Abstract
Diabetes mellitus is a common chronic condition worldwide, especially in the elderly population. Several epidemiologic studies in the last 2 years have consistently associated diabetes with physical disability, a condition that may profoundly affect the quality of life of older people. Although in older people with diabetes, the pathogenesis of functional limitation and disability has not been completely elucidated, it is certainly complex and involves multiple potential pathways. In this narrative review, we described the most recent epidemiologic and clinical evidence supporting the association between diabetes and impaired physical function in older persons focusing on emerging biological mechanisms explaining the excess risk of disability associated with diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes, the most common metabolic disease in older people, is highly prevalent in industrialized countries. Because of the ongoing demographic transition and progressive aging of the overall population, its prevalence will further increase in the next few decades [1], leading to different clinical presentations and different age-related complications. Indeed, in addition to the traditional cardiovascular complications, diabetes has been associated with excess risk of clinical conditions typical of the geriatric population such as physical disability that may profoundly affect the quality of life of older patients with this disease [2••]. Disability represents a major risk factor for loss of independence, recurrent hospitalization, falls, injuries, acute illnesses, and eventually institutionalization [3]. Despite the growing interest on the pathogenesis of diabetes-related disability, the biological mechanisms explaining this association are not completely understood.
This narrative review outlines and discusses epidemiological evidence for a causal association between diabetes and physical disability, focusing on hypothesized pathogenetic pathways that would explain the excess risk of disability associated with diabetes.
Epidemiology of Diabetes
According to the International Diabetes Federation, about 366 million people aged 20–79 years worldwide were estimated to have diabetes in 2011. If increasing trends continue, by 2030 some 552 million people will be affected by this metabolic condition [1]. For community-dwelling people in the United States, from 1980 through 2011, the percentage of people with diagnosed diabetes increased 167 % (from 0.6 % to 1.6 %) for those aged 0–44 years, 118 % (from 5.5 % to 12.0 %) for those aged 45–64 years, 140 % (9.1 % to 21.8 %) for those aged 65–74 years, and 125 % (8.9 % to 20.0 %) for those aged 75 years and older [4]. In 2012, an estimated 22.3 million people in the US were diagnosed with diabetes, representing about 7 % of the population [5•].
In the future, the diabetes epidemic is expected to expand; indeed, according to the US Census Bureau, total diabetes prevalence (diagnosed and undiagnosed cases) is estimated to increase from 14 % in 2010 to 21 % of the US adult population by 2050 [6].
Currently, besides the huge prevalence of people with clinically diagnosed diabetes, almost 10 % suffer from unrecognized disease, or meet a condition of impaired glucose tolerance (IGT) that also constitutes a major public health problem, both because of its association with diabetes incidence [7] and its own association with an increased risk of poorer physical function [8].
Diabetes imposes a substantial burden on the economy in the form of increased medical costs and indirect costs from work-related absenteeism, reduced productivity at work and at home, reduced labor force participation from chronic disability, and premature mortality. The estimated US national cost of diabetes in 2012 was $245 billion, of which $176 billion (72 %) represents direct health care expenditures attributed to diabetes and $69 billion (28 %) represents lost productivity from work-related absenteeism, reduced productivity at work and at home, unemployment from chronic disability, and premature mortality [5•].
Diabetes as a Risk Factor for Physical Disability
Diabetes and the Disablement Process
According to Verbrugge and Jette, “the disablement process is a progressive, multi-step, and dynamic pathway” in which different diseases and several predisposing conditions may trigger and facilitate the onset of physical disability [9]. This representation offers a speculative interpretation of the pathogenetic mechanisms, connecting diabetes, impairments, functional limitation, and disability, providing important information on postulated determinants and mediators of the course of the process. In agreement with the original definition suggested by Nagi, functional limitation is characterized by “limitation in performance at the level of the whole organism or person,” whereas disability denotes the presence of “limitation in performance of socially defined roles and tasks within a sociocultural and physical environment” [10]. In older people, the onset of disability may develop with different temporal trajectories (ie, progressive or catastrophic) and the level of difficulty may fluctuate over time and even regress.
