Abstract
Unfortunately, the only approved medical treatment for type 1 diabetes mellitus (DM) is insulin, despite the fact that tight control cannot be reached without some serious side effects such as hypoglycemia and weight gain. More and more importance is now shifted towards developing new drugs that can reach a better glycemic control with lesser side effects. Some of these promising drugs are the glucagon-like peptides 1 (GLP-1) and their agonists, which have been FDA approved for the treatment of type 2 DM. The purpose of this article is to review all of the relevant literature on the potential role of GLP-1 in the treatment of type 1 DM. The major source of data acquisition included Medline search strategies, using the words “type 1 diabetes mellitus” and “GLP-1.” Articles published in the last 20 years were screened. GLP-1 increases insulin secretion in humans with existing beta cells; it also decreases glucagon secretion, and blunts appetite. Of note, new animal studies demonstrate a role in beta cell-proliferation and decreased apoptosis. Because of all the effects mentioned above, GLP-1 seems to be a promising drug for type 1 DM treatment, but more studies are still needed before solid conclusions can be drawn.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As prevalence of diabetes is increasing worldwide and is still expected to rise, the urgency to control it and prevent its various complications have become more important than ever. This is especially urgent in type 1 DM, where insulin has been the only option for treatment ever since its discovery in 1921. Unfortunately, this only option has never been the ideal treatment. For instance, the attempt to achieve a tight glycemic control, which is necessary to prevent micro- and macrovascular complications and decrease mortality (as has been shown by many studies), might induce side effects as higher doses of insulin are associated with hypoglycemia and weight gain. Even the new insulin analogs did not decrease those side effects.
So it is ideal to introduce a treatment that can control glycemia without risking hypoglycemia or weight gain. GLP-1 and its analogues have been shown to improve glycemic control and induce weight loss in type 2 DM and have been approved by the FDA for its treatment. But do GLP-1 and its analogues have a role in type 1 DM? This review will focus on the physiology of GLP-1, its role, the wisdom behind its possible benefit in type 1DM, and all the studies that have been conducted on GLP-1 and type 1 DM.
Evidence Acquisition
The major source of data acquisition included Medline search strategies, using the words “type 1 diabetes mellitus,” and “GLP-1.” Articles published in the last 20 years were screened.
Discovery and Physiology of GLP-1
GLP-1 belongs to the family of incretins, defined as peptides secreted by the gut that can stimulate insulin secretion in response to nutrient intake. This action was referred to as the incretin effect (ie, the increase in insulin secretion that occurs after an oral carbohydrate load above and beyond that produced by an isoglycemic intravenous glucose challenge).
The concept behind incretin hormones goes back to the beginning of the 20th century, when Bayliss and Starling proposed the presence of a signal secreted by the gut in response to ingested nutrients to help dispose of the ingested carbohydrates [1]. Efforts to use incretins in the treatment of diabetes ensued, but they were prematurely terminated when 3 studies were published that questioned the existence of incretins [2–4]. It was not until the ability to measure hormones through development of radioimmunoassays (RIA) in the 1960s that interest in incretins was revived, especially after noticing that an oral load of glucose stimulated a higher insulin secretion than the same or even higher intravenous load [5, 6]. The renewed research led to the isolation of 2 incretin hormones with a strong insulinotropic effect: gastric inhibitory peptide (GIP) [7–10] and glucagon-like peptides or GLP-1 and 2 [11–13].
Several studies showed that in type 2 DM there was a resistance to GIP, so no further efforts were made to study GIP in the treatment of diabetes [14, 15].
GLP-2 was found to serve as an epithelial growth factor with cytoprotective properties as well as a suppressor of intestinal inflammation with no role in glycemic control [16, 17]. Therefore, research focused on a GLP-1 effect on glycemic control.
GLP-1 is secreted mainly from intestinal L-cells, located in the distal ileum, colon, and rectum, whereas a small quantity is secreted by alpha cells along with glucagon. GLP-1, GLP-2, and glucagon derive from the cleavage of a common precursor molecule called preproglucagon.
GLP-1 secretion is regulated mainly by ingestion of nutrients, specifically carbohydrates and fat [mainly monounsaturated long chain fats) [18, 19]. Proteins and amino acids have no effect on GLP-1 secretion. Other regulators of GLP-1 secretion are neuroendocrine modulators: somatostatin, insulin and atropine are inhibitors [20–22], whereas vagal input, epinephrine and beta-agonists are stimulators [23, 24].
Due to enzymatic degradation by dipeptidyl peptidase 4 (DPP 4) and renal clearance, GLP-1 has a very short half-life of around 2 minutes. This motivated the synthesis of analogues that were resistant to DPP4 action, such as exendin-4, a 39 amino acid peptide isolated from the saliva of the venomous lizard Heloderma suspectum or Gila monster [25].
