Abstract
Providing behavioral, biomarker, or disease risk feedback to patients is a key component of most behavioral interventions in diabetes, but it remains unclear what is necessary for such feedback to be truly engaging and effective. We sought to identify how personalized health-related feedback is most effectively designed and delivered, and how feedback may be tailored to meet the needs of individual patients with diabetes. To do so, we systematically reviewed recent findings concerning the effectiveness of feedback in eight health-related areas, including several specific to diabetes care (blood glucose monitoring and HbA1c) and others which touch on broader care dimensions (blood pressure, cholesterol, dietary intake, pedometer usage, self-weighing, and medical imaging). Five interdependent characteristics of health-related feedback were identified (clarity of the feedback message, personal meaningfulness of the feedback, frequency of feedback, guidance and support accompanying feedback, and interplay between feedback and patient characteristics) and applications for use in diabetes care were provided. Findings suggested that feedback will be most effective when it is easy for patients to understand and is personally meaningful, frequency of feedback is appropriate to the characteristics of the behavior/biomarker, guidance for using feedback is provided, and feedback is qualified by patient characteristics. We suggest that the effectiveness of feedback to promote better diabetes outcomes requires careful consideration of the feedback message, how it is delivered, and characteristics of the recipients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Personalized health feedback is widely regarded as a potent strategy for enhancing patient motivation and for promoting and maintaining behavior change [1–3]. The provision of behavioral, biomarker, or disease risk feedback to patients lies at the heart of many of the major health behavior change theories (health belief model, theory of reasoned action, health decision model) and serves as a key component of most health-related behavioral interventions in diabetes care and beyond, including those designed to reduce smoking and alcohol consumption, increase physical activity, improve diet, enhance medication adherence, and reduce substance abuse, risky sex, and obesity [4, 5]. But are such strategies effective? In particular, does feedback provided to patients with diabetes lead to positive behavior change and better clinical outcomes? Is the communication of personalized health-related information alone sufficient to improve diabetes self-care [6]?
Following DiClemente [7], we define personalized health-related feedback as the provision of personal information that has been derived from some type of assessment procedure (e.g., biological, behavioral, or condition-related health indicator) to prompt or maintain positive behavior change. To enhance change or to place it in context, the information provided often compares a person’s results either to a reference group (a normative comparison) or, in a more self-referential fashion, to their own results recorded at a different moment in time (an ipsative comparison) [8]. In the health care arena, personalized feedback may target behavior (e.g., pedometer feedback, daily calorie consumption), specific biomarkers (e.g., lipid levels, blood pressure), or broader constructs such as risk status (e.g., estimated likelihood that the individual will experience stroke within a defined time period).
Despite the ubiquity of personalized feedback in modern clinical care, studies that examine its direct impact on health outcomes are remarkably rare. The goal of this report is to examine recent findings in the scientific literature and to identify the ways in which feedback is most effectively delivered, which forms or types of feedback (e.g., intensity, frequency, modality) are most effective, and how feedback may need to be tailored to meet the personal needs of individuals with diabetes. Although many studies include feedback as a component of an intervention, it is rare that the specific impact of the feedback itself is evaluated as an independent contributor to change in outcomes. Thus, formal meta-analytic methods cannot easily be utilized to evaluate the relative effectiveness of different kinds or characteristics of feedback. Therefore, in an effort to summarize and integrate what is known about the effects of feedback on various outcomes, in this report we present a comprehensive narrative summary of the literature that identifies the key elements of feedback that should be taken into consideration to enhance patient motivation and to promote and/or sustain changes in diabetes self-care.
Linkages Between Personalized Feedback and Behavior Change
Information processing theory posits that providing appropriate feedback prompts systems to automatically adjust their functioning to maintain a steady state, as in homeostasis, or to alter their functioning in other ways to provide optimal outcomes (e.g., self-regulation and control theories [9, 10]). These theories work well when addressing biological processes, such as how a change in oxygen level leads to an adaptive shift in respiratory rate, but when applying this approach to more complex systems, such as those requiring human behavior, the impact of feedback is not at all straightforward [11]. Indeed, an examination of the recent literature indicates that health-related feedback often has beneficial effects on patient behavior and clinical outcomes [12–14], but feedback is sometimes found to be no better than no feedback [15, 16] and it is sometimes decidedly less beneficial than usual care, suggesting that feedback may at times be harmful [17, 18].
As a relevant diabetes-related illustration, consider the impact of providing glycosylated hemoglobin (HbA1c) feedback to patients. In the four major studies to date, computer-generated, tailored feedback regarding recent HbA1c and other metabolic indices were provided to patients with type 2 diabetes (T2D) and compared to a no feedback control [19–22]. Investigators then observed how HbA1c changed over the next 6–15 months. Of the four studies, two [19, 20] found that HbA1c feedback had a significant, positive impact on glycemic control compared to controls. In the first study, the intervention group evidenced a mean A1C drop of 0.9 %, while the control group dropped 0.2 % [19]. In the second study, A1C dropped 1.1 % in the intervention condition and only 0.6 % among controls [20]. In contrast, the remaining two studies [21, 22] found no significant between-group HbA1c difference. Furthermore, in one of the latter studies, those patients with poor baseline HbA1c (>8 %) displayed a significant worsening of glycemic control as a result of HbA1c feedback compared to controls [20]. Thus, the commonly held belief that feedback is uniformly helpful for improving health-related outcomes is not supported by recent studies.
