Abstract
Background
Currently, the standard management of locally advanced rectal cancer (LARC) is neoadjuvant chemoradiotherapy followed by resection. Despite the significant improvement in local recurrence, survival benefits are not gained due to distant failure and radiotherapy-associated toxicity. Compliance to adjuvant chemotherapy after preoperative chemoradiotherapy is also poor. Neoadjuvant chemotherapy alone followed by surgery may be an alternative. The objective of this review is to determine the efficacy of neoadjuvant chemotherapy alone in operable LARC.
Materials and Methods
Electronic databases searched (from database inception–December 2013) were Medline, PubMed, Embase, Scopus, Cochrane library, and the Clinical Trials Register. Specific journals were also hand searched. The selection criteria were studies published in English investigating stage II–III non-metastatic rectal cancer patients treated with neoadjuvant chemotherapy (oral, intravenous or rectal route) followed by curative resection. The primary outcome measure was tumour response. Secondary outcome measures included acute toxicity, operative morbidity, R0 resection, local recurrence, overall survival (OS) and disease-free survival (DFS).
Results
One randomised phase III trial, six single-arm phase II trials and one retrospective case series study were eligible for inclusion. Six studies administered fluoropyrimidine-based multiple agent regimens and two studies administered fluorouracil-based monotherapy. The studies with multiple agents and stronger chemotherapy regimens (intravenous and/or oral) followed by delayed surgery showed better tumour response rates. The overall objective response rate was good and ranged from 62.5 to 93.7 %. Pathological complete response ranged from 3.8 to 33.3 %. The R0 resection and compliance rates were also high ranging from 90 to 100 % and 72 to 100 %, respectively. Grade 3–4 toxicities ranged from 2.3 to 39 %. Four- to 5-year OS and DFS ranged from 67.2 to 91 % and 60.5 to 84 %, respectively.
Conclusion
This review demonstrates that neoadjuvant chemotherapy could be affectively administered in LARC and could provide a good alternative to chemoradiotherapy in moderate-risk rectal cancers without compromising short- and long-term outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction and Literature Review
Colorectal cancer (CRC) accounts for 10 % of all cancer cases worldwide and is the third and second most common cancer in men and women, respectively [1]. In the UK, CRC is the second highest cause of cancer-related deaths with 8574 men and 7134 women dying from it in 2010 (male/female ratio of 12/10) [2]. Rectal cancers account for 25 % of all colorectal cancers.
Management of rectal cancer has evolved in the last two decades from a mainly surgically treated disease to a multimodality treatment model. Traditional management of locally advanced rectal cancer (T3–T4 and/or N+ or any T with N+) had been immediate surgery followed by chemoradiation. A landmark randomised German trial by Sauer et al. [3] altered the stage II–III rectal cancer management into neoadjuvant chemoradiotherapy. There was a significant improvement in 5-year local recurrence rate (6 vs. 13 %, P = 0.006) and a significant reduction in acute and late adverse effects among patients in preoperative long-course chemoradiotherapy arm [3]. A Polish randomised trial [4] compared long- and short-course radiotherapy for T3–T4 mid- to low-resectable rectal cancer (n = 312). Specifically, there were significantly higher rates of complete pathological responses in the long-course radiotherapy arm compared to the short course (16 vs. 1 %, P < 0.001). In addition, long-course radiotherapy patients achieved less + circumferential resection margin (CRM) as compared to short-course radiotherapy (4 vs. 13 %, P = 0.017). Chemotherapy given concurrently with radiotherapy acts as a radio sensitizer and enhances the tumour downstaging and sphincter preservation rate. The results of a French trial [5] and a multicentre European trial (EORTC) [6] show significantly lower local recurrence among patients given combined modalities.
Limitations of Preoperative Chemoradiotherapy
Despite the significantly low recurrence rates, none of the above-mentioned trials [3, 4, 6] show any difference in terms of overall survival because of higher distant metastatic rates of up to 38 % [6]. In addition, pelvic radiotherapy can lead to long-term morbidity. A Dutch trial [7] that randomised patients into preoperative short-course chemoradiotherapy followed by surgery vs. surgery alone showed that patients in the radiotherapy arm experienced increased bowel frequency and significantly higher rates of anal blood loss and faecal incontinence. The same group also showed that radiotherapy had a negative effect on sexual functioning in males (P = 0.004) and females (P < 0.001) and irradiated males had more ejaculation disorders (P = 0.002), and erectile functioning deteriorated over time (P < 0.001) [8].
