Abstract
Purpose of Review
This review offers an evidence-based analysis of established and emerging cardiovascular magnetic resonance (CMR) techniques used to assess the severity of primary mitral regurgitation (MR), identify adverse cardiac remodeling and its prognostic effect. The aim is to provide different insights regarding clinical decision-making and enhance the clinical outcomes of patients with MR.
Recent Findings
Cardiac remodeling and myocardial replacement fibrosis are observed frequently in the presence of substantial LV volume overload, particularly in cases with severe primary MR. CMR serves as a useful diagnostic imaging modality in assessing mitral regurgitation severity, early detection of cardiac remodeling, myocardial dysfunction, and myocardial fibrosis, enabling timely intervention before irreversible damage ensues.
Summary
Incorporating myocardial remodeling in terms of left ventricular (LV) dilatation and myocardial fibrosis with quantitative MR severity assessment by CMR may assist in defining optimal timing of intervention.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mitral regurgitation (MR) is a common valvular heart disease both in the United States and worldwide with a prevalence of more than 10% in the population older than 75 years of age [1,2,3]. Population-based outcomes studies have demonstrated that MR independently increases the risk of heart failure, arrhythmia, and mortality [4,5,6,7]. The clinical prognosis associated with mitral regurgitation (MR) is directly related to the severity of valvular dysfunction [8]. Typically, MR is classified as either primary (degenerative) or secondary (functional). In primary MR, the defect is primarily in the mitral valve apparatus, whereas secondary MR is believed to be the result of ventricular or atrial dysfunction/dilation [9]. Primary MR has seen substantial changes in recent years. The worldwide prevalence of primary MR has grown, affecting approximately 24.2 million people with greater incidence due to population aging [10]. Chronic primary MR in developed nations is predominantly caused by mitral valve prolapse (MVP) [10]. MVP is defined as atrial displacement of one or both mitral valve leaflets by more than 2 mm above mitral annulus [11]. MVP can manifest as a spectrum ranging from fibroelastic deficiency (seen in older patients with deficiency of connective tissues leading to rupture of chordal attachments without marked myxomatous changes) to Barlow's disease (seen in younger patients with significant multi-scallop myxomatous degeneration involving one or both leaflets with marked tissue redundancy). Although MVP without significant MR was originally thought to be a benign condition with a prognosis more or less equivalent to the general population, emerging data suggested that MVP is associated with left ventricular (LV) remodeling leading to heart failure, ventricular arrhythmia, and even sudden cardiac death, independent of the severity of mitral regurgitation with an estimated annual risk of 0.2–1.9% [12].
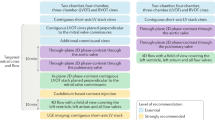
Importance and Indications of CMR in Primary MR Evaluation
When evaluating individuals with mitral valve disease, clinicians initially rely on transthoracic echocardiography (TTE) as the main imaging modality for both assessment and longitudinal follow up. The evaluation of mitral regurgitation can be effectively determined by gathering and combining data on valve morphology and function, cardiac chamber size, wall thickness, ventricular function, and estimates of pulmonary artery pressures. Interventional treatments such as MV repair or replacement can be effective at reducing mitral regurgitation, but long-term success and procedural risk depend primarily on optimal timing. Delayed intervention may increase perioperative mortality and impair procedural effectiveness due to LV or left atrial remodeling affecting mitral apparatus geometry or wall stress. As a result, recent guidelines recommend intervention for patients with severe primary MR if symptoms are present or, in the case of asymptomatic individuals, if LV dysfunction (ejection fraction ≤ 60%) or chamber dilation (end-systolic diameter ≥ 40 mm) are evident. However, despite these guidelines, one-fifth of individuals diagnosed with severe primary MR persistently have reduced ejection fraction and an elevated susceptibility to heart failure following surgical intervention [13]. The data presented in our review prompts inquiries regarding the exclusive reliance on 2D echocardiography-based measurements and ejection fraction for surgical intervention. The role of cardiac magnetic resonance (CMR) has been acknowledged in recent guidelines by the American Society of Echocardiography (ASE), in collaboration with the Society for Cardiovascular Magnetic Resonance (SCMR) [14].
In certain cases, variations in body habitus or the concurrent existence of lung disease can lead to suboptimal acoustic windows during echocardiography. This can subsequently result in technically challenging studies. Furthermore, it is worth noting that in certain individuals, the data obtained from clinical history, physical examination, or other diagnostic procedures may not align with the echocardiographic results and therefore the need for another tool to validate or interpret the findings.
