Abstract
Type 2 diabetes, a common metabolic disease in older people, is a major risk factor for functional limitation, impaired mobility, and loss of independence. In older people, the pathogenesis of functional limitation and disability is complex and multifactorial. A number of potential pathways are involved including cardiovascular disease, peripheral neuropathy, overweight, osteoarthritis, visual deficit, and cognitive impairment, conditions that are all more prevalent among patients with diabetes. Sarcopenia, a geriatric condition characterized by a progressive and generalized loss of skeletal muscle mass and strength, is also involved in the pathogenesis of functional limitations and disability. Recent research has shown that older patients with type 2 diabetes are often affected by skeletal muscle impairment, leading to reduced muscle strength and physical function. Insulin resistance, hyperglycemia, muscle fat infiltration, and peripheral neuropathies are hypothesized as the fundamental biological mechanisms leading to muscle impairment in people with diabetes. This review summarizes the current literature on the biological pathways responsible for skeletal muscle dysfunction in type 2 diabetes and analyzes the role of decline in muscle strength and quality on the association between diabetes and mobility disability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is common in older people, with a high prevalence in industrialized countries [1]. Recent statistics show that diabetes affects 382 million adults worldwide, and this number is estimated to rise to 592 million by 2035 due to the ongoing demographic transition and the progressive aging of the overall population [2]. Type 2 diabetes is the most common form of this disease, accounting for approximately 90 % of cases diagnosed [3], and it has been consistently reported as one of the strongest correlates of mobility limitation, especially in older people, and a potential risk factor for future mobility disability and loss of independence [4].
The American Diabetes Association and the American Geriatrics Society recently released a consensus report to emphasize the growing frequency of geriatric conditions in older adults with diabetes mellitus, highlighting the need for clinical studies to determine how functional decline may be prevented in this population [5]. Elucidating the specific contributors to functional decline in older adults with diabetes is important for patients and health-care systems in terms of quality of life and health-care costs. The mechanisms for loss of mobility and independence in type 2 diabetes are poorly understood. Long-term complications and diabetes-related comorbidities only partially explain the excess risk of disability associated with diabetes [6]. Changes in body composition, in particular progressive loss of muscle mass and, increase in fat mass, with decline in muscle strength and quality (defined as a composite measure of muscle strength standardized for muscle mass) have been proposed as additional potential mediators of the association between diabetes and disability [7]. This review analyzes the role of different biological mechanisms explaining the association between diabetes and mobility disability, focusing on decline in muscle strength and muscle quality.
Diabetes-related change in body composition
Type 2 diabetes is generally associated with overweight and obesity. These conditions can be considered not only important causes of type 2 diabetes, but also consequences of the disease itself that typically involve changes in fat distribution and muscle mass. Several studies have evaluated fat distribution in diabetic patients. A significantly higher trunk and visceral fat distribution [8] and a reduction in total leg fat mass caused by a lower subcutaneous adipose tissue [9] are associated with more intramuscular and intermuscular adipose tissue deposition [8, 9]. A number of epidemiological studies conducted in different populations have investigated the distribution of muscle mass according to diabetes status, using different analytic approaches, and provide conflicting results (Table 1) [7, 10]. Park et al. [10] demonstrated that in both sexes, the presence of diabetes was associated with a significantly higher appendicular (arms and leg) muscle mass. In the Invecchiare in Chianti (InCHIANTI) Study, an Italian population-based cohort study, older persons with diabetes had a larger cross-sectional calf muscle area, although this difference disappeared after standardization for body mass [7]. By contrast, data from the Look AHEAD trial indicated that participants with type 2 diabetes, despite having more trunk lean mass, had lower leg lean mass compared with controls and no differences in arm lean mass, highlighting that diabetes may affect not only the amount of muscle mass but also its distribution [11]. The Korean Sarcopenia Obesity Cohort Study showed that in men with diabetes total lean body mass and skeletal muscle index (SMI, lean mass standardized to body weight) were lower than in control subjects, even after adjustment for age, body mass index (BMI), health-related behaviors, medications, and metabolic parameters. In the women in this study, not only SMI but also appendicular lean mass was lower in patients with diabetes than in nondiabetic counterparts. In this study, prevalence of sarcopenia, defined as SMI <2 SD below the mean value of a young reference group, was significantly greater in participants with diabetes and this association was more robust in subjects older than 60 years (19.0 vs. 5.1 % in men and 27.0 vs. 14.0 % in women with and without diabetes, respectively) [12]. Participants without diabetes in the Baltimore Longitudinal Study of Aging, showed the presence of impaired fasting glycemia and hyperinsulinemia after an oral glucose tolerance test were associated with lower muscle mass, suggesting that glucose and insulin levels could have an early effect on muscle mass even in the absence of diabetes [13].