Ways to Improve Chemotherapy
Combining newer chemotherapeutic drugs such as oxaliplatin plus capacitabine (combination termed as XELOX or CAPOX) and irinotecan to conventional fluoropyrimidine-based chemotherapeutic schedules [9, 10] and the addition of targeted agents such as cetuximab and pantimumab [11] have shown promising results in patients with metastatic and locally advanced colorectal cancer. These newer agents are being extrapolated into the trials of recent approaches to neoadjuvant therapy in rectal cancer, but early results do not show any significant improvement on early pathological response [12]. Similarly, preliminary results from the multicentre randomised phase II EXPERT-C trial comparing neoadjuvant chemotherapy before chemoradiotherapy in high-risk rectal cancer with or without cetuximab did not show improved complete pathological response as a primary end point [13]. Several phase II single-arm studies have incorporated the approach of neoadjuvant chemotherapy prior to chemoradiotherapy in T3 rectal cancers (induction therapy). The results suggest that this approach is feasible with minimal risk of disease progression and compromise of subsequent radiotherapy and resection [14, 15].
Neoadjuvant Chemotherapy Without Chemoradiation
The concept of neoadjuvant chemotherapy without chemoradiation has not been assessed in resectable colorectal cancer until now because of the theoretical risk of disease progression on the one hand and the risk of over treating low-risk patients due to inaccurate radiological staging on the other hand. However, the potential advantages [16] for using neoadjuvant chemotherapy include:
-
Early systemic treatment of micrometastasis and circulating cancer cells.
-
Administration of chemotherapeutic agents at full systemic doses.
-
Improved compliance as compared to adjuvant chemotherapy.
-
Long-term morbidity associated with pelvic radiotherapy can be avoided.
-
Early recognition of non-responders who may benefit from intensive treatment strategies.
-
Better response in primary tumour due to a likely increase in drug distribution to the tumours with an intact blood supply.
Aims and Objectives of the Systematic Review
Primary
-
The primary objective of this systematic review is to assess tumour response to neoadjuvant chemotherapy alone in operable locally advanced rectal cancers.
Secondary
The secondary objectives of the study are as follows:
-
To determine the feasibility of neoadjuvant chemotherapy in locally advanced operable rectal cancer
-
To determine the safety and efficacy of neoadjuvant chemotherapy
-
To determine the surgical morbidity and outcomes after neoadjuvant chemotherapy
-
To determine the long-term oncological outcome in terms of recurrence, disease-free survival and overall survival
Methodology
The following methodology is in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines.
Eligibility Criteria
Types of Studies Included in the Review
As the concept of use of neoadjuvant chemotherapy without radiotherapy is an evolving one and on scoping review, no phase III randomised trials for neoadjuvant chemotherapy alone in rectal cancers were located; therefore, all study designs were included (phase II randomised or non-randomised clinical trials, cohort, case series). In addition, studies investigating the use of neoadjuvant chemotherapy alone in colorectal cancer were only included if separate end results would be provided for rectal cancers.
Participant Types
-
Inclusion Criteria
-
Patients with histologically proven rectal adenocarcinoma
-
Stage II–III rectal cancer undergoing neoadjuvant chemotherapy followed by surgery for curative intention
-
-
Exclusion Criteria
-
Metastatic disease
-
Unresectable cancer
-
Types of Intervention
-
This pertains to single agent preoperative chemotherapy or multiple preoperative chemotherapy agents including additional antiangiogenic agents followed by resection ± adjuvant chemotherapy.
Route of Neoadjuvant Chemotherapy Administration
The studies investigating oral, intravenous and rectal administration routes of chemotherapy administration were included. Various trials comparing capacitabine (oral pro-drug of 5-fluorouracil (5-FU))-based radiotherapy with intravenous 5-FU-based radiotherapy have demonstrated similar efficacy [17]. Similarly, tegafur (a pro-drug of 5-FU) when administered in the form of suppositories gets absorbed quickly through the rectal mucosa and reaches the level similar to intravenous tegafur administration [18].
Types of Outcome Measures
-
Primary Outcomes
-
Response rate to chemotherapy either radiological or pathological including histological downstaging, partial and complete pathological response
-
Histological downstaging of rectal tumour (based on the TNM system) in response to chemoradiation predicts long-term survival as an independent prognostic factor [19] and, therefore, is an important outcome. A meta-analysis [20] has shown that patients with partial tumour [21, 22] regression in response to neoadjuvant treatment in rectal cancer had a better disease-free survival than those with no response with decrease in hazard ratio of <50 %. Complete pathological response is defined as the absence of viable tumour cells in a resected specimen and is present in up to 25 % of rectal cancer patients operated following neoadjuvant chemoradiotherapy [21, 22]. Five-year disease-free survival is significantly higher for patients with complete pathological response than the patients without it as shown in a pooled analysis of several studies (83.3 vs. 65.6 %, P < 0.0001) [23].
-
Secondary Outcome Measures
-
Toxicity
-
Survival including overall survival and disease-free survival
-
Recurrence (local and distant)
-
Postoperative mortality and morbidity
-
R0 resection—complete resection with histologically negative margins [24]
-
Language
Only the studies published in English were included in the review.