In these scenarios, there exists a significant clinical application for CMR [15]. CMR offers the advantage of being free from ionizing radiation and, in some cases, does not require the use of contrast agents. The aims of a comprehensive CMR study in assessing mitral regurgitation can be categorized into three main objectives: first, to accurately measure the severity of regurgitation; second, to gain understanding of the underlying mechanism causing the regurgitation; and third, to determine the impact of the regurgitant lesion on cardiac remodeling (e.g. left ventricular volumes, left ventricular systolic function, left ventricular mass, left atrial volume, right ventricular volumes and function and myocardial fibrosis). Aside from myocardial fibrosis or late gadolinium enhancement (LGE) assessment, this information can be acquired without using intravenous (IV) contrast agents. Hence, CMR can be effectively conducted in individuals who have significant renal impairment.
Evaluation of Primary MR by CMR
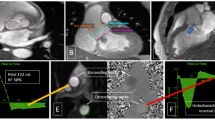
Quantification of Severity of Mitral Regurgitation (Central Illustration, Panel A, Fig. 1A)
For mitral regurgitation evaluation, a balanced steady-state free precession (b-SSFP) pulse sequence is used to obtain a full set of sequential short-axis (every 8–10 mm from base to apex) and long-axis cine images (usually conventional 2-chamber, 3-chamber, and 4-chamber views). With a typical spatial resolution of 1.5–2.0 mm per pixel and a slice thickness of 6–8 mm, this sequence has an elevated blood-to-myocardium contrast and signal-to-noise ratio. This ultrafast pulse sequence achieves temporal resolution of 25–35 ms (30–40 frames/s) in a 5–6 seconds breath hold that is tolerated by the majority of patients with severe valvular disease. Those who have difficulty holding their breath or have significant arrhythmias can undergo imaging using a "real-time" pulse sequence with parallel imaging. This dataset can calculate ventricular stroke volume and ejection fraction by planimetry of left and right ventricular volumes at end-diastole and end-systole and would be the first step in calculating MR severity [16].
The second step involves the velocity-encoded or phase-contrast cine CMR pulse sequence, which is the ideal imaging sequence for measuring flow and computing velocities. Protons moving along a magnetic field gradient experience a phase shift in comparison to stationary spins [17]. The net phase shift is directly proportional to the velocity of the moving protons. Magnitude images and phase velocity maps are produced using phase-contrast CMR. The magnitude image is used to anatomically align the imaging slice and to locate the vessel boundaries. The velocity within each pixel is encoded via the phase map. A region of interest can be traced on each time frame of the data set using both images. A commercially available post processing software solution can integrate the antegrade and retrograde flows through a location of interest using this data to determine flows. Phase-contrast CMR has been demonstrated to be particularly accurate for detecting antegrade and retrograde flow across semilunar valves and is thus the technique used to measure aortic or pulmonic insufficiency [16, 18, 19]. Because of the substantial movement of the mitral annulus during systole relative to the flow imaging plane, this approach is not as useful for mitral inflow and MR quantification. As a result, mitral regurgitation volume is typically quantified using the indirect method (detailed below).
In patients with mitral regurgitation, the total left ventricular stroke volume increases and is equal to the aortic forward stroke volume (antegrade flow) plus the mitral regurgitant volume (retrograde flow). Subtracting the aortic forward flow (step 2) from the left ventricular stroke volume (step 1) would result in the mitral regurgitation volume. This technique provides accurate calculations in the presence of isolated mitral regurgitation as well as coexisting aortic insufficiency, as the aortic insufficiency volume component is part of both the left ventricular stroke volume and the aortic forward flow, and hence would cancel out when we subtract those two values.
In a study of 109 asymptomatic patients with echocardiographic moderate or severe MR, CMR quantification of MR was associated with the development of symptoms or with future need for MV surgery and was superior to echocardiographic grading of regurgitation [20]. Quantitative measures of MR (mitral regurgitant volume and fraction) had a high area under the curve with good sensitivity and specificity to predict early surgery more than CMR derived LV volumetric indexes. Cox regression analysis showed independent associations for regurgitant volume and for regurgitant fraction if assessed separately from regurgitant volume. For regurgitant volume, the optimal threshold was 55 ml and for regurgitant fraction, the optimal threshold was 40% [20].
A more direct method to detect severity of MR was identified by Buchner et al. who revealed that cine CMR anatomical regurgitant orifice area (CMR-AROA) had a strong correlation with catheterization derived angiographic grading of mitral regurgitation severity (r = 0.81) with a CMR-ARO of more than 0.40 cm2 having a 94% sensitivity and specificity for identification of Sellers angiographic grade 3 or 4 mitral regurgitation [21].
