Abstract
Purpose of Review
The currently established standard of care treatment for locally advanced rectal cancer (LARC) is neoadjuvant chemoradiotherapy (CRT) followed by total mesorectal excision (TME) and adjuvant fluorouracil and oxaliplatin. The body of evidence that supports this treatment has grown over the last 20 years. However, recent advances and ongoing studies seek to further evaluate the role of neoadjuvant chemotherapy with and without radiation and total neoadjuvant therapy (TNT). In this article, we review the current literature as well as investigate the emerging role of TNT for patients with LARC and comment on updates utilizing combination neoadjuvant chemotherapy in early-stage rectal cancer.
Recent Findings
Evidence for the current standard of neoadjuvant CRT comes from well-established randomized phase III trials as well as emerging evidence on merits of TNT. There is a growing body of literature including retrospective analysis and ongoing clinical trials that look at upfront induction chemotherapy in addition to CRT prior to surgical resection leading to more effective delivery of systemic therapy and increases in response to treatment. Neoadjuvant combination chemotherapy is also being investigated in early-stage low rectal tumors to see if rates of local excision will increase compared to radical excision.
Summary
Current evidence continues to support neoadjuvant CRT as the standard treatment for LARC. There is an increasing body of evidence to support TNT in LARC as an effective treatment strategy that better ensures delivery of systemic therapy leading to higher rates of complete response (CR) and is encouraging for the development of non-operative protocols. There is ongoing evaluation looking at the benefit of novel sensitizers added to neoadjuvant therapy and ongoing investigation into upfront combination chemotherapy with selective use of radiation in upper rectal cancers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite advances in screening and treatment modalities over the last 20 years, an estimated 43,340 cases of rectal cancer will be diagnosed in 2020 with colorectal cancer remaining at the third highest incidence and mortality of all cancers in the USA [1]. These statistics continue to highlight the constant need to critically evaluate the current standards of treatment for rectal cancer and investigate novel treatment modalities. Disseminated disease remains the most common cause of death in patients but local recurrence (LR) leads to severe disabling symptoms, is difficult to treat, and is often fatal [2]. Over the last 20 years, the paradigm has shifted to neoadjuvant chemoradiotherapy (CRT) for locally advanced rectal cancer (LARC) with chemotherapy after surgery [3]. Although such multimodality therapy has markedly reduced local recurrence rates, there remains an estimated 5-year distant relapse rate of 35% representing the leading cause of death in this population [4•]. In many cases, planned adjuvant therapy cannot be fully completed bringing into the question the benefits of receiving a full-planned course of chemotherapy preoperatively. There have been significant advances in systemic chemotherapy for patients with colorectal cancer since 2002 with the introduction of combination regimens. Response rates with modern chemotherapy regimens such as 5-FU, leucovorin, oxaliplatin (FOLFOX) have routinely exceeded 50% and are frequently as high as 60–70% in the advanced setting. More recently, results of induction chemotherapy with CRT prior to surgery have been reported with promising results of higher percentages of completed courses of chemotherapy and higher rates of [4•]. These results have spurred to development of randomized prospective trials utilizing treatment arms separating total neoadjuvant therapy (TNT) and CRT + adjuvant chemotherapy.
Neoadjuvant combination therapy is under active investigation for early-stage rectal cancer as well. TME has been well-established to be a highly effective treatment for this disease with local recurrence of only 3–6% [5]. However, the postoperative mortality for these patients is significant at 3–4% and a permanent ostomy is needed in 25% of cases that is detrimental to quality of life [6]. Ongoing studies are evaluating the role of if upfront systemic therapy can lead to higher rates of rectal preservation in this subgroup.
There has been substantial groundwork leading to the current standard of care treatments for both LARC and early-stage rectal tumors. We will provide updates on novel treatment modalities and investigate updates in TNT for LARC and review ongoing clinical trials and explore the role of neoadjuvant combination chemotherapy for early-stage tumors. In the age of targeted and immunotherapy, a question that should be asked at every turn is what novel drugs and biomarkers can be added with the goal to improve pathologic complete response (pCR) and overall survival (OS).
Current Standard of Care
The standard of care for treatment of stage II and stage III LARC is well-established. Thus far, novel therapies have been ineffective for LARC [7, 8•, 9]. Treatment consists of neoadjuvant CRT followed by TME and adjuvant chemotherapy (fluorouracil- or capecitabine-based) leading to excellent local control with substantially lower rates of local recurrence compared to distant recurrence [10].
The defined regimen consisted of 50.4 Gy in 28 daily fractions concurrent with infusional fluorouracil (FU; 1000 mg/m2 daily for 5 days during the first and fifth weeks of RT. This was followed by four additional cycles of adjuvant single-agent FU (500 mg/m2 bolus daily for 5 days every 4 weeks) after TME.