Most traditional complications of diabetes are potentially associated with the multiple steps of the process leading to physical disability, one of the most relevant indicators of quality of life in older people. For example, amputation and blindness (impairments) may hinder from activities of daily living (ADLs), for example walking, nerve problems may put limitations on strengths and mobility activities (functional limitation), and the worsening cognitive function may impose limitations on instrumental activities of daily living (IADLs); for example managing money [11].
Diabetes and Disability
The relationship between diabetes and disability has been repeatedly investigated in the last few decades in multiple countries and different types of populations (Table 1). Globally taken, the results of these observational investigations demonstrated a constant excess rate of disability in multiple task of physical function in people with diabetes [2••]. More recently, other studies have confirmed and extended the results of the previous reports, addressing more innovative hypotheses underlying the biological relationship between diabetes and the early steps of the disablement process of older persons. For example, in a cross-sectional analysis of 835 people aged 65 and older enrolled in the InCHIANTI study, we have investigated the relationship between type 2 diabetes, lower extremity skeletal muscle characteristics, and performance in 2 objective measures of lower-extremity function, the 4-meter usual walking speed and 400-meters walking speed test. Compared with people without diabetes, participants with diabetes had worse muscle performance and were significantly slower on both the 4-meter and 400-meter walking test [12••].
In a study based on 156 patients recruited in 1 university and 4 district hospitals in the south of The Netherlands, people with diabetes mellitus, independently of the presence of polyneuropathy, were more likely to have a marked reduction in lower leg muscle strength and mobility, assessed by 6-minute walk test, a timed up-and-go test, and the Physical Activity Scale of the Elderly [13].
Among the more than 2500 adults aged 50 and older enrolled in the National Health and Nutrition Examination Survey (NHANES), diabetes mellitus was associated with significantly slower gait speed also after adjusting for demographic characteristics, weight, height, physical activity, C-reactive protein (CRP), and comorbidity [14].
A recent clinical investigation conducted on a small sample of well-functioning older men suggests that the detrimental effect of diabetes might play an important role also in the early steps of the disablement process. This study, conducted on 92 nondisabled men aged 70 ± 1 years, found that diabetic men had a poorer performance on the sit-to-stand performance test compared with those without diabetes. Furthermore, this difference was not explained by the presence of other clinical characteristics commonly associated with diabetes, suggesting a causal and direct relationship between diabetes and functional limitation [15]. The hypothesis of a causal association between diabetes and accelerated decline in physical function is further strengthened by several investigations with prospective design, including, but not limited to, the EPESE [16] and the Study of Osteoporotic Fractures [17]. Taken together, these studies have demonstrated an independent and consistent increased risk of incident disability over time in persons without disability at study inception. A more recent study conducted on 2143 older Brazilian adults enrolled in the Survey on Health and Well-being of the Elderly Study (SABE), showed that by itself, the presence of diabetes did not increase the risk of disability or the need for assistance. However, diabetes was related to increased risks when assessed in combination with stroke [18].
The Pathway from Diabetes to Disability
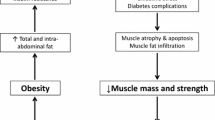
In order to recommend and implement new strategies for the prevention of disabling effect of type 2 diabetes in older persons, it is fundamental to fully elucidate the underlying biological mechanism that may explain the excess burden of physical disability associated with diabetes. In older patients diabetes is associated with multiple impairments and conditions including ischemic heart disease, congestive heart failure, peripheral arterial disease, visual impairment, chronic renal disease, neuropathies, obesity, mood disorders, and cognitive decline that may justify the increased burden of disability associated with the disease [2••].
Results of observational studies that formally investigated the mediating role of traditional diabetes complications demonstrated that these conditions explain only a part of diabetes-related disability [19]. For example, in the InCHIANTI Study, chronic conditions including cardiovascular and cerebrovascular diseases, peripheral arterial disease, peripheral neuropathy, overweight, and cognitive impairments, explained about 18 % and 30 % of the association between diabetes and functional limitation in the 4- and 400-meter tests, respectively [12••].
The Biological Mechanisms
In older persons, the detrimental effect of glucose metabolism dysregulation interacts with the multisystemic pathophysiological changes of the aging process fostering multiple and often interplaying potential pathways summarized in Table 2.