Search Strategy
Databases Searched
Electronic databases were searched (from database inception to 29 December 2013): Medline, PubMed, Embase, Scopus, Cochrane library and Clinical Trials Register. In addition, abstracts from international cancer meetings such as the American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO) were hand searched from relevant journals.
Search Terms with MeSH Headings: Medline
MeSH headings (1) rectal neoplasm, (2) preoperative period or preoperative care, (3) neoadjuvant therapy or combined modality therapy and (4) drug therapy were combined using Boolean operators ‘AND’ (Appendix 1). In addition, phrases (preoperative chemotherapy) AND (Rectal Cancer) and (neoadjuvant chemotherapy) AND (rectal cancer*) were also searched using Boolean operators ‘AND’ and ‘OR’, and synonyms for each component were also searched. In addition, ‘explode’ feature of the MeSH system was used to include the entire subtree of MeSH terms within a single world.
Selection of Studies and Quality Appraisal
Initial screening of studies based on titles and/or abstracts against the eligibility criteria was conducted (OJ). Appraisal of full text articles for the final selection of studies in the review was conducted by two authors (OJ and TA). The literature search of this review found quantitative studies with different methodologies, so a generic quantitative quality assessment tool developed by Effective Public Health Practice Project (EPHPP), Canada, was used which is suitable for multiple study designs (e.g. randomised controlled trials (RCTs), case-control, cohort (before and after designs): Appendix 2). This tool has been validated to be used in the effectiveness of systematic reviews and has been shown to have construct and content validity [25].
Data Extraction
The data extraction form used in this review was developed (Appendix 3) considering the review objectives and questions and has been adapted from the checklist of items to be considered in data collection and extraction by Higgins and Deeks [26].
Data Synthesis
A narrative synthesis was performed due to the different study designs and statistical heterogeneity.
Results
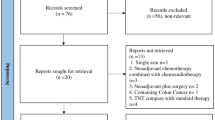
The total number of hits from all the resources was 2289. After the removal of duplicates and non-English articles, 1783 records were returned; 1682 records were excluded on the basis of screening of titles and/or abstracts. One hundred fifty-five full text articles were assessed for the eligibility. Of these, 146 did not fulfil the eligibility criteria and were excluded. A randomised study [27] comparing a control group (treated with radiotherapy and direct endolymphatic chemotherapy) with an experimental group (treated with intravenous ukrain chemotherapeutic agent) was excluded because differences in the outcomes between the two groups could not be attributed due to the confounder factor of endolymphatic chemotherapy in the control group. A total of eight studies were selected for the review. These include one randomised controlled trial, four single-arm published phase II trials, three unpublished single-arm phase II trials and one retrospective case series study. The randomised study included both colonic and rectal cancers but was included in the review because the patients were stratified for either colonic site or rectal cancer site and then randomly allocated to either group. Results of outcomes measured were provided separately for rectal cancers and included 5-year overall survival and DFS. Two ongoing trials were identified by searching the website www.clinicaltrials.gov: PROSPECT and BACCHUS. The four-phase flow diagram of literature search based on the PRISMA statement is shown in Fig. 1.
Description of Studies
The studies included in the review showed considerable heterogeneity clinically and methodologically.
Characteristics of the Patients Included in the Studies
The randomised trial by the chemotherapy study group of Japan [28] included 600 rectal cancer patients <70 years with at least T2–T3 or N+ disease and added preoperative chemotherapy with 5-FU IV infusion for 5 days up until the day before the surgery to postoperative chemotherapy in comparison with postoperative chemotherapy only. Two studies [29, 30] included only T3N− or T3N+ intermediate-risk mid-rectal cancer patients (n = 32 and 46, respectively). Patients were ineligible if the primary tumour was T4, encroaching on the mesorectal fascia, fixed or deemed unresectable. Contrary to this, Uehara et al. [31] only included MRI-defined high- or poor-risk cancers (n = 32) with CRM involved or threatened, tumour infiltrating ≥5 mm into peripheral fat, T4 or any TN2 tumours. Hasegawa et al. [32] included patients with stage either T4 (n = 18) and/or any T with N+ (n = 7). These two Japanese studies [31, 32] along with Fernandez-Martos et al. [29] administered the preoperative chemotherapy regimen—XELOX with bevacizumab. Two studies from the Memorial Sloan-Kettering Cancer Centre, New York [30, 33], administered the combination chemotherapy regimen—FOLFOX with bevacizumab. The later study by Cercek et al. [33] retrospectively reviewed six rectal cancer patients who received preoperative chemotherapy without radiotherapy because they had either refused radiotherapy or had contraindications to it. In addition, this study also included 14 patients with synchronous metastatic colon or rectal cancer treated with preoperative chemotherapy undergoing resection of primary tumour. The phase II trial by Ishii et al. [34] administered 2 cycles of irinotecan, 5-fluorouracil and leucovorin (IFL) neoadjuvant chemotherapy to T3–T4 and or N+ patients. In the trial by Ohwada et al. [35], neoadjuvant chemotherapy was administered in the form of tegafur suppositories—a pro-drug of 5-fluorouracil. Duration of treatment was at least 14 days before surgery and up until the day before surgery. Immediate surgery after the completion of chemotherapy was performed in two studies [28, 35]. Time interval to surgery after the completion of neoadjuvant treatment varied for the rest of the studies ranging from 2 to 8 weeks (Table 1).