There were three prospective randomized studies running simultaneously to compare the efficacy of neoadjuvant versus adjuvant CRT. These were the RTOG 94-01, NSABP R-03, and the CAO/ARO/AIO-94. Of these the RTOG 94-01 and NSABP R-03 were terminated early due to poor accrual. The German Rectal Cancer Study Group CAO/ARO/AIO-94 trial was completed with updated results reported after a median follow-up of 11 years published in 2012 [11]. Compared with patients randomized to the adjuvant arm, significantly lower rates of 5- and 10-year pelvic relapse (6% vs 13%; P ¼ .006 and 7% vs 10%; P ¼ .048, respectively) were seen in those allocated to neoadjuvant CRT, although there were no significant differences in disease-free survival (DFS) or OS between the 2 groups. Patients receiving neoadjuvant therapy also experienced considerably less acute grade 3 (27% vs 40%; P < .001) and chronic toxicities (14% vs 24%; P < .01). This established the current standard role of neoadjuvant CRT in stages II and III rectal cancer.
Capecitabine has also been compared to fluorouracil for LARC. Hofheinz et al. sought to show non-inferiority in a randomized, multicenter phase III study with results published in June 2012. This compared the two agents in both the neoadjuvant and adjuvant setting designed to examine non-inferiority of 5-year OS in the capecitabine versus that in the fluorouracil group. With median follow-up of 52 months, the 5-year OS in the capecitabine group was non-inferior to that in the fluorouracil group with HR 76% [95% CI 67–82] vs 67% [58–74]; P = 0·0004. The number of patients with local recurrences were similar in both groups (12 [6%] in the capecitabine group vs 14 [7%] in the fluorouracil group, P = 0·67). Notably, there were fewer patients that developed distant metastases in the capecitabine group (37 [19%] vs 54 [28%]; P = 0·04) [12].
It has been well-validated that preoperative therapy leads to significant tumor downsizing resulting in pathologic complete response (pCR) in a subset of patients and that the pathologic stage (which is heavily influenced by preoperative stage and response to treatment) has been found to be the best predictor of disease-free survival [13]. Prior literature suggests patients that reach pCR have improved recurrence and survival rates [14,15,16,17]. A systematic review of 16 LARC studies, compared with patients with residual pathologic disease, those achieving a pCR had significantly fewer local recurrences (odds ratio (OR) 0.25; P = 0.002), less frequent distant relapse (OR 0.23; P < .001), and higher 5-year DFS (OR 4.33; P < .001) [18]. However, multiple phase III trials have failed to validate pCR as an independent prognostic factor of OS. In search of better markers, the neoadjuvant rectal (NAR) score was developed as a short-term surrogate endpoint [19]. The NAR score is a weighted combination of post neoadjuvant therapy nodal stage (ypN) and downstaging of T. It has been tested as a surrogate endpoint in the NSABP R-04 study where it was shown to be closely associated with OS (P < .0001) and was a better predictor of OS than pCR (P < .0001) [19]. The NAR score has been approved by the National Cancer Institute as an acceptable surrogate primary endpoint in clinical trials assessing the impact of neoadjuvant therapy for rectal cancer.
Current consensus guidelines recommend 4 months of adjuvant fluoropyrimidine-based chemotherapy for all patients with LARC who receive neoadjuvant CRT followed by surgical resection, regardless of surgical pathologic findings [3]. Many studies have been conducted but unable to show benefit of adjuvant therapy in terms of DFS and OS [13]. These results led to the logical concluding question of how to more effectively implement systemic therapy to improve overall survival in the preoperative setting. Chemotherapy may have dual role in management of rectal cancer as it serves as a radiosensitizer and also tackles circulating micro metastases thus helping control local as well as distant relapses, ultimately prolonging DFS and OS.
Intensification of Therapy
Given the high risk of local and distant relapse associated with LARC, multiple studies have looked at the intensification of neoadjuvant therapy to improve disease control rates.
Chemotherapy as Radiosensitizer
The landmark study by Sauer et al. (CAO/ARO/AIO-94) established role of fluoropyrimidine-based CRT, as the standard of care. Oxaliplatin has activity in advanced colorectal cancer and has radiosensitizing properties, and by the virtue of these properties, its role in neoadjuvant CRT for rectal cancer, in combination with fluoropyrimidine and radiation has been explored extensively. Results from multiple trials adding oxaliplatin to standard CRT have all demonstrated increased toxicity with variable efficacy data but no clear benefit in terms of DFS and OS [20,21,22,23,24]. The NSABP R-04 study demonstrated nearly identical DFS and OS for LARC patients treated with either oral capecitabine or continuous 5-FU as a radiosensitizer. But, the addition of oxaliplatin failed to improve rates of pCR or rectal preservation but was associated with significantly higher rates of overall and grades 3–4 toxicities (P < .0001) [25]. The German CAO/ARO/AIO-04 published July 2015 was a multicenter, open-label, phase 3 study that randomly assigned patients to receive fluoropyrimidine-based CRT versus oxaliplatin added to both preoperative CRT and adjuvant chemotherapy. A 3-year DFS was 75.9% in the investigational group and 71.2% in the control group ((HR) 0.79 P = 0.03). Significantly more preoperative grades 3–4 toxicities occurred in 144 (24%) of 607 patients who received fluorouracil CRT and oxaliplatin compared with 128 (20%) of 625 patients who received fluorouracil CRT. Late grades 3–4 adverse event (AE) in protocol-specified preoperative and postoperative treatment was 25% of patients in the investigational group and 21% patients in the control group [26].