The Role of Glycemic Control
One of the most challenging questions is whether in older diabetic patients a strict glycemic control would translate in a less pronounced functional decline over time. For young and adult patients with type 1 and 2 diabetes, a large number of observational and experimental studies demonstrates that, an optimal and tight glycemic control, along with adequate treatment of associated metabolic conditions (hypertension and dyslipidemia), are the cornerstones for diabetes management and long-term prevention of chronic diabetes complications. The widely accepted recommendation that all patients pursue a glycated hemoglobin (HbA1c) <7.0 % is based largely on studies, such us the UK Prospective Diabetes Study [20], which actively excluded people aged >65 years [21]. Nevertheless, in older patients only a few long-term studies have demonstrated the beneficial effect of tight glycemic control. Furthermore, no studies have specifically assessed the effect of intensive anti-diabetic therapy on the future incidence of disability, falls, and frailty, although some observational studies reported better physical performance over time in older people with good glycemic control, compared with those with poor glycemic. The issue of whether aggressive diabetes treatment would be really beneficial and safe is particularly important in those complex patients with multiple comorbidities and reduced functional reserve that are at the highest risk of iatrogenic side effects and adverse drug reactions.
For example Kalyani et al. using data from the Women’s Health and Aging Study II, showed that, among 329 older diabetic women, HbA1c of 8.0 % or greater (vs reference category: HbA1c <5.5 %) was associated with development of poor physical performance, low walking speed, difficulty in walking, and incident frailty [22•].
In the San Antonio Longitudinal Study of Aging, a group of community older people aged 71–85, who met the American Diabetes Association criteria for the diagnosis of diabetes, was followed over a 36-month period and classified as a poor control (HbA1c >7 %), or good control (HbA1c <7 %). After 18 and 36 months, lower-extremity performance, assessed by the Short Physical Performance Battery (SPPB), was better in the optimal glycemic control class than in the poorer control class [23].
Conversely, more recent trials have generated controversy regarding the effects of pursuing very low glucose levels in older diabetic patients. In a longitudinal study on 367 community-dwelling and nursing home-eligible individuals with diabetes mellitus enrolled at On Lok Lifeways, HbA1c levels between 8.0 % and 8.9 % appeared to be associated with less functional decline or death at 2 years than HbA1c levels between 7.0 % and 7.9 %. Moreover, across all outcomes (death, functional decline, and combined outcome) of the study, participants with an HbA1c level less than 7.0 % had the highest risk of poor outcomes [24•].
Indeed, some studies suggested that in older patients, aggressive diabetic treatment and strict glycemic control may increase the incidence of hypoglycemia, falls, fractures, and eventually mortality [25]. For example, in the ACCORD-MIND study [26], intensive glycemic control was not associated with substantial benefit on cognitive function after 40 months of follow-up, whereas older patients randomized to the intensive glucose lowering group experienced a significantly higher rate of hypoglycemic events and death [27]. These studies suggest that the current American Geriatrics Society guideline [28] HbA1c target of 8.0 % or less for frail elderly adults may be lower than necessary to maintain function and delay death for this vulnerable population.
Diabetes and Sarcopenia
Another potential mediator of the association between diabetes and disability may be the diabetes related loss of skeletal muscle mass and muscle strength that has been referred to as sarcopenia.
Sarcopenia, a very frequent clinical trait in the geriatric population, is strongly associated with functional decline, disability, and mortality [29•]. Therefore, it might be hypothesized as a potential mediator of the association between diabetes and disability.