Effects of the Intervention
Response Rate
Objective Clinical (Radiological) Response
Three studies stated objective clinical response to neoadjuvant chemotherapy [29–31]. Objective response rate was assessed by measuring tumour diameter on either MRI images [29, 31] or both MRI and endoscopic rectal ultrasonography [30] before and after chemotherapy and reported according to the modified Response Evaluation Criteria in Solid Tumours (RECISTs). On the basis of intention to treat analysis, the objective response rates in these studies were 62.5 % [31], 78.6 % [29] and 93.7 % [30]. Though the protocol of the last two studies [29, 30] allowed chemoradiotherapy to be administered to non-responding patients, no disease progression was detected in both of these studies. In the study by Uehara et al. [31], there was no progression of disease detected in the patients who had surgery (30/32).
Histological Tumour Regression and Pathological Complete Response
The two studies from the Memorial Sloan-Kettering Cancer Centre, New York, reported a higher rate of complete pathological response (cPR) and categorised the tumour response based on the measure of residual viable cancer vs. the measure of fibrotic tissues within the tumour mass proposed by Dworak et al. [36]. One of the two studies by Cercek et al. [33] showed the highest rate of cPR, i.e. 33 % but total number of patients was small (n = 2/6 cases of localised rectal cancer). The other cases in this series had 99, 95 and 90 % response, while the sixth case had minimal response. Overall cPR rate in all 20 patients including synchronous metastatic colorectal cancers was 35 % (n = 7). The second study by Schrag et al. [30] along with the study by Fernandez-Martos et al. [29] reported cPR rates of 25 % (n = 8/32) and 19.6 % (95 % CI 9.4 to 33.9) (n = 9/46), respectively. Both these studies recruited moderate-risk patients. Schrag et al. [30] also reported regression of 80–90 % in 13 cases, 60–70 % in 4, 50 % in 2 cases and <50 % in 5 cases. The four Japanese trials [31, 32, 34, 35] assessed pathological response based on the Japanese Society for Cancer of colon and rectum guidelines. A moderate pathological complete response rate of 13.3 % (n = 4/30) was observed in the Japanese trial by Uehara et al. [31] despite recruiting poor-risk locally aggressive rectal cancers. Tumour regression on histological basis was seen in all 30 cases who underwent surgical resections. But grade 2 or above regression was seen in 11 cases (36.7 %). Ishii et al. [34], Ohwada et al. [35] and Hasegawa et al. [32] reported lower cPR rates of 3.8 % (n = 1/26), 3.9 % (n = 5/129) and 4 %, respectively. In addition to the lower rate of cPR, Ohwada et al. [35] also reported lower grade 2 regression rate of 6.2 %. Almost 90 % of cases in this trial showed either poor or no response. However, despite lower cPR, Hasegawa et al. [32] reported good histological regression (grade 2 or above) rate of 61 %.
Downstaging
Data for overall T downstaging was available for the five studies and ranged from 46 % in the study by Ishii et al. [34] to 69 % in the study by Schrag et al. Fernandez-Martos et al. [29], Uehara et al. [31] and Cercek et al. [33] reported T downstaging rate of 48, 60 and 66 %, respectively. In the study by Uehara et al. [31], 57 % of cases (17/30) were staged cT4 (T4a = 9/T4b = 8). All cT4a patients showed regression, but 63 % of cT4b showed stable disease ypT4b requiring combined resection of adjacent organs to enable R0 resection. Hasegawa et al. [32] reported individual T downstaging in 29, 63 and 50 % cases of T2–3, T4a and T4b tumours, respectively. Nodal downstaging (cN+ to ypN0) rate varied from around 65 % [30, 33, 34] to 83.3 % [31]. Hasegawa et al. [32] reported N downstaging in 86 and 63 % of N1 and N2 cancers, respectively.