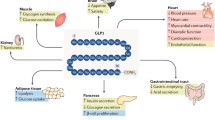
GLP-1 exerts its effects through its interaction with the GLP-1 receptor (GLP-1R), a member of the seven-transmembrane G-coupled receptor family which is mainly expressed in pancreatic islets, kidney, heart and brain. The main effects of GLP-1 are stimulation of insulin secretion and inhibition of glucagon secretion (both of which are glucose-dependent), delayed gastric emptying, appetite reduction, and induction of satiety. Advanced and recent studies have implicated GLP-1 on increased beta-cell proliferation and differentiation and decreased apoptosis. This effect will be further detailed later in this review.
Pathophysiology of Type 1 DM
Type 1 DM is a condition of absolute insulin deficiency secondary to auto-immune (both T- and B-lymphocyte mediated) destruction of pancreatic beta cells [26]. It becomes manifest when the number of beta cells fall below a threshold necessary for the secretion of adequate insulin to maintain normal glucose tolerance [26]. At the time of diagnosis, patients with type 1 DM still have a remnant of 20 %–30 % capacity of insulin secretion (honeymoon period), which means that there is still a potentially expandable beta-cell mass [27]. However, the autoimmune destruction continues and this reserve will continue to shrink until around only 10 % capacity is present at 5 years of diagnosis [28]. All this means that the beta-cell injury starts well before the appearance of diabetes, and a drug that can decrease beta-cell apoptosis and increase beta-cell proliferation and differentiation, if started early enough, may at least delay the occurrence of diabetes. Several anti-immune drugs such as anti-CD3, potential vaccines, and biological drugs were studied and used for this purpose, but they showed no major benefit, and they had several side effects. So efforts are shifted to GLP-1 analogues in this area, and studies done in this filed will be detailed later in the review.
Besides insulin deficiency, another essential abnormality in type 1 DM is the abnormal suppression of glucagon in response to hyperglycemia, which can lead to worsening of postprandial hyperglycemia [29]. Since GLP-1 can suppress glucagon secretion in a glucose-dependent manner, a possible added effect on insulin treatment can be expected, which can lead to lower postprandial hyperglycemia and maybe lower doses of insulin needed.
Only a few studies have addressed the secretion of GLP-1 under physiologic conditions in patients with type 1 DM, with controversial and ambiguous results. In patients predisposed to having type 1 DM (ie, positive islet cell antibodies, normal fasting, but elevated 2 hours OGTT glucose) there was an impaired first-phase insulin response, lack of glucagon suppression, and an impaired incretin effect, but with normal plasma levels of GLP-1 [30]. In another study, done on 16 patients with long-standing diabetes, fasting GLP-1 concentrations did not differ from those of normal subjects, but the GLP-1 secretion in response to a mixed meal was absent [31]. In contrast, Vilsbol et al showed that the fasting GLP-1 level was lower in subjects with C-peptide negative type 1 DM than in normal controls, whereas they had a normal postprandial GLP-1 response compared with normal controls [32]. A recent study that measured the activity of the DPP4 enzyme in patients with type 1 DM, type 2 DM, and normal controls showed that compared with type 2 DM and controls, patients with type 1 DM had a higher DDP4 enzymatic activity both in the fasting state and 60 and 180 min after a meal. Correlation was neither detected between the fasting plasma glucose nor between the HbA(1 C) and the DPP-4 values in any of the groups studied which suggests that it is not the hyperglycemia, rather the type of diabetes which determinates the serum DPP-4 enzymatic activity. This could lead to lower GLP-1 levels, thus affecting glucose control in type 1 DM. Therefore, GLP-1 agonists that are resistant to DPP4 may be able to compensate for this deficit [33•]. Despite the controversy and the need for further studies on the GLP-1 effect in type 1 DM, GLP-1 and its agonists have been studied in patients with type 1 DM.
GLP-1 Effect on Glycemic Control in Type 1 DM
Effect on Fasting Glucose Levels
Since any beneficial effect of GLP-1 in type 1 DM was postulated to occur mainly through the modulation of postprandial hyperglycemia, very few studies have examined its effect on fasting hyperglycemia. In one of these studies, a continuous infusion of GLP-1 at a dose of 1.2 pmol/kg/min resulted in reduction of fasting hyperglycemia from 13.4 to 10 mmol/L and a 50 % reduction in glucagon concentration, though it should be noted that participants had an induced fasting hyperglycemia and hyperglucagonemia by halving their night-time dose of intermediate insulin [34]. In contrast, in another study where patients were well insulinized and had no fasting hyperglycemia, GLP-1 infusion had no effect on insulin or on glucagon levels, and glucose levels remained stable at 8 mmol/L in both GLP-1 and saline infusion [35]. In view of the scarcity of the available studies and their contradictory results, more studies are still needed to test the effect of GLP-1 on fasting hyperglycemia in patients with type 1 DM.
Effect on Postprandial Hyperglycemia
Several studies have been done on the effect of GLP-1 on postprandial hyperglycemia with the majority showing beneficial effect.