Irinotecan has also been tested in combination with fluorouracil-based CRT in multiple phase II trials [27,28,29]. A study by Mohiuddin et al. enrolled 106 patients randomized to either 5-fluorouracil plus pelvic hyperfractionated radiation in arm 1 or 5-fluorouracil plus irinotecan weekly × 4, plus pelvic RT for arm 2. With a median follow-up of 6.4 years in arm 1 and 7.0 years in arm 2, pCR rates of 30% (95% CI 0.17, 0.43) and 26% (95% CI 0.15, 0.38) were observed respectively. Locoregional recurrence rates were similar at 16% in arm 1 and 17% in arm 2. Five-year OS rates were 61% (95% CI: 47%, 74%) in arm 1 and 75% (95% CI: 61%, 85%) in arm 2; however, OS and DFS data were complicated by five unrelated second primaries occurring in patients on arm 1, and 1 s primary occurred in arm 2. Gollins et al. evaluated 110 patients with LARC (high-risk T3 and T4 rectal tumors identified on MRI) in a single-arm phase II study. Radiotherapy was given to 45 Gy in 25 fractions over 5 weeks with concurrent oral capecitabine at 650 mg/m2 twice per day continuously days 1 through 35 and intravenous irinotecan at 60 mg/m2 once weekly weeks 1 to 4. Three-year local recurrence-free survival was 96.9%, metastasis-free survival was 71.1%, OS was 88.2%, and DFS was 63.5%. This demonstrated high response rates and promising long-term survival and further suggesting that downstaging to ypCR remained a significant predictor of OS (P = 0.005) and may be a short-term surrogate for long-term survival.
The phase III ARISTOTLE study comparing standard CRT with capecitabine- and irinotecan-based CRT has completed accrual and will provide further guidance about the use of irinotecan-based combinations in this setting (ISRCTN09351447) [30•].
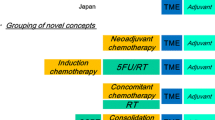
Total Neoadjuvant Therapy
There has been an increasing body of evidence to support delivery of systemic chemotherapy in the neoadjuvant setting in addition to CRT. The important goal of this multi-modal approach is to make optimal the delivery of systemic therapy targeting micrometastases. Phase II/III studies that have investigated CRT followed by TME and adjuvant chemotherapy have continued to find mediocre compliance with adjuvant chemotherapy resulting in only 50% of trial patients able to receive the full-planned course of post-surgical treatment most commonly due to toxicity or patient refusal [31].
Chau et al. published the results of a prospective single-arm study in 2006 that evaluated the effect of upfront combination chemotherapy followed by CRT on the rate of radiologic and symptomatic response as well as rate of pCR for patients with MRI-defined poor-risk rectal cancers. Patients received 12 weeks of neoadjuvant oxaliplatin with capecitabine followed by synchronous CRT and TME followed by 12 weeks of adjuvant capecitabine. Pathologic complete response was observed in 16 patients (24%; 95% CI, 14 to 36%), and additionally, 32 patients (48%) had only microscopic tumor foci. Eighty-eight percent of patients had radiologic response after receiving capecitabine/oxaliplatin and 86% had symptomatic responses with a median of only 32 days [32].
One of the early randomized studies to investigate the role of TNT was performed by the Grupo Cancer de Recto 3 by Fernandez-Martos et al. The phase II randomized study specifically looked at CRT followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) in arm A compared to CAPOX followed by CRT and then surgery in arm B. The rates of pCR were similar between the two groups (13.5% and 14.3% respectively). Notably, CAPOX treatment exposure was significantly higher in arm B compared to that in arm A (P < 0.0001). Grades 3–4 toxicities were similar during CRT but significantly higher during postoperative CAPOX in arm A compared with neoadjuvant in arm B [33]. The TNT group showed improved overall compliance. PANEX, a pooled analysis of EXPERT and EXPERT-C trials, the two largest trials of neoadjuvant CAPOX followed by CRT, TME, and adjuvant CAPOX ± cetuximab in MRI-defined, high-risk, LARC was presented at ASCO meeting in 2014. The analysis suggested a radiologic response rate of 62% after neoadjuvant chemotherapy and 80% after CRT. Surgery was performed in 91% and T/N downstaging was achieved in 56%/55% cases, and pCR rate was 19%. After a median follow-up of 69 months, 5-year local control and overall survival were 94% and 73% [34].