Compared with those without diabetes, older diabetic patients have been reported having similar muscle mass, as a consequence of their usually excessive total body mass. Nevertheless, observational studies have suggested that older people with diabetes, despite having “adequate” skeletal muscle mass, tended to have reduced muscle quality and more rapid decline in muscle mass and lower extremity strength over time [12••, 30••]. In a recent cross-sectional analysis based on 835 participants of the inCHIANTI study, we have revealed that participants with diabetes, despite having a larger muscle calf area, had lower knee and ankle muscle strength and muscle power, as well as worse muscle quality (defined as the ratio of lower limb muscle strength over muscle area) and slower gait speed on both 4-meter and 400-meter walking tests. In the multivariate regression models, lower-limb muscle characteristics accounted for 24.3 % and 15.1 % of walking speed difference comparing diabetic and nondiabetic subjects in the 4- and 400-meter walks, respectively [12••]. These results have been recently confirmed by Leenders et al. who investigated whether differences in muscle mass and/or strength may translate to differences in functional capacity in older people with and without diabetes. In accordance with the decline in leg extension strength, they reported a substantially longer sit-to-stand time in the older patients with type 2 diabetes when compared with the normoglycemic controls [15]. These findings suggest that standardized assessment of muscle characteristics and lower-extremity function, with early detection of sarcopenia and impaired muscle quality, might prompt appropriate lifestyle or medical intervention to postpone functional decline, prevent disability, and preserve independence and quality of life of older persons with diabetes [2••].
Why older adults with diabetes tend to have greater decline of skeletal muscle mass and performance is not clear and still under investigation. Indeed, in patients with long-standing disease the presence of classic diabetes complications including peripheral neuropathy and obstructive arterial disease, might functionally impair lower limb skeletal muscles. Epidemiological studies have clearly demonstrated a strong and independent association of these 2 classic chronic complications with muscle strength and walking speed [31, 32].
Additional supposed causal pathway leading to sarcopenia and reduced muscle strength in patients with diabetes is increased muscle fat infiltration [33] that results in a loss of muscle quality and in decreased muscle density. Increased muscle fat infiltration has been associated with both reduced oxidative activity and maximal aerobic capacity and, furthermore, in epidemiological studies of older persons, fat infiltration predicted the risk of mobility disability over time [34].
Recent studies have also reported that people with diabetes have increased serum levels of inflammatory markers, including interleukin-6 and CRP that have been associated with muscle strength decline and risk of disability in several older populations [35, 36]. Lastly, limited leisure time physical activity and physical inactivity are also frequent in older people with diabetes, particularly in those affected by overweight and obesity, and therefore might contribute to the well documented age-related reduction in strength and physical performance. Regardless of the underlying mechanisms, these findings suggest that standardized assessment of muscle characteristics and lower-extremity function, with early detection of sarcopenia and impaired muscle quality, might prompt appropriate lifestyle or medical intervention to postpone functional decline, prevent disability, and preserve independence and quality of life of older persons with diabetes.
Diabetes and Physical Activity
Among older people with diabetes regular physical exercise represents a powerful tool to protect the integrity and function of skeletal muscle and to reduce the risk of disability. Several longitudinal studies, conducted in different countries and populations, suggest that physical activity may decrease the influence of diabetes on the development of disability, improving glycemic control and weight loss. For example in older Mexican Americans, physical activity moderates the rate of decline in disability and physical function over a 10–12-year period in older people with diabetes [37]. Among the more than 5000 overweight or obese adults with diabetes enrolled in the Look AHEAD study, an intensive lifestyle intervention, including weight loss and improved fitness, resulted in a 48 % reduction in the risk of loss of mobility over a 4-year period, compared with the control group [38•].
There are several long-term effects of low-level physical activity on the progression of functional disability related to diabetes. Reduction of physical activity may be a risk factor for the development of some conditions that, in turn, contribute to the onset of disability such as sarcopenia, heart disease, and peripheral arterial disease. Aerobic exercise may also improve glucose tolerance [39], preventing the onset of obesity and diabetes mellitus and their complications [40], and finally, may have a direct positive effect on the inflammatory status that has been involved in the development of sarcopenia and functional limitation [30••, 41].
Other Biological Mechanisms
An important step toward preventing diabetes-related disability is a better understanding of biomarkers that can identify older adults with diabetes, not yet disabled, but at the highest risk of becoming disabled. Accelerated atherosclerosis is a common pathway for the majority of diabetes-related complications: albuminuria and high proinflammatory cytokines have been linked to endothelial dysfunction, as well as initiation and progression of atherosclerosis [36, 42].