How to make sense of these inconsistent findings? Careful examination of studies to date indicate that health-related feedback, though it is typically considered a single, uniform type of intervention, actually varies dramatically in form, time frame, frequency, and content from one study to another. Furthermore, as noted above, the actual provision of feedback is typically folded into larger, multi-component programs, often with different time frames and different feedback content, that make the true impact of feedback difficult to assess directly [23]. The impact of feedback on complex behavioral systems in real-world clinical settings, therefore, is heavily dependent on the subtle characteristics of the feedback message. This may include the ways in which the information is communicated, how often feedback is provided, to whom the feedback is directed, and more. The critical issue is how to identify the key conditions under which feedback increases patient engagement and positive diabetes-related health behaviors.
Five Elements of Feedback
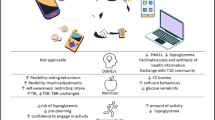
The two authors independently reviewed all of the recent studies extracted from the literature and identified those major elements that seemed to directly influence the impact of health-related feedback. The two authors then compared and integrated their findings, yielding five major elements: clarity, personal meaningfulness, frequency, guidance and support, and patient interpretation (Fig. 1). These five are neither mutually exclusive nor exhaustive, but they frame the different ways in which feedback can be characterized. Below, we define each and then, to the extent that data are available, review how each element influences patient behavior and might be used most effectively to shape feedback messages to enhance outcomes. This summary is based on our examination of eight distinct areas of health-related feedback, including those that are specific to diabetes care (blood glucose monitoring and HbA1c) and those that touch upon broader care dimensions (blood pressure, serum cholesterol, dietary intake, pedometer usage, self-weighing, and medical imaging). Unfortunately, there have been no systematic efforts to examine how the various characteristics, frequencies, and types of feedback delivery directly influence biobehavioral outcomes, and in many of the available studies, it is difficult to ascertain whether the feedback was directed at influencing patient behavior (e.g., medication adherence) or clinician behavior (e.g., medication titration).
1. Clarity of Feedback
The ways in which feedback are communicated and presented directly affect recipient comprehension [1]. Not surprisingly, when data are presented in a form that is clear, attractive, and not overly complex, they are likely to be interesting and engaging, thus enhancing use. For example, it has been suggested that one reason for the widespread use of pedometers is that the feedback provided is extremely simple, easy to understand, and easy to use. Pedometer feedback reflects an uncomplicated metric (number of steps taken), the tool is inexpensive and readily available and it is usable by low and high literacy patients. The data are easily summarized and change can be seen over time [24]. In contrast, when feedback is unappealing, too complicated, or cannot be easily interpreted by the recipient, a positive response is less likely [25].
We identify two keys for addressing clarity. The first concerns how well, or how poorly, the data are summarized for the patient. As an illustration, consider that intricate sets of numerical data, such as home blood pressure (HBP) measurements over a period of days or weeks, may be more welcomed and less likely to be dismissed when they are presented in a patient-friendly summary form, compared to a long list of raw HBP numbers. Kabutoya and colleagues [26], for example, found that patients asked to make daily use of a HBP device that displayed a graphical summary that helped them visualize the feedback data over time achieved adequate blood pressure control significantly more rapidly than patients equipped with a standard HBP monitor that did not present any summarized data.
The second major key is whether or not feedback is linked to “real-world” comparators that make sense to the patient. Appropriate comparators, anchored to a sensible metric, ground the feedback in a manner that is more relevant to the patient’s daily experience. For example, there is evidence that the benefits of providing calorie counts for recent meals may be greater when they are associated with easy-to-conceptualize referents, such as the amount of exercise needed to compensate for the food consumed, than when the referent is more abstract and less salient, such as the percent of daily calories recommended [27].
Although recent research suggests that patients often find some health-related feedback data, such as blood glucose monitoring results, difficult to understand [28], we are unaware of any feedback intervention studies that have directly evaluated how well patients are able to comprehend the information provided and how this information affects outcomes. Still, clarity is likely to be a key contributor to feedback efficacy, especially when there is an emphasis on summarizing and framing complex data in a patient-friendly, easily understood manner.
2. Personal Meaningfulness of the Feedback Message
The effectiveness of feedback varies directly as a function of its personal meaningfulness and relevance to the recipient. Our review suggests that meaningfulness increases when one or more of the following four critical dimensions are considered.
First, the effectiveness of the feedback message can be enhanced when the recipient’s data are compared with data from a relevant peer group. Presenting feedback in this way provides the patient with a sense of perspective, highlighting how his/her behavior or disease status is different from a particularly relevant norm or appropriate reference group. For example, attempts to reduce smoking are significantly enhanced when personalized health risk data are presented as “lung age” (the age of the average individual with similar spirometry scores), in contrast to a presentation that includes only raw spirometry data with no relevant referent [29]. Interestingly, the “lung age” effect occurred irrespective of the severity of patient disorder, suggesting the power of using a poignant referent for an entire patient population [30]. Thus, providing a comparative frame of reference that is personally meaningful can lead to greater behavioral change.