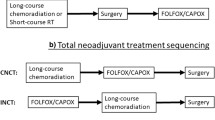
Recently reported studies directly compare TNT to CRT and induction versus consolidation chemotherapy prospectively. PRODIGE-23 is a phase III multicenter open-label randomized 2-arm phase III superiority trial by Conroy et al. that enrolled 461 patients [35•]. It sought to compare 3-year disease-free survival (DFS) of chemotherapy followed by CRT and TME versus CRT followed by TME and adjuvant chemotherapy. The TNT group received 4 cycles of mFOLFIRINOX followed by 5 weeks of CRT with capecitabine then proceeded with TME. The CRT arm used capecitabine with 5 weeks of radiation and adjuvant use of either mFolfox6 or capecitabine based on center choice. Final results were presented at ASCO 2020 where compliance to CRT was not hampered by neoadjuvant chemotherapy. Rates of pCR (27.5% vs 11.7, P < 0.001) and 3-year DFS favored the experimental arm (75.7% vs 68.5%, HR 0.69, 95% CI 0.49–0.97, P = 0.034). 3-year OS was 90.8 vs 87.7% (HR 0.65, CI 0.40–1.05, P = 0.077).
The role of short-course radiotherapy followed by systemic chemotherapy has also been evaluated in the TNT setting given promising phase II data [36]. This led to the RAPIDO trial, a phase III trial comparing short course followed by 18 weeks of CAPOX/FOLFOX chemotherapy before surgery with standard of care CRT followed by surgery in 920 patients with locally advanced tumors (T4a-b or N2 or radiographic evidence of vascular invasion or mesorectal fascia or involved pelvic side wall nodes) and M0 disease. In the recently presented results, rates of pCR were 27.7% vs 13.8% (OR 2.40 [1.70–3.39]; P < 0.001) in the experimental and standard arms, respectively. At 3 years, disease-related treatment failure rate was 23.7% in the experimental arm and 30.4% in the standard arm (HR 0.76 [0.60–0.96]; P = 0.02). Distant metastasis and locoregional failure rates were 19.8% vs 26.6% (HR 0.69 [0.53–0.89]; P = 0.004) and 8.7% vs 6.0% (HR 1.45 [0.93–2.25]; P = 0.10), in the experimental and standard arms respectively. Overall health (P = 0.192), quality of life (P = 0.125) and low anterior resection syndrome score (P = 0.136) were comparable between the two treatment arms [37•].
There is also continued effort to evaluate novel sensitizers in the neoadjuvant setting. The NRG-GI002, a phase II trial by T.J. George et al., is investigating the role of immunotherapy PD-1 inhibitor pembrolizumab and the PARP inhibitor veliparib given in conjunction with CRT after mFOLFOX6 therapy. The primary outcome is the change in the neoadjuvant rectal cancer (NAR) score. This is an easier to measure surrogate marker for overall survival (OS) and DFS [13]. Secondary measure outcomes are 3-year OS and DFS, rate of pCR, and rate of sphincter preservation. Two arms of the study have completed accrual with results awaited, and in light of the previously presented data, the study may undergo a redesign.
De-Intensification of Therapy
Based on the previous section, it is quite certain that TNT, consolidation or induction chemotherapy with CRT, provides several benefits in the treatment of locally advanced rectal cancer. One of the known concerns of neoadjuvant CRT is the undesirable side effects of radiation. Long-term morbidities include greater than 5 bowel movements per day, rectal bleeding, bowel obstruction, and development of bowel necrosis/perforation/fistula [38]. Given advances in radiation, surgery, and chemotherapy, there is ongoing investigation looking into chemotherapy alone with selective addition of CRT versus CRT alone to treat patients with LARC prior to surgery. Perhaps patients could be spared the side effects of pelvic radiation. This is especially true for upper rectal cancer, where circumferential resection margin is not a major risk factor.
The phase III FOWARC trial, with its 3-arm trial design, included 495 patients with LARC randomly allocated to neoadjuvant CRT with 5FU leucovorin (de Gramont’s regimen), neoadjuvant CRT with mFOLFOX6, or neoadjuvant chemotherapy (mFolFOX6) alone. Primary endpoint was 3-year DFS [39•]. There was no difference in the 3-year DFS (72.9%, 77.2%, and 73.5%, respectively P = 0.71) or the 3-year overall survival rate (91.3%, 89.1%, and 90.7% respectively, P = .97) between the arms. In this study, omitting radiotherapy did not lead to increase in rate of local recurrence in the chemotherapy alone arm. Exclusion of RT was associated with significantly less treatment-related toxicity and perioperative complications, while achieving comparable rates of recurrence-free and overall survival. The study provides only available prospective randomized data of relative benefit of chemotherapy alone in comparison with CRT.