Survival and Recurrence
Four studies reported late outcomes in terms of recurrence and survival—overall and disease-free. The randomised study [28] did not show significant differences for either 5-year survival rate (67.2 vs. 69.2 %) or DFS (60.5 vs. 63 %) between the treatment or control group. Ishii et al. [34] reported 5-year overall survival rate of 74 % and disease-free survival of 84 % with median follow-up of 75 months. Recurrence was observed in five cases (three local and two distant). The study by Schrag et al. [30] reported higher 4-year overall survival and disease-free survival in 91 and 84 % of cases, respectively. No local recurrence was reported until the mean follow-up of 53 months. Distant recurrence was observed in 4/32 cases (12.5 %). The study using tegafur suppositories [35] reported 4-year overall survival rate of 80.1 % (95 % CI 72.3–87.9) and disease-free survival rate of 67.6 % (95 % CI 59.4–76.8). This study showed the highest overall recurrence rate of 24 % (n = 31). Local recurrence rate was 6.2 % (n = 8) but distant metastasis occurred in 23.3 % (n = 30). OS and DFS for all these studies are comparable to the overall survival rate of 63.9 % and DFS rate of 57.5 % in non-metastatic rectal cancer patients treated with chemoradiotherapy [37]. However, this also suggests that these tumours were intermediate risk.
R0 Resection and Postoperative Morbidity
Four studies [30, 32, 34, 35] reported 100 % R0 resection rate. Despite recruiting MRI-defined poor-risk rectal cancers, the study by Uehara et al. [31] achieved R0 resection in 90 % (27/30) of cases. However, this study reported the highest overall incidence of postoperative complications in 13 patients (43.3 %) who develop anastomotic leak (n = 5/18, 27.8 %), wound infection (n = 7), pelvic sepsis (n = 3), ileus (n = 6) and UTI (n = 3). Hasegawa et al. [32] reported postoperative complication in 39 % of cases; however, there are no further details and data available for such complications. Ohwada et al. [35] reported overall postoperative morbidity rate of 32 %. The most frequent complication was wound infection (n = 17) followed by anastomotic leak (n = 7, 9 %), sexual dysfunction (n = 6), neurogenic bladder (n = 5) and intestinal obstruction (n = 3) and bleeding (n = 2). Higher rate of anastomotic leak (9 %) could be due to the fact that there was no construction of defunctioning stoma for sphincter sparing surgery.
Ishii et al. [34] reported the lowest complication rate of 15 %. Four patients developed postoperative complications: two had anastomotic leak and two had wound infection. Schrag et al. [30] reported one postoperative death 17 days after surgery due to renal failure attributed to high output from ileostomy.
Compliance
Compliance reported by five studies ranged from 72 % [32] to 100 % [34]. For the randomised trial [28], a completion rate of 5-FU continuous infusion was 96.8 %. In the trial by Uehara et al. [31] using XELOX-bevacizumab, 29 out of 32 patients (90.6 %) completed the scheduled chemotherapy. Two patients refused to continue because of grade 3 toxicity and the third patient had disease progression. In the study by Schrag et al. [30], 2/32 patients did not complete the neoadjuvant chemotherapy secondary to cardiovascular toxicity (one patient had arrhythmia and the other had angina).
Toxicity
Grade 3–4 toxicities were reported by the five studies and ranged from 2.3 to 39 % [29, 31, 32, 34, 35]. Two studies [31, 34] used the Common Toxicity Criteria of the National Cancer Institute (version 3.0) and one study [35] used the WHO scale. No information was available for the remaining two studies [29, 32]. Ohwada et al. [35] and Ishii et al. [34] reported lower rates of grade 3–4 adverse events (2.3 % n = 3/129 and 3.8 % n = 1/26, respectively). Fernandez-Martos et al. [29], Hasegawa et al. [32] and Uehara et al. [31] reported higher rates of grade 3–4 toxicities: 39, 28 and 25 %, respectively. All these three studies administered the same neoadjuvant regimen, i.e. XELOX with bevacizumab. In the first of these studies, the most frequent adverse event was diarrhoea (15 %) which was also responsible for the two deaths. In the second study, majority of toxicities were of haematological origin (12.5 %) and the last study reported appetite loss (12.5 %) being the most common adverse event.
Assessment of Robustness of the Synthesis
Table 2 shows the grading for the quality of studies in the review. None of the study was rated strong on global rating (where strong is defined as no weak ratings in all six domains). On the global rating, five studies were rated moderate (with one weak rating in any domain) and three studies were rated weak (two or more weak ratings). In addition, limited data and information were available regarding the three studies (all rated weak on global rating) that were only published as conference abstracts [29, 32, 33].
Discussion
The varying response to chemotherapy among the phase II trials included in the review could be attributed to the differences in neoadjuvant chemotherapy regimens and time interval to surgery after the completion of chemotherapy. The randomised trial [28] was unsuccessful in proving the efficacy of neoadjuvant chemotherapy which was its main aim. This could be due to the administration of the single agent 5-FU in a low dose and patients undergoing immediate surgery. Similarly, patients underwent immediate surgery in the trial using tegafur suppositories [35], and results showed low levels of cPR rate of 3.9 % and grade 2 response rates of 6.2 %. This could also be attributed to the different routes of administration as compared to contemporary chemotherapy regimens. Delaying the surgery in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy leads to increasing tumour response [38] and significant downstaging [39].