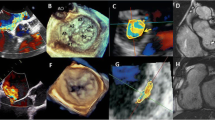
Evaluation of the Mechanism of Primary Mitral Rregurgitation (Central Illustration, Panel B, Fig. 1B)
Transesophageal echocardiography (TEE) is acknowledged for its effective evaluation of mechanism of valve dysfunction and is considered the most suitable technique to identify the specific structural lesion causing mitral regurgitation. However, it is operator dependent and is considered as a semi-invasive technique usually requiring patient sedation and therefore, it is less desirable by some patients. Hence, CMR, when used optimally, may play a role in assessing the mechanism of primary MR in patients in whom TTE has not provided adequate imaging or TEE was considered too invasive. The commonly obtained CMR imaging planes alone are seldom sufficient to determine the cause of MR, therefore necessitating a specific series of sequences that need both time and expertise [22]. A high resolution, small field of view cine-CMR of the mitral valve scallops is used to evaluate mechanism of MR using steady-state free-precession sequence with typical flip angle of 45 to 85 degrees, repetition time of 3.0 ms, echo time of 1.3 ms, reconstructed in-plane spatial resolution of 0.6 mm, slice thickness of 4 to 5 mm, and temporal resolution of ~ 25 ms to acquire a stack of high-resolution cine-CMR images in “en-face” views as well as a stack of 3-chamber views perpendicular to the short axis of the valve to completely visualize all individual mitral scallops (A1 to P1, A2 to P2, and A3 to P3) [22].
Few studies exist on assessing the accuracy and reproducibility of CMR in defining mechanism of primary MR compared to TTE and/or TEE. In a study comparing the sensitivity of CMR to 2DTTE to detect diseased or prolapsed leaflets using high resolution MV sequences, CMR could detect posterior MV leaflet prolapse with 100 percent sensitivity and anterior mitral valve leaflet prolapse with 78 percent sensitivity when compared with 2D TTE; however, the study did not compare the accuracy of these findings to surgical findings [11]. In a second study involving 27 patients, CMR correctly identified segmental defects in 82% of the time compared to 2D TTE [23]. CMR and 2D TEE results were concordant in diagnosing prolapse and identifying the abnormal leaflet in 43 patients using surgical findings as the reference standard, but no information was provided regarding the identification of the abnormal scallop [24]. Correct identification of not only the defective leaflet but also the specific scallop is now a prerequisite for preoperative assessment of primary MR, and the degree of accuracy and quantity of information provided by 3D TEE considerably surpasses that of CMR in this regard. In addition, for pre-operative planning, CMR cannot yet provide the same high-quality enface view as 2D and 3D echocardiography. Hence, 2D and 3D TEE remained the reference approach for the anatomic and functional assessment of the MV apparatus in primary MR, allowing for precise localization of the abnormality and determination of its etiology, which is essential for planning and guiding intervention.
Evaluation of Myocardial Remodeling (Central Illustration, Panel C, Fig. 1C) (Table 1)
Left Ventricular and Left Atrial Remodeling
CMR is the diagnostic imaging modality of choice for accurately assessing ventricular volumes in patients with primary MR patients, especially those with MVP. As previously mentioned, after obtaining the set of short and long axis cine images using b-SSFP pulse sequence covering both ventricles from the base to apex, endocardial and epicardial contours can be manually traced to measure LV/RV end systolic, end diastolic volumes, stroke volumes as well as total myocardial mass identifying any degree of chamber enlargement or myocardial hypertrophy. The basal slice at the end of systole should be at the mitral annulus (top of the muscle) and not at the mitral valve leaflets, particularly in patients with bileaflet mitral valve prolapse [25]. CMR is a well-established method for quantifying LV dimension and EF, with high reproducibility and without the need for geometric assumptions, considering the parameters that elicit MR intervention. In a series of 258 asymptomatic patients with at least moderate MR, MRI derived LV end-systolic volume (LVESV) index, regurgitant volume and MRI derived category (moderate vs severe) were independent predictors of all-cause mortality [26]. In the same study, MRI derived regurgitant volume showed the largest area under the curve to predict mortality, need for MV surgery or both [26]. CMR-derived LVESV index had a higher predictive value than echo-derived LV end- systolic diameter for survival without mitral surgery [27].