In a study by Gutniak et al [36], postprandial hyperglycemia excursions were significantly improved when GLP-1 infusions were combined with insulin infusions than when insulin infusions were given alone; although this finding suggested an improvement in insulin sensitivity, delayed gastric emptying was not taken into account. In 8 subjects with type 1 DM and residual C-peptide secretion, the continuous infusion of 1.2 pmol/kg/min of GLP-1 after a mixed meal almost abolished increments of plasma glucose. This was mostly explained by delayed gastric emptying and decreased glucagon secretion [37]. In another study on persons with type 1 DM and a wide range of residual insulin secretion conducted by the same investigators, a subcutaneous injection of 0.63 μg/kg of GLP-1 administered before meals with the usual dose of insulin led to a decrease in postprandial glucose and human pancreatic polypeptide (HPP). However, in this study, glucagon and C-peptide levels did not differ from control, highlighting delayed gastric emptying as a mechanism to explain the effect of GLP-1 on postprandial hyperglycemia [38]. In another study conducted on subjects with type 1 DM and no residual endogenous insulin secretion (negative C-peptide), 0.63 μg/kg of GLP-1 subcutaneously in addition to their dose of insulin was administered first before breakfast and lunch (8-hour study) and then before each meal for 5 days (5 day study). In the 8-hour study there was a decrease in the area under the curve (AUC) for plasma glucose, a decrease in human pancreatic polypeptide (a marker of afferent vagal activity) and glucagon. In the 5-day study, there was no effect on fasting glucose levels when compared with placebo, but there was a significant decrease in postprandial increments. There were no episodes of hypoglycemia and no change in insulin dosages. Thus the effect was mainly attributed to delayed gastric emptying and decreased glucagon secretion [39]. In another study performed by the same authors, this time using 0.03 μg/kg of exendin-4 given 15 min before each meal to persons with type 1 DM and negative C-peptide secretion, there was again a reduction in the postprandial glycemic excursions to levels comparable to those of normal subjects; there were also reductions in plasma HPP, glucagon, and acetaminophen levels (used as a marker for gastric emptying), whereas insulin levels were not affected. These findings again favor a main mechanism of delayed gastric emptying and decreased glucagon secretion [39].
In a recent double-blind randomized controlled study conducted on 8 adolescent volunteers with type 1 DM and negative C-peptide, treatment with 2 doses (1.25 and 1.5 μg) of the GLP-1 analogue exenatide (a derivative of exendin-4) pre-meals resulted in lower postprandial glucose excursions and greater delays in gastric emptying when compared with insulin monotherapy, but failed to suppress glucagon secretion [40••]. In another study, 9 C-peptide negative participants with type 1 DM received concomitant infusions of arginine and 1.2 pmol/kg/min of GLP-1 or saline. The active infusion caused a significant decrease in glucagon levels as well as a decreased arginine-stimulated glucagon release. Only 1 subject showed increased C-peptide levels, presumably due to induction of endogenous insulin secretion [41•].
In summary, it appears that GLP-1 and its agonists have a favorable effect on postprandial hyperglycemia with no major hypoglycemia, driven mainly by delayed gastric emptying but also by decreased glucagon secretion. Limitations of all the studies are mainly the short period of time and the small number of participants. Bigger and longer-term studies are still needed.
Effect on Insulin Resistance
Since GLP-1R is found in rat muscle, fat, and liver [42–44], it was postulated that GLP-1 can influence peripheral glucose uptake. In vitro studies have shown that GLP-1 can increase glucose uptake in each of these tissues [45, 46]. Whether this can be observed in vivo and in humans is still controversial. The previously mentioned study done by Gutniak et al. [36] showed increased glucose uptake but did not account for the observed delay in gastric emptying. A more recent study showed no effect on peripheral glucose uptake [47]. A study done by Vella et al [48] that examined the effect of GLP-1 on splanchnic glucose uptake showed that there was no difference in total glucose uptake in the first 3 hours after infusion, but this was higher after the fourth hour, whereas splanchnic glucose uptake was lower in GLP-1 infused patients. The authors concluded that GLP-1 increases total body uptake in a time-dependent manner in people with type 1 DM through unknown mechanisms. More recently, the same authors examined the effect of both GLP-1 and exendin-4 in healthy nonobese volunteers using hyperinsulinemic-euglycemic clamps, but this time they concluded that neither GLP-1 nor exendin-4 have insulinomimetic effect [49].
In conclusion, it is still not clear whether GLP-1 has any direct effect on glucose uptake and insulin sensitivity.