Studies which use multiple agents were more effective. Specifically, a randomised phase III study [40] comparing the different combinations of irinotecan, 5-FU and oxaliplatin in the treatment of advanced colorectal cancer found significantly superior time to progression, response rate and survival time for fluorouracil, leucovorin and oxaliplatin (FOLFLOX) regimen compared to the IFL combination. There was only one patient out of 26 with cPR in the study using the IFL regimen [34] compared to 8/32 (25 %) [30] and 2/6 (33.3 %) [33] in the two studies by the Memorial Sloan-Kettering Cancer Centre using powerful neoadjuvant chemotherapy comprised of FOLFLOX. Similarly, one of these studies [30] showed T downstaging in 73 % of cases compared to 46 % in the study using IFL regimen.
The rates of grade 3–4 toxicities for the review studies are comparable to those of large randomised phase III trials treating rectal cancers with chemoradiotherapy. Two German trials [3, 41] reported 23–27 % grade 3–4 toxicities in the preoperative group. A pilot phase of an ongoing randomised controlled trial [42] comparing neoadjuvant systemic chemotherapy for 6 weeks with fluorouracil and oxaliplatin vs. no preoperative chemotherapy in operable locally advanced colon cancer reported 34 % grade 3–4 adverse events with neoadjuvant treatment. In the current review, two studies [31, 32] reported high overall postoperative morbidity in 43.3 and 39 % of patients. Anastomotic leak rate was also high in the first study (5/18, 27.8 %). Both these studies administered bevacizumab with chemotherapeutic agents. Many phase II trials have used bevacizumab in varying combinations with chemoradiotherapy/chemotherapy regimens in rectal cancer. Concerns have been raised that bevacizumab may increase postoperative morbidity due to its inhibitory effect on vascular endothelial growth factor in physiological wound healing. However, this evidence is not conclusive and the available phase II trials do not show a significant association between bevacizumab and postoperative morbidity [43].
Limitations of the Literature Review
This review provides an up-to-date synthesis of the best available evidence. However, the main limitation of this review is that only eight English language studies with varying trial designs currently report on this topic as identified by a comprehensive search strategy. Study designs varied from RCT, cohort (before and after) to case series/case reports. Sources of variability among the studies were related to the staging of patients; a lack of standardised neoadjuvant chemotherapeutic regimes among the studies in terms of doses, route and duration; and differences in time interval to surgery after completion of chemotherapy. In addition, there were differences in the tools to assess response rate and toxicity.
Conclusion
This review demonstrates that compliance with neoadjuvant chemotherapy treatment is good, and with acceptable tolerance, it can be effectively administered in moderate-risk rectal cancers achieving a high rate of R0 resection and comparable pathological complete responses. Neoadjuvant systemic chemotherapy alone followed by surgery could provide a good alternative to chemoradiotherapy in moderate-risk rectal cancers without compromising survival outcomes. In addition, this could avoid unnecessary radiotherapy-associated adverse effects. Multiple agent and stronger chemotherapy regimens contribute to further improvement in tumour response, local and distant control. However, these conclusions are mainly based upon a few single-arm phase II trials when patient selection is likely much more relevant for the results than the given therapy. These findings need to be validated by large randomised phase III trials comparing neoadjuvant chemotherapy alone and preoperative chemoradiotherapy for locally advanced rectal cancers.
Abbreviations
- APR:
-
Abdominoperineal resection
- CAPOX:
-
Oxaliplatin and capecitabine
- CIV:
-
Continuous intravenous infusion
- cPR:
-
Complete pathological response
- CRC:
-
Colorectal cancer
- CRM:
-
Circumferential resection margin
- CRT:
-
Chemoradiotherapy
- DFS:
-
Disease-free survival
- 5-FU:
-
5-Fluorouracil
- FLOFOX:
-
Oxaliplatin, fluorouracil (5-FU) and folinic acid
- IFL:
-
Irinotecan, 5-fluorouracil and leucovorin
- ITT:
-
Intention to treat
- IV:
-
Intravenous
- LAR:
-
Low anterior resection
- MRI:
-
Magnetic resonance imaging
- OS:
-
Overall survival
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- RCT:
-
Randomised controlled trial
- RECISTs:
-
Response evaluation criteria in solid tumours
- TPE:
-
Total pelvic exenteration
- XELOX:
-
Oxaliplatin and capacitabine
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C and Parkin DM. GLOBOCAN 2008 v2.0, Cancer incidence and mortality worldwide: IARC Cancer Base No. 10. 2010.
Cancer Research UK. Cancer statistics report: cancer incidence in the UK in 2010. 2013.
Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–40.
Bujko K, Nowacki M, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M. Long-term results of a randomised trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215–23.