Patients with primary MR frequently suffer from left atrial (LA) remodeling, caused by volume overload and subsequent atrial dilation triggering a cascade of pathways that end up in formation of atrial fibrosis as part of atrial remodeling process. Using CMR, LA remodeling will be assessed by measuring LA volume and LA geometry. LA volume (ml) is calculated from two- and four-chamber cine images using the biplanar method at end-systole. Accordingly, LA volumes are divided by body surface area (BSA) to calculate LA volume index (LAVI) [28].
The prolapse volume (PV) is a dome-shaped systolic blood volume between the mitral annulus and the prolapsed leaflets, causing a non-regurgitant volume overload in the LV and the LA that can account for the LV enlargement out of proportion to the MR in patients with MVP, particularly in Barlow's disease. Our lab first demonstrated the importance of PV in MVP where it was identified as an independent and significant predictor of LA and LV dilation in a multivariate analysis [29]. Levy et al. also had similar results independently, except for unexpected significant remodeling of RV size and function irrespective of PV [30] warranting further research in that field. Given this information, this gives CMR an extra advantage on 2D TTE in accurately assessing MR severity and LV volume load. In 2D TTE, proximal isovelocity surface area (PISA) method is mostly used capturing only the transvalvular MR jet crossing the mitral valvular plane and ignoring the prolapse volume, leading to underestimation of LV volume load.
CMR to Assess Myocardial Tissue Composition in Primary Mitral Regurgitation
Chronic volume overload induced by primary mitral regurgitation causes remodeling of the LV. Myocardial fibrosis, the defining characteristic of LV remodeling, is a consequence of extracellular matrix alterations involving collagen I deposition and loss of myofibers at a later stage [31]. The ability to non-invasively assess the myocardial composition is a unique feature of CMR. CMR imaging techniques permit direct and indirect evaluation of myocardial fibrosis. T1 mapping and late gadolinium enhancement (LGE) permit myocardial tissue characterization and provide measures of direct myocardial fibrosis, Recent studies had identified two types of myocardial fibrosis occurring in patients with primary MR. Replacement fibrosis is more common in MVP patients than those without MVP, and it is more commonly located close to papillary muscle traction sites such as the inferior, inferolateral, and anterolateral LV walls. There is growing evidence of association of replacement myocardial fibrosis with malignant ventricular arrhythmia (MVA) and sudden cardiac death, as would be mentioned later in the review. Diffuse interstitial fibrosis had similar incidence in both MVP and non-MVP patients. Interstitial fibrosis had a direct relationship to the severity of MR and can be quantified by myocardial extracellular volume (ECV) by T1 mapping.
A) Late Gadolinium Contrast Enhanced CMR
LGE CMR is the most potent non-invasive modality for quantifying myocardial scar and replacement fibrosis. The increased extracellular space results in a greater volume of distribution and a protracted wash-out of gadolinium [32]. Ten to twenty minutes after intravenous administration of gadolinium, mid- to late-diastole 2D segmented inversion recovery (Gradient Echo GRE or Phase Sensitive Inversion Recovery PSIR) images are acquired. The inversion time is selected to null the normal myocardium and provide optimal tissue contrast between fibrous tissue, which appears bright, and normal myocardium, which appears black. In cases of irregular rhythm or difficulties in breath holding, single shot imaging can be acquired because it is less susceptible to arrhythmias and breathing artifacts as well as shorter acquisition times for patients with difficulties with breath holding. The caveat is that single shot imaging has lower spatial and temporal resolution compared to segmented images which are acquired along multiple cardiac cycles. Due to the low scar-to-blood contrast of these conventional sequences, it can be challenging to detect subtle papillary muscle fibrosis/ hyperenhancement adjacent to the blood pool which is frequently seen with MVP. Various novel “dark-blood” LGE approaches have been proposed to increase scar-to-blood contrast and improve subendocardial scar imaging, particularly that of papillary muscle. Most of these methods use additional magnetization preparation mechanisms to either suppress the blood pool signal partly (gray-blood techniques) or null the signal completely (black-blood techniques). These mechanisms include T2 preparation, magnetization transfer, and spin-locking in concert with the standard inversion pulse, and utilization of multiple inversion pulses [33,34,35].