GLP-1 Effect on Pancreatic Β-Cells in Type 1 DM
Sine type 1 DM is an autoimmune disease with persistent destruction of beta cells that leads to absolute insulin deficiency, most efforts in the field of treatment are focused toward identification of drugs that can stop or delay this process, or that can induce beta-cell proliferation or differentiation. GLP-1 is one of these promising drugs. Most studies done on type 2 DM and nondiabetic models of rodents have generally concluded that GLP-1 treatment increases beta-cell mass mainly through enhanced beta-cell proliferation, inhibition of beta-cell apoptosis, and induction of differentiation of new beta cells from pancreatic duct cells [35, 50–54]. Moreover GLP-1 seems to have an effect on beta-cell function, since GLP-1R-deficient mice were found to have fasting hyperglycemia and impaired insulin secretion in response to parenteral glucose [55]. Recent studies in vitro [56] and in humans [57] suggest that GLP-1 levels play a role in beta-cell function not only by stimulating insulin secretion but also by increasing insulin biosynthesis (by enhancing proinsulin gene transcription [58]) and stimulation of the transcription of other beta-cell genes, such as glucokinase and GLUT2 glucose transporters [59]. GLP-1 also increases the capacity of individual beta cells to respond to glucose; this ability to activate glucose-insensitive cells has been called induction of glucose competence [60].
Several recent animal studies have tested the effect of GLP-1 on rodent models of type 1 DM such as the non-obese diabetic (NOD) mouse, which spontaneously develops autoimmune diabetes similar to human autoimmune DM [61].
In a study done on 8-week-old female NOD mice, human GLP-1 was administered for 4–8 weeks subcutaneously. Those mice showed increased beta-cell mass (both through proliferation of preexisting islet beta cells and through differentiation of pancreatic duct cells into beta cells) as well as a 54.2 % decrease in the relative number of apoptotic cells. This was associated with a transient decrease in an insulitis score, improved glucose tolerance tests, and a delayed onset of diabetes. Mice that were treated for 8 weeks did not develop diabetes compared with a 60 % diabetes incidence in control animals [62].
In another study, exendin-4 was given to 4-week old NOD prediabetic mice. Diabetes onset was delayed from age 21 to 29 weeks, and treatment was associated with lower insulitis scores, and increases in beta-cell mass and islet number compared with controls [63].
Three-week combination therapy with gastrin and GLP-1 restored normoglycemia in diabetic NOD mice, an effect that was not seen with either agent alone (CITE). There was also increased pancreatic insulin content, beta-cell mass (ascribed to pancreatic duct differentiation rather than islet beta-cell proliferation), and insulin-positive cells in pancreatic ducts, as well as decreased beta-cell apoptosis and insulin antibodies [64]. The latter finding suggests that combination of gastrin and GLP-1 alters the autoimmune response through yet unknown mechanisms.
Given the reported decline in beta-cell function after the first year of a single course of anti-CD3 in human studies (CITE), the authors reasoned that a major determinant of the successful response to treatment is the extent of beta-cell function at diagnosis. Hence, GLP-1 (which stimulates beta-cell proliferation and inhibits apoptosis in rodents) was combined with several immunomodulators in many studies, to search for any potential additive effect. In one of these studies, new-onset diabetic NOD mice were treated with the monoclonal antibody anti-CD3 in addition to daily injections of exendin-4 and compared with those treated with anti-CD3 alone or exendin-4 alone. Mice that were treated with the combination showed a higher remission rate (44 %) than exendin-4 alone (0 %) or anti-CD3 alone (37 %). This was associated with increased pancreatic insulin content and recovery of residual islets that was only seen in mice with residual functional beta-cell mass upon initiation of treatment (glucose level <350 mg/dL). In contrast exendin-4 alone had no effect the immune reaction, nor on beta-cell mass [65].
Exendin-4 was also combined with the immunomodulator lisofylline (LSF), a blocker of T cell activation and cytokine production. This study also showed control of hyperglycemia that was completely absent with either drug alone [66].
Another combination with the immunosuppressant anti-lymphocyte serum (ALS) in NOD overtly diabetic mice led to complete remission in 23 out of 26 mice treated vs 6 out of 15 treated with ALS alone and 0 remission in those treated with exendin alone. Beta-cell mass was not measured directly in this study [67].
Combination of exendin-4 with anti-interferon-γ monoclonal antibody in Bio-breeding rats (another animal model of type 1 DM) led to increases in beta-cell proliferation at higher rates than either drug alone whereas exendin-4 alone induced a notable decrease in beta-cell apoptosis that was not modified by the combination [68••].
In a very recent study designed to investigate whether the local production of GLP-1 in the intestine can differentiate intestinal stem cells into insulin-producing cells, a recombinant adenovirus expressing GLP-1 (rAd-GLP-1) injected was intra-intestinally into diabetic mice. The injected mice showed intestinal insulin mRNA expression, insulin and glucagon-positive cells in the intestine, and significantly increased serum insulin but not glucagon. This was associated with a significant reduction in blood glucose levels and improved glucose tolerance. Expression of transcription factors related to beta-cell differentiation was detected in the intestine at 2 weeks after injection [69••]. This study is particularly relevant, since islet transplantation has proven to be hard and limited by scarcity of donors: thus, an alternative would be to induce differentiation of stem cells (such as pancreatic duct cells, or possibly intestinal cells) into insulin-secreting cells.