Longitudinal studies have found that older adults with type 2 diabetes experience an accelerated loss of muscle mass compared to normoglycemic counterpart. In a study among 3153 older Chinese adults, participants with type 2 diabetes showed an accelerated appendicular lean mass loss over a period of 4 years, independently of the diabetes-related conditions studied [14]. Park et al. [15] using data from the Health ABC Study demonstrated that older adults with either diagnosed or undiagnosed type 2 diabetes showed excessive loss of appendicular lean mass and trunk lean fat mass compared with nondiabetic subjects. The decline in muscle mass was higher in previously undiagnosed diabetic participants suggesting that the most important loss of lean mass might happen in the early stages of the disease or when diabetes is untreated [13, 15]. Similarly, data from the Osteoporotic Fractures in Men (MrOS) Study showed that men with untreated diabetes, diabetes treated without insulin sensitizers, or impaired fasting glycemia had greater loss in total and appendicular lean mass even after adjustment for medical comorbidities or lifestyle factors. In contrast, the relative loss in total and appendicular lean mass in men with diabetes treated with insulin sensitizers was significantly lower than that in normoglycemic men supporting a pivotal role of insulin resistance in the pathogenesis of muscle mass loss [16].
Diabetes and muscle dysfunction
The biological mechanisms accounting for muscle strength decline can arise from skeletal muscle factors, such as loss of muscle mass, changes in muscle architecture and fiber type, but also from neurological factors, such as decreased cortical and spinal excitability, decreased maximal motor unit discharge rate, and slowed nerve conduction. It follows that loss of muscle mass is only one of the many potential factors responsible for the amount of voluntary force output [17].
Epidemiological evidence
A number of population-based cohort studies have suggested that older people with type 2 diabetes, despite having adequate muscle mass because of their increased overall body mass, tended to have lower muscle strength [10] and steeper age-related decline in both muscle mass [15] and lower extremity strength [18] (Table 2). As a consequence, the concept of muscle quality that defines a composite measure of muscle strength standardized for an indicator of muscle mass has been introduced. As demonstrated using data from the InCHIANTI Study, people with type 2 diabetes on pharmacological therapy had statistically significant higher muscle fat infiltration, lower muscle strength, muscle power, and muscle quality (operationalized as the ratio of ankle strength to the calf muscle area) compared with the nondiabetic counterparts (all P values <0.05) [7]. Among the 485 older adults with type 2 diabetes of the Health ABC Study, male participants with diabetes showed higher arm and leg appendicular muscle mass but significantly lower muscle strength in both upper and lower extremities (P < 0.05) compared to controls. In women with diabetes, absolute arm and leg strength were not significantly different from those without diabetes, despite greater arm and leg regional muscle mass. Muscle quality was therefore consistently lower in both upper and lower extremities in all diabetic participants compared with nondiabetic counterparts. These differences were more robust in those with longer duration of the disease and poor glycemic control, further supporting the negative role of glycemic disregulation [10]. In a group of well-functioning nondiabetic adults enrolled in the same population study, Barzilay et al. [19] analyzed the association between lower extremity strength and insulin resistance. They demonstrated, in agreement with prior analyses, that quadriceps strength per kilogram of muscle mass was inversely associated with HOMA-IR, independently of other factors negatively associated with strength such as age, female sex, low physical activity, impaired fasting glucose, and increased total body fat. Based on the National Health and Nutrition Examination Survey (NHANES) data from 1999 to 2002, Kalyani et al. [20] found that diabetes was associated with significantly lower quadriceps strength and quadriceps power, diabetes duration in men and women was inversely associated with age-adjusted quadriceps strength and power (all P ≤ 0.001), whereas hemoglobin A1c was not associated with muscle performance.
Similar results were found in South Asian Surinamese, African Surinamese, Ghanaian, Dutch, Turkish, and Moroccan people enrolled in the Healthy Life in an Urban Setting (HELIUS) study. van der Kooi et al. [21] demonstrated that in all these ethnic groups, handgrip strength was lower among diabetic participants than in normoglycemic counterpart.
Most of the above-mentioned studies were designed to investigate the relationship between type 2 diabetes and mobility disability and were therefore focused on the assessment of lower extremity muscle strength. Only a few studies specifically investigated the association between diabetes and upper extremity muscle strength, with controversial results. In a case–control study of 72 individuals, participants with type 2 diabetes, despite having significantly lower knee and ankle muscles strength, had very similar isokinetic strength of extensor and flexor muscles at the wrist and elbow [22]. Similarly, older women with diabetes enrolled in the Study of Osteoporotic Fractures (SOF) tended to have a greater, although not significant, grip strength compared to women without diabetes [23]. By contrast, both in the Hertfordshire Cohort Study and in a more recent case–control study conducted in 92 Dutch subjects, diabetic patients had significant lower handgrip strength compared to normoglycemic controls [24, 25].