Several recent studies have highlighted the prognostic implication of myocardial fibrosis in the setting of primary MR in patients with MVP (Table 1). Basso et al. revealed a prevalence of 93% of LV fibrosis in patients with MVP-related SCD and MVP with complex ventricular arrhythmias [12]. In a study conducted by Kitkungvan et al., patients with MVP and evidence of LGE experienced a 7.7% rate of fatal arrhythmic events as compared to 2.7% for MVP patients without myocardial fibrosis, and 0.6% for group with primary MR due to non-MVP etiology, highlighting that mitral valve apparatus alterations in MVP are associated with myocardial abnormalities and are considered a pro-fibrotic milieu [36••]. Similarly, in a French study [37••], patients with MVP and replacement fibrosis had a 2.6-fold higher rate of cardiac death, heart failure, atrial fibrillation and arterial embolism or life-threatening ventricular arrhythmias than patients without fibrosis. In a recent study, 41% of patients with MVP and replacement fibrosis developed ventricular arrhythmias after a median follow up period of 1008 days [38]. In a retrospective multicenter study with a total of 474 patients, the authors found that LGE was a predictor of adverse clinical outcome (sustained ventricular tachycardia, SCD or unexplained syncope) [39].
B) T1 Mapping and Extracellular Volume (ECV)
By using T1 mapping sequences, CMR allows quantification of the myocardial extracellular volume (ECV), a quantitative marker of diffuse myocardial fibrosis that correlates with the severity of histological fibrosis [40, 41]. Before and 15 to 20 min after contrast administration, modified look-locker inversion recovery (MOLLI), shortened MOLLI, or equivalent pulse sequences with motion correction are conducted in a single breath hold at the level of the mid-basal and mid-LV short axis [42]. Changes in the myocardial and blood T1 signal (before and after contrast) are used to derive the ECV, which represents the cellular fraction of the blood. The ECV is less dependent on the intensity of the magnetic field than the T1 signal. Treibel et al. [43] have proposed a way to estimate ECV (synthetic ECV) in the absence of a hematocrit sample based on the correlation between the hematocrit and the longitudinal relaxation rate of the blood and it has proven a good correlation with cardiovascular outcomes [43].
Studies have shown myocardial ECV to be correlated with histologically quantified diffuse interstitial myocardial fibrosis in valvular heart diseases [40]. In a large study of 424 patients, diffuse interstitial fibrosis identified by ECV was associated with MR severity, regardless of the etiology of primary MR and was independently associated with symptoms and clinical events either MV surgery or cardiovascular death (e.g., patients with an ECV of ≥ 30% had a higher likelihood of death or need for MV surgery) [44•, 45].
A prospective multicenter study enrolled 104 patients with primary MR who underwent CMR prior to and an average of 8 months after mitral valve repair [46]. A significant decrease in ECV fraction and indexed ECV (surrogates of diffuse interstitial fibrosis DIF), proportional to their preoperative results, was observed after surgery, but not in LGE (replacement fibrosis). These results imply that interstitial fibrosis may be reversible, whereas replacement fibrosis is not. While the preoperative ECV% and LGE burden did not predict LV reverse remodeling after repair, a greater indexed ECV was linked to worse postoperative LV systolic function, characterized by higher indexed LVESV (LVESVi) and lower LVEF [47]. These data highlighted the prognostic value of fibrosis imaging in primary MR and are supportive of early intervention in asymptomatic patients with chronic severe MR.
C) CMR Tagging and Feature Tracking
CMR tagging and feature tracking CMR are newer techniques allowing evaluation of myocardial strain, a functional parameter that indirectly correlates with myocardial fibrosis via assessing the mechanics and the deformation of the myocardium without using contrast agents [48]. CMR tagging is based on modifying the magnetization of myocardial tissue to produce trackable markers within the myocardium, which are visualized as dark lines arranged in a grid pattern. This allows for an immediate visual assessment of myocardial deformation, but a more objective approach and evaluation requires additional post-processing. Recent advances in pulse sequences and image processing have led to an abundance of new labelling techniques [47]. Similar to 2D and 3D echocardiographic speckle tracking, feature tracking CMR relies on the post-processing of standard SSFP cine images. Feature tracking CMR algorithms focus on the endocardial and epicardial borders and detect inward and outward motion at the cavity-tissue interface [49, 50]. From the basic long- and short-axis views, global and segmental longitudinal, circumferential, and radial strain, strain rates, and LV rotational mechanics can be derived. Global strain values appear to be more reproducible and more consistent than segmental values [51,52,53]. The primary advantage of CMR tagging over feature tracking CMR is that the imposed tags are more clearly defined and simpler to track than natural features and are not subject to through-plane displacements, resulting in more reproducible measurements [49], whereas, the primary drawbacks are the requirement for additional complex sequences with limited accuracy when applied to thin myocardium (such as the remodeled, thin-walled LV, the right ventricle, and the atria) and the time-consuming post-processing. However, it is worth mentioning that neither of these novel techniques have been studied for prediction of outcomes of primary MR and additional methodological standardization is a crucial prerequisite for this technique's widespread adoption in clinical practice.