Among 1729 not institutionalized elderly adults with diabetes enrolled in the NHANES study, increased urinary albumin excretion was associated with functional disability, independent of chronic comorbidities, systolic blood pressure, glycemic control, renal function, total cholesterol, and chronic inflammation. Furthermore subjects with albuminuria and increased CRP values were significantly more likely to have higher odds of disability than individuals with albuminuria and normal CRP values, suggesting that the presence of subclinical inflammation may amplify the effect of albuminuria on functional disability in older diabetic adults [36]. Albuminuria and low-grade systemic inflammation, are related to cerebral atherosclerosis and lower limb arterial disease, that in turn have both been associated with slow gait speed, decreased muscle strength, and physical disability. Cerebral atherosclerosis precedes both cerebral macroangiopathy and subcortical white matter lesions. These cerebral lesions, thus, interrupt the integrity of frontal-subcortical circuits that are associated with cognitive impairment, functional decline, and slow walking speed [43].
Regardless of the presence of overt neurological signs and brain pathology, cognitive impairment and depressive disorders are common risk factors for functional decline in older people. In the last few years a number of epidemiological and clinical studies strongly supported a causal linking between diabetes and these disabling conditions [44–46], suggesting additional biological pathways.
Conclusions and Future Directions
Diabetes prevalence increases with age and imposes a substantial burden both to patients and health care systems. The etiology of physical disability in older patients with diabetes is multifactorial. Nevertheless most of the mechanisms that have been postulated as important mediators of the disablement process are, at least partially, modifiable and should be considered as potential targets for feasible intervention program designed to buffer and reduce the level of disability related to diabetes. Estimates of the current and future burden of diabetes are important in order to allocate community and health resources, to emphasize the role of lifestyle, and to encourage measures to counteract trends for increasing prevalence.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
International Diabetes Federation (IDF). The Global Burden: Diabetes. Available at: http://www.idf.org/diabetesatlas/5e/Update2012. Accessed April 30, 2013.
•• Volpato S, Maraldi C, Fellin R. Type 2 diabetes and risk for functional decline and disability in older persons. Curr Diabetes Rev. 2010;6:134–43. An exhaustive review defining the nature of the relationship between diabetes and physical disability.
Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology and risk. J Am Geriatr Soc. 1997;45:92–100.
U.S. Centers for Disease Control and Prevention. Diabetes data & trends: percentage by age of civilian, noninstitutionalized persons with diagnosed diabetes, United States, 1980–2011. Available at: http://www.cdc.gov/diabetes/statistics/prev/national/figbyage.html. Accessed April 30, 2013.
• Economic costs of diabetes in the U.S. in 2012. American Diabetes Association. Diabetes Care. 2013;36:1033–46. This paper estimates the economic impact of diabetes in the U.S.
Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Population Health Metrics. 2010;22:8–29.
Shaw J, Zimmet P, de Courten M, Dowse G, Chitson P, Gareeboo H, et al. Impaired fasting glucose or impaired glucose tolerance. What best predicts future diabetes in Mauritius? Diabetes Care. 1999;22:399–402.
Hiltunen LA. Does glucose tolerance affect elderly persons’ balance, gait or muscle strength? Cent Eur J Public Health. 2001;9:22–5.
Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38:1–14.
Guralnik JM, Ferrucci L. Assessing the building blocks of function: utilizing measures of functional limitation. Am J Prev Med. 2003;25:112–21.
Chiu CJ, Wray LA. Physical disability trajectories in older Americans with and without diabetes: the role of age, gender, race or ethnicity, and education. Gerontologist. 2011;51:51–63.
•• Volpato S, Bianchi L, Lauretani F, et al. Role of muscle mass and muscle quality in the association between diabetes and gait speed. Diabetes Care. 2012;35:1672–9. This study found that older people with diabetes have higher muscle mass, lower muscle strength, worse muscle quality, and slower gait speed compared with participants without diabetes.
Izerman TH, Schaper NC, Melai T, Meijer K, Willems PJ, Savelberg HH. Lower extremity muscle strength is reduced in people with type 2 diabetes, with and without polyneuropathy, and is associated with impaired mobility and reduced quality of life. Diabetes Res Clin Pract. 2012;95:345–51.