Second, the feedback message may be more impactful when it shows in graphic and understandable ways how one’s physical health is directly affected by the targeted behavior. When the feedback is an image of bodily function, for example, rather than something less immediate (such as a calculated risk score), enhanced patient interest and activation might be expected. For example, greater improvement in glycemic control was seen in type 1 diabetes adults with non-proliferative retinopathy and suboptimal glycemic control (HbA1c > 7.0 %) who viewed pictures of their own retinal images compared to similar patients who received only an abstract risk score [31•]. Similarly [32], smokers shown ultrasound images of both their own carotid arteries, indicating atherosclerotic plaque, and images of disease-free arteries were more likely to initiate smoking cessation behaviors than smokers who did not view these images [33]. In sum, feedback is more likely to be engaging when there is a meaningful, tangible referent.
Third, effectiveness is enhanced when the feedback message includes how the feedback can be put to good use [8]. Unfortunately, many patients come to believe that certain forms of health-related feedback have no functional value. Consider the patient with type 2 diabetes who is asked to self-monitor his/her blood glucose (SMBG) each day, but never learns what to do with the results. In such cases, SMBG is not viewed as providing usable information, leading to poor adherence to SMBG recommendations [34•]. In contrast, when feedback is collected, structured, and communicated in a manner that is linked to corrective action, the probability of positive behavior change is increased [35]. For example, when SMBG is structured in a “paired testing” format (testing blood glucose before and after a specific event, such as daily exercise), the patient can more easily see that what he/she does really makes a difference [28, 36]. This phenomenon, known as “perceived treatment efficacy”, is recognized as a key contributor to patient activation and motivation because it demonstrates in vivid form that the patient’s own behavior has a tangible impact on health-related indices [37].
Regularly monitoring and updating key data can enhance the utility of feedback even further by providing patients with a presentation of progress over time and, potentially, a tangible record of success [23]. When shared with a health care professional, such data may also contribute to an enhanced level of accountability [24]. Therefore, feedback may have the most beneficial effects when multiple data points over time can be presented and reviewed, especially with a clinician, that clearly links how personal behavior influences the attainment of well-defined health outcomes or goals [1, 38].
Fourth, the time interval between when feedback information is collected and when it is delivered to the recipient is critical. To the extent possible, feedback is more effective when it closely follows when the behavior occurred [39]. When feedback is long delayed, it may seem less “real”, be of less critical value, and perhaps be less usable. For example, the positive impact on patients with diabetes of receiving HbA1c feedback might be quite different depending on whether the data communicated to the patient came from an in-office, point-of-care device (representing the most recent data possible) or from a mailed notice about a laboratory HbA1c completed several weeks or months earlier.
Each of these critical feedback characteristics addresses how feedback is configured in ways that enhance perceived meaningfulness and relevance. It is apparent that the simple provision of information and feedback to patients does not necessarily lead directly to awareness, engagement, and effective action. Information alone can be easily ignored or seen as irrelevant and unusable. To be most effective, feedback needs to be crafted in ways that enhance its personal value, including comparisons to relevant peer groups and salient ties to health consequences.
3. Frequency of Feedback
The frequency of delivery is often dependent on the specific behavior or biomarker. For example, real-time continuous glucose monitoring (RT-CGM) yields data every few minutes and can help a patient see how specific lifestyle behaviors are linked to glucose levels. In contrast, lipid levels are typically assessed only once or twice yearly, making lifestyle behaviors and lipid levels relatively more difficult to connect.
In general, greater benefits are seen with more, rather than less, frequent feedback. For example, providing at-risk patients with personalized coronary risk information at several points over time is linked to a significant reduction in predicted risk, whereas presenting such information at only one point in time has little or no effect on risk indices [40]. More frequent at-home weighing and more frequent diet and physical activity monitoring are linked to better weight loss outcomes over time [3, 41, 42]. For people with diabetes, more frequent SMBG is associated with greater glycemic benefits [43, 44]. More frequent RT-CGM use is also linked to better HbA1c outcomes [45].
Several factors, however, qualify the “more is better” rule. In some cases, more frequent feedback may be the result, not the cause, of the noted health benefits. Some patients, for example, may choose to check their weight more often only when they believe that they are successfully losing weight. Furthermore, frequent feedback may lead to feeling overwhelmed, bored, or discouraged, especially if positive change is not seen over time or if changes appear unrelated to patient behavior [37].
Importantly, the effectiveness of feedback varies along a gradient of time in which recipients may benefit from feedback initially, but then become disinterested, discouraged, or unresponsive to feedback later [46, 47]. There is typically a significant drop-off in use of pedometers, online behavioral monitoring programs [48], and home BP monitors over time [49], though there are no consistent data as to when drop-off typically begins. In addition, the long-term effects of feedback programs, and the factors that contribute to maintaining responsiveness to feedback, have not been well documented [50]. Changes in the form, frequency, and structure of feedback may need to occur over time to maintain interest and engagement, especially for patients with a chronic disease like diabetes.