Conclusion
CMR is the modality of choice for accurate assessment of patients with primary MR. CMR can provide information regarding the quantification of MR severity, LV/ LA volumes, LV systolic function and myocardial fibrosis. The prognosis of MR patients is worsened once they have developed symptoms even with normal LV size and systolic function, as surgical mortality is increased. In addition, MV intervention may not ameliorate the pre-formed myocardial fibrosis, emphasizing the significance of early intervention prior to the onset of symptoms. Thus, incorporating myocardial remodeling in terms of left ventricular (LV) dilatation and myocardial fibrosis with quantitative MR severity assessment by CMR may assist in stratifying patients who may benefit from earlier intervention.
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Nishimura RA, Vahanian A, Eleid MF, Mack MJ. Mitral valve disease–current management and future challenges. Lancet. 2016;387(10025):1324–34. https://doi.org/10.1016/S0140-6736(16)00558-4.
Dziadzko V, Clavel MA, Dziadzko M, et al. Outcome and undertreatment of mitral regurgitation: a community cohort study. Lancet. 2018;391:960–9. https://doi.org/10.1016/S0140-6736(18)30473-2.
Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet. 2009;373:1382–94. https://doi.org/10.1016/S0140-6736(09)60692-9.
Ling LH, Enriquez-Sarano M, Seward JB, et al. Clinical outcome of mitral regurgitation due to flail leaflet. N Engl J Med. 1996;335:1417–23. https://doi.org/10.1056/NEJM199611073351902.
Grigioni F, Avierinos JF, Ling LH, et al. Atrial fibrillation complicating the course of degenerative mitral regurgitation: determinants and long-term outcome. J Am Coll Cardiol. 2002;40:84–92. https://doi.org/10.1016/s0735-1097(02)01922-8.
Tribouilloy C, Rusinaru D, Grigioni F, et al. Long-term mortality associated with left ventricular dysfunction in mitral regurgitation due to flail leaflets: a multicenter analysis. Circ Cardiovasc Imaging. 2014;7:363–70. https://doi.org/10.1161/CIRCIMAGING.113.001251.
Rossi A, Dini FL, Faggiano P, et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart. 2011;97:1675–80. https://doi.org/10.1136/hrt.2011.225789.
Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. 2005;352:875–83. https://doi.org/10.1056/NEJMoa041451.
Filsoufi F, Carpentier A. Principles of reconstructive surgery in degenerative mitral valve disease. Semin Thorac Cardiovasc Surg. 2007;19:103–10. https://doi.org/10.1053/j.semtcvs.2007.04.003.
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, Bonny A, Brauer M, Brodmann M, Cahill TJ, Carapetis J, Catapano AL, Chugh SS, Cooper LT, Coresh J, Criqui M, DeCleene N, Eagle KA, Emmons-Bell S, Feigin VL, Fernández-Solà J, Fowkes G, Gakidou E, Grundy SM, He FJ, Howard G, Hu F, Inker L, Karthikeyan G, Kassebaum N, Koroshetz W, Lavie C, Lloyd-Jones D, Lu HS, Mirijello A, Temesgen AM, Mokdad A, Moran AE, Muntner P, Narula J, Neal B, Ntsekhe M, Moraes de Oliveira G, Otto C, Owolabi M, Pratt M, Rajagopalan S, Reitsma M, Ribeiro ALP, Rigotti N, Rodgers A, Sable C, Shakil S, Sliwa-Hahnle K, Stark B, Sundström J, Timpel P, Tleyjeh IM, Valgimigli M, Vos T, Whelton PK, Yacoub M, Zuhlke L, Murray C, Fuster V; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76(25):2982–3021. https://doi.org/10.1016/j.jacc.2020.11.010. Erratum in: J Am Coll Cardiol. 2021 Apr 20;77(15):1958–1959. PMID: 33309175; PMCID: PMC7755038.
Han YC, Peters DC, Salton CJ, Bzymek D, Nezafat R, Goddu B, et al. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC Cardiovasc Imaging. 2008;1:294–303. https://doi.org/10.1016/j.jcmg.2008.01.013.
Basso C, Perazzolo Marra M, Rizzo S, De Lazzari M, Giorgi B, Cipriani A, et al. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation. 2015;132:556–66. https://doi.org/10.1161/CIRCULATIONAHA.115.016291.
Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease The task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2017;38:2739. https://doi.org/10.1093/eurheartj/ehx391.
Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30:303–71. https://doi.org/10.1016/j.echo.2017.01.007.
Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C; ACC/AHA Joint Committee Members; O'Gara PT, Beckman JA, Levine GN, Al-Khatib SM, Armbruster A, Birtcher KK, Ciggaroa J, Deswal A, Dixon DL, Fleisher LA, de Las Fuentes L, Gentile F, Goldberger ZD, Gorenek B, Haynes N, Hernandez AF, Hlatky MA, Joglar JA, Jones WS, Marine JE, Mark D, Palaniappan L, Piano MR, Spatz ES, Tamis-Holland J, Wijeysundera DN, Woo YJ. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Thorac Cardiovasc Surg. 2021;162(2):e183–e353. https://doi.org/10.1016/j.jtcvs.2021.04.002.
Pennell DJ, Sechtem UP, Higgins CB, et al. Clinical indications for cardiovascular magnetic resonance (CMR): consensus panel report. Eur Heart J. 2004;25:1940–65. https://doi.org/10.1016/j.ehj.2004.06.040.
Masci PG, Dymarkowski S, Bogaert J. Valvular heart disease: what does cardiovascular MRI add? Eur Radiol. 2008;18:187–208. https://doi.org/10.1007/s00330-007-0731-x.
Cawley PJ, Maki JH, Otto CM. Cardiovascular magnetic resonance imaging for valvular heart disease: technique and validation. Circulation. 2009;119:468–78. https://doi.org/10.1161/CIRCULATIONAHA.107.742486.
Nayak KS, Nielsen JF, Bernstein MA, et al. Cardiovascular magnetic resonance phase contrast imaging. J Cardiovasc Magn Reson. 2015;17:71. https://doi.org/10.1186/s12968-015-0172-7.
Myerson SG, d’Arcy J, Christiansen JP, Dobson LE, Mohiaddin R, Francis JM, Prendergast B, Greenwood JP, Karamitsos TD, Neubauer S. Determination of Clinical Outcome in Mitral Regurgitation With Cardiovascular Magnetic Resonance Quantification. Circulation. 2016;133(23):2287–96. https://doi.org/10.1161/CIRCULATIONAHA.115.017888.
Buchner S, Debl K, Poschenrieder F, et al. Cardiovascular magnetic resonance for direct assessment of anatomic regurgitant orifice in mitral regurgitation. Circ Cardiovasc Imaging. 2008;1:148–55. https://doi.org/10.1161/CIRCIMAGING.107.753103.
Chan KMJ, Wage R, Symmonds K, Rahman-Haley S, Mohiaddin RH, Firmin DN, et al. Towards comprehensive assessment of mitral regurgitation using cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2008;10:61. https://doi.org/10.1186/1532-429X-10-61.
Gabriel RS, Kerr AJ, Raffel OC, Stewart RA, Cowan BR, Occleshaw CJ. Mapping of mitral regurgitant defects by cardiovascular magnetic resonance in moderate or severe mitral regurgitation secondary to mitral valve prolapse. J Cardiovasc Magn Reson. 2008;10:16. https://doi.org/10.1186/1532-429X-10-16.
Wolff R, Uretsky S. Defining the left ventricular base in mitral valve prolapse: impact on systolic function and regurgitation. Int J Cardiovasc Imaging. 2020;36:2221–7. https://doi.org/10.1007/s10554-020-01927-0.
Hudsmith LE, Petersen SE, Francis JM, Robson MD, Neubauer S. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson. 2005;7:775–82. https://doi.org/10.1080/10976640500295516.
Penicka M, Vecera J, Mirica DC, Kotrc M, Kockova R, Van Camp G. Prognostic implications of magnetic resonance-derived quantification in asymptomatic patients with organic mitral regurgitation: comparison with Doppler echocardiography-derived integrative approach. Circulation. 2018;137:1349–60. https://doi.org/10.1161/CIRCULATIONAHA.117.029332.
Romero Daza A, Chokshi A, Pardo P, Maneiro N, Guijarro Contreras A, Larrañaga-Moreira JM, et al. Mitral valve prolapse morphofunctional features by cardiovascular magnetic resonance: more than just a valvular disease. J Cardiovasc Magn Reson. 2021;23:107. https://doi.org/10.1186/s12968-021-00800-w.
Cameli M, Lisi M, Righini FM, Massoni A, Natali BM, Focardi M, et al. Usefulness of atrial deformation analysis to predict left atrial fibrosis and endocardial thickness in patients undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol. 2013;111(4):595–601. https://doi.org/10.1016/j.amjcard.2012.10.049.