Kalyani RR, Tra Y, Yeh HC, Egan JM, Ferrucci L, Brancati FL. Quadriceps strength, quadriceps power, and gait speed in older U.S. adults with diabetes mellitus: results from the National Health and Nutrition Examination Survey, 1999–2002. J Am Geriatr Soc. 2013. [Epub ahead of print].
Leenders M, Verdijk LB, van der Hoeven L, Adam JJ, van Kranenburg J, Nilwik R, et al. Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J Am Med Dir Assoc. 2013 [Epub ahead of print].
Guralnik JM, LaCroix AZ, Abbott RD, et al. Maintaining mobility in late life. I. Demographic characteristics and chronic conditions. Am J Epidemiol. 1993;137:845–57.
Gregg EW, Mangione CM, Cauley JA, et al. Diabetes and incidence of functional disability in older women. Diabetes Care. 2002;25:61–7.
Andrade FC, Guevara PE, Lebrão ML, Duarte YA. Correlates of the incidence of disability and mortality among older adult Brazilians with and without diabetes mellitus and stroke. BMC Publ Health. 2012;12:361.
Volpato S, Blaum C, Resnick H, Ferrucci L, Fried LP, Guralnik JM. Comorbidities and impairments explaining the association between diabetes and lower extremity disability: the women's health and aging study. Diabetes Care. 2002;25:678–83.
UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–65. Erratum, Lancet 1998;352:1558.
Huang ES, Zhang Q, Gandra N, Chin MH, Meltzer DO. The effect of comorbid illness and functional status on the expected benefits of intensive glucose control in older patients with type 2 diabetes: a decision analysis. Ann Intern Med. 2008;149:11–9.
• Kalyani RR, Tian J, Xue QL, Walston J, Cappola AR, Fried LP, et al. Hyperglycemia and incidence of frailty and lower extremity mobility limitations in older women. J Am Geriatr Soc. 2012;60:1701–7. This paper clearly demonstrates that higher levels of glycated hemoglobin are associated with development of poor physical performance.
Wang CP, Hazuda HP. Better glycemic control is associated with maintenance of lower-extremity function over time. Diabetes Care. 2011;34:268–73.
• Yau CK, Eng C, Cenzer IS, Boscardin WJ, Rice-Trumble K, et al. Glycosylated hemoglobin and functional decline in community-dwelling nursing home–eligible elderly adults with diabetes mellitus. J Am Geriatr Soc. 2012;60:1215–21. This paper introduced a controversy regarding the effects of pursuing very low glucose levels in older diabetic patients.
Schwartz AV, Vittinghoff E, Sellmeyer DE, et al. Diabetes-related complications, glycemic control, and falls in older adults. Diabetes Care. 2008;31:391–6.
Launer LJ, Miller ME, Williamson JD, Lazar RM, Gerstein HC, Murray AM, et al. ACCORD MIND investigators. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomized open-label substudy. Lancet Neurol. 2011;10:969–77.
Gerstein HC, Miller ME, Byington RP, Goff Jr DC, Bigger JT, Buse JB, et al. Action to control cardiovascular risk in diabetes study group, Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59.
Brown AF, Mangione CM, Saliba D, et al. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc. 2003;5(Suppl Guidelines):265–80.
• Fielding R, Vellas B, Evans W, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc. 2011;12:249–56. This paper provides new data on the definition, prevalence, etiology, and consequences of Sarcopenia.
•• Park SW, Goodpaster BH, Lee JS, et al. Health, aging, and body composition study. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;1993–7. This is one of the first papers in which type 2 diabetes has been associated with excessive loss of skeletal muscle mass in community-dwelling older adults overtime.
Martinelli AR, Mantovani AM, Nozabieli AJ, Ferreira DM, Barela JA, Camargo MR, et al. Muscle strength and ankle mobility for the gait parameters in diabetic neuropathies. Foot. 2013;23:317–21.
McDermott MM, Guralnik JM, Albay M, Bandinelli S, Miniati B, Ferrucci L. Impairments of muscles and nerves associated with peripheral arterial disease and their relationship with lower extremity functioning: the InCHIANTI study. J Am Geriatr Soc. 2004;52:405–10.