A longitudinal analysis of the Health ABC Study demonstrated that older men and women with diabetes had a steeper decline in lower extremity muscle strength and quality over time [18]. Data from the Baltimore Longitudinal Study of Aging confirmed these results, finding a statistically significant trend of decreasing muscle strength and quality across higher HbA1c categories during the follow-up [26]. Conversely, older women with diabetes mellitus enrolled in the SOF Study did not show any significantly steeper decline in handgrip strength compared to participants without diabetes mellitus [23]. Taken globally these results indicate that patients with diabetes or unrecognized diabetes are more likely to experience accelerated loss of muscle strength over time, particularly in the lower extremities, compared with those without diabetes.
Biological mechanisms
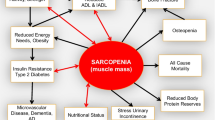
Although ample evidence suggests that older patients with diabetes have increased risks of muscle impairment and disability, the underlying mechanisms of these associations are not clear and still under investigation. Traditional long-term diabetes complications, including peripheral neuropathy and peripheral arterial disease, only partially explain the diabetes-related impairment of skeletal muscles, suggesting a direct impact of the metabolic disregulation on the intrinsic structure and functional properties of the muscle [27]. A number of potential mechanisms have been investigated (Table 3), elucidating, at least partially, the complex and multiple pathways involved (Fig. 1).
-
1.
Insulin resistance and hyperglycemic muscle fat infiltration
It is well established that high fasting and post-challenge concentrations of both glucose and insulin are independently associate with muscle loss and weakness in individuals without diabetes [13, 19, 28]. These findings suggest that, since the early stages of type 2 diabetes (preclinical phase), dysglycemia, insulin resistance, and hyperinsulinemia might act as powerful risk factors for accelerated loss of both muscle mass and strength.
Human skeletal muscle consists of slow-twitch oxidative (type 1) and fast-twitch (type 2) fibers. According to their metabolic properties, type 2 fibers can be further categorized into type 2A, or fatigue-resistant/fast-twitch oxidative fibers, type 2B, or fast fatigable/fast-twitch glycolytic fibers, and 2X fibers that have twitch properties similar to those of 2A and 2B units and a resistance to fatigue intermediate between those of 2A and 2B [29]. Slow-twitch fibers are more insulin-sensitive and more insulin-responsive compared with fast-twitch fibers [30]. In patients with type 2 diabetes, the fraction of slow fiber has been reported to be lower compared with either obese or healthy control subjects [30] and GLUT4 expression that is normally higher in slow fibers [31] was found to be reduced in obese subject and further decreased in type 2 diabetic patients [30]. These specific changes in fiber type, concentration, and GLUT distribution may contribute to the reduced insulin-stimulated glucose uptake in skeletal muscle in type 2 diabetes. Since aging is also associated with reduction in the number and size of fast-twitch fibers [32], older people with diabetes might be affected by a negative synergistic effect of the age-related pathophysiological changes and diabetes-mediated impairments. Insulin resistance is more common in older than in younger individuals with diabetes, and it is directly linked to slow walking speed [33] and frailty [34]. Reduced insulin signaling leads to decreased protein synthesis and increased activation of protein degradation pathways that might ultimately lead to muscle loss. A complex intracellular insulin signaling cascade [35] may trigger a vicious circle that, through autophagy, muscle protein degradation and mitochondrial dysfunction eventually lead to muscle impairment [36]. The resulting loss of muscle mass leads to a decreased surface area for glucose transport that may potentially exacerbate insulin resistance. The progression of mitochondrial dysfunction may also worsen insulin resistance [37]. Individuals with type 2 diabetes may be genetically predisposed to skeletal muscle impairment. Important individual variability can be detected in the fiber type composition of human skeletal muscles. Results of a large study including 270 healthy sedentary and 148 physically active individuals of both sexes suggested that the proportion of type 1 fibers in the human vastus lateralis may vary from 15 to 85 % [38]. Analyses of muscle biopsies from monozygotic and dizygotic twins indicate that almost 50 % of this variance is associated with genetic factors [39].
Hyperglycemia itself and in particular high fluctuations of glycemia over time may be related to decreased skeletal muscle mass through multiple pathways. Potential explanations include the relationship of hyperglycemia with elevated inflammatory factors, decreased physical activity, and comorbidities, such as neuropathy [26]. Kalyani et al. [26] found that among the 5434 older adults without known diabetes enrolled in the NHANES Study, hyperglycemia was independently associated with lower lean mass even after adjustment for these potential confounders. A growing body of evidence supports the concept that hyperglycemia could directly affect the intrinsic abilities of the muscle to generate force [27]. The mechanisms may be a toxic effect on skeletal muscle mitochondrial activity [40] or glycation of skeletal muscle myosin [41].
-
2.