These animal studies have several limitations; first, inaccurate interpretation of data can result from the use of an antibody that only recognizes the amidated form of GLP-1, which is predominant in humans but not rodents [70]; second, animal studies cannot be extrapolated to humans because rodent beta cells have around 5- to 10-fold higher capacity for replication after partial pancreatectomy [71], and third, there are several major differences in both innate and adaptive immunity between rodents and humans [72].
Potential mechanisms explaining the effect of GLP-1 on the beta-cell mass are still poorly defined. It seems that several transduction pathways are involved, but the critical step seems to be the activation of the pancreatic and duodenal homeobox factor-1 by GLP-1. PDX-1 is an important transcription factor for pancreas development and beta-cell survival, and it has been shown that GLP-1 directly stimulates the expression of PDX-1 homeodomain protein in mice, and that it is essential for many of its proliferative and cytoprotective effects [73, 74].
Effect of GLP-1 on Islet Function After Islet Cell Transplantation in Patients with Type 1 DM
Given the beneficial effects of GLP-1 on beta-cell proliferation and decreased apoptosis in rodents, it was reasonable to test possible benefits after islet cell transplantation. Animal studies were very promising. Exendin-4 was able to promote revascularization and the formation of insulin-producing cells in mice transplanted with fetal islet cell clusters [75]. In streptozocin-induced NOD mice, human islet cells were transplanted under the renal capsule. Combination of GLP-1 and gastrin improved correction of hyperglycemia, increased insulin content in human cell grafts and increased plasma level of C-peptide [73].
Some human studies have also shown promise. Sixteen patients with islet allograft dysfunction requiring exogenous insulin received exendin-4 for approximately 7 months. Three of the 16 patients were able to discontinue insulin and overall need for insulin was significantly decreased by about 27 %. Unfortunately, beta-cell function was not tested at the end of treatment [76]. In another study, 3-month treatment with exendin-4 led to increased insulin secretion and decreased insulin needs by about 40 % in 11 of 12 patients after islet transplantation, but this effect was only transient and disappeared after 1 month [77]. In 5 subjects with type 1 DM treated with islet cells transplantation and exenatide, there was no need for exogenous insulin during and 18-month follow-up compared with only 1out of 5 subjects with type 1 DM who did not receive exenatide [78].
Conclusions
GLP-1 and its agonists seem to have a promising and beneficial effect in patients with type 1 DM. GLP-1 regulates glucose mainly by both delaying gastric emptying and decreasing glucagon secretion, and in rodents it has proliferative and anti-apoptotic effects on beta-cell mass that are independent of glucose control. Of course these latter effects need to be studied more in humans, but if proven to be beneficial, GLP-1 could delay the onset of type 1 DM, especially if started early enough, when residual beta-cell function is still present. In addition further long-term larger studies on the effect of GLP- 1 after islet cell transplantation are still needed.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of outstanding importance
Bayliss WM, Starling EH. Mechanism of pancreatic secretion. J Physiol. 1902;28:235–334.
Loew ER, Gray JS, Ivy AC. Is a duodenal hormone involved in carbohydrate metabolism? Am J Physiol. 1940;129:659–63.
Loew ER, Gray JS, Ivy AC. The effect of duodenal instillation of hydrochloric acid upon the fasting blood sugar of dogs. Am J Physiol. 1939;126:270–6.
Loew ER, Gray JS, Ivy AC. The effect of acid stimulation of the duodenum upon experimental hyperglycemia and utilization of glucose. Am J Physiol. 1940;128:298–308.
Elrick H, Stimmler L, Hlad CJ, Arai Y. Plasma insulin responses to oral and intravenous glucose administration. J Clin Endocrinol Metab. 1964;24:1076–82.
Mcintryre N, Holdsworth CD, Turner DA. New interpretation of oral glucose tolerance. Lancet. 1964;II:20–1.
Brown JC. A gastric inhibitory polypeptide. I. The amino acid composition and the tryptic peptides. Can J Biochem. 1971;49:255–61.
Brown JC, Dryburgh JR. A gastric inhibitory polypeptide. II. The complete amino acid sequence. Can J Biochem. 1971;49:867–72.
Brown JC, Mutt V, Pederson RA. Further purification of a polypeptide demonstrating enterogastrone activity. J Physiol. 1970;209:57–64.
Dupreo J, Ross SA, Watson D, Brown JC. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab. 1973;37:826–28.
Bell GI, Sanchez-Pescador R, Laybourn PJ, Najarian RC. Exon duplication and divergence in the human preproglucagon gene. Nature. 1983;304:368–71.
Schmidt WE, Siegel EG, Creutzfeldt W. Glucagonlike peptide-1 but not glucagon-like-peptide-2 stimulates insulin release from isolated rat pancreatic islets. Diabetologia. 1985;28:704–7.