In 2014, there was a single institutional pilot study from Memorial Sloan Kettering Cancer Center (MSK) that evaluated and treated 32 patients with stages II/III LARC using induction FOLFOX with selective use of CRT [11]. One hundred percent of the trial patients had R0 resections and 30 of 32 patients had tumor regression and went for TME without preoperative chemoradiotherapy. At 4 years, local recurrence was 0% and DFS was 84% indicating neoadjuvant chemotherapy followed by selective CRT did not adversely affect outcomes. These results warranted further investigation.
The PROSPECT trial (NCT01515787) is a multicenter phase II/III study looking to first ensure that neoadjuvant chemotherapy with 5FU and oxaliplatin followed by selective use of CRT in non-responders maintains a high rate of R0 resection and non-inferior for time to local recurrence (TLR). The phase III component directly compares neoadjuvant FOLFOX followed by selective CRT to standard neoadjuvant CRT. The trial has completed accrual but results are awaited. Similar to the PROSPECT trial, another study using the selective radiation approach is being conducted in China (FORTUNE, NCT02217020) but it uses FOLFOXIRI (5FU, leucovorin, oxaliplatin and Irinotecan) as the chemotherapy backbone. The primary endpoint for this study is the rate of tumor downstaging, and it has completed accrual but final results are pending.
TNT is beneficial as it gives the opportunity to assess chemosensitivity and tumor response prior to surgery and can help stratify patients needing surgery. In fact, several studies reporting a non-operative approach have suggested that patients who achieved a complete clinical response could be safely left with the rectum and have good long-term outcomes in localized rectal cancer [40,41,42]. To date, no prospective data is available comparing surgery or non-operative management; however, accumulating evidence reports favorable long-term outcomes with this approach. Majority of this evidence comes from the Brazilian institutional-level studies from Habr-Gama et al. where patients with stages I–III (>/=T2) rectal cancer who achieved a cCR after neoadjuvant CRT were allowed to skip surgery and follow a wait and watch (W&W) approach of intense local surveillance with good long-term outcomes. The inclusion of stage I tumors (up to 20% in initial study) limits application of the data. More recently, the International Watch & Wait Database published the outcomes of this strategy through a large-scale registry of pooled individual patient data from 1009 rectal cancer patients who did not undergo definitive surgery after neoadjuvant CRT [43]. All patients skipped definitive surgery and 889 patients (87%) had a cCR after neoadjuvant CRT. The 2-year cumulative incidence of local recurrence was 25·2% (95% CI 22.2–28.5%), most occurring within the first 2 years, and 97% of these were located in the bowel wall. Rate of distant failure (8%), 5-year OS (85%, 95% CI 80·9–87.7%), and 5-year disease-specific survival (94%, 95% CI 91–96%) was comparable to that seen with standard of care therapy.
Looking at prospective studies, the recently reported results of OPRA trial support this idea. In this trial, patients with stages II and III rectal adenocarcinoma were randomized to 4 months of FOLFOX or CAPOX before (induction) or after (consolidation) fluoropyrimidine-based CRT. Patients with complete or near-complete clinical response were offered watchful waiting and outcomes followed. The disease-free survival (primary endpoint) was comparable between induction and consolidation chemotherapy arms (78% vs 77%, P = 0.90) but the consolidation strategy was able to provide a 58% rate of organ preservation [44•]. The authors concluded that omitting surgery and adopting a wait and watch approach for patients that achieve a clinical complete response to TNT results in organ preservation without compromising survival in a high percentage of patients.
Despite the advances in the treatment of LARC to improve local control of disease, there has been a significant drive to establish treatment modalities to reduce incidence of distal recurrence as the 5-year distant relapse remains at around 30% [45,46,47].
Early-Stage Rectal Cancer
The role of tri-modality therapy for early-stage distal T1-2 N0 tumors to increase the rate of organ preservation is not well-established. For mid- and lower-third rectal cancers, the standard of care is total mesorectal excision (TME) done either by low anterior resection or by abdominoperineal resection with or without preoperative CRT. A proportion of these patients will require either a temporary or permanent stoma leading to an overall diminished quality of life [48]. More than half of patients will go on to experience a degree of fecal incontinence and may also have autonomic nerve damage leading to either urinary continence or retention (25–34%) and sexual dysfunction [49]. This leads to concerns that radical surgery—which evolved to treat locally advanced and symptomatic tumors—may not be the optimal method of treatment for early-stage tumors.