Muscle fat infiltration
Overweight and obesity are common among older persons with type 2 diabetes, and elevated BMI has been related to increased fat infiltration into the skeletal muscle [42]. Several epidemiological studies suggest that skeletal muscle fat infiltration influences muscle strength, resulting in both decreased muscle density and loss of muscle quality [42, 43]. Intermuscular adipose tissue, defined as visible adipose tissue beneath the muscle fascia and between muscle groups, is also negatively associated with insulin sensitivity in individuals with type 2 diabetes mellitus and with reduction in both oxidative activity and maximal aerobic capacity [44]. As a consequence, it has been demonstrated that in older persons, fat infiltration increases the risk of mobility disability over time [45].
-
3.
Chronic inflammation and oxidative stress.
Recent studies have reported that people with diabetes have increased circulating levels of inflammatory markers, including interleukin-6 (IL-6), tumor necrosis factor (TNF)-alpha, and C-reactive protein (CRP) [46–48]. These inflammatory markers have been related to both insulin resistance and other conditions associated with insulin resistance such as obesity, hypertension, or dyslipidemia [47]. TNF-alpha plays a mechanistic role in insulin resistance through the down-regulation of GLUT-4 and inhibition of insulin receptor activity [49]. IL-6 could affect insulin sensitivity directly, through the inhibition of insulin transcription factor, or indirectly, inducing liver CRP synthesis [47, 50].
Hyperglycemia is one of the most important factors responsible for the development of oxidative stress in diabetes mellitus that may cause damage to cells, tissues, and biomolecules by means of the increased generation of reactive oxygen species [51]. Oxidative stress and molecular inflammation by themselves or combined with IR play an important role in age-related muscle atrophy. These factors may interfere with the balance between protein synthesis and breakdown, may cause mitochondrial dysfunction, and may induce apoptosis leading to fiber atrophy and fiber loss, and eventually to sarcopenia [51].
-
4.
Physical inactivity.
Older people with diabetes, and in particular those simultaneously affected by overweight and obesity, often have limited leisure physical activity and physical inactivity may contribute to the age-related reduction in muscle mass and strength and to the development of disability [52, 53]. Reduced muscle mass in the lower extremities has been associated with a more sedentary lifestyle that in turn may contribute to the onset and progression of sarcopenia. Furthermore physical inactivity results in weight gain and worse glycemic control, culminating in a higher risk of diabetic complications [54] that could further exacerbate physical inactivity.
Aerobic exercise might improve insulin resistance and glucose tolerance [55], preventing the onset of obesity and diabetes mellitus complications [56], and may have a direct positive effect on the inflammatory status that has been involved to the development of sarcopenia and functional limitation [57]. Reduced physical activity has been associated with greater insulin resistance and increase in intermuscular adipose tissue [58].
In addition to aerobic exercise, resistance training exercise has been associated in several studies with weight loss, adipose tissues reduction, increase in fat free mass, and eventually in amelioration of insulin resistance [59, 60].
-
5.
Diabetic complications.
Chronic long-term complications of diabetes have been implicated in the pathogenesis of muscle impairment in type 2 diabetic patients. Lower extremity peripheral arterial disease (PAD) may functionally impair lower limb skeletal muscles by means of decreased blood flow that could lead to muscle atrophy, fewer muscle cells, and worse oxidative metabolism [61]. Arterial stiffening, a dysfunction in blood vessel dynamics, has been related to reduced lower extremity blood flow volume in type 2 diabetic patients as well as to reduced muscle mass decline in the general population [62, 63]. PAD is also associated with poor nerve conduction velocity (NCV) and with impaired lower extremity functioning in persons with and without symptoms of intermittent claudication [61]. In addition to PAD, the autonomic nervous system plays a major role in capillary recruitment, and in patients with diabetes, subclinical autonomic nervous system alterations might affect contraction by reducing blood supply to the exercising muscle [64].
Diabetic peripheral neuropathy (DPN) is another long-term detrimental complication of type 2 diabetes that directly predisposes diabetic patients to disability in daily life activities [6]. DPN, through sensory impairment, affects position sense leading to ataxia [65] and reduces movement perception at the ankle, which is thought to contribute impaired dynamic balance control, slow walking speed and increased risk of falling [66, 67]. DPN, by means of sensory and motor impairment, is involved in foot ulceration that is a common cause of lower extremity disability and amputation [68].
In addition to these effects on postural stability and gait, DPN may facilitate the development of muscle atrophy and strength reduction through muscle denervation caused by loss of motor axons combined with insufficient re-innervation [69]. Numerous clinical and experimental research studies have demonstrated that diabetes is responsible for an accelerated decline in muscle mass [27] that occurs first in the foot muscles and successively progresses to the lower legs [67]. This decline is related to the severity of DPN and is more pronounced distally, supporting the concept that the neuropathic process might depend on the length of the nerve [70]. DPN has also been shown to be responsible for a significant reduction in strength of the proximal muscle groups, in particular the flexor and extensor muscles of the knee [22]. Andersen et al. [22] found a reduction in muscle strength at the ankle and knee in diabetic patients with peripheral neuropathy; this reduction was related to severity of DPN and was independent of the degree of nephropathy, retinopathy, or the metabolic abnormalities associated with diabetes. Long-term diabetic patients with symptomatic neuropathy are subject to a progressive decline of muscle strength at the ankle, whereas diabetic patients without neuropathy preserve their muscle strength [71].