Mojsov S, Weir GC, Habener JF. Insulinotropin glucagon-like peptide-1 (7–36): co-encoded in the glucagons gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest. 1987;79:616–19.
Meier JJ, Hucking K, Holst JJ, Deacon CF, Schmiegel WH, Nauck MA. Reduced insulinotropic effect of gastric inhibitory polypeptide in first-degree relatives of patients with type 2 diabetes. Diabetes. 2001;50:2497–504.
Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301–7.
Drucker DJ, Erlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci USA. 1996;93:7911–16.
Drucker DJ, Yusta B, Boushey RP, Deforest L, Brubaker PL. Human [Gly2]GLP-2 reduces the severity of colonic injury in a murine model of experimental colitis. Am J Physiol. 1999;276:G79–91.
Rocca AS, Lagreca J, Kalitsky J, Brubaker PL. Monounsaturated fatty acid diets improve glycemic tolerance through increased secretion of glucagon-like peptide-1. Endocrinology. 2001;142:1148–55.
Thomsen C, Storm H, Holst JJ, Hermansen K. Differential effects of saturated and monounsaturated fats on postprandial lipemia and glucagon-like peptide 1 responses in patients with type 2 diabetes. Am J Clin Nutr. 2003;77:605–11.
Sakurai H, Dobbs RE, Unger RH. The effect of somatostatin on the response of GLI to the intraduodenal administration of glucose, protein, and fat. Diabetologia. 1975;11:427–30.
Matsuyama T, Hoffman WH, Dunbar JC, Foa NL, Foa PP. Glucose, insulin, pancreatic glucagon and glucagon-like immunoreactive materials in the plasma of normal and diabetic children. Effect of the initial insulin treatment. Horm Metab Res. 1975;7:452–6.
Balks HJ, Holst JJ. Von Zur Muhlen A, Brabant G. Rapid oscillations in plasma glucagon-like peptide-1 (GLP-1) in humans: cholinergic control of GLP-1 secretion via muscarinic receptors. J Clin Endocrinol Metab. 1997;82:786–90.
Dumoulin V, Dakka T, Plaisancie P, Chayvialle JA, Cuber JC. Regulation of glucagon-like peptide-1-(7-36) amide, peptide YY, and neurotensin secretion by neurotransmitters and gut hormones in the isolated vascularly perfused rat ileum. Endocrinology. 1995;136:5182–88.
Lickley HL, Kemmer FW, Gray DE, et al. Chromatographic pattern of extrapancreatic glucagons and glucagon-like immunoreactivity before and during stimulation by epinephrine and participation of glucagons in epinephrine-induced hepatic glucose overproduction. Surgery. 1981;90:186–94.
Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. J Biol Chem. 1992;267:7402–5.
Devendra D, Liu E, Eisenbarth GS. Type 1 diabetes: recent developments. BMJ. 2004;328:750–4.
Madsbad S, Krarup T, Regeur L, Faber OK, Binder C. Insulin secretory reserve in insulin dependent patients at time of diagnosis and the first 180 days of insulin treatment. Acta Endocrinol (Copenh). 1980;95:359–63.
Madsbad S. Prevalence of residual B cell function and its metabolic consequences in Type 1 (insulin-dependent) diabetes. Diabetologia. 1983;24:141–7.
Dinneen S, Alzaid A, Turk D, Rizza R. Failure of glucagon suppression contributes to postprandial hyperglycaemia in IDDM. Diabetologia. 1995;38:337–43.
Greenbaum CJ, Prigeon RL, D’Alessio DA. Impaired beta-cell function, incretin effect, and glucagon suppression in patients with type 1 diabetes who have normal fasting glucose. Diabetes. 2002;51:951–7.
Lugari R, Dell’Anna C, Ugolotti D, et al. Effect of nutrient ingestion on glucagon-like peptide 1 (7-36 amide) secretion in human type 1 and type 2 diabetes. Horm Metab Res. 2000;32:424–8.
Vilsboll T, Krarup T, Sonne J, et al. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88:2706–13.
• Varga T, Firneisz G, Nagy G, Somogyi A. Elevated serum DPP4 activity in type 1 diabetes mellitus: a direct comparison. Orv Hetil 2010 151: 899-902. This study showed that compared with type 2 DM and normal controls, patients with type 1 DM had an elevated DPP4 activity that can affect GLP-1 activity.
Creutzfeldt WO, Kleine N, Willms B, Orskov C, Holst JJ, Nauck MA. Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon-like peptide I (7-36) amide in type I diabetic patients. Diabetes Care. 1996;19:580–6.
Meier JJ, Nauck MA. Glucagon-like peptide 1(GLP-1) in biology and pathology. Diabetes Metab Res Rev. 2005;21:91–117.
Gutniak M, Orskov C, Holst JJ, Ahren B, Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7-36) amide in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992;326:1316–22.
Dupre J, Behme MT, Hramiak IM, et al. Glucagon-like peptide I reduces postprandial glycemic excursions in IDDM. Diabetes. 1995;44:626–30.
