreased ASIP protein levels [71]. In addition, the effects on coat color induced by maternal methyl-donor supplemented diet are also inherited in the F2 generation, indicating a germline propagation of the epigenetic modifications [51]. On the other hand, maternal exposure to the environmental pollutant bisphenol A, which is commonly present in many items such as food and plastic beverage containers and baby bottles, shifted the coat color of the offspring toward yellow by decreasing DNA methylation of CpG sites within the Agouti promoter [68]. Maternal supplementation with methyl donors abolished the bisphenol A-induced hypo-methylation of the Agouti gene in the offspring, demostrating the potential protective effect of simple dietary interventions against effect of an unhealthy environment effects on the fetal epigenome [68]. In humans, DNA methylation of the Pre-proopiomelanocortin (POMC) gene which encodes the anorexigenic hormone α-MSH produced by hypothalamic arcuate nucleus neurons has been associated with the individual risk of childhood obesity [72]. In particular, using peripheral blood cells, Kuenen et al. have found hyper-methylation at the Intron 2/Exon 3 boundary of the POMC gene in obese compared with normal weight children. In particular, in these obese children, the Alu elements, which are known to influence methylation in their genomic proximity at the Intron 2, trigger a default state methylation at the Intron 2/Exon 3 boundary, interfering with binding of the histone acetyltransferase/transcriptional coactivator p300 and reducing POMC expression [72]. In addition, several studies suggest a critical role of epigenetic marks also as predictors of susceptibility to obesity and metabolic disease in humans and animal models [73, 74]. A further example of this concept in humans has been provided by studies on the Retinoid X receptor-alpha (RXRA) gene. Godfrey et al., designed a perinatal epigenetic analysis of the methylation status of CpG sites at the promoters of 78 selected candidate genes in DNA from umbilical cord tissue of children who were assessed for adiposity at age 6 and 9 years. These authors have established that the variation of adiposity and the onset of obesity in pre-pubertal children were associated with the specific hyper-methylation of a CpG site at the RXRA chr9:1363558885+ at birth [74]. Furthermore, in the same population, it was demonstrated that this neonatal epigenetic mark was associated with lower maternal carbohydrate intake in pregnancy first trimester, providing a further example of how epigenetic processes may link the early prenatal life with the predisposition to obesity and other phenotypic outcomes [74]. In the future, perinatal identification of individuals that present DNA methylation changes at specific genes may help in preventing later obesity. In accordance to this concept, a recent bioinformatic analysis, performed to search for epigenetic obesity biomarkers, has established potential regions of interest which have a high density of CGIs in the promoter of several obesity-related genes (epiobesigenes), such as Leptin, Phosphatase and tensin homolog (PTEN), and Fibroblast growth factor 2 (FGF2) or genes implicated in adipogenesis, such as Peroxisome proliferator-activated receptor gamma (PPARG), in inflammation, such as Suppressors of cytokine signaling 1 and 3 (SOCS1/SOCS3), and insulin signaling, like Lipoprotein lipase (LPL), Fatty acid binding protein 4 (FABP4), and Insulin-like growth factor binding protein-3 (IGFBP3) [75].
