Abstract
Glucagon-like Peptide-1 (GLP-1) receptor agonists (GLP-1RAs) emerged as a primary treatment for type-2 diabetes mellitus (T2DM), however, their multifaceted effects on various target organs beyond glycemic control opened a new era of treatment. We conducted a comprehensive literature search using databases including Scopus, Google Scholar, PubMed, and the Cochrane Library to identify clinical, in-vivo, and in-vitro studies focusing on the diverse effects of GLP-1 receptor agonists. Eligible studies were selected based on their relevance to the varied roles of GLP-1RAs in T2DM management and their impact on other physiological functions. Numerous studies have reported the efficacy of GLP-1RAs in improving outcomes in T2DM, with demonstrated benefits including glucose-dependent insulinotropic actions, modulation of insulin signaling pathways, and reductions in glycemic excursions. Additionally, GLP-1 receptors are expressed in various tissues and organs, suggesting their widespread physiological functions beyond glycemic control potentially include neuroprotective, anti-inflammatory, cardioprotective, and metabolic benefits. However, further scientific studies are still underway to maximize the benefits of GLP-1RAs and to discover additional roles in improving health benefits. This article sought to review not only the actions of GLP1RAs in the treatment of T2DM but also explore its effects on potential targets in other disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type-2 diabetes mellitus (T2DM) is primarily recognized by the inability of the human body to control the quantity of glucose (sugar) present in the blood with the help of insulin hormone [1]. Shockingly, the incidences of diabetes are expected to rise from 415 million (2015) to 640 million (2040) worldwide [2]. The incretin hormonal axis is created by the combination of gastrointestinal and endocrine pathways, and any abnormalities in this axis can potentially initiate the onset of T2DM [3]. A majority of the incretin function is constituted by GLP-1 and gastro-inhibitory intestinal peptide (GIP) [3]. GLP-1 exerts its mechanism of action through GLP-1 receptor (GLP-1R), a G-protein coupled receptor (GPCR), generally found extensively in organs including the brain, lung, pancreatic islets, lung, heart, vascular smooth cells, pancreas, macrophages, endothelial cells, central nervous system, kidney, peripheral chemoreceptors such as carotid body, and GI tract [4,5,6].
Glucagon-like peptide-1 (GLP-1) is a peptide hormone, typically composed of 30 amino acids, released from lower intestinal enteroendocrine L-cells and specific neurons located within the solitary tract in the brainstem, primarily in response to food intake [7]. The active structure of the GLP-1 protein includes two α-helices spanning amino acid positions 13–20 and 24–35, separated by a linker region [3, 8, 9]. Naturally occurring GLP-1 is rapidly cleaved at position 2 (alanine) by dipeptidyl peptidase-4 (DPP-4) along with neutral endopeptidase 24.11 (NEP 24.11) and renal clearance. Hence, this degradation of GLP-1 leads to a short half-life of about 2 min, resulting in only a small fraction (10–15%) of intact GLP-1 reaching circulation, resulting in fasting plasma levels typically within the range of 0–15 pmol/l [7, 9]. To preserve the concentrations of GLP-1, DPP-4 inhibitors are periodically used in patients with Type-2 diabetes mellitus (T2DM) [10]. To address this limitation and maximize the utilization, GLP-1 receptor agonists (GLP-1RAs) and DPP-4 inhibitors were developed to enhance GLP-1 efficacy.
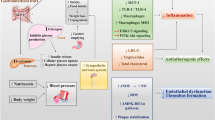
In contrast to conventional treatments like insulin and sulfonylureas, GLP-1-based therapies have been linked to weight loss and a reduced risk of hypoglycemia, making them particularly advantageous for diabetic patients [11]. Currently, the efficacy of GLP-1RAs is most commonly associated with their pivotal role in managing T2DM [12]. The ability of GLP-1RAs to enhance insulin secretion, suppress glucagon release, slow gastric emptying, and promote satiety fundamentally transformed the landscape of diabetes care [13] and is currently, considered a potential ally in the ongoing battle against the global epidemic of diabetes [14]. From the clinical point of view, the narrative of GLP-1RAs has taken an unexpected twist; GLP-1RAs are now captivating the attention of clinicians, researchers, and patients by revealing an astonishing array of their multifaceted roles extending far beyond diabetes [11]. This review embarks on an exciting and transformative journey of GLP-1RAs and their gradual increase in diverse applications in a spectrum of treatments. We delve into the expanding body of knowledge that uncovers the potential of these agents in metabolic health, cardiovascular wellness, hepatic and renal functions, and even the enigmatic scope of neuroprotection. Hence, we aim to explore the latest research findings, clinical insights, and emerging trends that underscore the multifaceted roles of GLP-1RAs in reshaping the future of medicine, offering new hope and possibilities to individuals facing a spectrum of health challenges. GLP-1RA can exhibit various roles beyond just treating T2DM and some of these functions are elucidated in Fig. 1 and discussed in this review.
Current clinical guidelines for diabetes management
The current treatment guidelines are based on a large number of evidence-based information and expert opinions on achieving end glucose level goals [Normal range: fasting plasma glucose < 5.5 mmol/l; Glycosylated hemoglobin (HbA1c: < 5.6%); Prediabetic range: fasting plasma glucose—5.5 to 7 mmol/l (HbA1c: 5.7 to 6.4%); Diabetic range: fasting plasma glucose > 7 mmol/l (HbA1c: > 6.5%)]. To minimize complications, the treatment goal is to achieve glycated hemoglobin (HbA1c) of 6.5% or less, recognizing the need to reduce the chances of hypoglycemia. Current types of anti-diabetic therapies include monotherapy, dual therapy, and triple therapy, which incorporates eight major classes of medications (biguanides, DPP-4 inhibitors, thiazolidinediones, sulfonylureas, incretin mimetics, bile acid sequestrants, α-glucosidase inhibitors, meglitinides), and insulin-based therapy [15]. Management of hyperglycemia in T2DM recommends a patient-centered approach for selecting appropriate pharmacologic treatment recommended by clinicians. Traditionally, metformin is a safe, effective, and inexpensive start at diagnosis and is considered the first-line treatment. However, if hyperglycemia is severe or any catabolic features (weight loss, hypertriglyceridemia, ketosis) are present, insulin can be used as part of any combination regimen. When blood glucose levels are above 300 mg/dL or HbA1C > 10% or any of the above two characteristics are present, then insulin therapy should be generally initiated [16]. Similarly, sulfonylurea, considered second-line agents, reduces HbA1c by 1–2%. Non-sulfonyl urea secretagogues (repaglinide and nateglinide) can be used in patients with renal insufficiency. The other class, α-glucosidase inhibitors, reduces postprandial blood glucose (PPBG); however, its long-term compliance and higher cost are significant issues. Thiazolidinediones (rosiglitazone and pioglitazone) reduce insulin resistance and HbA1c by 0.5–1.4% when used as monotherapy. DPP-4 inhibitors are the newer class of medicines in which sitagliptin is the only Food and Drugs Administration (FDA)-approved drug showing a reduction in HbA1c by 0.5–0.8%. Patients treated with sodium-glucose cotransporter-2 (SGLT2) inhibitors (empagliflozin, canagliflozin, dapagliflozin) or GLP-1RAs (liraglutide, semaglutide, dulaglutide) have shown a reduction in cardiovascular events along with improvements in glucose levels [17, 18]. As T2DM is a progressive disease, monotherapy with metformin is not sufficient in many patients, and other drugs are optimized stepwise to achieve the ideal HbA1c target [19].
How GLP-1RAs reduce high blood sugar?
GLP-1RAs are available as injectables and in oral form to achieve glycemic targets in diabetic patients [20]. GLP-1RAs are designed to mimic the actions of the naturally occurring GLP-1 hormone, which plays a crucial role in blood glucose homeostasis and satiety [3]. Upon GLP-1RA administration, they stimulate the GLP-1 receptor on pancreatic beta cells, prompting the secretion of insulin in a glucose-dependent manner without risking hypoglycemia [21,22,23,24,25]. Furthermore, GLP-1RAs slow down gastric emptying and suppress glucagon secretion, which eventually controls post-meal glucose spikes [26,27,28,29]. Beyond their immediate impact on glycemic control, these analogs have demonstrated benefits for weight management due to their appetite-suppressing effects and promotion of satiety via modifying eating behavior, which leads to reducing energy intake by approximately 12% interacting with the peripheral nervous system [13]. With these dual actions on both glucose regulation and weight management, GLP-1RAs can be a versatile and attractive option for individuals with T2DM, particularly those who struggle with obesity [30]. These benefits of GLP-1 analogs set the stage for a deeper exploration of their clinical applications and the evolving landscape of diabetes care [3].
GLP-1 directly suppresses glucagon secretion in the pancreas and indirectly enhances meal-induced insulin secretion in synergy with the glycemic stimulus, which modulates glucose levels [7]. The presence of histidine at position 7 in the GLP-1 amino acid structure is essential for the hormone's ability to stimulate insulin production and inhibit the secretion of glucagon [3, 11]. As shown in Fig. 2, the insulinotropic effect mainly comes from increased intracellular cAMP levels and then followed by serine/threonine kinase protein kinase A (PKA), cyclic adenosine monophosphate (cAMP)-regulated guanine nucleotide exchange factor 2 (cAMP-GEF2) also called EPAC2 and activated protein kinase A. PKA leads to the closure of Adenosine triphosphate (ATP)-sensitive K+ channels, causing membrane depolarization, and activation of L-type voltage-dependent calcium channel (VDCC) leads to an increase in intracellular Ca2+ causing insulin release [23]. EPAC2 activates Rap1 leading to calcium-induced calcium release, all of which increases Ca2+ thereby inducing mitochondrial ATP synthesis and exocytotic insulin release from insulin granules [31, 32]. The insulinotropic effect of GLP-1, mediated by increased intracellular cAMP levels and subsequent activation of PKA and EPAC2 pathways, is depicted in Fig. 2. multiple intracellular pathways, including protein kinase B and extracellular signal-related kinase (Erk), and epidermal growth factor receptor (EGFR) transactivation through the c-src kinase are responsible for the proliferative effects of GLP-1 [33, 34].