Gerard JP, Conroy T, Bonnetain F, Bouche O, Chapet O, Closon-Dejardin M, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24(28):4620–5.
Bosset J, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. EORTC Radiotherapy Group Trial 22921. N Engl J Med. 2006;14(355):1114–23.
Kapiteijn E, Marijnen C, Nagtegaal I, Putter H, Steup W, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345(9):638–46.
Marijnen C, van de Velde C, Putter H, van den Brink M, Maas C, Martijn H, et al. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol. 2005;23(9):1847–58.
Seymour MT, Ferry DR, Meade AM, Thompson L, Griffiths GO, Parmar MK, et al. Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomised controlled trial. Lancet. 2007;370(9582):143–52.
de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18(16):2938–47.
Ribero D, Wang H, Donadon M, Zorzi D, Thomas MB, Eng C, et al. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin‐based chemotherapy for colorectal liver metastases. Cancer. 2007;110(12):2761–7.
Glynne-Jones R, Chau I. Neoadjuvant therapy before surgical treatment. Eur J Cancer Suppl. 2013;11(2):45–59.
Dewdney A, Cunningham D, Tabernero J, Capdevila J, Glimelius B, Cervantes A, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C). J Clin Oncol. 2012;30(14):1620–7.
Chau I, Allen M, Cunningham D, Tait D, Brown G, Hill M, et al. Neoadjuvant systemic fluorouracil and mitomycin C prior to synchronous chemoradiation is an effective strategy in locally advanced rectal cancer. Br J Cancer. 2003;88(7):1017.
Chua YJ, Barbachano Y, Cunningham D, Oates JR, Brown G, Wotherspoon A, et al. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol. 2010;11(3):241–8.
Glynne-Jones R, Anyamene N, Moran B, Harrison M. Neoadjuvant chemotherapy in MRI-staged high-risk rectal cancer in addition to or as an alternative to preoperative chemoradiation? Ann Oncol. 2012;23(10):2517–26.
Fernandez-Martos C, Nogue M, Cejas P, Moreno-Garcia V, Machancoses AH, Feliu J. The role of capecitabine in locally advanced rectal cancer treatment: an update (clinical report). Drugs. 2012;72(8):1057.
Galandiuk S, Wrightson W, Marr L, Myers S, LaRocca R. Suppository delivery of 5-fluorouracil in rectal cancer. Ann Surg Oncol. 1996;3(3):270–6.
Dhadda AS, Dickinson P, Zaitoun AM, Gandhi N, Bessell EM. Prognostic importance of Mandard tumour regression grade following pre-operative chemo/radiotherapy for locally advanced rectal cancer. Eur J Cancer. 2011;47(8):1138–45.
Lee Y, Hsieh C, Chuang J. Prognostic significance of partial tumor regression after preoperative chemoradiotherapy for rectal cancer: a meta-analysis. Dis Colon Rectum. 2013;58(9):1093–101.
Smith F, Waldron D, Winter D. Rectum-conserving surgery in the era of chemoradiotherapy. Br J Surg. 2010;97(12):1752–64.
Garcia-Aguilar J, Smith D, Avila K, Bergsland E, Chu P, Krieg RM. Optimal timing of surgery after chemoradiation for advanced rectal cancer: preliminary results of a multicenter, nonrandomized phase II prospective trial. Ann Surg. 2011;254(1):97–102.
Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo L, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11(9):835–44.
Greene F, Page D, Fleming I, et al., editors. Colon and rectum. In: AJCC cancer staging manual. 6th ed. Springer, New York: American Joint Committee on Cancer; 2002. p. 113–123.
Armijo‐olivo S, Stiles CR, Hagen NA, Biondo PD, Cummings GG. Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: methodological research. J Eval Clin Pract. 2012;18(1):12–8.
Higgins J, Deeks JJ. Selecting studies and collecting data. In: Higgins J, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 ed.: The Cochrane Collaboration; 2011.
Bondar G, Borota A, Yakovets Y, Zolotukhin S. Comparative evaluation of the complex treatment of rectal cancer patients (chemotherapy and X-ray therapy, Ukrain monotherapy). Drugs Exp Clin Res. 1998;24(5–6):221–6.
Colorectal Cancer Chemotherapy Study Group of Japan. Results of a randomized trial with or without 5-FU-based preoperative chemotherapy followed by postoperative chemotherapy in resected colon and rectal carcinoma. Jpn J Clin Oncol. 2003;33(6):288–96.
Fernandez-Martos C, Estevan R, Salud A, Pericay C, Gallen M, Sierra E, et al. Neoadjuvant capecitabine, oxaliplatin, and bevacizumab (CAPOX-B) in intermediate-risk rectal cancer (RC) patients defined by magnetic resonance (MR): GEMCAD 0801 trial. Journal of Clinical Oncology, 2012 ASCO Annual Meeting Abstracts 2012; vol 30 (No 15_suppl (May 20 Supplement)): Abstract no. 3586.