Epigenetics and nutrition: lessons from recent studies
Nutritional epigenetics has become an attractive field of study since it associates nutrients and bioactive food components with epigenetic modifications of gene function. As reported in this review, a variety of evidence, in both humans and animal models, supports the association between changes in nutritional status, epigenetic modifications and predisposition to T2D and obesity [76]. Shen et al. have demonstrated that high fat diet (HFD) feeding impacts on the Leptin gene by inducing promoter CGI hyper-methylation in murine white adipose tissue (AT) [66]. Consistent with this observation, data obtained by our own group have further highlighted the role of over-nutrition in contributing to the gene function de-regulations occurring in obesity through epigenetic modification. By methylated DNA immuno-precipitation sequencing (MeDIP-seq), we have recently revealed that, HFD triggers a massive DNA methylation reprogramming in AT [77]. In particular, about 14.8 × 103 regions were found to be differentially methylated (DMRs) in mice fed a HFD. Interestingly, we have demonstrated that prolonged HFD regimen promotes a specific DMR distribution in mice [77]. DMR occurrence was increased in the gene-associated elements, particularly introns, and in the CGIs, while the number of DMRs identified in genomic repeat elements including long terminal repeats (LTRs) and long interspersed elements (LINEs) was decreased in obese compared with lean mice. Gene ontology analysis indicates that HFD feeding promotes DMRs enrichment in genes involved in developmental, metabolic and transcriptional processes in mice. In this same study, it has also been revealed that, among several differentially methylated pathways, the Hox family of transcription factors was highly enriched in differentially methylated genes in HFD-fed compared with STD-fed mice. In particular, the Hoxa5 gene, which is implicated in fat tissue differentiation and remodeling [78, 79], was highly methylated at its 5′UTR and transcriptionally repressed in AT from obese compared with lean mice. In addition, the exposure of murine 3T3-L1 adipocytes to palmitate, a major component of the HFD, enhances methylation at the Hoxa5 5′UTR and causes Hoxa5 mRNA down-regulation, suggesting that in AT the epigenetic silencing of Hoxa5 gene may be dependent, at least in part, on the effect of saturated fats rather than on obesity per se [79]. Interestingly, when obese mice exposed to chronic HFD treatment were returned to standard chow diet for two further months, Hoxa5 DNA methylation and expression levels returned to values similar to those of mice maintained under STD, emphasizing the plasticity of these epigenetic events [79] (Fig. 2).
High fat feeding: DNA methylation at the Hoxa5 gene in vivo. DNA methylation analysis performed on the AT from mice fed chow diet or high fat diet reveals the presence of a DMR relative to the Hoxa5 locus among high fat fed- and chow diet fed-mice. This event is associated with a strong reduction of the Hoxa5 mRNA expression levels in obese mice compared with lean mice. Interestingly, re-feeding obese mice a chow diet for 2 further months reverted the Hoxa5 DNA methylation and its expression to levels similar to those in lean control fed a standard chow diet
This same MeDIP-seq approach has also identified the Ankyrin repeat domain 26 (Ankrd26) as a gene sensitive to nutrition-induced epigenetic changes. ANKRD26, a gene highly expressed in different areas of the hypothalamus, has been related to specific forms of hereditary obesity in humans [80] and was demonstrated to be involved in the regulation of feeding behavior and in the development of both obesity and diabetes in mice [81–83]. Mice with a partial inactivation of this gene show an obese phenotype which results from a marked hyperphagia rather than a reduction of the energy expenditure and activity [81]. When deleted at its C-terminus, Ankrd26 leads to excessive food intake and obesity due to severe region-specific changes in primary cilia in the brain [83]. In addition to its function in appetite control, the Ankrd26 gene has a role in the regulation of adipocyte differentiation in mouse embryonic fibroblasts and in 3T3-L1 cells [84, 85]. We have demonstrated that hyper-methylation of the Ankrd26 promoter occurs in obese mouse AT upon prolonged HFD feeding compared to age- and sex-matched STD-fed mice and directly interferes with the binding of the histone acetyltransferase/transcriptional coactivator p300 to this same region. These events result in the down regulation of the Ankrd26 gene expression [unpublished observations]. We have further reveled that Ankrd26 silencing alters secretion of pro-inflammatory adipokines in vitro. These findings indicate that the epigenetic silencing of the Ankrd26 gene might be one of the mechanisms responsible for AT inflammation in response to HFD [unpublished observations]. In humans, computational data from a genome-wide DNA methylation analysis in subcutaneous white AT revealed that ANKRD26 gene is included in a list of 2825 genes where both DNA methylation and mRNA expression levels significantly correlate with BMI [86]. According to these findings, our preliminary data in human peripheral blood leukocytes underline a negative correlation between ANKRD26 mRNA expression levels and BMI, supporting the hypothesis that an epigenetic regulation of ANKRD26 gene may occur in humans, as well as in mice, and represent a pathogenic mechanism by which environmental exposures to nutrients contribute to disease susceptibility through epigenetic modifications.