Dupre J, Behme MT, Hramiak IM, McDonald TJ. Subcutaneous glucagon-like peptide I combined with insulin normalizes postcibal glycemic excursions in IDDM. Diabetes Care. 1997;20:381–4.
Behme MT, Dupre J, McDonald TJ. Glucagon-like peptide 1 improved glycemic control in type 1 diabetes. BMC Endocr Disord. 2003;10:1–9.
•• Raman VS, Mason KJ, Rodriguez LM, Hassan K, Yu Xi, Bomgaars L, et al. The role of adjunctive exenatide therapy in pediatrics type 1 DM. Diabetes Care 2010;33(6):1294–6. This randomized controlled trial showed that compared with insulin monotherapy treatment with 2 different doses of exenatide (both low and high dose) led to a better controlled postprandial hyperglycemia in adolescent patients with type 1 DM.
• Kielgast A, Madsbad H. Effect of GLP-1 on alfa and beta-cell function in c-peptide negative type 1 diabetic patients. J Clin Endocrin Metab 2010;95(5):2492–6. In this study done on C-peptide negative type 1 DM patients, GLP-1 agonists decreased both non-stimulated and arginine-stimulated glucagon release.
Villanueva-Penacarrillo ML, Alcantara AI, Clemente F, Delgado E, Valverde I. Potent glycogenic effect of GLP-1(7-36)amide in rat skeletal muscle. Diabetologia. 1994;37:1163–6.
Merida E, Delgado E, Molina LM, Villanueva-Penacarrillo ML, Valverde I. Presence of glucagon and glucagon-like peptide-1-(7-36) amide receptors in solubilized membranes of human adipose tissue. J Clin Endocrinol Metab. 1993;77:1654–7.
Villanueva-Penacarrillo ML, Delgado E, Trapote MA, Alcantara A, Clemente F, Luque MA, et al. Glucagon-like peptide-1 binding to rat hepatic membranes. J Endocrinol. 1995;46:183–9.
Morales M, Lopez-Delgado MI, Alcantara AI, Luque MA, Clemente F, Marquez L, et al. Preserved GLP-1 effects on glycogen synthase a activity and glucose metabolism in isolated hepatocytes and skeletal muscle from diabetic rats. Diabetes. 1997;46:1264–9.
Lopez-Delgado MI, Morales M, Villanueva-Penacarrillo ML, Malaisse WJ, Valverde I. Effects of glucagon-like peptide 1 on the kinetics of glycogen synthase a in hepatocytes from normal and diabetic rats. Endocrinology. 1998;139:2811–17.
Meneilly GS, McIntosh CH, Pederson RA, et al. Effect of glucagon- like peptide 1 (7-36 amide) on insulin-mediated glucose uptake in patients with type 1 diabetes. Diabetes Care. 2003;26:837–42.
Vella A, Shah P, Basu R, Basu A, Camilleri M, Schwenk FW, et al. Effect of glucagon-like peptide-1(7-36)-amide on initial splanchnic glucose uptake and insulin action in humans with type 1 diabetes. Diabetes. 2001;50:565–72.
Vella A, Shah P, Reed AS, Adkins AS, Basu R, Rizza RA. Lack of effect of exendin-4 and glucagon-like peptide-1-(7-36)-amide on insulin action in nondiabetic humans. Diabetologia. 2002;45:1410–15.
Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–57.
Brubaker PL, Drucker DJ. Minireview. Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004;145:2653–9.
Deacon CF. Therapeutic strategies based on glucagon-like peptide. Diabetes. 2004;53:2181–9.
Egan JM, Bulotta A, Hui H, Perfetti R. GLP-1 receptor agonists are growth and differentiation factors for pancreatic islet beta cells. Diabetes Metab Res Rev. 2003;19:115–23.
List JF, Habener JF. Glucagon-like peptide 1 agonists and the development and growth of pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2004;286:E875–81.
Scrocchi LA, Brown TJ, MacLusky N, Brubaker PL, Auerbach AB, Joyner AL, et al. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med. 1996;2:1254–8.
Serre V, Dolci W, Schaerer E, Scrocchi L, Drucker D, Efrat S, et al. Exendin-(9-39) is an inverse agonist of the murine glucagon- like peptide-1 receptor: implications for basal intracellular cyclic adenosine 3,5-monophosphate levels and beta-cell glucose competence. Endocrinology. 1998;139:4448–54.
Schirra J, Sturm K, Leicht P, Arnold R, Goke B, Katschinski M. Exendin(9-39)amide is an antagonist of glucagon-like peptide-1(7-36)amide in humans. J Clin Invest. 1998;01:1421–30.
Fehmann HC, Habener JF. Insulinotropic hormone glucagon- like peptide-I(7-37) stimulation of proinsulin gene expression and proinsulin biosynthesis in insulinoma beta TC-1 cells. Endocrinology. 1992;130:159–66.