4. Guidance and Support Accompanying the Feedback Message
Feedback interventions vary dramatically by the quality and intensiveness of accompanying support and guidance provided by clinicians [50, 51]. Guidance can vary from frequent, intensive, nurse-led reviews of SMBG results [52, 53] to occasional, cursory, automated emails from a patient’s electronic health record [5]. Many types of feedback, however, require ongoing professional support and direction to be effective [8, 54]. Without such support, feedback may have little impact. For example, there is a large body of data suggesting that initiation of SMBG in patients with type 2 diabetes not using insulin has little or no impact on glycemic control when there is no collaborative support from their diabetes clinician (e.g., little guidance regarding how best to respond to worrisome SMBG patterns and/or lack of positive feedback when SMBG results have substantially improved over time) [28]. A recent Canadian study showed that although 63 % of primary care physicians encouraged their hypertensive patients to monitor their blood pressure at home, only 8 % were given specific training and ongoing support. Interestingly, the inclusion of training by a clinician was the strongest predictor of sustained home blood pressure use over time [55, 56].
Many studies demonstrate that feedback interventions accompanied by close guidance and support can enhance health outcomes. For example, feedback on lipid levels coupled with highly specific, personalized actions to improve cholesterol profiles led to significant improvements in cholesterol, relative to standard care [57]. Despite these encouraging findings, to date there have been no carefully controlled trials documenting the impact of specific kinds (and/or frequencies) of support and guidance on health-related outcomes. We suggest, however, that several factors may be operative. Outcomes are likely to be better when the patient is guided to interpret feedback as encouraging rather than discouraging (i.e., compensatory action is possible and worthwhile), when there is an absence of blaming and shaming (worrisome feedback is not linked to the person being labeled “good” or “bad”) [1, 29], when an atmosphere of trust and collaboration is cultivated (the feedback and the clinician are seen as trustworthy) [58•], and when there is collaborative decision-making regarding how best to respond to the presented results [28, 59]. Compared to web-based, mobile, or mailed forms, feedback is more effective when a live, supportive clinician with whom the patient has a trusting relationship is somehow involved [22, 60]. Without a sense of being accountable and of being of interest to another person, feedback data—especially if it is complex, repetitive, hard to act upon, or aversive—is too easy to ignore [8, 21].
5. Patient Interpretation of the Feedback Message
The effectiveness of feedback is directly related to the dynamic interplay between the qualities of the feedback message and the characteristics of individuals receiving that feedback [61, 62]. Of the patient characteristics that need to be considered, four seem critical: affective state, health beliefs, interest or readiness to receive feedback, and demographics. First, problematic affective states, such as clinical or subclinical depression, anxiety disorders, or a high level of life stress, reduce a patient’s attentiveness and responsiveness to health-related messages [63]. There may just be insufficient cognitive bandwidth and/or personal energy to respond to feedback in a productive way when a patient is distracted by other life problems or is experiencing high levels of emotional distress. Second, feedback is more likely to be accepted and used when the individual feels confident that the data are accurate and believes that positive change is achievable and worthwhile. In addition, the impact will be enhanced when the patient has an underlying coping style that supports such beliefs. This may include such key traits as conscientiousness, internal locus of control, self-efficacy [64], and an ability to set realistic and achievable expectations [38]. Third, the impact of feedback will be influenced by the patient’s interest, awareness, or readiness to receive feedback, which is related to the individual’s perceived need for change [35, 65]. Finally, common demographic characteristics, such as patient age, gender, and educational level, qualify the effectiveness of feedback interventions. To illustrate, short-term utilization of pedometers has been found to be higher among men than women, and frequency of pedometer usage among youth and young adults is far lower than among older adults [24, 66]. In sum, individual differences in patient demographics, attitudes, affective status, and personal styles are likely to qualify the impact of feedback on consequent behavior.
Making Use of the Five Critical Feedback Elements: Diabetes Care Examples
Our overview of the literature suggests that the effectiveness of health-related feedback is increased when attention is given to the critical feedback elements we have identified. In Table 1, we summarize these elements, list their key characteristics, and then present several questions that can aid developers of feedback programs to address feedback in a comprehensive, patient-targeted way. As examples, below we consider how these five feedback characteristics can be applied in the use of RT-CGM and in the more episodic use of SMBG, especially for T2D patients.
Regarding RT-CGM, there are two feedback mechanisms to consider: immediate feedback viewed in real time on the receiver screen and delayed feedback that is summarized electronically in various composite forms. Two of the five feedback components are likely to be of critical importance for both aspects of RT-CGM. First, meaningfulness is critical since the burden of using an intrusive device over time must be balanced by the belief that it will be of personal value. Second, the incorporation of support/guidance in RT-CGM is crucial since the overwhelming amount of data provided by RT-CGM, the less-than-perfect accuracy of the current technology, and the oft-reported confusion regarding how best to make sense of and respond to the results can lead to frustration, discouragement, and poor persistence over time [67].