Conclusions and future perspectives
The epigenome undergoes continuous transformations throughout our own lifetime leading to changes in genome function. The epigenetic hypothesis argues that, in addition to genetic variation, epigenetics provides an additional set of mechanisms mediating the relationship between genotype and the external environment and potentially contributing to the individual susceptibility to different disorders. During the past decades, the study of epigenetic modifications has been one of the most emerging and novel areas in fundamental as well as in clinical research and currently represents a very productive field of study, which has already provided a framework for the search of etiological factors in environment-associated diseases such as T2D and obesity. It is, indeed, clear that genetic variability only marginally contributes to the pathogenesis and family risk of these disorders. In this review, we have presented important acquisitions on the epigenetic network in T2D and obesity, mostly focusing on changes of the DNA methylation status of specific genes. However, understanding of the epigenetics of these two complex diseases is still limited. Further work is needed to clarify the molecular mechanisms responsible for the epigenetic control of gene activity and their interactions and alterations, and to establish the role of epigenetics in the risk stratification of these diseases. In the near future, further hints on how epigenetic changes are involved in the etiopathogenesis of T2D and obesity will be attained from studies accomplished on other epigenetic modifications, such as histone modifications and ncRNAs, which may selectively affect the expression of specific genes, and from epigenome-wide analysis extended to specific human cell types, e.g., stem, precursor and differentiated cells, or to particular tissues, e.g., fat, skeletal muscle, liver and pancreatic islets. It is expected that these studies will pave the way to novel and more effective strategies aimed at diabetes and obesity prevention and at personalized epigenetic treatment. Indeed, it becomes clear that getting a full picture of the epigenetic events involved in these two diseases will (1) represent a powerful tool for predicting and preventing future disease onset in the population; (2) provide additional stimuli for the development of clinical epigenetic biomarkers, which will generate novel relevant information for diagnosis, prognosis and therapy optimization; and (3) drive advancement in epigenetic drug discovery with the generation of more effective epi-drugs, selective for specific epi-targets, which in the forthcoming future might be used in combination with conventional therapeutics and/or might get the opportunity to develop epigenetic treatment personalized to the patient’s epigenetic traits.
References
Rappaport SM, Smith MT (2010) Epidemiology. Environment and disease risks. Science 330:460–461
Holliday R (2006) Epigenetics: a historical overview. Epigenetics 1:76–80
Waddington CH (1942) The epigenotype. Endeavour 1:18–20
Dupont C, Armant DR, Brenner CA (2009) Epigenetics: definition, mechanisms and clinical perspective. Semin Reprod Med 27:351–357
Davis TL, Yang GJ, McCarrey JR, Bartolomei MS (2000) The H19 methylation imprint is erased and re-established differentially on the parental alleles during male germ cell development. Hum Mol Genet 9:2885–2894
Bollati V, Baccarelli A (2010) Environmental epigenetics. Heredity 105(1):105–112
Tyson FL, Heindel J (2005) Environmental Influences on Epigenetic Regulation. Environ Health Perspect 113:A839
Jaenisch R, Bird A (2003) Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 33(Suppl):245–254
Raciti GA, Nigro C, Longo M et al (2014) Personalized medicine and type 2 diabetes: lesson from epigenetics. Epigenomics 6:229–238