Miljkovic-Gacic I, Wang X, Kammerer CM, Gordon CL, Bunker CH, Kuller LH, et al. Fat infiltration in muscle: new evidence for familial clustering and associations with diabetes. Obesity. 2008;16:1854–60.
Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–33.
Badawi A, Klip A, Haddad P, Cole DE, Bailo BG, El-Sohey A, et al. Type 2 diabetes mellitus and inflammation: prospects for biomarkers of risk and nutritional intervention. Diabetes Metab Syndr Obes. 2010;3:173–86.
Kuo HK, Al Snih S, Kuo YF, Raji MA. Cross-sectional associations of albuminuria and C-reactive protein with functional disability in older adults with diabetes. Diabetes Care. 2011;34:710–7.
Palmer RF, Espino DV, Dergance JM, Becho J, Markides K. The role of physical activity and diabetes status as a moderator: functional disability among older Mexican Americans. Age Ageing. 2012;41:752–8.
• Rejeski WJ, Ip EH, Bertoni AG, Bray GA, Evans G, Gregg EW, et al. Look AHEAD Research Group. Lifestyle change and mobility in obese adults with type 2 diabetes. N Engl J Med. 2012;366:1209–17. An important outcome paper from Look AHEAD showing that lifestyle intervention appears to impact the loss of mobility in people with type 2 diabetes.
Umpierre D, Ribeiro PA, Kramer CK, Leitão CB, Zucatti AT, Azevedo MJ, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790–9.
Fagour C, Gonzalez C, Pezzino S, Florenty S, Rosette-Narece M, Gin H, et al. Low physical activity in patients with type 2 diabetes: the role of obesity. Diabetes & Metabolism. 2013;39:85–7.
Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Williams GR, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI Study. J Gerontol. 2004;59:242–8.
Libby P, Ridker PM, Hansson GK. Leducq Transatlantic Network on atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–38.
Pugh KG, Lipsitz LA. The microvascular frontal-subcortical syndrome of aging. Neurobiol Aging. 2002;23:421–31.
Maraldi C, Volpato S, Penninx BW, Yaffe K, Simonsick EM, Strotmeyer ES, et al. Diabetes mellitus, glycemic control, and incident depressive symptoms among 70- to 79-year-old persons: the health, aging, and body composition study. Arch Intern Med. 2007;167:1137–44.
Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42:484–91.
Nouwen A, Winkley K, Twisk J, Lloyd CE, Peyrot M, Ismail K, et al. European Depression in Diabetes (EDID) Research Consortium. Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia. 2010;53:2480–6.
Malhotra C, Chan A, Malhotra R, Ostbye T. Prevalence, correlates and perceived causes of limitations in activities of daily living among older Singaporeans. Aging Clin Exp Res. 2012;24:56–61.
Dybicz SB, Thompson S, Molotsky S, Stuart B. Prevalence of diabetes and the burden of comorbid conditions among elderly nursing home residents. Am J Geriatr Pharmacother. 2011;9:212–23.
Chiu CJ, Wray LA, Ofstedal MB. Diabetes-related change in physical disability from midlife to older adulthood: evidence from 1996–2003. Survey of health and living status of the elderly in Taiwan. Diabetes Res Clin Pract. 2011;91:413–23.
Martinez-Huedo MA, Hernandez-Barrera V, Palacios-Ceña D, Carrasco-Garrido P, Hernandez DM, Jiménez-Garcia R. Trends in the prevalence of physical and functional disability among Spanish elderly suffering from diabetes (2000–2007). Diabetes Res Clin Pract. 2011;94:30–3.
Chau PH, Woo J, Lee CH, et al. Older people with diabetes have higher risk of depression, cognitive and functional impairments: implications for diabetes services. J Nutrit Health Aging. 2011;15:751–5.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Lara Bianchi declares that she has no conflicts of interest. Giovanni Zuliani declares that he has no conflicts of interest. Stefano Volpato declares that he has no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bianchi, L., Zuliani, G. & Volpato, S. Physical Disability in the Elderly with Diabetes: Epidemiology and Mechanisms. Curr Diab Rep 13, 824–830 (2013). https://doi.org/10.1007/s11892-013-0424-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11892-013-0424-6