Diabetes and physical function
Several epidemiological studies conducted in different populations have shown that diabetes is associated with functional limitation and physical disability, defined as difficulty in performing routine physical tasks [72, 73].
Cross-sectional and longitudinal studies demonstrated that older diabetic adults show greater difficulty in walking one-quarter of a mile, climbing stairs, raising from a chair five times, and standing in a tandem position, compared with normoglycemic people [74]. With regard to physical disability, several studies have shown a strong association between diabetes and difficulty in both basic (ADL) and instrumental activity of daily life (IADL) [73]. A recent meta-analysis showed that having diabetes was associated with an increased odds of difficulties with ADL and IADL compared with those without diabetes (OR 1.82, 95 % CI 1.63–2.04 and OR 1.65 95 % CI 1.55–1.74, respectively) [73]. These associations were also confirmed in two longitudinal studies that demonstrated an increased risk of incident disability for person with diabetes free of ADL disability at baseline (RR 1.82, 95 % CI 1.40–2.36) [75, 76].
The higher prevalence of functional limitation and disability in older adults with diabetes may be the result of a multifactorial process including interaction between coexisting medical conditions, diabetes-related comorbidities [6], poor glycemic control [77], and classical diabetes complications, like PAD or DPN [6, 64].
However, considered together, these factors cannot fully explain the association between diabetes, functional limitation, and physical disability. As suggested by Volpato and colleagues using data from the Women’s Health and Aging Study, chronic conditions (including cardiovascular diseases, peripheral arterial disease, peripheral neuropathy, overweight, depression, and visual impairment) explained <60 % of the diabetes-related excess risk of severe walking limitation, whereas they explained about the 85 % of the risk of ADL disability [6]. These data were confirmed also in the NHANES Study in which comorbidities, like cardiovascular diseases, obesity, leg ulcer, chronic kidney disease, visual impairment, hearing impairment, memory problems, hip fracture, arthritis, chronic obstructive pulmonary disease, cancer, and level of glycemic control, were associated with 59 and 72 % of the excess odds for ADL and IADL disability observed in older adults with diabetes [74]. The same researchers using data from the InCHIANTI Study found that diabetes-related and associated comorbidities explain only about 18 and 30 % of the association between diabetes and functional limitation in the 4- and 400-m tests, respectively [7].
Based on this evidence, it has been proposed that sarcopenia might play an additional pathogenetic role in the multiple steps of disablement process of people with diabetes. A cross-sectional analysis of the InCHIANTI Study showed that participants with diabetes had a slower gait speed on both 4- and 400-m walking tests compared to participants without diabetes. Adjustment for lower limb muscle characteristics accounted for 24.3 and 15.1 % of walking speed difference comparing diabetic and nondiabetic subjects in the 4- and 400-m walks, respectively, suggesting an important mediating role of sarcopenia in the determination of functional limitation [7]. Van Sloten et al [78]. also showed that, among patients with type 2 diabetes, decreased muscle strength was associated with worse outcome of functional capacity tests such as 6-min walk test, the timed “up and go” test, and the stair climbing test. These results have been also confirmed by Leenders et al. [25] who found that, among older patients with type 2 diabetes compared with the normoglycemic controls, the decline in leg extension strength was paralleled by a poorer performance in the sit-to-stand test.
In addition to muscle strength and quality, another potential mediator for functional limitation in type 2 diabetic patients is muscular endurance. In parallel with impaired muscle strength, diabetic patients can also experience premature muscle fatigue, with consequent reduction in work capacity [79]. A few studies reported a significant reduction in muscular endurance in both lower and upper limbs muscular groups [80]. It remains to be clarified if and how these results depend on the type of muscle contraction, on the body region considered, and on the presence of comorbidities affecting neuromuscular function [27].
Conclusion and future direction
In the last two decades, a large amount of epidemiological and clinical data demonstrated that lower extremity muscle dysfunction and impaired mobility are more common in older people with type 2 diabetes. These conditions strongly affect the ability to maintain independence threatening the quality of life of these patients and their relatives. As a consequence, muscle dysfunction and mobility impairment should be considered as long-term complication of diabetes. Duration of the disease, age of the patient, and obesity are well-established risk factors for muscle impairment, whereas more recent data suggest a direct potential role for hyperglycemia and poor glycemic control.