Egger G, Liang G, Aparicio A, Jones PA (2004) Epigenetics in human disease and prospects for epigenetic therapy. Nature 429:457–463
Sun C, Burgner DP, Ponsonby AL et al (2013) Effects of early-life environment and epigenetics on cardiovascular disease risk in children: highlighting the role of twin studies. Pediatr Res 73:523–530
Esteller M (2008) Epigenetics in cancer. N Engl J Med 358:1148–1159
Coulondre C, Miller JH, Farabaugh PJ, Gilbert W (1978) Molecular basis of base substitution hotspots in Escherichia coli. Nature 274:775–780
Gonzalgo ML, Jones PA (1997) Mutagenic and epigenetic effects of DNA methylation. Mutat Res 386:107–118
Li E (2002) Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet 3:662–673
Reik W, Dean W, Walter J (2001) Epigenetic reprogramming in mammalian development. Science 293:1089–1093
Klose RJ, Bird AP (2006) Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci 31:89–97
Smith ZD, Meissner A (2013) DNA methylation: roles in mammalian development. Nat Rev Genet 14:204–220
Deaton AM, Bird A (2011) CpG islands and the regulation of transcription. Genes Dev 25:1010–1022
Mohn F, Weber M, Rebhan M et al (2008) Lineage-specific polycomb targets and de novo DNA methylation define restriction and potential of neuronal progenitors. Mol Cell 30:755–766
Payer B, Lee JT (2008) X chromosome dosage compensation: how mammals keep the balance. Annu Rev Genet 42:733–772
Luger K (2001). Nucleosomes: Structure and Function. Encyclopedia of Life Sciences 1–8
Cooper GM (2000) The Cell: A Molecular Approach. 2nd edition. Sunderland (MA): Sinauer Associates. Chromosomes and Chromatin. Available from: http://www.ncbi.nlm.nih.gov/books/NBK9863/
Ma J (2005) Crossing the line between activation and repression. Trends Genet 21:54–59
Richards EJ, Elgin SC (2002) Epigenetic codes for heterochromatin formation and silencing: rounding up the usual suspects. Cell 108:489–500
Kouzarides T (2007) Chromatin modifications and their function. Cell 128:693–705
Tamaru H (2010) Confining euchromatin/heterochromatin territory: jumonji crosses the line. Genes Dev 24:1465–1478
Seol JH, Kim HJ, Yang YJ et al (2006) Different roles of histone H3 lysine 4 methylation in chromatin maintenance. Biochem Biophys Res Commun 349:463–470
Costa FF (2008) Non-coding RNAs, epigenetics and complexity. Gene 410:9–17
Mattick JS, Makunin IV (2006) Non-coding RNA. Hum Mol Genet 15 Spec No 1:R17–29
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Londin E, Loher P, Telonis AG et al (2015) Analysis of 13 cell types reveals evidence for the expression of numerous novel primate- and tissue-specific microRNAs. Proc Natl Acad Sci USA 112:E1106–E1115
Mattick JS, Makunin IV (2005) Small regulatory RNAs in mammals. Hum Mol Genet 14 Spec No 1:R121–132
Guo X, Zhang Z, Gerstein MB, Zheng D (2009) Small RNAs originated from pseudogenes: cis- or trans-acting? PLoS Comput Biol 5:e1000449
Wienholds E, Plasterk RH (2005) MicroRNA function in animal development. FEBS Lett 579:5911–5922
Vrba L, Muñoz-Rodríguez JL, Stampfer MR, Futscher BW (2013) miRNA gene promoters are frequent targets of aberrant DNA methylation in human breast cancer. PLoS One 8:e54398
Sato F, Tsuchiya S, Meltzer SJ, Shimizu K (2011) MicroRNAs and epigenetics. FEBS J 278:1598–1609
International Diabetes Federation. Diabetes Atlas 6th ed, 2013 Brussels, Belgium: International Diabetes Federation, 2014
Ng M, Fleming T, Robinson M et al (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384:766–781
InterAct Consortium et al (2013) The link between family history and risk of type 2 diabetes is not explained by anthropometric, lifestyle or genetic risk factors: the EPIC-InterAct study. Diabetologia 56:60–69
Meigs JB, Cupples LA, Wilson PW (2000) Parental transmission of type 2 diabetes: the Framingham Offspring Study. Diabetes 49:2201–2207
Klein BE, Klein R, Moss SE, Cruickshanks KJ (1996) Parental history of diabetes in a population-based study. Diabetes Care 19:827–830