Early rectal tumors may be locally excised either by local excision (LE) or by transanal endoscopic microsurgery (TEMS). These procedures seek to omit TME and preserve the rectum. However, this increases the risk of residual microscopic lymph node metastasis leading to local failure. Prior studies have explored the use of pelvic chemoradiation followed by transanal microsurgery as a means to increase organ preservation but have shown high complication rates with LE and TEMS after CRT including 30-day readmissions, wound dehiscence, grades 3–4 complications, and adverse effects on bowel, sexual, and urinary function [49, 50]. Also, patients who develop recurrence following this strategy are difficult to salvage as re-irradiation is not usually an option. The role of chemotherapy to downstage tumor and address micrometastatic disease in early-stage disease has not been explored much. Given the significant advances in combination chemotherapy, investigators have hypothesized that the use of combination chemotherapy in the neoadjuvant setting will help downsize primary low rectal tumors leading to improved local excision rates and reduce the number of patients that need radical surgery and not compromise oncologic outcomes.
The Canadian Cancer Trials Group is investigating the effects of upfront neoadjuvant chemotherapy with either FOLFOX or CAPOX in early-stage rectal cancer prior to tumor excision. The NEO: Neoadjuvant Chemotherapy, Excision and Observation for Early Rectal Cancer (NCT03259035) is a single-arm phase II trial that is two-staged with the primary endpoint of organ (rectum) preservation rate of 65%. Neoadjuvant regimen selection is either six 2-week cycles of FOLFOX or four 3-week cycles of CAPOX prior to surgery. Secondary outcomes include 3-year measurements of locoregional recurrence, distant relapse rate, DFS, and rate of postoperative complications. This trial has completed accrual, and final results are awaited (CO.28-NCT03259035). Another ongoing trial, GI-116: Phase II Study of Organ Preservation in Early Rectal Cancer Patients (NCT03548961), is a single-arm phase II study investigating neoadjuvant combination chemotherapy followed by local excision and postoperative chemoradiotherapy in patients with early-stage, low rectal adenocarcinoma. The primary endpoint is the number of patients whose tumor can be resected by local excision with negative margins. Eligible patients will undergo 12 weeks of FOLFOX followed by restaging of the primary tumor with pelvic MRI and/or sigmoidoscopy 2–4 weeks after completing therapy. Those who respond will proceed with local excision 6–12 weeks after completing neoadjuvant chemotherapy and 4–12 weeks after local excision will undergo 5-FU-based chemoradiotherapy.
Conclusions
There have been substantial advances in treatment of rectal cancers in the last 20 years. The role of neoadjuvant chemotherapy continues to advance in the setting of early-stage and locally advanced rectal cancers. In a disease process where the pathologic stage at the time of surgery is the best predictor of DFS, there is strong incentive to improve upon optimal delivery of systemic therapy prior to surgical resection. The current standard of care for LARC remains either neoadjuvant CRT followed by surgery and adjuvant chemotherapy or TNT; however, these regimens have not been compared prospectively. There remains a lack of evidence of the effectiveness of novel sensitizing agents in LARC. Table 1 provides a summary of ongoing or recently completed studies in this area. The NRG-GI002 phase II trial is investigating the role of immunotherapy PD-1 inhibitor pembrolizumab and the PARP inhibitor veliparib given in conjunction with CRT after mFOLFOX6 therapy. In regard to early-stage low rectal cancers, the GI-116 and NEO phase II clinical trials will assess the role of neoadjuvant combination chemotherapy leading increased rates of less invasive surgery that spares the rectum.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
American Cancer Society Annual Cancer Facts and Figures. 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf. Accessed 4 Mar 2020.
Zorcolo L, Rosman AS, Restivo A, Pisano M, Nigri GR, Fancellu A, et al. Complete pathologic response after combined modality treatment for rectal cancer and long-term survival: a meta-analysis. Ann Surg Oncol. 2012;19:2822–32.
Benson AB, Al-Hawary MM, Arain MA, et al. 2020. NCCN Guidelines Version 2.2020 Rectal Cancer Continue NCCN Guidelines Panel Disclosures. Accessed 3 Mar 2020.
Cercek A, Roxburgh CSD, Strombom P, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol. 2018;4:e180071 A retrospective cohort analysis revealing higher rates of completion of planned treatment in patients undergoing TNT versus traditional adjuvant chemotherapy.
Peeters KCMJ, Marijnen CAM, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693–701.
Tekkis PP, Heriot AG, Smith J, Thompson MR, Finan P, Stamatakis JD. Comparison of circumferential margin involvement between restrorative and nonrestorative resections for rectal cancer. Color Dis. 2005;7:369–74.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54.
Leichman CG, McDonough SL, Smalley SR, et al. Cetuximab combined with induction oxaliplatin and capecitabine, followed by neoadjuvant chemoradiation for locally advanced rectal cancer: SWOG 0713. Clin Colorectal Cancer. 2018;17:e121–5 Phase II study showing the addition of EGFR-targeted antibody cetuximab to neoadjuvant CRT in KRAS wild-type patients does not improve pCR.
Dewdney A, Cunningham D, Tabernero J, et al. Multicenter randomized phase ii clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C). 2012. https://doi.org/10.1200/JCO.2011.39.6036.
Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, et al. Colorectal cancer. Lancet. 2010;375:1030–47.
Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–33.
Hofheinz RD, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann JT, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012;13:579–88.
Schrag D, Weiser MR, Goodman KA, Gon̈en M, Hollywood E, Cercek A, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol. 2014;32:513–8.
Sainato A, Cernusco Luna Nunzia V, Valentini V, de Paoli A, Maurizi ER, Lupattelli M, et al. No benefit of adjuvant fluorouracil leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): long term results of a randomized trial (I-CNR-RT). Radiother Oncol. 2014;113:223–9.
Breugom AJ, van Gijn W, Muller EW, Berglund Å, van den Broek CBM, Fokstuen T, et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol. 2015;26:696–701.
Glynne-Jones R, Counsell N, Quirke P, Mortensen N, Maraveyas A, Meadows HM, et al. Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol. 2014;25:1356–62.
Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15:184–90.
Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg. 2012;99:918–28.
George TJ, Allegra CJ, Yothers G. Neoadjuvant rectal (NAR) score: a new surrogate endpoint in rectal cancer clinical trials. Curr Colorectal Cancer Rep. 2015;11:275–80.
Aschele C, Cionini L, Lonardi S, Pinto C, Cordio S, Rosati G, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773–80.
Gérard JP, Azria D, Gourgou-Bourgade S, Martel-Lafay I, Hennequin C, Etienne PL, et al. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol. 2012;30:4558–65.
Schmoll H-J, Haustermans K, Price TJ, Nordlinger B, Hofheinz R, Daisne JF, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with capecitabine and oxaliplatin versus capecitabine alone in locally advanced rectal cancer: disease-free survival results at interim analysis. J Clin Oncol. 2014;32:3501–3501.
Allegra CJ, Yothers G, O’Connell MJ, Beart RW, Wozniak TF, Pitot HC, et al. Neoadjuvant 5-FU or capecitabine plus radiation with or without oxaliplatin in rectal cancer patients: a phase III randomized clinical trial. J Natl Cancer Inst. 2015;107:djv248. https://doi.org/10.1093/jnci/djv248.
Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, et al. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC multicenter, open-label, randomized three-arm phase III trial. J Clin Oncol. 2016;34:3300–7.
O’Connell MJ, Colangelo LH, Beart RW, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from national surgical adjuvant breast and bowel project trial R-04. J Clin Oncol. 2014;32:1927–34.
Rödel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold D, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16:979–89.
Gollins S, Sun Myint A, Haylock B, Wise M, Saunders M, Neupane R, et al. Preoperative chemoradiotherapy using concurrent capecitabine and irinotecan in magnetic resonance imaging-defined locally advanced rectal cancer: impact on long-term clinical outcomes. J Clin Oncol. 2011;29:1042–9.
Navarro M, Dotor E, Rivera F, Sánchez-Rovira P, Vega-Villegas ME, Cervantes A, et al. A phase II study of preoperative radiotherapy and concomitant weekly irinotecan in combination with protracted venous infusion 5-fluorouracil, for resectable locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2006;66:201–5.
Mohiuddin M, Paulus R, Mitchell E, Hanna N, Yuen A, Nichols R, et al. Neoadjuvant chemoradiation for distal rectal cancer: 5-year updated results of a randomized phase 2 study of neoadjuvant combined modality chemoradiation for distal rectal cancer. Int J Radiat Oncol Biol Phys. 2013;86:523–8.
Sebag-Montefiore DA. Phase III trial comparing standard versus novel CRT as pre-operative treatment for MRI defined locally advanced rectal cancer. http://www.ctc.ucl.ac.uk/TrialDetails.aspx?Trial=82&TherA=7. Accessed 27 Mar 2020. A phase III randomized controlled trial that has finished accrual investigating irinotecan as a radiosensitizer directly compared to standard CRT.
Mengual-Ballester M, García-Marín JA, Pellicer-Franco E, Guillén-Paredes MP, García-García ML, Cases-Baldó MJ, et al. Ileostomías de protección: complicaciones y mortalidad asociadas a su cierre. Rev Esp Enferm Dig. 2012;104:350–4.
Chau I, Brown G, Cunningham D, Tait D, Wotherspoon A, Norman AR, et al. Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J Clin Oncol. 2006;24:668–74.
Fernández-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo Cáncer de Recto 3 study. J Clin Oncol. 2010;28:859–65.
Sclafani F, Peckitt C, Cunningham D, Evans J, Brown G, Tabernero J, et al. Panex: a pooled analysis of EXPERT and EXPERT-C, two trials of neoadjuvant chemotherapy (NACT) and chemoradiotherapy (CRT) in high-risk locally advanced rectal cancer (LARC). J Clin Oncol. 2014;32:3575–3575.