A figure depicting the intracellular mechanism of GLP-1RAs on insulin secretion Insulin release takes place after several processes: (1) Closure of KATP channels; (2) Opening of L-type VDC channels; (3) Inhibition of voltage-gated K+ channels; (4) PKA- and EAPC2-dependent mechanisms increases the intracellular Ca2+ concentrations; (5) Ca2+-induced Ca2+ mobilization stimulates ATP synthesis intracellularly which further enhances KATP channel closure; (6) accumulation of insulin-containing granules near the plasma membrane, ultimate insulin secretion into the circulation. ATP adenosine triphosphate, cAMP cyclic adenosine monophosphate, EPAC2 exchange protein activated by cAMP, ER endoplasmic reticulum, Kv voltage-gated K+ channels, PKA protein kinase A, RYR ryanodine receptors
GLP-1RAs—An emerging superclass of drugs for diabetes management
Exenatide was the first GLP-1RA approved for clinical use in 2005 by the USFDA and in 2006 by the European Union (EU) for the treatment of T2DM. It is a synthetic form of exendin-4, a naturally occurring peptide in Gila monster [35]. A triple-blind, placebo-controlled study, AMIGO, showed that exenatide maintained the long-term HbA1c below ≤ 7 and optimum body weight reduction [36]. Lixisenatide showed a greater reduction in body weight and 2-h post-prandial glucose when compared with sitagliptin. However, more frequent gastrointestinal (GI) side effects, such as nausea, were seen with lixisenatide than with sitagliptin [37]. Liraglutide, another GLP-1RA, is an acylated analog of GLP-1, with a plasma half-life of 10–18 h, [55] showed HbA1c reduction of up to 1.6% and weight loss of up to 2.5 kg over 30 weeks [38]. Liraglutide has been approved for reducing T2DM and has shown promising evidence in the reduction of risk of major cardiovascular (CV) events, obesity, liver disease, and other metabolic dysfunctions [39, 40]. American Diabetes Association (ADA) recommended liraglutide as a second-line drug after metformin for patients suffering from atherosclerotic cardiovascular disease [41]. Semaglutide is structurally similar to liraglutide but has less susceptibility to DPP-4 degradation. These structural modifications improved its binding with albumin and extended its half-life up to 7 days, allowing for once-weekly administration given subcutaneously [42]. SUSTAIN-1, a 30-week clinical study comparing semaglutide with placebo, showed a significant reduction in HbA1c and 0.2% weight reduction than the placebo group [43]. Albiglutide, a long-acting GLP-1 mimetic, is currently in phase 3 trials and is expected to provide a more patient-friendly dosing profile compared to available GLP-1 analogs [44]. Albiglutide has the characteristic to fuse with human albumin with DPP-4 resistant properties which increases its half-life up to 5–8 days and makes it suitable for once-weekly dosing as well [45]. Dulaglutide, a long-acting and large-size GLP-1RA, has a slower renal clearance which results from its prolonged half-life for 5–6 days allowing its once-a-week administration [46, 47]. The AWARD trial, using dulaglutide, showed an HbA1c reduction of 0.7% to 1.6% from its baseline. In the AWARD-1 study, dulaglutide was compared with twice-daily exenatide over 52 weeks which showed superior HbA1c reductions at 26 weeks with no significant difference in weight loss [48]. Overall, these promising evidence and characteristics suggest that GLP-1RAs have the efficiency to play a major role in diabetic management. Next, we explore the emerging role of GLP-1RAs and their potential benefits in other disorders.
GLP-1RAs in obesity management
In the ever-evolving landscape of obesity management, GLP-1 analogs have emerged as a revolutionary therapeutic option. While initially developed to address the complexities of diabetes care, these drugs have shown remarkable potential in the battle against obesity [49]. Unlike traditional weight loss medications that often come with a range of side effects and limited efficacy, GLP-1RAs offer a multifaceted approach to weight management [50]. GLP-1RAs have been documented to induce weight loss in a dose-dependent and progressive manner. An average weight reduction of 5.8 pounds (lbs.) is seen with long-acting exenatide [3]. The Liraglutide Effect and Action in Diabetes (LEAD) program observed weight reductions in more than 4000 participants, suggesting its potency in obesity management [51, 52]. Along with weight loss, GLP-1RAs have been demonstrated to reduce body mass index (BMI) and waist circumference in overweight or obese people with or without diabetes [53, 54]. Other GLP-1RA potentially works similarly in weight reduction; however, more systematic clinical studies need to be conducted to determine their extended role in weight reduction [55]. A novel dual GIP and GLP-1 receptor agonist Tirzepatide (15 mg) demonstrated dose-dependent reductions in body weight, with a significant difference of − 10.7 kg (SE 0.4; -13.9% reduction) outperforming dulaglutide in glycemic control and body weight reduction in Japanese patients with T2DM [56]. Conclusively, the majority of patients were able to get higher benefits with less adverse responses caused by GLP-1RAs, making them the preferred medication for the treatment of obesity.
Appetite regulation and weight loss effects in obesity management
The central nervous system, which regulates satiety, receives information from the digestive tract via afferent impulses to control eating behavior [57]. GLP-1 has been shown to reduce gut motility and stomach emptying, through which its association has been proposed in appetite regulation. Intravenous infusion of GLP-1 in male Sprague–Dawley rats effectively inhibits food intake in a dose-dependent manner. Neuroimaging studies demonstrated that peripherally injected GLP-1 alters brain activity in regions implicated in the control of food. Several studies in animals have revealed that administration of GLP-1RAs (Dulaglutide, Exenatide, Liraglutide, Exendin-4) resulted in the suppression of food intake mediated by direct GLP-1R activation in the brain and vagal afferents through several signaling pathways [58]. For instance, they stimulate adipocyte development by activating the Wnt signaling pathway and rely on SIRT1 to mediate lipolysis and fatty acid oxidation in adipose tissues [14]. GLP-1RAs encourage the transformation of visceral white adipose tissue (WAT) into brown adipose tissue (BAT), enhancing the thermogenesis of BAT and hence increasing energy expenditure under the control of AMP-activated protein kinase (AMPK) in the ventral medial hypothalamus [14]. These mechanisms are to be investigated further to accurately determine the precise role of GLP-1RAs and food intake in weight reduction.
Clinical trials and real-world evidence
Three notable clinical trials shed light on the interplay between pharmaceutical interventions and patient well-being. The first study (Phase-4; NCT03361098), a randomized and placebo-controlled trial on 65 participants, was conducted to investigate the effect of a dual approach involving exenatide and dapagliflozin (SGLT2 inhibitor) on appetite regulation. This study found that responsiveness to palatable food consumption underscores the synergistic effects of combining these agents, offering great insight into novel approaches for managing T2DM [59]. The combination therapy of GLP-1RAs with SGLT-2 inhibitors has progressively shown improvement in patients suffering from T2DM. Another trial (NCT00375492), a randomized, placebo-controlled trial involving 196 participants, focused on weight loss in diabetic patients. By administering exenatide alongside lifestyle modifications, this study examined the improvement in weight management in individuals with T2DM, measuring the impact on calorie intake and participant weight [60]. Lastly, the third trial (NCT05136287) presents a multicentric, randomized clinical trial assessing the weight loss outcomes with 140 participants investigating the efficacy of various GLP-1RAs (dulaglutide, exenatide, liraglutide, and lixisenatide) found significant reduction in body weight with minimizing adverse events [61]. Additionally, the combination therapy of liraglutide with sulfonylurea analog (glimepiride) was found to be effective in weight reduction compared to that of a placebo when given over 26 weeks [62]. If GLP-1RAs monotherapy or combination therapy with other anti-diabetic agents fails to provide satisfactory glycemic control and weight modulation, the addition of basal insulin to GLP-1RAs had been recommended and evaluated in late randomized control trials (RCTs). It was observed that the insulin titration used in conjunction with the GLP-1RAs had a beneficial impact on glycemic and appetite control and weight reduction [63, 64].
Table 1 shows the result of several clinical investigations performed to evaluate the role of GLP-1RAs to modulate weight and appetite. Such insights provide reasonable evidence to healthcare practitioners with valuable options to tailor treatments for individuals living with T2DM, ultimately improving their quality of life.
Evolution of GLP-1 analogs beyond diabetes and obesity
GLP-1 analogs, such as exenatide and liraglutide, were primarily designed to aid in glycemic control with the vision of a growing global diabetes epidemic. Patients taking these medications started experiencing unexpected weight loss, prompting further investigation [11, 70]. Additionally, studies began to highlight their cardiovascular benefits, particularly in reducing the risk of major adverse cardiovascular events (MACE). These serendipitous discoveries led to investigations into the therapeutic potential of GLP-1RAs in conditions beyond diabetes [71]. Subsequent regulatory approvals and label expansions reflected the shift in the medical paradigm, recognizing these agents as versatile tools in the arsenal of modern medicine. This historical context justifies the need for the review and highlights the urgency of synthesizing the latest research and clinical insights into the evolving landscape of GLP-1RA applications beyond diabetes.
Cardiovascular benefits of GLP-1RAs
The cardiovascular benefits associated with GLP-1RAs have emerged as a groundbreaking revelation in recent years. Beyond their primary function of glycemic control, GLP-1RAs have demonstrated a remarkable capacity to mitigate cardiovascular risk factors and reduce the incidence of MACE in individuals with T2DM [64]. In this discussion, we will delve into the multifaceted cardiovascular advantages offered by GLP-1RAs, exploring the mechanisms behind these benefits, the clinical evidence supporting their use, and the broader implications for the management of T2DM and cardiovascular disease.
Reduction of major adverse cardiovascular events (MACE)
Patients suffering from T2DM are at an increased susceptibility to developing cardiovascular complications that can also prove to be fatal. Hence, the prevention of these complications should be considered while choosing a course of treatment [72]. Most GLP-1RAs have shown benefits in lowering cardiovascular disease (CVD) complications such as dyslipidemia and high blood pressure (BP) [73]. GLP-1RAs were found to cause a decrease in the systolic blood pressure (SBP) by 2 to 6 mmHg and eventually a considerable reduction in MACE [74, 75]. Liraglutide and Semaglutide were observed to benefit CV outcomes in clinical studies; however, the precise mechanisms behind this benefit are yet to be discovered [76,77,78,79]. Clinical trials such as LEADER, SUSTAIN-6, and EXSCEL demonstrated that GLP-1RAs reduced cardiovascular events in CV patients with acute coronary syndrome and T2DM [80]. In the LEADER trial, liraglutide exhibited a 13% reduction in MACE with a hazard ratio (HR) of 0.87 (95% CI: 0.78; 0.97) compared to placebo, involving 8,121 patients with T2DM. Similarly, in the SUSTAIN-6 trial, semaglutide demonstrated a 26% reduction in MACE with an HR of 0.74 (95% CI: 0.58; 0.95) among 3,297 patients with T2DM, showcasing significant cardiovascular risk reduction [78, 81] Other trial using Lixisenatide, Liraglutide, and Semaglutide lowers the MACE symptoms and promotes positive CV outcomes in patients with T2DM [82].
Impact on atherosclerosis and vascular health
The majority of the population of patients suffering from diabetes may develop myocardial ischemia and heart failure in the future [74]. The SOUL trial revealed improvements using GLP-1Ras in heart failure outcomes, including reduced hospitalization rates and enhanced cardiac function [83]. In clinical practice, the implications of these findings are profound and encouraging. GLP-1RAs are now considered a critical component in individuals who have T2DM with established cardiovascular disease or those at high risk of cardiovascular events [11, 84]. Findings from animal studies revealed that GLP-1RAs had been shown to reduce atherosclerotic plaque development by exerting their anti-inflammatory effects in the endothelial cells and vascular smooth muscle cells and causing a more stabilized and less vulnerable plaque [85]. Based on the data received from clinical trials to evaluate the impact of GLP-1RAs in CV events, a consistent decrease in atherothrombotic events was observed which suggests the beneficial outcomes using GLP-1RAs in patients suffering from T2DM and atherosclerosis [75, 85].
Potential mechanisms of GLP-1RAs to reduce cardiovascular risks
GLP-1RAs have emerged as a pivotal component in managing T2DM due to their multifaceted implications for cardiovascular risk reduction via key mechanisms contributing to the regulation of BP (Fig. 3) [11]. GLP-1RAs have been associated with a consistent reduction in SBP and diastolic blood pressure (DBP), primarily by influencing the central nervous system possibly by reducing sympathetic nervous system activity [5, 6, 86]. These BP-lowering effects alleviate the strain on the heart and further reduce the risk of adverse cardiovascular events [86]. Furthermore, GLP-1RAs contribute to favorable changes in lipid profiles, characterized by lowered triglyceride levels and increased high-density lipoprotein cholesterol. These alterations promote a more cardioprotective lipid profile, reducing the risk of atherosclerosis and related cardiovascular complications [87, 88]. As discussed earlier, weight loss, often observed as a secondary effect of GLP-1RAs, plays a pivotal role in mitigating associated cardiovascular risk. Weight reduction improves insulin sensitivity, reduces inflammation, and contributes to overall cardiovascular well-being [89]. In essence, the cardiovascular benefits of GLP-1RAs have ushered in a new era of diabetes management, focusing on glucose regulation and the holistic health of individuals with associated disorders [90].