Stunkard AJ, Harris JR, Pedersen NL, McClearn GE (1990) The body-mass index of twins who have been reared apart. N Engl J Med 322:1483–1487
Rice T, Pérusse L, Bouchard C, Rao DC (1999) Familial aggregation of body mass index and subcutaneous fat measures in the longitudinal Québec family study. Genet Epidemiol 16:316–334
Segal NL, Allison DB (2002) Twins and virtual twins: bases of relative body weight revisited. Int J Obes Relat Metab Disord 26:437–441
Grarup N, Sandholt CH, Hansen T, Pedersen O (2014) Genetic susceptibility to type 2 diabetes and obesity: from genome-wide association studies to rare variants and beyond. Diabetologia 57:1528–1541
Al-Azzam SI, Khabour OF, Alzoubi KH, Alzayadeen RN (2014) The effect of leptin promoter and leptin receptor gene polymorphisms on lipid profile among the diabetic population: modulations by atorvastatin treatment and environmental factors. J Endocrinol Invest 37(9):835–842
Drong AW, Lindgren CM, McCarthy MI (2012) The genetic and epigenetic basis of type 2 diabetes and obesity. Clin Pharmacol Ther 92:707–715
Bloom JS, Ehrenreich IM, Loo WT, Lite TL, Kruglyak L (2013) Finding the sources of missing heritability in a yeast cross. Nature 494(7436):234–237
Jirtle RL, Skinner MK (2007) Environmental epigenomics and disease susceptibility. Nat Rev Genet 8:253–262
Cropley JE, Suter CM, Beckman KB, Martin DI (2006) Germ-line epigenetic modification of the murine A vy allele by nutritional supplementation. Proc Natl Acad Sci U S A 103:17308–17312
Saad MI, Abdelkhalek TM, Haiba MM, Saleh MM, Hanafi MY, Tawfik SH, Kamel MA (2016) Maternal obesity and malnourishment exacerbate perinatal oxidative stress resulting in diabetogenic programming in F1 offspring. J Endocrinol Invest 39(6):643–655
Speakman JR, O’Rahilly S (2012) Fat: an evolving issue. Dis Model Mech 5:569–573
Castillo-Fernandez JE, Spector TD, Bell JT (2014) Epigenetics of discordant monozygotic twins: implications for disease. Genome Med 6:60
Poulsen P, Esteller M, Vaag A, Fraga MF (2007) The epigenetic basis of twin discordance in age-related diseases. Pediatr Res 61:38R–42R
Lyssenko V, Laakso M (2015) Genetic Screening for the Risk of Type 2 Diabetes: worthless or valuable? Diabetes Care 36:S120–S126
Raciti GA, Longo M, Parrillo L et al (2015) Understanding type 2 diabetes: from genetics to epigenetics. Acta Diabetol 52(5):821–827
Ling C, Del Guerra S, Lupi R et al (2008) Epigenetic regulation of PPARGC1A in human type 2 diabetic islets and effect on insulin secretion. Diabetologia 51:615–622
Barrès R, Osler ME, Yan J et al (2009) Non-CpG methylation of the PGC-1alpha promoter through DNMT3B controls mitochondrial density. Cell Metab 10:189–198
Barres R, Kirchner H, Rasmussen M et al (2013) Weight loss after gastric bypass surgery in human obesity remodels promoter methylation. Cell Rep 3:1020–1027
Velho G, Robert JJ (2002) Maturity-onset diabetes of the young (MODY): genetic and clinical characteristics. Horm Res 57(Suppl 1):29–33
Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H (1998) beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev 12:1763–1768
Yang BT, Dayeh TA, Volkov PA et al (2012) Increased DNA methylation and decreased expression of PDX-1 in pancreatic islets from patients with type 2 diabetes. Mol Endocrinol 26:1203–1212
Toperoff G, Aran D, Kark JD et al (2012) Genome-wide survey reveals predisposing diabetes type 2-related DNA methylation variations in human peripheral blood. Hum Mol Genet 21:371–383
Jung UJ, Choi MS (2014) Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci 15(4):6184–6223
Shen W, Wang C, Xia L et al (2014) Epigenetic modification of the leptin promoter in diet-induced obese mice and the effects of N-3 polyunsaturated fatty acids. Sci Rep 4:5282