Wang Y, Perfetti R, Greig NH, Holloway HW, DeOre KA, Montrose-Rafizadeh C, et al. Glucagon-like peptide-1 can reverse the age-related decline in glucose tolerance in rats. J Clin Invest. 1997;99:2883–9.
Holz GG, Chepurny OG. Glucagon-like peptide-1 synthetic analogs: new therapeutic agents for use in the treatment of diabetes mellitus. Curr Med Chem. 2003;10:2471–83.
Anderson MS, Bluestone JA. The NOD mouse. A model of immune dysregulation. Annu Rev Immunol. 2005;23:447–85.
Zhang J, Tokui Y, Yamagata K, et al. Continuous stimulation of human glucagon-like peptide-1 (7-36) amide in a mouse model (NOD) delays onset of autoimmune type 1 diabetes. Diabetologia. 2007;50:1900–9.
Hadjiyanni I, Baggio LL, Poussier P, Drucker DJ. Exendin-4 modulates diabetes onset in nonobese diabetic mice. Endocrinology. 2008;149:1338–49.
Suarez-Pinzon WL, Power RF, Yan Y, Wasserfall C, Atkinson M, Rabinovitch A. Combination therapy with glucagon-like peptide-1 and gastrin restores normoglycemia in diabetic NOD mice. Diabetes. 2008;57:3281–8.
Sherry NA, Chen W, Kushner JA, et al. Exendin-4 improves reversal of diabetes in NOD mice treated with Anti-CD3 monoclonal antibody by enhancing recovery of β-cells. Endocrinology. 2007;148:5136–44.
Yang Z, Chen M, Carter JD, et al. Combined treatment with lisofylline and exendin-4 reverses autoimmune diabetes. Biochem Biophys Res Commun. 2006;344:1017–22.
Ogawa N, List JF, Habener JF, Maki T. Cure of overt diabetes in NOD mice by transient treatment with anti-lymphocyte serum and exendin-4. Diabetes. 2004;53:1700–5.
•• Perez-Arana G, Blandino-Rosano M, Prada-Oliveira A, Aguilar-Diosdado M, Segundo C. Decrease in beta-cell proliferation precedes apoptosis during diabetes development in Bio-Breeding/Worcester rat: beneficial role of Exendin-4. Endocrinology. 2010;151:2538–46. In this study comparing combination of exendin-4 and monoclonal anti-INF gamma monoclonal antibody to each drug alone, it was shown that exendin-4 monotherapy decreased beta-cell apoptosis and the combination increased beta-cell proliferation better than each drug alone.
•• Liu MJ, Han J, Lee YS, Park MS, Shin S, Jun HS. Amelioration of hyperglycemia by intestinal overexpression of GLP-1 in mice. J Mol Med. 2010;88:351–8. This study showed that local intra-intestinal production of GLP-1 in diabetic mice can induce intestinal stem cell differentiation into insulin secreting cells, and showed a high insulin level with better glycemic control in these mice.
Burcelin R. EuCSGLP-1. What is known, new and controversial about GLP-1? Minutes of the 1st European GLP-1 club meeting, Marseille 28-29 May 2008. Diabetes Metab. 2008;34(6 Pt 1):627–30.
Meier JJ. Beta-cell mass in diabetes: a realistic therapeutic target? Diabetologia. 2008;51(5):703–13.
Roep BO. Are insights gained from NOD mice sufficient to guide clinical translation? Another inconvenient truth. Ann NY Acad Sci. 2007;1103:1–10.
Li Y, Cao X, Li LX, Brubaker PL, Edlund H, Drucker DJ. Beta-cell Pdx1 expression is essential for the glucoregulatory, proliferative, and cytoprotective actions of glucagon-like peptide-1. Diabetes. 2005;54:482–91.
Stoffers DA, Kieffer TJ, Hussain MA, et al. Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes. 2000;49:741–8.
Suen PM, Li K, Chan JC, Leung PS. In vivo treatment with glucagon- like peptide 1 promotes the graft function of fetal islet-like cell clusters in transplanted mice. Int J Biochem Cell Biol. 2006;38:951–60.
Froud T, Faradji RN, Pileggi A, et al. The use of exenatide in islet transplant recipients with chronic allograft dysfunction: safety, efficacy and metabolic effects. Transplantation. 2008;86:36–45.
Ghofaili KA, Fung M, Ao Z, et al. Effect of exenatide on beta-cell function after islet transplantation in type 1 diabetes. Transplantation. 2007;15(83):24–8.
Faradji RN, Tharavanij T, Messinger S, et al. Long-term insulin independence and improvement in insulin secretion after supplemental islet infusion under exenatide and etanercept. Transplantation. 2008;27(86):1658–65.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Issa, C.M., Azar, S.T. Possible Role of GLP-1 and Its Agonists in the Treatment of Type 1 Diabetes Mellitus. Curr Diab Rep 12, 560–567 (2012). https://doi.org/10.1007/s11892-012-0291-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11892-012-0291-6