To enhance meaningfulness, one strategy might be to create a secondary screen display that presents a once-weekly estimation of HbA1c, which could provide a useful translation of RT-CGM data into an easily understandable and relevant form. Furthermore, a secondary screen could also display the past week’s glucose variability (in an easy-to-understand, summarized format) and compare it to values collected during an earlier period of time or to others who are currently using the same device. Regarding support and guidance, patients’ effective use of RT-CGM feedback could be enhanced through the development of live or online classes that provide guidance with respect to how best to understand RT-CGM data and to problem solve as needed.
Regarding episodic SMBG usage among T2D patients, the two critical feedback components are likely to be clarity and support/guidance. As described earlier, many T2D patients cannot make sense of their BG values and/or pattern of BG results [28, 34•]; therefore, devising methods for recording and displaying BG values in a systematic manner that can help patients to more clearly understand their values and to ground those values in a manner that is relevant to their own personal actions can be potentially quite illuminating. For example, dispensing with the traditional logbook, several studies have shown that asking patients to check BG levels intensively (e.g., before and after meals) over a limited period of time and record them in a graphical display can help patients better understand their BG patterns and see how their own lifestyle choices affect those values [52, 68]. Such displays may be simple paper forms or algorithms built into a BG monitor. Regarding support/guidance, even when BG data patterns are well understood by the patient, such information is essentially useless unless the patient knows how to respond effectively. When the patient has the opportunity to review his/her blood glucose data with a trusted healthcare professional, especially when pre-post meal BG data are available, BG feedback can promote greater engagement and, as a result, better glycemic control [53]. For example, professional guidance and data interpretation may help the patient understand that his/her regular BG spikes in the late evening may be due to consuming a high carbohydrate meal, or that a brisk walk following a large meal may reduce the chances of a BG spike. Prompting the patient to repeat a time-limited BG assessment periodically and then reviewing the data with the patient regularly provides an opportunity for BG feedback to be meaningful and useable.
Conclusions and Implications
In sum, we suggest that feedback provided to patients with diabetes (and to the broader audience of patients with chronic health conditions) will be more effective when:
-
it is not overly complicated and easy for the patient to understand
-
it is personally meaningful
-
the frequency of feedback is appropriate to the message and the characteristics of the behavior or biomarker
-
targeted guidance and support for using the feedback content is provided
-
the individual receiving the feedback is ready and able to make good use of it.
Several considerations about these five feedback elements and of the feedback literature in general are worthy of note. First, we suspect that although these five elements operate independently, they also operate interactively with one another. For example, to enhance outcomes, the clarity of feedback may need to be given greater attention as the frequency of feedback presentation increases, and the meaningfulness of the feedback message may be heavily influenced by the degree and quality of the available support and guidance.
Second, we note the almost complete absence of studies that evaluate the effect of repeated episodes of feedback over time. There seems to be an assumption that the effectiveness of feedback is the same after the first time it is delivered as after the 20th or 30th time. Varying the frequency of feedback and taking breaks from feedback are rarely considered in the design of feedback programs. This becomes especially important for patients with a chronic condition like diabetes where the same activities are required repeatedly over time.
Third, we are struck by the almost universal use of a “one-size-fits-all” approach to the delivery of feedback: there seems to be a belief that a single mode, frequency, form, and/or level of each of the five elements of feedback will work equally well for all patients without consideration of different patient needs, preferences, skills, and experiences. This uniform approach precludes patient tailoring and de-emphasizes designing feedback programs in ways that meet the needs of individual patients. Furthermore, such an approach does not provide clinicians and patients with a menu of easily selected options for use in designing feedback programs that address specific clinical problems or questions in patient-tailored ways.
Fourth, there is a striking lack of comprehensiveness and perspective when considering the frequent inconsistent or contradictory findings across studies. For example, among the four HbA1c feedback studies described above, a crucial feedback element that seems to have been omitted from consideration is the presence/absence of direct feedback support from a health care professional. Interestingly, direct support was provided as part of the protocol in the two studies with positive glycemic findings [19, 20], whereas no direct support was provided in the two studies with negative findings [21, 22]. Thus, it may be important to take into account the multiple elements of feedback when evaluating the results of a feedback program.
Last, as technology advances and as feedback devices for diabetes self-management become smaller, more easily used, and less expensive, we expect that the number and complexity of these devices will increase dramatically. There is a danger that a focus on technological sophistication will wow consumers with “cool apps” and devices will become more complex simply because we will have the technology to make them so [69] and not because they offer improvements in clinical outcomes over time. Many of these devices may serve a clinical need, but their emergence into the market must be accompanied by an equal emphasis on patient preference, the clinical problem they address, and the behavioral processes that link feedback to health-related outcomes. This interactive process between patient and feedback, taking into account all five elements of feedback, has not been well attended to in the current literature. We call for greater integration of the psychology of behavioral change into the development of new devices and the utilization of effective feedback in diabetes care.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
McClure JB. Are biomarkers a useful aid in smoking cessation? A review and analysis of the literature. Behav Med. 2001;27:37–47.