Schrag D, Weiser M, Goodman K, Gonen M, Hollywood E, Cercek A, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol. 2014;32(6):513–8.
Uehara K, Hiramatsu K, Maeda A, Sakamoto E, Inoue M, Kobayashi S, et al. Neoadjuvant oxaliplatin and capecitabine and bevacizumab without radiotherapy for poor-risk rectal cancer: N-SOG 03 phase II trial. Jpn J Clin Oncol. 2013;43(10):964–71.
Hasegawa J, Tsunekazu M, Ho MK, Yasuhiro M, Hiroyoshi T, Hiroshi T, et al. Neoadjuvant capecitabine and oxaliplatin (XELOX) with bevacizumab for locally advanced rectal cancer. J Clin Oncol (meeting abstracts). 2013;31(No 4_suppl (February 1 Supplement)): Abstract number:566.
Cercek A, Weiser M, Goodman K, Reidy D, Wong W, Guillem J, et al. Complete pathological response in the primary of rectal or colon cancer treated with FOLFOX without radiation. J Clin Oncol (ASCO meeting abstracts). 2010;28(28 (15S Suppl, May 20 Supplement)):Abstract no. 3649.
Ishii Y, Hasegawa H, Endo T, Okabayashi K, Ochiai H, Moritani K, et al. Medium-term results of neoadjuvant systemic chemotherapy using irinotecan, 5-fluorouracil, and leucovorin in patients with locally advanced rectal cancer. Eur J Surg Oncol. 2010;36(11):1061–5.
Ohwada S, Sato Y, Izumi M, Kashiwabara K, Ogawa T, Hamada K, et al. Preoperative tegafur suppositories for resectable rectal cancer: phase II trial. Dis Colon Rectum. 2005;49(10):1602–10.
Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Color Dis. 1997;12(1):19–23.
Ceelen W, Fierens K, Van Nieuwenhove Y, Pattyn P. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer: a systematic review and meta‐analysis. Int J Cancer. 2009;124(12):2966–72.
Johnson C, Wei C, Ensor J, Smolenski D, Amos C, Levin B, et al. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control. 2013;24(6):1207–22.
Francois Y, Nemoz C, Baulieux J, Vignal J, Grandjean J, Partensky C, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17(8):2396.
Goldberg R, Sargent D, Morton R, Fuchs C, Ramanathan R, Williamson S, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22(1):23–30.
Rödel C, Liersch T, Becker H, Fietkau R, Hohenberger W, Hothorn T, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13(7):679–87.
Foxtrot Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol. 2012;13(11):1152–60.
Cheon EC, Small W, Strouch MJ, Krantz SB, Rademaker A, Mulcahy MF, et al. The effects of bevacizumab on postoperative complications in patients undergoing colorectal and pancreatic cancer resection. J Surg Oncol. 2010;102(5):539–42.
Conflict of Interest
All the authors declare no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1 Search Terms with MeSH Headings: Medline
-
1.
Rectal neoplasm (used for tumour rectal, neoplasm rectal, neoplasms rectal, rectal tumours, rectal neoplasms, cancers rectal, cancer rectum, rectum neoplasms, rectal neoplasm, rectal cancer, neoplasm rectum, rectum neoplasm, cancer of rectum, cancer rectal, cancer of the rectum, tumours rectal, neoplasms rectum, rectum cancer, rectal cancers, cancers rectum, rectum cancers and rectal tumour)
-
2.
Preoperative period (used for period preoperative) or preoperative care (used for preoperative procedure, procedures preoperative, procedure preoperative, preoperative procedure, preoperative care, care preoperative)
-
3.
Neoadjuvant therapy (therapies neoadjuvant, treatments neoadjuvant, neoadjuvant treatments, therapy neoadjuvant, neoadjuvant treatment, neoadjuvant therapy, treatment neoadjuvant and neoadjuvant therapies) or combined modality therapy (treatment multimodal, therapy combined modality, treatments multimodal, multimodal treatment, combined modality therapy, combined modality therapies, modality therapy combined, multimodal treatments, modality therapies combined, therapies combined modality)
-
4.
Drug therapy (used for chemotherapies, therapy drug, chemotherapy, drug therapy, therapies drug pharmacotherapy, drug therapies, pharmacotherapies)
Appendix 2 Quality Assessment Tool for Quantitative Studies




Appendix 3
Rights and permissions
About this article
Cite this article
Jalil, O., Claydon, L. & Arulampalam, T. Review of Neoadjuvant Chemotherapy Alone in Locally Advanced Rectal Cancer. J Gastrointest Canc 46, 219–236 (2015). https://doi.org/10.1007/s12029-015-9739-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-015-9739-7