Physical exercise has been proposed as a potential strategy for preserving muscle mass and function deterioration in patients with diabetes. For example, it has been widely demonstrated that resistance exercise training can increase total fat free mass and both muscle strength and quality in patients with type 2 diabetes [81]. Among the 415 diabetic participants enrolled in the Lifestyle Interventions and Independence for Elders (LIFE) Study, a moderate-intensity physical activity intervention reduced significantly the incidence of major mobility disability [82]. Although insulin sensitizer agents may potentially help in preserving muscle mass and function, their benefit/risk profile has not been fully established so far, and therefore, further studies are needed in order to establish their efficacy and effectiveness for the prevention and therapy of muscle impairment in older patients with diabetes.
References
Danaei G, Finucane MM, Lu Y et al (2011) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 378:31–40
Guariguata L, Whiting DR, Hambleton I et al (2014) Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 103:137–149
Expert Canadian Diabetes Association Clinical Practice Guidelines, Goldenberg R, Punthakee Z (2013) Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes 37(Suppl 1):S8–S11
Bianchi L, Zuliani G, Volpato S (2013) Physical disability in the elderly with diabetes: epidemiology and mechanisms. Curr Diabetes Rep 13:824–830
Kirkman MS, Briscoe VJ, Clark N et al (2012) Diabetes in older adults. Diabetes Care 35:2650–2664
Volpato S, Blaum C, Resnick H et al (2002) Comorbidities and impairments explaining the association between diabetes and lower extremity disability: The Women’s Health and Aging study. Diabetes Care 25:678–683
Volpato S, Bianchi L, Lauretani F et al (2012) Role of muscle mass and muscle quality in the association between diabetes and gait speed. Diabetes Care 35:1672–1679
Goodpaster BH, Krishnaswami S, Resnick H et al (2003) Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care 26(2):372–379
Gallagher D, Kelley DE, Yim JE et al (2009) Adipose tissue distribution is different in type 2 diabetes. Am J Clin Nutr 89:807–814
Park SW, Goodpaster BH, Strotmeyer ES et al (2006) Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes 55:1813–1818
Heshka S, Ruggiero A, Bray GA et al (2008) Altered body composition in type 2 diabetes mellitus. Int J Obes (Lond) 32:780–787
Kim TN, Park MS, Yang SJ et al (2010) Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean sarcopenic obesity study (KSOS). Diabetes Care 33:1497–1499
Kalyani RR, Metter EJ, Ramachandran R et al (2012) Glucose and insulin measurements from the oral glucose tolerance test and relationship to muscle mass. J Gerontol A Biol Sci Med Sci 67:74–81
Lee JSW, Auyeung TW, Leung J et al (2010) The effect of diabetes mellitus on age-associated lean mass loss in 3153 older adults. Diabetes Med 27:1366–1371
Park SW, Goodpaster BH, Lee JS et al (2011) Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 32:1993–1997
Lee CG, Boyko EJ, Barrett-Connor E et al (2011) Insulin sensitizers may attenuate lean mass loss in older men with diabetes. Diabetes Care 34:2381–2386
Clark BC, Manini TM (2008) Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci 63:829–834
Park SW, Goodpaster BH, Strotmeyer ES et al (2007) Accelerated loss of skeletal muscle strength in older adults with type diabetes: the health, aging, and body composition study. Diabetes Care 30:1507–1512
Barzilay JI, Cotsonis GA, Walston J et al (2009) Insulin resistance is associated with decreased quadriceps muscle strength in nondiabetic adults aged > or = 70 years. Diabetes Care 32:736–738
Kalyani RR, Tra Y, Yeh HC et al (2013) Quadriceps strength, quadriceps power, and gait speed in older US. adults with diabetes mellitus: results from the national health and nutrition examination survey, 1999–2002. J Am Geriatr Soc 61:769–775
van der Kooi AL, Snijder MB, Peters RJ et al (2015) The association of handgrip strength and type 2 diabetes mellitus in six ethnic groups: an analysis of the HELIUS study. PLoS One 10(9):e0137739
Andersen H, Nielsen S, Mogensen CE et al (2004) Muscle strength in type 2 diabetes. Diabetes 53:1543–1548
Lee CG, Schwartz AV, Yaffe K et al (2013) Changes in physical performance in older women according to presence and treatment of diabetes mellitus. J Am Geriatr Soc 61:1872–1878
Sayer AA, Dennison EM, Syddall HE et al (2005) Type 2 diabetes, muscle strength, and impaired physical function: the tip of the iceberg? Diabetes Care 28:2541–2542
Leenders M, Verdijk LB, van der Hoeven L et al (2013) Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J Am Med Dir Assoc 14:585–592
Kalyani RR, Metter EJ, Egan J et al (2015) Hyperglycemia predicts persistently lower muscle strength with aging. Diabetes Care 38:82–90