Cappuccio FP, Kerry SM, Forbes L. Blood pressure control by home monitoring: meta-analysis of randomized trials. BMJ. 2004;329:145–51.
Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82:222–5.
Ford AL, Bergh C, Sabin MA, Hollinghurst S, Hunt LP, Shield JP. Treatment of childhood obesity by retraining eating behavior: randomized controlled trial. BMJ. 2010;340:5388–92.
Fitzsimons CF, Baker G, Gray SR, Nimmo MA, Mutrie N. Does physical activity counseling enhance the effects of a pedometer-based intervention over the long-term: 12-month findings from the Walking for Wellbeing in the West Study. BMJ Public Health. 2012;12:206–18.
Hollands GJ, Hankins M, Marteau TM. Visual feedback of individual’s medical imaging results for changing health behavior. Cochrane Database Systematic Reviews 2010.
DiClemente CC, Marinilli AS, SIngh M, Bellino LE. The role of feedback in the process of health behavior change. Am J Health Behav. 2001;25:217–27.
Conroy MB, Kyeongra Y, Elci OU, Gabriel KP, Styn MA, Wang J, et al. Physical activity self-monitoring and weight loss: 6-month results of the SMART Trial. Med Sci Sports Exerc. 2011;43:1568–74.
Carver CS, Scheier MF. Control theory: a useful conceptual framework for personality-social, clinical and health psychology. Psychol Bull. 1982;92:111–35.
Kanfer FH, Duerfeldt PH. Motivational properties of self-reinforcement. Elmsford: Pergamon Press; 1990.
Bize R, Burnand B, Mueller Y, Comuz J. Biomedical risk assessments as an aid for smoking cessation. Cochrane Database Syst Rev. 2005;4, CD004705.
Agarwal R, Bills JE, Hecht TJ, Light RP. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57:29–38.
Marquez-Contreras E, Martell-Claros N, Gil-Guillen V, Figuera-Von M, Casado-Martinez J. Efficacy of a home blood pressure monitoring programme on therapeutic compliance in hypertension: the EAPACUM-HTA study. J Hypertens. 2006;24:169–75.
Vrijens B, Goetghebeur E. Comparing compliance patterns between randomized treatments. Controlled Clin Trials. 1997;18:187–203.
Ogedegbe G, Schoenthaler A. A systematic review of the effects of home blood pressure monitoring on medication adherence. J Clin Hypertens. 2006;8:176–81.
Onzenoort HA, Verberk WJ, Kroon AA, Kessels AG, Nelemans PJ, van der Kuy PM, et al. Effect of self-measurement of blood pressure on adherence to treatment in patients with mild-to-moderate hypertension. J Hypertens. 2010;28:622–7.
Staessen JA, Hond ED, Celis H, Fagard R, Keary L, Vanderhoven G, et al. Antihypertensive treatment based on blood pressure management at home or in the physician’s office: a randomized controlled trial. JAMA. 2004;291:955–64.
Mahler H, Kulik J, Gerrard M, Gibbons F. Long-term effects of appearance-based interventions on sun protection behaviors. Health Psychol. 2007;26:350–60.
Chapin RB, Williams DC, Adair RF. Diabetes control improved when inner-city patients received graphic feedback about glycosylated hemoglobin levels. J Gen Intern Med. 2003;18:120–4.
Levetan CS, Dawn KR, Robbins DC, Ratner RE. Impact of computer-generated personalized goals on HbA1C. Diabetes Care. 2002;25:2–8.
O’Connor PJ, Sperl-Hillen J, Johnson PE, Rush WA, Crain AL. Customized feedback to patients and providers failed to improve safety or quality of diabetes care. Diabetes Care. 2009;32:1158–63.
Sherifali D, Greb JL, AMirthavasar G, Hunt D, Haynes RB, Harper W, et al. Effect of computer-generated tailored feedback on glycemic control in people with diabetes in the community. Diabetes Care. 2011;34:1794–8.
Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, et al. Using pedometers to increase physical activity and improve health. JAMA. 2007;298:2296–304.
Tudor-Locke C, Lutes L. Why do pedometers work? A reflection upon the factors related to successfully increasing physical activity. Sports Med. 2009;39:981–93.
Tudor-Locke C, Corbin CB. Taking steps toward increased physical activity; using pedometers to measure and motivate. Pres Counc Phys Fit Sports. 2002;3:1–8.
Kabutoya T, Ishikawa J, Hoshide S, Eguchi K, Shimada K, Kario K. A home blood pressure monitor equipped with a graphic function facilitates faster blood pressure control than conventional home blood pressure monitor. J Clin Hypertens. 2009;11:422–5.
Dowray S, Swartz JJ, Braxton D, Viera AJ. Potential effect of physical activity based menu labels on the calorie content of selected fast food meals. Appetite. 2013;62:173–81.
Polonsky WH, Fisher L. Right answer, but wrong question: SMBG can be clinically valuable for non-insulin users. Diabetes Care 2012;35.