Cifani C, Micioni Di Bonaventura MV et al (2015) Regulation of hypothalamic neuropeptides gene expression in diet induced obesity resistant rats: possible targets for obesity prediction? Front Neurosci 9:187
Dolinoy DC (2008) The agouti mouse model: an epigenetic biosensor for nutritional and environmental alterations on the fetal epigenome. Nutr Rev 66(Suppl 1):S7–11
Cooney CA, Dave AA, Wolff GL (2002) Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr 132:2393S–2400S
Wolff GL, Kodell RL, Moore SR, Cooney CA (1998) Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J 12:949–957
Ling C, Groop L (2009) Epigenetics: a molecular link between environmental factors and type 2 diabetes. Diabetes 58:2718–2725
Kuehnen P, Mischke M, Wiegand S et al (2012) An Alu element-associated hypermethylation variant of the POMC gene is associated with childhood obesity. PLoS Genet 8:e1002543
Reddy MA, Natarajan R (2015) Recent developments in epigenetics of acute and chronic kidney diseases. Kidney Int 88:250–261
Godfrey KM, Sheppard A, Gluckman PD et al (2011) Epigenetic gene promoter methylation at birth is associated with child’s later adiposity. Diabetes 60(5):1528–1534
Campión J, Milagro FI, Martínez JA (2009) Individuality and epigenetics in obesity. Obes Rev 10:383–392
Seki Y, Williams L, Vuguin PM, Charron MJ (2012) Minireview: epigenetic programming of diabetes and obesity: animal models. Endocrinology 153:1031–1038
Parrillo L, Costa V, Raciti GA, et al (2016) Hoxa5 undergoes dynamic DNA methylation and transcriptional repression in the adipose tissue of mice exposed to high-fat diet. Int J obesity. (in press)
Cowherd RM, Lyle RE, Miller CP, Mcgehee RE Jr (1997) Developmental profile of homeobox gene expression during 3T3-L1 adipogenesis. Biochem Biophys Res Commun 237:470–475
Charlier C, Segers K, Karim L et al (2001) The callipyge mutation enhances the expression of coregulated imprinted genes in cis without affecting their imprinting status. Nat Genet 27:367–369
Dong C, Li WD, Geller F et al (2005) Possible genomic imprinting of three human obesity-related genetic loci. Am J Hum Genet 76:427–437
Bera TK, Liu XF, Yamada M et al (2008) A model for obesity and gigantism due to disruption of the Ankrd26 gene. Proc Natl Acad Sci USA 105:270–275
Raciti GA, Bera TK, Gavrilova O, Pastan I (2011) Partial inactivation of Ankrd26 causes diabetes with enhanced insulin responsiveness of adipose tissue in mice. Diabetologia 54:2911–2922
Acs P, Bauer PO, Mayer B et al (2015) A novel form of ciliopathy underlies hyperphagia and obesity in Ankrd26 knockout mice. Brain Struct Funct 220:1511–1528
Fei Z, Bera TK, Liu X, Xiang L, Pastan I (2011) Ankrd26 gene disruption enhances adipogenesis of mouse embryonic fibroblasts. J Biol Chem 286:27761–27768
Liu XF, Bera TK, Kahue C et al (2012) ANKRD26 and its interacting partners TRIO, GPS2, HMMR and DIPA regulate adipogenesis in 3T3-L1 cells. PLoS ONE 7:e38130
Rönn T, Volkov P, Gillberg L et al (2015) Impact of age, BMI and HbA1c levels on the genome-wide DNA methylation and mRNA expression patterns in human adipose tissue and identification of epigenetic biomarkers in blood. Hum Mol Genet 24:3792–3813
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the European Foundation for the Study of Diabetes (EFSD), by the Ministero dell’Università e della Ricerca Scientifica (grants PRIN and FIRB-MERIT, and PON 01_02460) and by the Società Italiana di Diabetologia (SID-FO.DI.RI). This work was further supported by the P.O.R. Campania FSE 2007-2013, Project CREMe.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study informed consent is not required.
Rights and permissions
About this article
Cite this article
Desiderio, A., Spinelli, R., Ciccarelli, M. et al. Epigenetics: spotlight on type 2 diabetes and obesity. J Endocrinol Invest 39, 1095–1103 (2016). https://doi.org/10.1007/s40618-016-0473-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-016-0473-1