Schematic illustration of the effects of GLP-1RAs on satiety, cardiovascular outcomes, and non-alcoholic fatty liver disease (NAFLD). GLP-1RAs enhance satiety by reducing body weight and caloric intake along with causing improvements in cardiovascular parameters including blood pressure, heart rate, and myocardial contractility. These effects thereby facilitate improved blood flow to the heart and mitigate the risk of atherosclerosis, stroke, and major adverse cardiovascular events (MACE). Furthermore, they yield favorable outcomes in non-alcoholic fatty liver disease (NAFLD) by optimizing liver fat utilization and diminishing inflammatory markers within the liver
Pre-clinical and clinical findings
Here, we discuss the pre-clinical evidence (Table 2) that serves as the foundational knowledge upon which clinical trials are built, providing a strong rationale for testing these compounds in humans [86].
Table 3 depicts clinical findings that support the implementation of GLP-1RAs in cardiovascular disorders.
GLP-1RAs in non-alcoholic fatty liver disease (NAFLD)
In recent years, GLP-1RA has emerged as a promising avenue of research and treatment in the context of NAFLD [102]. NAFLD encompasses a spectrum of liver conditions, ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), characterized by inflammation and liver cell damage, which can progress to fibrosis, cirrhosis, and even hepatocellular carcinoma [103]. With the global prevalence of NAFLD on the rise, investigations into the potential therapeutic role of GLP-1RAs have gained momentum for their potential to mitigate liver fat accumulation, inflammation, and fibrosis. We assess the intricate interplay between GLP-1RAs and NAFLD by exploring the mechanisms, pre-clinical and clinical evidence, and the evolving treatment landscape for complex liver disorders.
Effects on liver fat accumulation
Currently, lifestyle modifications, including weight loss, remain the existing alternatives to cure NAFLD; however, these alternatives are difficult to maintain in patients who cannot adhere to them [104]. The prevalence of NAFLD significantly increased in patients pre-existing with T2DM, with up to 65% in patients suffering from Non-alcoholic steatohepatitis (NASH) [105]. It has been observed that liraglutide also improves the hepatic enzyme lipase activity, thereby modulating liver fat to improve the outcome of liver fatty disease [106]. Recent research has shown that GLP-1RAs influence liver fat processing either directly (impacting hepatocyte fat metabolism) or indirectly (incretin action) due to the ultimate effect of reversing insulin resistance [107, 108]. Another study utilizing exendin-4 revealed that the liver fat content was decreased when this drug was administered to NAFLD-induced mice, along with improved insulin signaling [109]. A recent meta-analysis of 25 trials concluded that GLP-1RAs caused at least 2.8 kg weight reduction in people with or without diabetes, contributing to reducing NAFLD symptoms. Therefore, GLP-1RAs may play a crucial role in regulating liver fat accumulation and, contribute to the treatment of NAFLD.
Improvement in liver function
GLP-1RAs may lead to improvements in liver function for a variety of reasons. They decrease the de novo lipogenesis, which further reduces the lipolysis-induced free fatty acid formation and toxic substances due to triglycerides (Fig. 3) [110]. Several animal studies using GLP-1RAs showed the repair of the dysfunctional adipose tissue, regulate the destructive effects of hepatic fatty acids by maintaining their oxidative processes via controlling the effects of acetyl-CoA carboxylase and fatty acid synthase, and ultimately, alleviating the hepatic toxicity [111, 112]. GLP-1RAs also modulate the liver inflammation in NAFLD by decreasing the levels of inflammatory mediators, including c-Jun-N-terminal kinase (JNK), Interleukin-1 (IL-1), Intracellular cell adhesion molecule (ICAM-1) in the liver and preventing processes such as liver fibrosis, necrosis [91, 113]. However, clinical studies into this context are currently lacking, and further insights may help to adequately prove the role of GLP-1RAs in liver function restoration [104]. Gu and colleagues carried out a meta-analysis combining the results of nine RCTs comparing the effects of GLP-1RAs in contrast to other antidiabetic drugs (pioglitazone) considered as placebo in the improvement of liver histology from steatosis, inflammation, fibrosis, or necrosis [114]. Further clinical investigation may be required to understand more benefits to support the clinical significance of GLP-1RAs in liver disease with or without T2DM [115, 116].
Reno-protective effects of GLP-1RAs
GLP-1RAs have also unveiled a remarkable facet of their pharmacological prowess in renoprotection [3]. Chronic kidney disease (CKD) is a prevalent and debilitating complication of T2DM, with a substantial impact on patient morbidity and mortality [117]. In this discussion, we delve into the link between GLP-1RAs and renal protection with evolving underlying mechanisms in published articles and the promising implications for individuals at risk of diabetic nephropathy and other renal disorders.
Impact on kidney function using GLP-1RAs
Diabetic nephropathy is most commonly associated with patients with T2DM whose kidney functions are negatively affected [118]. In models of diabetic nephropathy, exendin-4 treatment prevented glomerular macrophage infiltration in glomeruli, significantly decreased oxidative stress, inflammation in tubular cells, and gene expression of cluster of differentiation 14 (CD14), ICAM-1, and transforming growth factor-1 (TGF-1) in the renal cortex in streptozotocin (STZ)-induced diabetic rats [119]. Therefore, by lowering renal leukocyte infiltration and proinflammatory mediators, GLP-1RAs may benefit in improving nephropathy [120]. GLP-1R is expressed in the proximal tubules [121], and this expression possibly leads to the inhibition of renal inflammation and oxidative stress using GLP-1 therapy on diabetic nephropathy and acute kidney damage [122]. The direct and indirect effects of GLP-1RAs are illustrated in Fig. 44.
A figure displaying the mechanisms through which GLP-1RAs can cause renoprotection. Stimulating GLP-1 receptors by GLP-1RAs potentially be directly or indirectly involved in the restoration of kidney functions leading to reno-protective effects. The direct effects include maintenance or reduction of oxidative stress, inflammation, natriuresis, and glomerular hypertension, whereas the indirect effects include regulation of hypertension, dyslipidemia, and other CV risk factors [126]
Studies suggest that the reno-protective effects of GLP-1 may be mediated by two signaling pathways: (1) Increasing natriuresis and diuresis in a dose-dependent manner by functioning on the gut-renal (natriuretic) axis, and (2) Reducing the activity of the Na+ /H+ exchanger isoform NHE3 to reduce proximal sodium reabsorption, and possibly by boosting glomerular filtration rate. A study also showed that glomerular mesangial proliferation, a characteristic feature of diabetic patients, was remarkably reduced by GLP-1RAs [123]. Recombinant human GLP-1 reduces oxidative stress in the glomeruli and glomerular microvascular endothelial cells in diabetic rats by inhibiting protein kinase C and activating PKA [124]. Another study reported that liraglutide significantly decreased albuminuria and oxidative stress in Type-1 DM rats produced by STZ [125].
Clinical studies of GLP-1RAs impacting renal outcomes
A journey through the clinical investigations using GLP-1RAs on renal outcomes has been categorized in Yin W et al. studied recombinant human GLP-1RAs' effect on kidney function and revealed that these agents improved renal tubules and tubulointerstitial lesions in diabetic nephropathy rats [127]. GLP-1 also inhibits the activity of multiple proteins that have been associated with diabetic nephropathy, notably collagen I, alpha-smooth muscle actin (a-SMA), fibronectin (FN), and tubulointerstitial TNF-α [127, 128]. It also effectively inhibits the level of C-peptide, which is majorly responsible for the inflammation of tubulointerstitial fibrosis [129, 130]. In patients with diabetic kidney disease, GLP-1RAs, namely liraglutide, and lixisenatide, were observed to prolong the decline of renal function towards end-stage renal disease along with a reduction in albuminuria [40, 131]. This response was due to increased cAMP levels and PKA activity while decreasing NADPH oxidase activity, interfering with the expression of advanced glycation end product (AGE) receptors, and suppressing the NF-κβ mediated signaling pathway. This mechanism prevents oxidative damage and the production of reactive oxygen species (ROS). This mechanistic view indicates that GLP-1RAs play a sensible role in renal protection.
Table 4 In the majority of trials, GLP-1 analogs demonstrated enhanced effectiveness in improving serum creatinine (Sr.Cr) levels and glomerular filtration rate (GFR) in patients with T2DM who were at risk of developing CKD or already diagnosed with CKD. Yin W et al. studied recombinant human GLP-1RAs' effect on kidney function and revealed that these agents improved renal tubules and tubulointerstitial lesions in diabetic nephropathy rats [127]. GLP-1 also inhibits the activity of multiple proteins that have been associated with diabetic nephropathy, notably collagen I, alpha-smooth muscle actin (a-SMA), fibronectin (FN), and tubulointerstitial TNF-α [127, 128]. It also effectively inhibits the level of C-peptide, which is majorly responsible for the inflammation of tubulointerstitial fibrosis [129, 130]. In patients with diabetic kidney disease, GLP-1RAs, namely liraglutide, and lixisenatide, were observed to prolong the decline of renal function towards end-stage renal disease along with a reduction in albuminuria [40, 131]. This response was due to increased cAMP levels and PKA activity while decreasing NADPH oxidase activity, interfering with the expression of advanced glycation end product (AGE) receptors, and suppressing the NF-κβ mediated signaling pathway. This mechanism prevents oxidative damage and the production of reactive oxygen species (ROS). This mechanistic view indicates that GLP-1RAs play a sensible role in renal protection.
Exploring the neuroprotective potential of GLP-1RAs
GLP-1RAs have emerged as a beacon of hope, offering a potential advantage for neuroprotection in neurodegenerative diseases. Neurodegenerative disorders, such as Alzheimer’s disease (AD), and Parkinson’s disease (PD) represent some of the most challenging and devastating health conditions in the modern era [138, 139]. As the global population ages, the burden of these diseases continues to grow, underscoring the urgent need for innovative therapies. GLP-1RAs have expanded their therapeutic role from T2DM and obesity to preserving and restoring neuronal health [11].
Pre-clinical and clinical findings in neurodegenerative disorders
GLP-1R is known to be expressed in the brain, primarily affecting the brain function regarding satiety and appetite via the autonomic nervous system [140]. GLP-1 plays an important role in a variety of neural functions, including hippocampus circuit activity, neurite outgrowth stimulation, cell survival enhancement, and up-regulation of enzyme and neurotransmitter production (Fig. 5) [7, 141]. GLP-1R expressions have been identified on neurons, specifically pyramidal neurons in the hippocampus and neocortex, where they are found on dendrites and cell bodies. This indicates that these receptors are crucial for the movement of synaptic signals among neurons [142, 143]. Novel GLP-1RAs have a significantly greater biological half-life (Val8GLP-1, liraglutide, exendin-4) and have been demonstrated to impact memory formation and synaptic plasticity in the brain significantly. Also, along with such effects, GLP-1RAs can cross the blood–brain barrier, imparting CNS effects unlike most neuroprotective growth factors [138, 144, 145]. Additionally, mice that overexpressed GLP-1R in the hippocampus displayed enhanced learning and increased neurite development [146]. Recent findings show that co-activation of GIP and GLP-1 receptors is neuroprotective in a model of PD and enhances cognitive performance in a rat model of AD [147]. GLP-1R has been found in astrocytes and microglia, suggesting that the glia may be crucial in the inflammatory reactions of the central nervous system [148]. The pathogenesis of both the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyidine (MPTP)-induced PD model and human PD is strongly influenced by microglial activation [149, 150]. In this study, exendin-4 (50mcg/kg) showed significant effectiveness in mitigating the activation of microglial cells induced by MPTP. Moreover, it effectively curbed the production of inflammatory cytokines such as TNF-α and IL-1 triggered by MPTP. A study was conducted with results that showed that the usage of GLP-1RA for more than 3–6 months helped individuals with PD and AD with their motor and cognitive symptoms, respectively. GLP-1 injections daily for eight weeks showed a substantial improvement in the recognition index in mice measuring with an object recognition test, indicating improved learning and memory, while mice feeding on a high-fat diet led to a decline in cognitive performance [151]. These findings suggest that the inhibitory impact of exendin-4 on microglial activation holds promise as a therapeutic approach for the management of PD. In summary, GLP-1RAs exhibit protection in synaptogenesis, neurogenesis, cell repair, and reduced inflammation in the brain [152].