Orlando G, Balducci S, Bazzucchi I et al (2016) Neuromuscular dysfunction in type 2 diabetes: underlying mechanisms and effect of resistance training. Diabetes Metab Res Rev 32:40–50
Abbatecola AM, Ferrucci L, Ceda G et al (2005) Insulin resistance and muscle strength in older persons. J Gerontol A Biol Sci Med Sci 60(10):1278–1282
Reggiani C, Schiaffino S (2011) Fiber types in mammalian skeletal muscles. Physiol Rev 91(4):1447–1531
Gaster M, Staehr P, Beck-Nielsen H et al (2001) GLUT4 is reduced in slow muscle fibers of type 2 diabetic patients: is insulin resistance in type 2 diabetes a slow, type 1 fiber disease? Diabetes 50:1324–1329
Gaster M, Poulsen P, Handberg A et al (2000) Direct evidence of fibertype dependent GLUT-4 expression in human skeletal muscle. Am J Physiol 278:E910–E916
Lexell J (1995) Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50:11–16
Kuo CK, Lin LY, Yu YH et al (2009) Inverse association between insulin resistance and gait speed in nondiabetic older men: results from the US. national health and nutrition examination survey (NHANES) 1999–2002. BMC Geriatr 9:49
Kalyani RR, Varadhan R, Weiss CO et al (2012) Frailty status and altered glucose-insulin dynamics. J Gerontol A Biol Sci Med Sci 67:1300–1306
Kalyani RR, Corriere M, Ferrucci L (2014) Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol 2:819–829
Kaushik S, Singh R, Cuervo AM (2010) Autophagic pathways and metabolic stress. Diabetes Obes Metab 12(suppl 2):4–14
Phielix E, Mensink M (2008) Type 2 diabetes mellitus and skeletal muscle metabolic function. Physiol Behav 94:252–258
Simoneau JA, Bouchard C (1989) Human variation in skeletal muscle fiber-type proportion and enzyme activities. Am J Physiol Endocrinol Metab 257:E567–E572
Simoneau JA, Bouchard C (1995) Genetic determinism of fiber type proportion in human skeletal muscle. FASEB J 9:1091–1095
Kelley DE, He J, Menshikova EV et al (2002) Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51:2944–2950
Ramamurthy B, Höök P, Jones AD et al (2001) Changes in myosin structure and function in response to glycation. FASEB J 15:2415–2422
Hilton TN, Tuttle LJ, Bohnert KL et al (2008) Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther 88:1336–1344
Goodpaster BH, Carlson CL, Visser M et al (2001) Attenuation of skeletal muscle and strength in the elderly: the Health ABC study. J Appl Physiol 90:2157–2165
Goodpaster BH, Thaete FL, Kelley DE (2000) Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 71:885–892
Visser M, Goodpaster BH, Kritchevsky SB et al (2005) Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well functioning older persons. J Gerontol A Biol Sci Med Sci 60:324–333
Pedersen M, Bruunsgaard H, Weis N et al (2003) Circulating levels of TNF-alpha and IL-6-relation to truncal fat mass and muscle mass in healthy elderly individuals and in patients with type-2 diabetes. Mech Ageing Dev 124:495–502
Abbatecola AM, Ferrucci L, Grella R et al (2004) Diverse effect of inflammatory markers on insulin resistance and insulin-resistance syndrome in the elderly. J Am Geriatr Soc 52(3):399–404
Fiorentino TV, Hribal ML, Perticone M et al (2015) Unfavorable inflammatory profile in adults at risk of type 2 diabetes identified by hemoglobin A1c levels according to the american diabetes association criteria. Acta Diabetol 52(2):349–356
Hotamisligil GS (1999) The role of TNF-alpha and TNF receptors in obesity and insulin resistance. J Intern Med 245:621–625
Bastard JP, Maachi M, Van Nhieu JT et al (2002) Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab 87(5):2084–2089
Meng SJ, Yu LJ (2010) Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci 11:1509–1526
Booth FW, Laye MJ, Roberts MD (2011) Lifetime sedentary living accelerates some aspects of secondary aging. J Appl Physiol 111:1497–1504
Hamasaki H, Kawashima Y, Adachi H et al (2015) Associations between lower extremity muscle mass and metabolic parameters related to obesity in Japanese obese patients with type 2 diabetes. Peer J 3:e942
Sigal RJ, Kenny GP, Wasserman DH et al (2006) Physical activity/exercise and type 2 diabetes: a consensus statement from the american diabetes association. Diabetes Care 29:1433–1438
Umpierre D, Ribeiro PA, Kramer CK et al (2011) Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA 305:1790–1799
Fagour C, Gonzalez C, Pezzino S et al (2013) Low physical activity in patients with type 2 diabetes: the role of obesity. Diabetes Metab 39:85–87
Cesari M, Penninx BW, Pahor M et al (2004) Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sc 59:242–248
Manini TM, Clark BC, Nalls MA et al (2007) Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr 85:377–384
Tresierras MA, Balady GJ (2009) Resistance training in the treatment of diabetes and obesity: mechanisms and outcomes. J Cardiopulm Rehabil Prev 29(2):67–75