Parkes G, Greenhalgh T, Griffen M, Dent T. Effect on smoking quit rate of telling patients their lung age: the Step 2 quit randomized controlled trial. BMJ 2008.
Schultz PW, Nolan JM, Cialdini RB, Goldstein NJ, Griskevicius V. The constructive, destructive and reconstructive power of social norms. Psychol Sci. 2007;18:429–34.
Rees G, Lamoureux EL, Nicolaou TE, Hodgson LA, Weinman J, Speight J. Feedback of personal retinal images appears to have a motivational impact in people with non-proliferative diabetic retinopathy and suboptimal HbA1C: findings of a pilot study. Diabetic Med. 2013;30:1122–5. In this small but intriguing pilot study, T1D patients with non-proliferative retinopathy who were exposed to personal retinal images demonstrated greater improvement in glycemic control than control patients over a 3-month period.
Gibbons F, Gerrard M, Lane DJ, Mahler H, Kulik J. Using UV photography to reduce use of tanning booths: a test of cognitive mediation. Health Psychol. 2005;24:358–63.
Shahab L, Hall S, Marteau T. Showing smokers with vascular disease images of the arteries to motivate cessation: a pilot study. Br J Health Psychol. 2007;12:275–83.
Polonsky WH, Fisher L, Hessler DH, Edelman SV. What is so tough about self-monitoring of blood glucose? Perceived obstacles among patients with type 2 diabetes. Diabet Med. 2014;31:40–6. Three key obstacles to self-monitoring of blood glucose were identified—Burden, Avoidance, and Pointlessness. Higher levels of Avoidance and Pointlessness, but not Burden, were associated with less frequent self-monitoring use.
Wilson TD, Dunn DS, Kraft D, Lisle DJ. Introspection, attitude change and attitude-behavior consistency: the disruptive effects of explaining why we feel the way we do. In: Berkowitz L, editor. Advances in social psychology. New York: Academic Press; 1989. p. p. 287–343.
Parkin CG, Hinnen D, Campbell RK, Geil P, Tetrick DL, Polonsky WH. Effective use of paired testing in type 2 diabetes: practical applications in clinical practice. Diabetes Educ. 2009;35:915–27.
Polonsky WH, Skinner CS. Perceived treatment efficacy: an overlooked opportunity in diabetes care. Clin Diabet. 2010;28:89–92.
Richardson CR, Newton TL, Abraham JJ, Sen A, Jimbo M, Swartz AM. A meta-analysis of pedometer-based walking interventions and weight loss. Ann Fam Med. 2008;6:69–77.
Cagliero E, Levina EV, Nathan DM. Immediate feedback of HbA1C levels improves glycemic control in type 1 and insulin-dependent type 2 diabetic patients. Diabetes Care. 1999;22:1785–9.
Sheridan SL, Viera AJ, Krantz MJ, Ice CL, Steinman LE, Peters KE, et al. The effect of giving global risk information to adults: a systematic review. Arch Intern Med. 2010;170:230–9.
Casazza KBA, Astrup A, Bertz F, Baum C, Brown MB, et al. Weighing the evidence of common beliefs in obesity research. Crit Rev Food Sci Nutr. 2014. doi:10.1080/10408398.2014.922044.
Linde JA, Jeffery RW, French SA, Pronk NP, Boyle RG. Self-weighing in weight gain prevention and weight loss trials. Ann Behav Med. 2005;30:210–6.
Karter AJ, Parker MM, Moffet HH, Spence MM, Chan J, Ettner SL. Longitudinal study of new and prevalent use of self-monitoring of blood glucose. Diabetes Care. 2006;29:1757–63.
Schutt M, Kern W, Krause U, Busch P, Dapp A. Is the frequency of self-monitoring of blood glucose related to long-term metabolic control? Multicenter analysis including 24,500 patients from 191 centers in Germany and Austria. Clin Endocrinol Diabetes. 2006;114:384–8.
Group JDRFCGMS. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464–76.
Jakicic JM, Winters C, Lang W. Effect of exercise duration and intensity on weight loss in overweight, sedentary women: a randomized trial. JAMA. 2003;1999:1323–30.
Shay LE, Seibert D, Watts D, Sbrocco T, Pagliara C. Adherence and weight loss outcomes associated with food-exercise diary preference in a military weight management program. Eat Behav. 2009;10:220–7.
Baker G, Mutrie N, Lowry R. Using pedometers as motivational tools: are goals set in steps more effective than goals set in minutes for increased walking? J Health Promot Educ. 2008;46:21–6.
Fung CS, Wong WC, Wong CK, Lee A, Lam CL. Home blood pressure monitoring: a trial on the effect of a structured education program. Aust Fam Physician. 2013;42:233–8.
Uhlig K, Balk EM, Patel K, Ip S, Kitsios GD, Obadab NO, Haynes SM, Rao M, Chang L, Gaylor J, Iovin RC: Self-measured blood pressure monitoring: comparative effectiveness. In Effective health care program: comparative effectiveness review. Rockville, MD, AHRQ, 2012.