Visual representation of the beneficial roles of GLP-1RA in neuroprotection. GLP-1RAs facilitate neuronal repair by regulating hippocampal circuit activity, stimulating neurite outgrowth, enhancing cell survival, and increasing the production of enzymes and neurotransmitters. They inhibit neuronal apoptosis, the release of proinflammatory cytokines, and oxidative stress. Conversely, they promote the synaptic formation and improve mitochondrial function, thereby supporting neurogenesis
Therapeutic potential of GLP-1RAs in stroke
As the global population ages, the prevalence of stroke continues to rise, necessitating novel and effective interventions. GLP-1RAs recently emerged as promising candidates for stroke therapy due to their multifaceted neuroprotective properties [153].
GLP-1RAs reduce neuroinflammation and improve cognition in stroke
GLP-1R expression within the brain increases the level of intracellular cAMP via its signaling pathways, which also serve as the target for neuroprotection in ischemic stroke [154]. It has been hypothesized that the effect of GLP-1RAs via cAMP/PKA signaling stimulation may contribute to the anti-neuroinflammatory activity, given that brain inflammation is an immunological response mediated by microglia and astrocytes [155].
GLP-1R expression was found when embryonic primary cerebral cortical and ventral mesencephalic (dopaminergic) neurons were experimentally studied. Hypoxia and formation of 6-hydroxydopamine cause cell death, and GLP-1 and exendin-4 protected hypoxia-induced cell death, and this effect disappeared in the cells from GLP-1R knockout (−/−) mice [156]. These findings show that exendin-4 can defend neurons from oxidative and metabolic stresses and provide therapeutic potential in the management of stroke. Exendin-4 had a strong dilatory effect on cortical arterioles in acute brain slices of the rat cerebral cortex and effectively reversed arteriolar constrictions brought on by metabolite lactate and glucose deprivation in an ex-vivo model of ischemic stroke. Exendin-4 caused significant increases in brain tissue pO2, a sign of elevated cerebral blood flow via strong dilation of cortical arterioles, in rats under anesthesia. These findings show that a pathway involving GLP-1R signaling mediates the neuroprotection against ischemic stroke created by distant ischemia training [157]. Another study utilizing liraglutide prevented brain edema, and neurologic deficits and reduced the inflammatory response produced by intracerebral hemorrhage (ICH) in mice. This protection was mechanistically medicated via activation of AMPK, which can reduce the expression of proinflammatory mediators like ICAM-1 and E-selectin [158,159,160]. In a rat model of middle cerebral artery occlusion (MCAO) stroke, liraglutide exhibited comparable protective qualities by reducing apoptosis and oxidative stress in the affected brain region [161]. In another study, animals treated with semaglutide had lower neurological impairment scores on a variety of motor and grip strength measures along with reduced extent of cerebral infarction [159, 162]. GLP-1RAs exhibit neuroprotection, but the definite mechanism that mediates this response is yet to be known, which invites further studies [163]. The effect of GLP-1RAs in improving cognitive behavior, motor skills, and neuroprotection in neurodegenerative diseases and stroke opened up new ways to repurpose drugs as therapeutic interventions for neurological diseases [164].
Safety profile and adverse effects of GLP-1RAs
During clinical trials using GLP-1RAs, gastrointestinal issues were the most reported side effects among all participants. Vomiting, constipation, abdominal discomfort, and dyspepsia were all reasonably prevalent (1/10 to 1/100), but nausea and diarrhea were highly common (1/10) [165]. At the onset of the therapy, these side effects appeared more frequent, but as the therapy proceeded, gastrointestinal issues gradually subsided. The peak of the GLP-1 effects, which is visible in conjunction with the injection, is thought to be the cause of the activation of the brain regions responsible for controlling appetite, satiety, and nausea [166, 167]. Transient nausea may be clinically insignificant but attributed to about 15% of the cases upon administration of GLP-1RAs, which can be due to delayed emptying of gastric contents. When compared exenatide with liraglutide, exenatide shows 15% more cases of nausea and gastric discomfort [168]. Diarrhea may also result using GLP-1RAs in 10% to 20% of patients [169]. It is also postulated that continuous usage of these agents can cause a significant decrease in gastric acid and lipase secretion. Along with gastric disturbances, usage of GLP-1RAs in animals and humans has reported several long-term safety concerns although there are few reliable epidemiological data available on the prevalence of acute pancreatitis in people with T2DM. Exenatide patients suffered pancreatitis at the incidence of 27 occurrences per 100,000 patients [170]. A numerically higher incidence of benign adenomas was seen in preclinical experiments on female rats exposed to exenatide. It was not statistically different when this increase in adenoma incidence was corrected for the rat life span. In humans, only five thyroid neoplasm cases in the clinical studies were reported [171]. Regarding GLP-1 effects on the colon, GLP-1 may decrease intestinal motility via reduced circular contractions in full-thickness muscular colon strips. Hence, gastrointestinal symptoms are common but short-lived, and they do not pose a significant barrier or risks to using these drugs comparing their benefits [172].
Conclusion and future perspectives
GLP-1RAs emerged as a great hope for individuals grappling with the intricate relation of chronic glucose regulation. GLP-1RAs have recently gained global attention for their role in blood glucose control in diabetes, as well as their impact on other diseases. It is well-known that diabetes and other comorbidities may increase the likelihood of other complications, including cardiovascular, hepatic, renal, and cerebrovascular diseases in the patients, and the multifaceted roles of GLP-1RAs in these pathologies have been highlighted in the present review. However, the amount of evidence that supports the comprehensive roles of GLP-1RAs and their mechanisms has not yet been fully explored. Further investigations are warranted considering the expansion of GLP-1RAs in potential benefits from diabetes and associated disorders.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
We confirm that EndNote Version 9, a freely available software application, was utilized for reference management, and Biorender, another freely available tool, was employed for the creation of figures in this manuscript.
Change history
03 August 2024
The e-mail addresses of both corresponding authors are swapped.
Abbreviations
- (Camp-GEF2):
-
(CAMP)-regulated guanine nucleotide exchange factor 2
- (MPTP):
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyidine
- (ATP):
-
Adenosine triphosphate
- (AGE):
-
Advances glycation end-product
- (ALT):
-
Alanine aminotransferase
- (ALD):
-
Alcoholic liver disease
- (AGI):
-
Alpha-glucosidase inhibitors
- (a-SMA):
-
Alpha-smooth muscle actin
- (AD):
-
Alzheimer's disease
- (ADA):
-
American diabetes association
- (AMPK):
-
AMP-activated protein kinase
- (APP/PS1):
-
Amyloid polypeptide/Presenilin 1
- (ApoE):
-
Apolipoprotein E
- (AST):
-
Aspartate aminotransferase levels
- (ANP):
-
Atrial natriuretic peptide
- (BP):
-
Blood pressure
- (BMI):
-
Body mass index
- (BAT):
-
Brown adipose tissue
- (CICR):
-
Calcium-induced calcium release
- (C-terminus):
-
Carboxyl terminal
- (CV):
-
Cardiovascular
- (CVDs):
-
Cardiovascular diseases
- (CVOT):
-
Cardiovascular outcome trials
- (CNS):
-
Central nervous system
- (CKD):
-
Chronic kidney disease
- (JNK):
-
C-Jun-N-terminal kinase
- (CD14):
-
Cluster of differentiation 14
- (CRP):
-
C-reactive protein
- (cAMP):
-
Cyclic adenosine monophosphate
- (DCCT):
-
Diabetes control and complications trial
- (DM):
-
Diabetes mellitus
- (DN):
-
Diabetic nephropathy
- (DBP):
-
Diastolic blood pressure
- (DPP-4):
-
Dipeptidyl peptidase-4
- (eGFR):
-
Electronic glomerular filtration rate
- (ER):
-
Endoplasmic reticulum
- (EC):
-
Endothelial cells
- (ESRD):
-
End-stage renal disease
- (EGFR):
-
Epidermal growth factor receptor
- (EU):
-
European union
- (ELIXA):
-
Evaluation of lixisenatide in acute coronary syndrome
- (EXSCEL):
-
Exenatide study of cardiovascular event lowering
- (EX-4):
-
Exendin-4
- (ER):
-
Extended-release
- (Erk):
-
Extracellular signal-related kinase
- (FN):
-
Fibronectin
- (FDA):
-
Food and drugs administration
- (FFA):
-
Free fatty acid
- (GIP):
-
Gastro-inhibitory intestinal peptide
- (GI):
-
Gastrointestinal
- (GLP-1RA’s):
-
GLP-1 receptor agonists
- (GLP-1):
-
Glucagon-like peptide-1
- (HbA1c):
-
Glycosylated haemoglobin
- (GPCR):
-
G-protein-coupled receptor
- (HR):
-
Hazard ratio
- (IGT):
-
Impaired glucose tolerance
- (IP3):
-
Inositol 1,4,5-triphosphate
- (IR):
-
Insulin receptors
- (ICAM-1):
-
Intercellular cell adhesion molecule
- (IL-1):
-
Interleukin 1
- (ICAM-1):
-
Intracellular cell adhesion molecule
- (ICH):
-
Intracerebral haemorrhage
- (LV):
-
Left ventricular
- (LPS):
-
Lipopolysaccharide
- (LEAD):
-
Liraglutide effect and action on diabetes
- (LDL):
-
Low-density lipoprotein
- (MACE):
-
Major adverse cardiovascular events
- (MAP):
-
Mean arterial blood pressure
- (MCAO):
-
Middle cerebral artery occlusion
- (MCP-1):
-
Monocyte chemoattractant protein-1
- (MS):
-
Multiple Sclerosis
- (NEP 24.11):
-
Neutral endopeptidase 24.11
- (NPH):
-
Neutral protamine hagedorn
- (NO):
-
Nitric oxide
- (NAFLD):
-
Non-alcoholic fatty liver disease
- (NASH):
-
Non-alcoholic steatohepatitis
- (NF-κB):
-
Nuclear factor-kappa beta
- (NTS):
-
Nucleus tractus solitarii
- (OD):
-
Once in a day
- (ox-LDL):
-
Oxidized low-density lipoprotein
- (PD):
-
Parkinson's disease
- (PPBG):
-
Postprandial blood glucose
- (Lbs):
-
Pounds
- (PC):
-
Propeptide convertase
- (PKA):
-
Protein kinase A
- (PKC):
-
Protein kinase C
- (RCTs):
-