Conroy T, Lamfichekh N, Etienne P-L, et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: final results of PRODIGE 23 phase III trial, a UNICANCER GI trial. J Clin Oncol. 2020;38:4007–4007 Investigated and found compliance to CRT was not negatively impacted by neoadjuvant chemotherapy and that TNT significantly increased ypCR rate as well as 3-year DFS compared to traditional CRT and adjuvant chemotherapy.
van Dijk TH, Tamas K, Beukema JC, Beets GL, Gelderblom AJ, de Jong KP, et al. Evaluation of short-course radiotherapy followed by neoadjuvant bevacizumab, capecitabine, and oxaliplatin and subsequent radical surgical treatment in primary stage IV rectal cancer. Ann Oncol. 2013;24:1762–9.
Hospers G, Bahadoer RR, Dijkstra EA, et al. Short-course radiotherapy followed by chemotherapy before TME in locally advanced rectal cancer: the randomized RAPIDO trial. J Clin Oncol. 2020;38:4006–4006 An investigation of short-course radiotherapy followed by chemotherapy prior to TME compared to standard CRT. Results with significantly lower disease-related treatment failure rate in high-risk LARC patients.
Feeney G, Sehgal R, Sheehan M, Hogan A, Regan M, Joyce M, et al. Neoadjuvant radiotherapy for rectal cancer management. World J Gastroenterol. 2019;25:4850–69.
Deng Y, Chi P, Lan P, et al. Neoadjuvant modified folfox6 with or without radiation versus fluorouracil plus radiation for locally advanced rectal cancer: final results of the Chinese FOWARC trial. J Clin Oncol. 2019;37:3223–33 A phase III prospective study showing no difference in 3-year DFS and OS and less treatment-related toxicity with neoadjuvant chemotherapy alone compared to CRT.
Havenga K, Enker WE, Norstein J, Moriya Y, Heald RJ, van Houwelingen HC, et al. Improved survival and local control after total mesorectal excision or D3 lymphadenectomy in the treatment of primary rectal cancer: an international analysis of 1411 patients. Eur J Surg Oncol. 1999;25:368–74.
Bosset J-F, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–23.
Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620–5.
van der Valk MJM, Hilling DE, Bastiaannet E, Meershoek-Klein Kranenbarg E, Beets GL, Figueiredo NL, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391:2537–45.
Garcia-Aguilar J, Patil S, Kim JK, Yuval JB, Thompson H, Verheij F, et al. Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial. J Clin Oncol. 2020;38:4008–4008 Prospective study that showed comparable DFS between induction and consolidation chemotherapy + CRT in addition to high organ preservation rate in the consolidation arm allowing adoption of a watch and wait approach in patients who achieve clinical complete response with TNT.
Marijnen CAM, Kapiteijn E, van de Velde CJH, Martijn H, Steup WH, Wiggers T, et al. Acute side effects and complications after short-term preoperative radiotherapy combined with total mesorectal excision in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol. 2002;20:817–25.
Hendren SK, O’Connor BI, Liu M, Asano T, Cohen Z, Swallow CJ, et al. Prevalence of male and female sexual dysfunction is high following surgery for rectal cancer. Ann Surg. 2005;242:212–23.
Cercek A, Goodman KA, Hajj C, Weisberger E, Segal NH, Reidy-Lagunes DL, et al. Neoadjuvant chemotherapy first, followed by chemoradiation and then surgery, in the management of locally advanced rectal cancer. JNCCN J Natl Compr Cancer Netw. 2014;12:513–9.
Verseveld M, de Graaf EJR, Verhoef C, van Meerten E, Punt CJA, de Hingh IHJT, et al. Chemoradiation therapy for rectal cancer in the distal rectum followed by organ-sparing transanal endoscopic microsurgery (CARTS study). Br J Surg. 2015;102:853–60.
Garcia-Aguilar J, Shi Q, Thomas CR, Chan E, Cataldo P, Marcet J, et al. A phase II trial of neoadjuvant chemoradiation and local excision for T2N0 rectal cancer: preliminary results of the ACOSOG Z6041 trial. Ann Surg Oncol. 2012;19:384–91.
Pucciarelli S, de Paoli A, Guerrieri M, la Torre G, Maretto I, de Marchi F, et al. Local excision after preoperative chemoradiotherapy for rectal cancer: results of a multicenter phase II clinical trial. Dis Colon Rectum. 2013;56:1349–56.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Namrata Vijayvergia has received consultancy fees from Lexicon, Novartis, HalioDx, and Sun Pharma and grants from Merck and Bayer. Thomas Holden declares that he has no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Systemic Therapies in Colorectal Cancer
Rights and permissions
About this article
Cite this article
Holden, T., Vijayvergia, N. Review and Updates on Approaches to Neoadjuvant Chemotherapy in Rectal Cancer. Curr Colorectal Cancer Rep 17, 1–9 (2021). https://doi.org/10.1007/s11888-020-00462-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11888-020-00462-3