Pomerleau J, Lock K, Knai C, McKee M. Interventions designed to increase adult fruit and vegetable intake can be effective: a systematic review of the literature. J Nutr. 2005;135:2486–95.
Franciosi M, Lucisano G, Pellegrini F, Cantarello A, Consoli A, Cucco L. ROSES: role of self-monitoring of blood glucose and intensive education in patients with type 2 diabetes not receiving insulin: a pilot randomized clinical trial. Diabet Med. 2011;21:999–106.
Duran A, Martin P, Runkle I, Perez N, Abad R, Fernandedz M, et al. Benefits of self-monitoring of blood glucose in the management of new-onset type 2 diabetes mellitus: The St Carlos Study, a prospective randomized clinic-based interventional study with parallel groups. J Diabet. 2010;2:203–11.
Shaw R, Fenwick E, Baker G, McAdam C, Fitzsimons CF, Mutrie N. “Pedometers cost buttons”: the feasibility of implementing a pedometer-based walking programme within the community. BMC Public Health. 2011;11:200–8.
Logan AG, Dunai A, Mclassac WJ, Irvine MJ, Tisler A. Attitudes of primary care physicians and their patients about home blood pressure monitoring in Ontario. J Hypertens. 2008;26:446–52.
Bancej CM, Campbell N, McKay DW, Nichol M, Walker RL, Kaczorowski J. Home blood pressure monitoring among Canadian adults with hypertension: results from the 2009 survey on Living with Chronic Diseases in Canada. Can J Cardiol. 2010;26:152–7.
Levetan CS, Dawn KR, Murray JF, Popma JJ, Ratner RE, Robbins DC. Impact of computer-generated personalized goals on cholesterol lowering. Value Health. 2005;8:639–46.
Polonsky WH, Hessler DH. What are the quality of life-related benefits and losses associated with real-time continuous glucose monitoring? A survey of current users. Diabetes Technol Ther. 2013;15:295–301. Diabetes-specific QOL benefits resulting from RT-CGM were found to be common; those benefits were associated with satisfaction with device accuracy and usability and trust in one’s ability to use RT-CGM data, suggesting that “perceived efficacy,” for both device and self, are key QOL determinants.
Heisler M, Bouknight RR, Hayward RA, Smith DM, Kerr EA. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. J Gen Intern Med. 2000;17:243–52.
Murray E, Burns J, See TS, Lai R, Nazareth I. Interactive health communication applications for people with chronic disease. Cochrane Database Syst Rev. 2005;4, CD004274.
Bosworth HB, Olsen MK, Oddone EZ. Improving blood pressure control by tailored feedback to patients and clinicians. Am Heart J. 2005;149:795–803.
Krueter MW, Skinner CS. Tailoring: what’s in a name? Health Educ Res. 2000;15:1–4.
Gonzalez JS, Fisher L, Polonsky WJ. Depression in diabetes: have we been missing something important? Diabetes Care. 2011;34:236–9.
Fisher L, Hessler DH, Masharani U, Stryker LA. The impact of baseline patient characteristics on interventions to reduce diabetes distress: the role of personal conscientiousness and diabetes self-efficacy. Diabetic Med. 2014;31:739–46.
Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011;111:92–102.
Butcher Z, Fairclough S, Stratton G, Richardson D. The effect of feedback and information on children’s pedometer step counts at school. Pediatr Exerc Sci. 2007;19:29–38.
Chamberlain J, Dopita D, Gilgen E. Persistence of continuous glucose monitoring use in a community setting one year after purchase. Clin Diabetes. 2013;31:106–9.
Polonsky WH, Fisher L, Schikman C, Hinnen D, Parkin C, Jelovsky Z, et al. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care. 2011;34:262–7.
Fisher L, Dickinson WP. New technologies to support self-management support in diabetes: not just a bunch of cool apps. Diabetes Care. 2011;34:240–3.
Acknowledgments
This research was funded by an unrestricted educational grant from Abbott Diabetes Care.
The authors gratefully acknowledge the contributions of the following colleagues in the preparation of this manuscript: Jeffrey S. Gonzales, PhD; Linda Gonder-Frederick, PhD; Richard A. Jackson, MD; Julie A. Wagner, PhD; and John D. Piette, PhD.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
William H. Polonsky has served as a consultant for Roche Diabetes Care, Abbott Diabetes Care, Johnson & Johnson Diabetes Care, Dexcom, Eli Lilly, Novo Nordisk, Sanofi Diabetes, and Takeda. Lawrence Fisher has served as a consultant for Roche Diabetes Care, Abbott Diabetes Care, and Eli Lilly.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Psychosocial Aspects
Rights and permissions
About this article
Cite this article
Polonsky, W.H., Fisher, L. When Does Personalized Feedback Make A Difference? A Narrative Review of Recent Findings and Their Implications for Promoting Better Diabetes Self-Care. Curr Diab Rep 15, 50 (2015). https://doi.org/10.1007/s11892-015-0620-7
Published:
DOI: https://doi.org/10.1007/s11892-015-0620-7