Randomized control trials
- (ROS):
-
Reactive oxygen species
- (RhGLP-1 RA’s):
-
Recombinant human GLP-1RAs
- (RYR):
-
Ryanodine receptors
- (SUSTAIN-6):
-
Semaglutide in subjects with type 2 diabetes
- (Sr.Cr):
-
Serum creatinine
- (SGLT2):
-
Sodium-glucose cotransporter 2
- (STZ):
-
Streptozotocin
- (SBP):
-
Systolic blood pressure
- (HOMA-beta):
-
The homeostasis model for β-cell function
- TGF-1:
-
Transforming growth factor-1
- (TGF-β1):
-
Transforming growth factor-beta 1
- (TBI):
-
Traumatic brain injury
- (TNF-α):
-
Tumor necrosis factor-alpha
- (T2DM):
-
Type-2 diabetes mellitus
- (UACR):
-
Urine albumin to creatinine ratio
- (VCAM-1):
-
Vascular cell adhesion molecule
- (VSMCs):
-
Vascular smooth muscle cells
- (VDCC):
-
Voltage-dependent calcium channel
- (WAT):
-
White adipose tissue
References
Suryasa IW, Rodríguez-Gámez M, Koldoris T (2021) Health and treatment of diabetes mellitus. Int J Health Sci. https://doi.org/10.53730/ijhs.v5n1.2864
Unnikrishnan R, Anjana RM, Mohan V (2016) Diabetes mellitus and its complications in India. Nat Rev Endocrinol 12(6):357–370. https://doi.org/10.1038/nrendo.2016.53
Gupta V (2013) Glucagon-like peptide-1 analogues: an overview. Indian J Endocrinol Metab 17(3):413–421. https://doi.org/10.4103/2230-8210.111625
Mao D, Cao H, Shi M, Wang CC, Kwong J, Li JJX et al (2021) Increased co-expression of PSMA2 and GLP-1 receptor in cervical cancer models in type 2 diabetes attenuated by exendin-4: a translational case-control study. EBioMedicine 65:103242. https://doi.org/10.1016/j.ebiom.2021.103242
Pauza AG, Thakkar P, Tasic T, Felippe I, Bishop P, Greenwood MP et al (2022) GLP1R attenuates sympathetic response to high glucose via carotid body inhibition. Circ Res 130(5):694–707. https://doi.org/10.1161/circresaha.121.319874
Thakkar P, Pauza AG, Murphy D, Paton JFR (2023) Carotid body: an emerging target for cardiometabolic co-morbidities. Exp Physiol 108(5):661–671. https://doi.org/10.1113/ep090090
Müller TD, Finan B, Bloom SR, D’Alessio D, Drucker DJ, Flatt PR et al (2019) Glucagon-like peptide 1 (GLP-1). Mol Metab 30:72–130. https://doi.org/10.1016/j.molmet.2019.09.010
Donnelly D (2012) The structure and function of the glucagon-like peptide-1 receptor and its ligands. Br J Pharmacol 166(1):27–41. https://doi.org/10.1111/j.1476-5381.2011.01687.x
Manandhar B, Ahn JM (2015) Glucagon-like peptide-1 (GLP-1) analogs: recent advances, new possibilities, and therapeutic implications. J Med Chem 58(3):1020–1037. https://doi.org/10.1021/jm500810s
Livesey G, Taylor R, Livesey HF, Buyken AE, Jenkins DJA, Augustin LSA et al (2019) Dietary glycemic index and load and the risk of type 2 diabetes: assessment of causal relations. Nutrients. https://doi.org/10.3390/nu11061436
Nauck MA, Quast DR, Wefers J, Meier JJ (2021) GLP-1 receptor agonists in the treatment of type 2 diabetes-state-of-the-art. Mol Metab 46:101102. https://doi.org/10.1016/j.molmet.2020.101102
Gallwitz B (2011) Glucagon-like peptide-1 analogues for type 2 diabetes mellitus: current and emerging agents. Drugs 71(13):1675–1688. https://doi.org/10.2165/11592810-000000000-00000
Maselli DB, Camilleri M (2021) Effects of GLP-1 and its analogs on gastric physiology in diabetes mellitus and obesity. Adv Exp Med Biol 1307:171–192. https://doi.org/10.1007/5584_2020_496
Zhao X, Wang M, Wen Z, Lu Z, Cui L, Fu C et al (2021) GLP-1 receptor agonists: beyond their pancreatic effects. Front Endocrinol (Lausanne) 12:721135. https://doi.org/10.3389/fendo.2021.721135
Rodbard HW, Jellinger PS, Davidson JA, Einhorn D, Garber AJ, Grunberger G et al (2009) Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 15(6):540–559. https://doi.org/10.4158/ep.15.6.540
Babu A, Mehta A, Guerrero P, Chen Z, Meyer PM, Koh CK et al (2009) Safe and simple emergency department discharge therapy for patients with type 2 diabetes mellitus and severe hyperglycemia. Endocr Pract 15(7):696–704. https://doi.org/10.4158/ep09117.Orr
Rolek B, Haber M, Gajewska M, Rogula S, Pietrasik A, Gąsecka A (2023) SGLT2 Inhibitors vs GLP-1 agonists to treat the heart, the kidneys and the brain. J Cardiovasc Dev Dis. https://doi.org/10.3390/jcdd10080322
Deng R, Mei K, Song T, Huang J, Wu Y, Yu P et al (2024) First-line treatment with sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide-1 receptor agonists in type 2 diabetic population at low risk of cardiovascular disease: a meta-analysis. Front Endocrinol (Lausanne) 15:1289643. https://doi.org/10.3389/fendo.2024.1289643
Cahn A, Cefalu WT (2016) Clinical considerations for use of initial combination therapy in type 2 diabetes. Diabetes Care 39:S137–S145. https://doi.org/10.2337/dcS15-3007
Pratley R, Amod A, Hoff ST, Kadowaki T, Lingvay I, Nauck M et al (2019) Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet 394(10192):39–50. https://doi.org/10.1016/s0140-6736(19)31271-1
MacDonald PE, Salapatek AM, Wheeler MB (2002) Glucagon-like peptide-1 receptor activation antagonizes voltage-dependent repolarizing K(+) currents in beta-cells: a possible glucose-dependent insulinotropic mechanism. Diabetes 51(Suppl 3):S443–S447. https://doi.org/10.2337/diabetes.51.2007.s443
Herzberg-Schäfer S, Heni M, Stefan N, Häring HU, Fritsche A (2012) Impairment of GLP1-induced insulin secretion: role of genetic background, insulin resistance and hyperglycaemia. Diabetes Obes Metab 14(Suppl 3):85–90. https://doi.org/10.1111/j.1463-1326.2012.01648.x
Doyle ME, Egan JM (2007) Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther 113(3):546–593. https://doi.org/10.1016/j.pharmthera.2006.11.007
Drucker DJ (2018) Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab 27(4):740–756. https://doi.org/10.1016/j.cmet.2018.03.001
Ja’arah D, Al Zoubi MS, Abdelhady G, Rabi F, Tambuwala MM (2021) Role of glucagon-like peptide-1 (GLP-1) receptor agonists in hypoglycemia. Clin Med Insights Endocrinol Diabetes. https://doi.org/10.1177/11795514211051697
Nadkarni P, Chepurny OG, Holz GG (2014) Regulation of glucose homeostasis by GLP-1. Prog Mol Biol Transl Sci 121:23–65. https://doi.org/10.1016/b978-0-12-800101-1.00002-8
Tong J, D’Alessio D (2014) Give the receptor a brake: slowing gastric emptying by GLP-1. Diabetes 63(2):407–409. https://doi.org/10.2337/db13-1764
Suganuma Y, Shimizu T, Sato T, Morii T, Fujita H, Harada Sassa M et al (2020) Magnitude of slowing gastric emptying by glucagon-like peptide-1 receptor agonists determines the amelioration of postprandial glucose excursion in Japanese patients with type 2 diabetes. J Diabetes Investig 11(2):389–399. https://doi.org/10.1111/jdi.13115
Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M et al (2009) Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 374(9701):1606–1616. https://doi.org/10.1016/s0140-6736(09)61375-1
Rizvi AA, Rizzo M (2022) The emerging role of dual GLP-1 and GIP receptor agonists in glycemic management and cardiovascular risk reduction. Diabetes Metab Syndr Obes 15:1023–1030. https://doi.org/10.2147/dmso.S351982
Idevall-Hagren O, Jakobsson I, Xu Y, Tengholm A (2013) Spatial control of Epac2 activity by cAMP and Ca2+-mediated activation of Ras in pancreatic β cells. Sci Signal. https://doi.org/10.1126/scisignal.2003932
Yosida M, Dezaki K, Uchida K, Kodera S, Lam NV, Ito K et al (2014) Involvement of cAMP/EPAC/TRPM2 activation in glucose- and incretin-induced insulin secretion. Diabetes 63(10):3394–3403. https://doi.org/10.2337/db13-1868
Mayendraraj A, Rosenkilde MM, Gasbjerg LS (2022) GLP-1 and GIP receptor signaling in beta cells—a review of receptor interactions and co-stimulation. Peptides 151:170749. https://doi.org/10.1016/j.peptides.2022.170749
Kalra S, Das AK, Sahay RK, Baruah MP, Tiwaskar M, Das S et al (2019) Consensus recommendations on GLP-1 RA use in the management of type 2 diabetes mellitus: South Asian task force. Diabetes Ther 10(5):1645–1717. https://doi.org/10.1007/s13300-019-0669-4
Bhavsar S, Mudaliar S, Cherrington A (2013) Evolution of exenatide as a diabetes therapeutic. Curr Diabetes Rev 9(2):161–193. https://doi.org/10.2174/1573399811309020007
Jose B, Tahrani AA, Piya MK, Barnett AH (2010) Exenatide once weekly: clinical outcomes and patient satisfaction. Patient Prefer Adherence 4:313–324. https://doi.org/10.2147/ppa.s7494
Van Gaal L, Souhami E, Zhou T, Aronson R (2014) Efficacy and safety of the glucagon-like peptide-1 receptor agonist lixisenatide versus the dipeptidyl peptidase-4 inhibitor sitagliptin in young (< 50 years) obese patients with type 2 diabetes mellitus. J Clin Transl Endocrinol 1(2):31–37. https://doi.org/10.1016/j.jcte.2014.03.001
Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G (2017) Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter. Open-Label Randomized Controlled Trial Diabetes Ther 8(1):55–73. https://doi.org/10.1007/s13300-016-0223-6
Lv X, Dong Y, Hu L, Lu F, Zhou C, Qin S (2020) Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) for the management of nonalcoholic fatty liver disease (NAFLD): a systematic review. Endocrinol Diabetes Metab 3(3):e00163. https://doi.org/10.1002/edm2.163
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA et al (2016) Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 375(4):311–322. https://doi.org/10.1056/NEJMoa1603827
Shi L, Ji Y, Jiang X, Zhou L, Xu Y, Li Y et al (2015) Liraglutide attenuates high glucose-induced abnormal cell migration, proliferation, and apoptosis of vascular smooth muscle cells by activating the GLP-1 receptor, and inhibiting ERK1/2 and PI3K/Akt signaling pathways. Cardiovasc Diabetol 14:18. https://doi.org/10.1186/s12933-015-0177-4
Lau J, Bloch P, Schäffer L, Pettersson I, Spetzler J, Kofoed J et al (2015) Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J Med Chem 58(18):7370–7380. https://doi.org/10.1021/acs.jmedchem.5b00726
Sorli C, Harashima SI, Tsoukas GM, Unger J, Karsbøl JD, Hansen T et al (2017) Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol 5(4):251–260. https://doi.org/10.1016/s2213-8587(17)30013-x
St Onge EL, Miller SA (2010) Albiglutide: a new GLP-1 analog for the treatment of type 2 diabetes. Expert Opin Biol Ther 10(5):801–806. https://doi.org/10.1517/14712598.2010.481281