McGinley SK, Armstrong MJ, Boulé NG et al (2015) Effects of exercise training using resistance bands on glycaemic control and strength in type 2 diabetes mellitus: a metaanalysis of randomised controlled trials. Acta Diabetol 52(2):221–230
McDermott MM, Guralnik JM, Albay M et al (2004) Impairments of muscles and nerves associated with peripheral arterial disease and their relationship with lower extremity functioning: the InCHIANTI study. J Am Geriatr Soc 52:405–410
Abbatecola AM, Chiodini P, Gallo C et al (2012) Pulse wave velocity is associated with muscle mass decline: Health ABC study. Age (Dordr) 34(2):469–478
Suzuki E, Kashiwagi A, Nishio Y et al (2001) Increased arterial wall stiffness limits flow volume in the lower extremities in type 2 diabetic patients. Diabetes Care 24:2107–2114
Womack L, Peters D, Barrett EJ et al (2009) Abnormal skeletal muscle capillary recruitment during exercise in patients with type 2 diabetes mellitus and microvascular complications. J Am Coll Cardiol 53:2175–2183
Resnick HE, Vinik AI, Schwartz AV et al (2000) Independent effects of peripheral nerve dysfunction on lower-extremity physical function in old age. The Women’s Health and Aging study. Diabetes Care 23:1642–1647
Van Deursen RW, Simoneau GG (1999) Foot and ankle sensory neuropathy, proprioception, and postural stability. J Orthop Sports Phys Ther 29:718–726
Volpato S, Leveille SG, Blaum C et al (2005) Risk factors for falls in older disabled women with diabetes: The Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci 60(12):1539–1545
Bild DE, Selby JV, Sinnock P et al (1989) Lower-extremity amputation in people with diabetes: epidemiology and prevention. Diabetes Care 12:24–31
Andreassen CS, Jakobsen J, Ringgaard S et al (2009) Accelerated atrophy of lower leg and foot muscles—a follow-up study of long-term diabetic polyneuropathy using magnetic resonance imaging (MRI). Diabetologia 52:1182–1191
Andersen H, Poulsen PL, Mogensen CE et al (1996) Isokinetic muscle strength in long-term IDDM patients in relation to diabetic complications. Diabetes 45:440–445
Andreassen CS, Jakobsen J, Ringgaard S et al (2009) Muscle weakness: a progressive late complication in diabetic distal symmetric polyneuropathy. Diabetes 55:806–812
de Rekeneire N, Volpato S (2015) Physical function and disability in older adults with diabetes. Clin Geriatr Med 31:51–65
Wong E, Backholer K, Gearon E et al (2013) Diabetes and risk of physical disability in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 1:106–114
Kalyani RR, Saudek CD, Brancati FL et al (2010) Association of diabetes, comorbidities, and A1C with functional disability in older adults: results from the National Health and Nutrition Examination survey (NHANES), 1999–2006. Diabetes Care 33:1055–1060
Volpato S, Ferrucci L, Blaum C et al (2003) Progression of lower-extremity disability in older women with diabetes: The Women’s Health and Aging study. Diabetes Care 26:70–75
Al Snih S, Fisher MN, Raji MA et al (2005) Diabetes mellitus and incidence of lower body disability among older mexican americans. J Gerontol A Biol Sci Med Sci 60A:1152–1156
Bossoni S, Mazziotti G, Gazzaruso C et al (2008) Relationship between instrumental activities of daily living and blood glucose control in elderly subjects with type 2 diabetes. Age Ageing 37:222–225
van Sloten TT, Savelberg HH, Duimel-Peeters IG et al (2011) Peripheral neuropathy, decreased muscle strength andobesity arestrongly associated with walking in persons with type 2 diabetes without manifest mobility limitations. Diabetes Res Clin Pract 91:32–39
Fritschi C, Quinn L (2010) Fatigue in patients with diabetes: a review. J Psychosom Res 69:33–41
Shah S, Sonawane P, Nahar P et al (2011) Are we ignoring diabetic disability: a cross sectional study of diabetic myopathy. Indian J Med Sci 65:186–192
Sénéchal M, Johannsen NM, Swift DL (2015) Association between changes in muscle quality with exercise training and changes in cardiorespiratory fitness measures in individuals with type 2 diabetes mellitus: results from the HART-D study. PLoS One 10(8):e0135057
Pahor M, Guralnik JM, Ambrosius WT et al (2014) Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 311:2387–2396
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
All procedures were in accordance with the ethical standards of the institutional research committee and with the Helsinky Declaration.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, informed consent is not required.
Additional information
Managed by Antonio Secchi.
Rights and permissions
About this article
Cite this article
Bianchi, L., Volpato, S. Muscle dysfunction in type 2 diabetes: a major threat to patient’s mobility and independence. Acta Diabetol 53, 879–889 (2016). https://doi.org/10.1007/s00592-016-0880-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-016-0880-y