Sharma D, Verma S, Vaidya S, Kalia K, Tiwari V (2018) Recent updates on GLP-1 agonists: current advancements & challenges. Biomed Pharmacother 108:952–962. https://doi.org/10.1016/j.biopha.2018.08.088
Barrington P, Chien JY, Showalter HD, Schneck K, Cui S, Tibaldi F et al (2011) A 5-week study of the pharmacokinetics and pharmacodynamics of LY2189265, a novel, long-acting glucagon-like peptide-1 analogue, in patients with type 2 diabetes. Diabetes Obes Metab 13(5):426–433. https://doi.org/10.1111/j.1463-1326.2011.01364.x
Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z (2014) Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care 37(8):2149–2158. https://doi.org/10.2337/dc13-2761
Wysham C, Blevins T, Arakaki R, Colon G, Garcia P, Atisso C et al (2014) Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care 37(8):2159–2167. https://doi.org/10.2337/dc13-2760
Lafferty RA, Flatt PR, Irwin N (2023) GLP-1/GIP analogs: potential impact in the landscape of obesity pharmacotherapy. Expert Opin Pharmacother 24(5):587–597. https://doi.org/10.1080/14656566.2023.2192865
Li M, Jeeyavudeen MS, Arunagirinathan G, Pappachan J (2023) Is type 2 diabetes mellitus a behavioural disorder? An evidence review for type 2 diabetes mellitus prevention and remission through lifestyle modification. touchREV Endocrinol 19(1):7–15. https://doi.org/10.17925/ee.2023.19.1.7
Vilsbøll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, Courrèges JP et al (2007) Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care 30(6):1608–1610. https://doi.org/10.2337/dc06-2593
Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL (2012) Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 344:d7771. https://doi.org/10.1136/bmj.d7771
Zhang F, Tong Y, Su N, Li Y, Tang L, Huang L et al (2015) Weight loss effect of glucagon-like peptide-1 mimetics on obese/overweight adults without diabetes: a systematic review and meta-analysis of randomized controlled trials. J Diabetes 7(3):329–339. https://doi.org/10.1111/1753-0407.12198
Ryan D, Acosta A (2015) GLP-1 receptor agonists: nonglycemic clinical effects in weight loss and beyond. Obesity (Silver Spring) 23(6):1119–1129. https://doi.org/10.1002/oby.21107
Deol H, Lekkakou L, Viswanath AK, Pappachan JM (2017) Combination therapy with GLP-1 analogues and SGLT-2 inhibitors in the management of diabesity: the real world experience. Endocrine 55(1):173–178. https://doi.org/10.1007/s12020-016-1125-0
Inagaki N, Takeuchi M, Oura T, Imaoka T, Seino Y (2022) Efficacy and safety of tirzepatide monotherapy compared with dulaglutide in Japanese patients with type 2 diabetes (SURPASS J-mono): a double-blind, multicentre, randomised, phase 3 trial. Lancet Diabetes Endocrinol 10(9):623–633. https://doi.org/10.1016/s2213-8587(22)00188-7
Strandwitz P (2018) Neurotransmitter modulation by the gut microbiota. Brain Res 1693:128–133. https://doi.org/10.1016/j.brainres.2018.03.015
Xu F, Lin B, Zheng X, Chen Z, Cao H, Xu H et al (2016) GLP-1 receptor agonist promotes brown remodelling in mouse white adipose tissue through SIRT1. Diabetologia 59:1059–1069
Nowak C, Lind M, Sumnik Z, Pelikanova T, Nattero-Chavez L, Lundberg E et al (2022) Intralymphatic GAD-Alum (Diamyd®) improves glycemic control in Type 1 diabetes with HLA DR3-DQ2. J Clin Endocrinol Metab 107(9):2644–2651. https://doi.org/10.1210/clinem/dgac343
Pencek R, Blickensderfer A, Li Y, Brunell SC, Anderson PW (2012) Exenatide twice daily: analysis of effectiveness and safety data stratified by age, sex, race, duration of diabetes, and body mass index. Postgrad Med 124(4):21–32. https://doi.org/10.3810/pgm.2012.07.2567
Seijas-Amigo J, Salgado-Barreira Á, Castelo-Domínguez R, Pereira-Pía M, Rodríguez-Mañero M, González-Juanatey JR (2022) Semaglutide versus GLP-1 agonists. Effectiveness, safety, and quality of life in patients with diabetes mellitus 2. The SEVERAL study. Farm Hosp 46(6):372–379
Balena R, Hensley IE, Miller S, Barnett AH (2013) Combination therapy with GLP-1 receptor agonists and basal insulin: a systematic review of the literature. Diabetes Obes Metab 15(6):485–502. https://doi.org/10.1111/dom.12025
Camilleri M, Acosta A (2018) Combination therapies for obesity. Metab Syndr Relat Disord 16(8):390–394. https://doi.org/10.1089/met.2018.0075
Del Olmo-Garcia MI, Merino-Torres JF (2018) GLP-1 receptor agonists and cardiovascular disease in patients with type 2 diabetes. J Diabetes Res 2018:4020492. https://doi.org/10.1155/2018/4020492
van Ruiten CC, Veltman DJ, Schrantee A, van Bloemendaal L, Barkhof F, Kramer MHH et al (2022) Effects of dapagliflozin and combination therapy with exenatide on food-cue induced brain activation in patients With type 2 diabetes. J Clin Endocrinol Metab 107(6):e2590–e2599. https://doi.org/10.1210/clinem/dgac043
Apovian CM, Bergenstal RM, Cuddihy RM, Qu Y, Lenox S, Lewis MS et al (2010) Effects of exenatide combined with lifestyle modification in patients with type 2 diabetes. Am J Med 123(5):468.e9–17. https://doi.org/10.1016/j.amjmed.2009.11.019
Seijas-Amigo J, Salgado-Barreira Á, Castelo-Dominguez R, Pérez-Álvarez MT, Ponce-Piñón B, Fernández-Silva M et al (2023) Differences in weight loss and safety between the glucagon-like peptide-1 receptor agonists: a non-randomized multicenter study from the titration phase. Prim Care Diabetes 17(4):366–372. https://doi.org/10.1016/j.pcd.2023.05.004
Wadden TA, Chao AM, Machineni S, Kushner R, Ard J, Srivastava G et al (2023) Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: the SURMOUNT-3 phase 3 trial. Nat Med 29(11):2909–2918. https://doi.org/10.1038/s41591-023-02597-w
Aronne LJ, Sattar N, Horn DB, Bays HE, Wharton S, Lin WY et al (2024) Continued treatment with tirzepatide for maintenance of weight reduction in adults with obesity: the SURMOUNT-4 randomized clinical trial. JAMA 331(1):38–48. https://doi.org/10.1001/jama.2023.24945
Collins L, Costello RA. (2023) Glucagon-like peptide-1 receptor agonists. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Ryan Costello declares no relevant financial relationships with ineligible companies.: StatPearls Publishing. Copyright © 2023, StatPearls Publishing LLC.
Stenberg E, Näslund E (2023) Major adverse cardiovascular events among patients with type-2 diabetes, a nationwide cohort study comparing primary metabolic and bariatric surgery to GLP-1 receptor agonist treatment. Int J Obes (Lond) 47(4):251–256. https://doi.org/10.1038/s41366-023-01254-z
Leon BM, Maddox TM (2015) Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes 6(13):1246–1258. https://doi.org/10.4239/wjd.v6.i13.1246
Ma X, Liu Z, Ilyas I, Little PJ, Kamato D, Sahebka A et al (2021) GLP-1 receptor agonists (GLP-1RAs): cardiovascular actions and therapeutic potential. Int J Biol Sci 17(8):2050–2068. https://doi.org/10.7150/ijbs.59965
Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ (2017) Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation 136(9):849–870. https://doi.org/10.1161/circulationaha.117.028136
Marx N, Husain M, Lehrke M, Verma S, Sattar N (2022) GLP-1 receptor agonists for the reduction of atherosclerotic cardiovascular risk in patients with type 2 diabetes. Circulation 146(24):1882–1894. https://doi.org/10.1161/circulationaha.122.059595
Nauck MA, Quast DR (2021) Cardiovascular safety and benefits of semaglutide in patients with type 2 diabetes: findings from SUSTAIN 6 and PIONEER 6. Front Endocrinol (Lausanne) 12:645566. https://doi.org/10.3389/fendo.2021.645566
Schnell O, Rydén L, Standl E, Ceriello A (2016) Current perspectives on cardiovascular outcome trials in diabetes. Cardiovasc Diabetol 15(1):139. https://doi.org/10.1186/s12933-016-0456-8
Verma S, Bain SC, Monk Fries T, Mazer CD, Nauck MA, Pratley RE et al (2019) Duration of diabetes and cardiorenal efficacy of liraglutide and semaglutide: a post hoc analysis of the LEADER and SUSTAIN 6 clinical trials. Diabetes Obes Metab 21(7):1745–1751. https://doi.org/10.1111/dom.13698
Verma S, Poulter NR, Bhatt DL, Bain SC, Buse JB, Leiter LA et al (2018) Effects of liraglutide on cardiovascular outcomes in patients with type 2 diabetes mellitus with or without history of myocardial infarction or stroke. Circulation 138(25):2884–2894. https://doi.org/10.1161/circulationaha.118.034516
Røder ME (2018) Major adverse cardiovascular event reduction with GLP-1 and SGLT2 agents: evidence and clinical potential. Ther Adv Chronic Dis 9(1):33–50. https://doi.org/10.1177/2040622317735283
Hinton W, Feher M, Munro N, Walker M, de Lusignan S (2019) Real-world prevalence of the inclusion criteria for the LEADER trial: data from a national general practice network. Diabetes Obes Metab 21(7):1661–1667. https://doi.org/10.1111/dom.13710
McLean BA, Wong CK, Campbell JE, Hodson DJ, Trapp S, Drucker DJ (2021) Revisiting the complexity of GLP-1 action from sites of synthesis to receptor activation. Endocr Rev 42(2):101–132. https://doi.org/10.1210/endrev/bnaa032
McGuire DK, Busui RP, Deanfield J, Inzucchi SE, Mann JFE, Marx N et al (2023) Effects of oral semaglutide on cardiovascular outcomes in individuals with type 2 diabetes and established atherosclerotic cardiovascular disease and/or chronic kidney disease: design and baseline characteristics of SOUL, a randomized trial. Diabetes Obes Metab 25(7):1932–1941. https://doi.org/10.1111/dom.15058
Honigberg MC, Chang LS, McGuire DK, Plutzky J, Aroda VR, Vaduganathan M (2020) Use of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes and cardiovascular disease: a review. JAMA Cardiol 5(10):1182–1190. https://doi.org/10.1001/jamacardio.2020.1966
Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M et al (2008) Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358(24):2560–2572. https://doi.org/10.1056/NEJMoa0802987
Okerson T, Chilton RJ (2012) The cardiovascular effects of GLP-1 receptor agonists. Cardiovasc Ther 30(3):e146–e155. https://doi.org/10.1111/j.1755-5922.2010.00256.x
Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS (2023) Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res 118(17):3272–3287. https://doi.org/10.1093/cvr/cvac013
Patel VJ, Joharapurkar AA, Shah GB, Jain MR (2014) Effect of GLP-1 based therapies on diabetic dyslipidemia. Curr Diabetes Rev 10(4):238–250. https://doi.org/10.2174/1573399810666140707092506
Ma Z, Cai M, Yang K, Liu J, Guo T, Liu X et al (2023) Predicting the risk of autoimmune thyroid disease in patients with vitiligo: development and assessment of a new predictive nomogram. Front Endocrinol (Lausanne) 14:1109925. https://doi.org/10.3389/fendo.2023.1109925
Liao MT, Sung CC, Hung KC, Wu CC, Lo L, Lu KC (2012) Insulin resistance in patients with chronic kidney disease. J Biomed Biotechnol 2012:691369. https://doi.org/10.1155/2012/691369
Rizzo M, Nikolic D, Patti AM, Mannina C, Montalto G, McAdams BS et al (2018) GLP-1 receptor agonists and reduction of cardiometabolic risk: potential underlying mechanisms. Biochim Biophys Acta Mol Basis Dis 1864:2814–2821. https://doi.org/10.1016/j.bbadis.2018.05.012
Tashiro Y, Sato K, Watanabe T, Nohtomi K, Terasaki M, Nagashima M et al (2014) A glucagon-like peptide-1 analog liraglutide suppresses macrophage foam cell formation and atherosclerosis. Peptides 54:19–26. https://doi.org/10.1016/j.peptides.2013.12.015
Arakawa M, Mita T, Azuma K, Ebato C, Goto H, Nomiyama T et al (2010) Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes 59(4):1030–1037. https://doi.org/10.2337/db09-1694
Rakipovski G, Rolin B, Nøhr J, Klewe I, Frederiksen KS, Augustin R et al (2018) The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE(-/-) and LDLr(-/-) mice by a mechanism that includes inflammatory pathways. JACC Basic Transl Sci 3(6):844–857. https://doi.org/10.1016/j.jacbts.2018.09.004
Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME et al (2002) Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest 110(1):43–52. https://doi.org/10.1172/jci15595
Liu Q, Anderson C, Broyde A, Polizzi C, Fernandez R, Baron A et al (2010) Glucagon-like peptide-1 and the exenatide analogue AC3174 improve cardiac function, cardiac remodeling, and survival in rats with chronic heart failure. Cardiovasc Diabetol 9:76. https://doi.org/10.1186/1475-2840-9-76
Patti AM, Giglio RV, Allotta A, Bruno A, Di Bella T, Pantea Stoian A et al (2023) Effect of semaglutide on subclinical atherosclerosis and cardiometabolic compensation: a real-world study in patients with type 2 diabetes. Biomedicines. https://doi.org/10.3390/biomedicines11051362
Hamal S, Cherukuri L, Shaikh K, Kinninger A, Doshi J, Birudaraju D et al (2020) Effect of semaglutide on coronary atherosclerosis progression in patients with type II diabetes: rationale and design of the semaglutide treatment on coronary progression trial. Coron Artery Dis 31(3):306–314. https://doi.org/10.1097/mca.0000000000000830
Sun L, Yuan Y, Li Y, Rao X (2023) Effect of liraglutide on atherosclerosis in patients with impaired glucose tolerance: a double-blind, randomized controlled clinical trial. Exp Ther Med 25(6):249. https://doi.org/10.3892/etm.2023.11948
Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P et al (2019) Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394(10193):121–130. https://doi.org/10.1016/s0140-6736(19)31149-3
Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR et al (2019) Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 381(9):841–851. https://doi.org/10.1056/NEJMoa1901118
Godoy-Matos AF, Silva Júnior WS, Valerio CM (2020) NAFLD as a continuum: from obesity to metabolic syndrome and diabetes. Diabetol Metab Syndr 12:60. https://doi.org/10.1186/s13098-020-00570-y
Cariou B, Byrne CD, Loomba R, Sanyal AJ (2021) Nonalcoholic fatty liver disease as a metabolic disease in humans: a literature review. Diabetes Obes Metab 23(5):1069–1083. https://doi.org/10.1111/dom.14322
Seghieri M, Christensen AS, Andersen A, Solini A, Knop FK, Vilsbøll T (2018) Future perspectives on GLP-1 receptor agonists and GLP-1/glucagon receptor co-agonists in the treatment of NAFLD. Front Endocrinol (Lausanne) 9:649. https://doi.org/10.3389/fendo.2018.00649
Mantovani A, Byrne CD, Bonora E, Targher G (2018) Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: a meta-analysis. Diabetes Care 41(2):372–382. https://doi.org/10.2337/dc17-1902
He Q, Sha S, Sun L, Zhang J, Dong M (2016) GLP-1 analogue improves hepatic lipid accumulation by inducing autophagy via AMPK/mTOR pathway. Biochem Biophys Res Commun 476(4):196–203. https://doi.org/10.1016/j.bbrc.2016.05.086
Liu J, Wang G, Jia Y, Xu Y (2015) GLP-1 receptor agonists: effects on the progression of non-alcoholic fatty liver disease. Diabetes/Metab Res Rev 31(4):329–335
Liu J, Wang G, Jia Y, Xu Y (2015) GLP-1 receptor agonists: effects on the progression of non-alcoholic fatty liver disease. Diabetes Metab Res Rev 31(4):329–335. https://doi.org/10.1002/dmrr.2580
Ding X, Saxena NK, Lin S, Gupta NA, Anania FA (2006) Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology 43(1):173–181. https://doi.org/10.1002/hep.21006
Cuthbertson DJ, Irwin A, Gardner CJ, Daousi C, Purewal T, Furlong N et al (2012) Improved glycaemia correlates with liver fat reduction in obese, type 2 diabetes, patients given glucagon-like peptide-1 (GLP-1) receptor agonists. PLoS ONE 7(12):e50117. https://doi.org/10.1371/journal.pone.0050117
Chen Z, Yu R, Xiong Y, Du F, Zhu S (2017) A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis 16(1):203. https://doi.org/10.1186/s12944-017-0572-9
Kim ER, Park JS, Kim JH, Oh JY, Oh IJ, Choi DH et al (2022) A GLP-1/GLP-2 receptor dual agonist to treat NASH: targeting the gut-liver axis and microbiome. Hepatology 75(6):1523–1538. https://doi.org/10.1002/hep.32235
Rizzo M, Nikolic D, Patti AM, Mannina C, Montalto G, McAdams BS et al (2018) GLP-1 receptor agonists and reduction of cardiometabolic risk: potential underlying mechanisms. Biochimica et Biophysica Acta-Mol Basis Dis 1864(9):2814–2821
Gu Y, Sun L, He Y, Yang L, Deng C, Zhou R et al (2023) Comparative efficacy of glucagon-like peptide 1 (GLP-1) receptor agonists, pioglitazone and vitamin E for liver histology among patients with nonalcoholic fatty liver disease: systematic review and pilot network meta-analysis of randomized controlled trials. Expert Rev Gastroenterol Hepatol 17(3):273–282. https://doi.org/10.1080/17474124.2023.2172397
Roeb E, Weiskirchen R (2021) Fructose and non-alcoholic steatohepatitis. Front Pharmacol 12:634344. https://doi.org/10.3389/fphar.2021.634344
Nevola R, Epifani R, Imbriani S, Tortorella G, Aprea C, Galiero R et al (2023) GLP-1 receptor agonists in non-alcoholic fatty liver disease: current evidence and future perspectives. Int J Mol Sci. https://doi.org/10.3390/ijms24021703
Kovesdy CP (2022) Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011). 12(1):7–11. https://doi.org/10.1016/j.kisu.2021.11.003
Gheith O, Farouk N, Nampoory N, Halim MA, Al-Otaibi T (2016) Diabetic kidney disease: world wide difference of prevalence and risk factors. J Nephropharmacol 5(1):49–56
Kodera R, Shikata K, Kataoka HU, Takatsuka T, Miyamoto S, Sasaki M et al (2011) Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia 54(4):965–978. https://doi.org/10.1007/s00125-010-2028-x
Chen YT, Tsai TH, Yang CC, Sun CK, Chang LT, Chen HH et al (2013) Exendin-4 and sitagliptin protect kidney from ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med 11:270. https://doi.org/10.1186/1479-5876-11-270
Choi JH, Kim SJ, Kwon SK, Kim HY, Jeon H (2019) Renal Tubular Glucagon-Like Peptide-1 Receptor Expression Is Increased in Early Sepsis but Reduced in Chronic Kidney Disease and Sepsis-Induced Kidney Injury. Int J Mol Sci. https://doi.org/10.3390/ijms20236024
Docherty NG, le Roux CW (2014) Improvements in the metabolic milieu following Roux-en-Y gastric bypass and the arrest of diabetic kidney disease. Exp Physiol 99(9):1146–1153. https://doi.org/10.1113/expphysiol.2014.078790
Yang S, Lin C, Zhuo X, Wang J, Rao S, Xu W et al (2020) Glucagon-like peptide-1 alleviates diabetic kidney disease through activation of autophagy by regulating AMP-activated protein kinase-mammalian target of rapamycin pathway. Am J Physiol Endocrinol Metab 319(6):E1019–E1030. https://doi.org/10.1152/ajpendo.00195.2019
Yin W, Jiang Y, Xu S, Wang Z, Peng L, Fang Q et al (2019) Protein kinase C and protein kinase A are involved in the protection of recombinant human glucagon-like peptide-1 on glomeruli and tubules in diabetic rats. J Diabetes Investig 10(3):613–625. https://doi.org/10.1111/jdi.12956
Yu JH, Park SY, Lee DY, Kim NH, Seo JA (2022) GLP-1 receptor agonists in diabetic kidney disease: current evidence and future directions. Kidney Res Clin Pract 41(2):136–149. https://doi.org/10.23876/j.krcp.22.001
Mosterd CM, Bjornstad P, van Raalte DH (2020) Nephroprotective effects of GLP-1 receptor agonists: where do we stand? J Nephrol 33(5):965–975. https://doi.org/10.1007/s40620-020-00738-9
Yin W, Xu S, Wang Z, Liu H, Peng L, Fang Q et al (2018) Recombinant human GLP-1(rhGLP-1) alleviating renal tubulointestitial injury in diabetic STZ-induced rats. Biochem Biophys Res Commun 495(1):793–800. https://doi.org/10.1016/j.bbrc.2017.11.076
Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB et al (2020) Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. https://doi.org/10.3390/ijms21176275
Hills CE, Squires PE (2011) The role of TGF-β and epithelial-to mesenchymal transition in diabetic nephropathy. Cytokine Growth Factor Rev 22(3):131–139. https://doi.org/10.1016/j.cytogfr.2011.06.002
Ninčević V, Omanović Kolarić T, Roguljić H, Kizivat T, Smolić M, Bilić ĆI (2019) Renal benefits of SGLT 2 inhibitors and GLP-1 receptor agonists: evidence supporting a paradigm shift in the medical management of type 2 diabetes. Int J Mol Sci. https://doi.org/10.3390/ijms20235831
Granata A, Maccarrone R, Anzaldi M, Leonardi G, Pesce F, Amico F et al (2022) GLP-1 receptor agonists and renal outcomes in patients with diabetes mellitus type 2 and diabetic kidney disease: state of the art. Clin Kidney J 15(9):1657–1665. https://doi.org/10.1093/ckj/sfac069
Aviles Bueno B, Soler MJ, Perez-Belmonte L, Jimenez Millan A, Rivas Ruiz F, Garcia de Lucas MD (2022) Semaglutide in type 2 diabetes with chronic kidney disease at high risk progression-real-world clinical practice. Clin Kidney J 15(8):1593–1600. https://doi.org/10.1093/ckj/sfac096
Idorn T, Knop FK, Jørgensen MB, Jensen T, Resuli M, Hansen PM et al (2016) Safety and efficacy of liraglutide in patients with type 2 diabetes and end-stage renal disease: an investigator-initiated, placebo-controlled, double-blind, parallel-group. Randomized Trial Diabetes Care 39(2):206–213. https://doi.org/10.2337/dc15-1025
Idorn T, Knop FK, Jørgensen M, Jensen T, Resuli M, Hansen PM et al (2013) Safety and efficacy of liraglutide in patients with type 2 diabetes and end-stage renal disease: protocol for an investigator-initiated prospective, randomised, placebo-controlled, double-blinded, parallel intervention study. BMJ Open. https://doi.org/10.1136/bmjopen-2013-002764
Tuttle KR, Lakshmanan MC, Rayner B, Busch RS, Zimmermann AG, Woodward DB et al (2018) Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol 6(8):605–617. https://doi.org/10.1016/s2213-8587(18)30104-9
Tuttle KR, McKinney TD, Davidson JA, Anglin G, Harper KD, Botros FT (2017) Effects of once-weekly dulaglutide on kidney function in patients with type 2 diabetes in phase II and III clinical trials. Diabetes Obes Metab 19(3):436–441. https://doi.org/10.1111/dom.12816
Mann JFE, Fonseca VA, Poulter NR, Raz I, Idorn T, Rasmussen S et al (2020) Safety of liraglutide in type 2 diabetes and chronic kidney disease. Clin J Am Soc Nephrol 15(4):465–473. https://doi.org/10.2215/cjn.11881019
Hölscher C (2012) Potential role of glucagon-like peptide-1 (GLP-1) in neuroprotection. CNS Drugs 26(10):871–882. https://doi.org/10.2165/11635890-000000000-00000
Salcedo I, Tweedie D, Li Y, Greig NH (2012) Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br J Pharmacol 166(5):1586–1599. https://doi.org/10.1111/j.1476-5381.2012.01971.x
Hölscher C (2019) Insulin signaling impairment in the brain as a risk factor in Alzheimer’s disease. Front Aging Neurosci 11:88. https://doi.org/10.3389/fnagi.2019.00088
Eren-Yazicioglu CY, Yigit A, Dogruoz RE, Yapici-Eser H (2020) Can GLP-1 be a target for reward system related disorders? A qualitative synthesis and systematic review analysis of studies on palatable food, drugs of abuse, and alcohol. Front Behav Neurosci 14:614884. https://doi.org/10.3389/fnbeh.2020.614884
Du H, Meng X, Yao Y, Xu J (2022) The mechanism and efficacy of GLP-1 receptor agonists in the treatment of Alzheimer’s disease. Front Endocrinol (Lausanne) 13:1033479. https://doi.org/10.3389/fendo.2022.1033479
Athauda D, Foltynie T (2016) The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: mechanisms of action. Drug Discov Today 21(5):802–818. https://doi.org/10.1016/j.drudis.2016.01.013
Wang XH, Li L, Hölscher C, Pan YF, Chen XR, Qi JS (2010) Val8-glucagon-like peptide-1 protects against Aβ1-40-induced impairment of hippocampal late-phase long-term potentiation and spatial learning in rats. Neuroscience 170(4):1239–1248. https://doi.org/10.1016/j.neuroscience.2010.08.028
Hunter K, Hölscher C (2012) Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci 13:33. https://doi.org/10.1186/1471-2202-13-33
Li QX, Gao H, Guo YX, Wang BY, Hua RX, Gao L et al (2021) GLP-1 and underlying beneficial actions in Alzheimer’s disease, hypertension, and NASH. Front Endocrinol (Lausanne) 12:721198. https://doi.org/10.3389/fendo.2021.721198
Pathak NM, Pathak V, Gault VA, McClean S, Irwin N, Flatt PR (2018) Novel dual incretin agonist peptide with antidiabetic and neuroprotective potential. Biochem Pharmacol 155:264–274. https://doi.org/10.1016/j.bcp.2018.07.021
Cui QN, Stein LM, Fortin SM, Hayes MR (2022) The role of glia in the physiology and pharmacology of glucagon-like peptide-1: implications for obesity, diabetes, neurodegeneration and glaucoma. Br J Pharmacol 179(4):715–726. https://doi.org/10.1111/bph.15683
Kim S, Moon M, Park S (2009) Exendin-4 protects dopaminergic neurons by inhibition of microglial activation and matrix metalloproteinase-3 expression in an animal model of Parkinson’s disease. J Endocrinol 202(3):431–439. https://doi.org/10.1677/joe-09-0132
Meredith GE, Rademacher DJ (2011) MPTP mouse models of Parkinson’s disease: an update. J Parkinsons Dis 1(1):19–33. https://doi.org/10.3233/jpd-2011-11023
Longo M, Di Meo I, Caruso P, Muscio MF, Scappaticcio L, Maio A et al (2023) Circulating levels of endothelial progenitor cells are associated with better cognitive function in older adults with glucagon-like peptide 1 receptor agonist-treated type 2 diabetes. Diabetes Res Clin Pract 200:110688. https://doi.org/10.1016/j.diabres.2023.110688
Reich N, Hölscher C (2022) The neuroprotective effects of glucagon-like peptide 1 in Alzheimer’s and Parkinson’s disease: an in-depth review. Front Neurosci 16:970925. https://doi.org/10.3389/fnins.2022.970925
Yang X, Qiang Q, Li N, Feng P, Wei W, Hölscher C (2022) Neuroprotective mechanisms of glucagon-like peptide-1-based therapies in ischemic stroke: an update based on preclinical research. Front Neurol 13:844697. https://doi.org/10.3389/fneur.2022.844697
Zhang H, Liu Y, Guan S, Qu D, Wang L, Wang X et al (2016) An orally active allosteric GLP-1 receptor agonist is neuroprotective in cellular and rodent models of stroke. PLoS ONE 11(2):e0148827. https://doi.org/10.1371/journal.pone.0148827
Parthsarathy V, Hölscher C (2013) The type 2 diabetes drug liraglutide reduces chronic inflammation induced by irradiation in the mouse brain. Eur J Pharmacol 700(1–3):42–50. https://doi.org/10.1016/j.ejphar.2012.12.012
Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW et al (2009) GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A 106(4):1285–1290. https://doi.org/10.1073/pnas.0806720106
Nizari S, Basalay M, Chapman P, Korte N, Korsak A, Christie IN et al (2021) Glucagon-like peptide-1 (GLP-1) receptor activation dilates cerebral arterioles, increases cerebral blood flow, and mediates remote (pre)conditioning neuroprotection against ischaemic stroke. Basic Res Cardiol 116(1):32. https://doi.org/10.1007/s00395-021-00873-9
Zhang L, Zhang W, Tian X (2023) The pleiotropic of GLP-1/GLP-1R axis in central nervous system diseases. Int J Neurosci 133(5):473–491. https://doi.org/10.1080/00207454.2021.1924707
Hölscher C (2014) Central effects of GLP-1: new opportunities for treatments of neurodegenerative diseases. J Endocrinol 221(1):T31-41. https://doi.org/10.1530/joe-13-0221
Yuan Z, Li D, Feng P, Xue G, Ji C, Li G et al (2017) A novel GLP-1/GIP dual agonist is more effective than liraglutide in reducing inflammation and enhancing GDNF release in the MPTP mouse model of Parkinson’s disease. Eur J Pharmacol 812:82–90. https://doi.org/10.1016/j.ejphar.2017.06.029
Briyal S, Shah S, Gulati A (2014) Neuroprotective and anti-apoptotic effects of liraglutide in the rat brain following focal cerebral ischemia. Neuroscience 281:269–281. https://doi.org/10.1016/j.neuroscience.2014.09.064
Zhang L, Zhang L, Li L, Hölscher C (2018) Neuroprotective effects of the novel GLP-1 long acting analogue semaglutide in the MPTP Parkinson’s disease mouse model. Neuropeptides 71:70–80. https://doi.org/10.1016/j.npep.2018.07.003
Darsalia V, Nathanson D, Nyström T, Klein T, Sjöholm Å, Patrone C (2014) GLP-1R activation for the treatment of stroke: updating and future perspectives. Rev Endocr Metab Disord 15(3):233–242. https://doi.org/10.1007/s11154-014-9285-9
Victorino DB, Nejm M, Guimarães-Marques M, Scorza FA, Scorza CA (2021) Repurposing GLP-1 receptor agonists for Parkinson’s disease: current evidence and future opportunities. Pharmaceut Med 35(1):11–19. https://doi.org/10.1007/s40290-020-00374-5
Wharton S, Davies M, Dicker D, Lingvay I, Mosenzon O, Rubino DM et al (2022) Managing the gastrointestinal side effects of GLP-1 receptor agonists in obesity: recommendations for clinical practice. Postgrad Med 134(1):14–19. https://doi.org/10.1080/00325481.2021.2002616
Nauck MA, Kemmeries G, Holst JJ, Meier JJ (2011) Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes 60(5):1561–1565. https://doi.org/10.2337/db10-0474
Holst JJ, Andersen DB, Grunddal KV (2022) Actions of glucagon-like peptide-1 receptor ligands in the gut. Br J Pharmacol 179(4):727–742. https://doi.org/10.1111/bph.15611
Filippatos TD, Panagiotopoulou TV, Elisaf MS (2014) Adverse effects of GLP-1 receptor agonists. Rev Diabet Stud 11(3–4):202–230. https://doi.org/10.1900/rds.2014.11.202
Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D et al (2008) Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 372(9645):1240–1250. https://doi.org/10.1016/s0140-6736(08)61206-4
Aroda VR, Ratner R (2011) The safety and tolerability of GLP-1 receptor agonists in the treatment of type 2 diabetes: a review. Diabetes Metab Res Rev 27(6):528–542. https://doi.org/10.1002/dmrr.1202
Jespersen MJ, Knop FK, Christensen M (2013) GLP-1 agonists for type 2 diabetes: pharmacokinetic and toxicological considerations. Expert Opin Drug Metab Toxicol 9(1):17–29. https://doi.org/10.1517/17425255.2013.731394
Fineman MS, Shen LZ, Taylor K, Kim DD, Baron AD (2004) Effectiveness of progressive dose-escalation of exenatide (exendin-4) in reducing dose-limiting side effects in subjects with type 2 diabetes. Diabetes Metab Res Rev 20(5):411–417. https://doi.org/10.1002/dmrr.499
Acknowledgement
Dr. Prajapati, extends his sincere appreciation to the Faculty of Pharmacy, Silpakorn University, Thailand, for their generous financial support that enabled the completion of this work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
PT initiated the topic conception and design of the index of this paper. PT and BGP provided constructive feedback, some new ideas edited the text, and approved the final version of the manuscript. MRC, BPD, IVS, NNS, and SUB drafted and subsequently revised the review article with significant literature data collection, and figures. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest that could have influenced the submitted work.
Ethical approval
Not applicable.
Consent to participant
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The e-mail addresses of both corresponding authors are swapped.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dave, B.P., Chorawala, M.R., Shah, I.V. et al. From diabetes to diverse domains: the multifaceted roles of GLP-1 receptor agonists. Mol Biol Rep 51, 835 (2024). https://doi.org/10.1007/s11033-024-09793-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09793-y