El-Tallawi KC, Kitkungvan D, Xu J, Cristini V, Yang EY, Quinones MA, et al. Resolving the disproportionate left ventricular enlargement in mitral valve prolapse due to barlow disease: insights from cardiovascular magnetic resonance. JACC Cardiovasc Imaging. 2021;14:573–84. https://doi.org/10.1016/j.jcmg.2020.08.029.
Levy F, Iacuzio L, Marechaux S, Civaia F, Dommerc C, Wautot F, et al. Influence of prolapse volume in mitral valve prolapse. Am J Cardiol. 2021;157:64–70. https://doi.org/10.1016/j.amjcard.2021.07.019.
Ahmed MI, Andrikopoulou E, Zheng J, Ulasova E, Pat B, Kelley EE, Powell PC, Denney TS Jr, Lewis C, Davies JE, Darley-Usmar V, Dell’Italia LJ. Interstitial Collagen Loss, Myocardial Remodeling, and Function in Primary Mitral Regurgitation. JACC Basic Transl Sci. 2022;7(10):973–81. https://doi.org/10.1016/j.jacbts.2022.04.014.
Croisille P, Revel D, Saeed M. Contrast agents and cardiac MR imaging of myocardial ischemia: from bench to bedside. Eur Radiol. 2006;16:1951–63. https://doi.org/10.1007/s00330-006-0244-z.
Holtackers RJ, Van De Heyning CM, Chiribiri A, et al. Dark-blood late gadolinium enhancement cardiovascular magnetic resonance for improved detection of subendocardial scar: a review of current techniques. J Cardiovasc Magn Reson. 2021;23:96. https://doi.org/10.1186/s12968-021-00777-6.
Van De Heyning CM, Holtackers RJ, Nazir MS, Grapsa J, Demetrescu C, Pype L, et al. Dark-blood late gadolinium enhancement CMR improves detection of papillary muscle fibrosis in patients with mitral valve prolapse. Eur J Radiol. 2022;147: 110118. https://doi.org/10.1016/j.ejrad.2021.110118.
Kim, et al. Flow-Independent Dark-blood DeLayed Enhancement (FIDDLE): validation of a novel black blood technique for the diagnosis of myocardial infarction. J Cardiovasc Magn Reson. 2016;18(Suppl 1). https://doi.org/10.1186/1532-429X-18-S1-O55.
•• Kitkungvan D, Nabi F, Kim RJ, Bonow RO, Khan MA, Xu J, et al. Myocardial fibrosis in patients with primary mitral regurgitation with and without prolapse. J Am Coll Cardiol. 2018;72:823–34. https://doi.org/10.1016/j.jacc.2018.06.048. Findings from this study showed higher prevalence of LV fibrosis in patients with MVP compared to those without MVP and LV fibrosis may represent a risk marker for cardiac events.
•• Constant Dit Beaufils A-L, Huttin O, Jobbe-Duval A, Senage T, Filippetti L, Piriou N, et al. Replacement myocardial fibrosis in patients with mitral valve prolapse: relation to mitral regurgitation, ventricular remodeling, and arrhythmia. Circulation. 2021;143:1763–74. https://doi.org/10.1161/CIRCULATIONAHA.120.050214. This study showed that replacement myocardial fibrosis detected by LGE on CMR was independently associated with an increase in cardiovascular events (cardiac death, HF, new-onset AF, arterial embolism, and life-threatening ventricular arrhythmia) during follow-up reinforcing the prognostic interest of CMR in MVP/MR.
Nagata Y, Bertrand PB, Baliyan V, Kochav J, Kagan RD, Ujka K, Alfraidi H, van Kampen A, Morningstar JE, Dal-Bianco JP, Melnitchouk S, Holmvang G, Borger MA, Moore R, Hua L, Sultana R, Calle PV, Yum B, Guerrero JL, Neilan TG, Picard MH, Kim J, Delling FN, Hung J, Norris RA, Weinsaft JW, Levine RA. Abnormal Mechanics Relate to Myocardial Fibrosis and Ventricular Arrhythmias in Patients With Mitral Valve Prolapse. Circ Cardiovasc Imaging. 2023;16(4):e014963. https://doi.org/10.1161/CIRCIMAGING.122.014963.
Figliozzi S, Georgiopoulos G, Lopes PM, Bauer KB, Moura-Ferreira S, Tondi L, et al. Myocardial fibrosis at cardiac mri helps predict adverse clinical outcome in patients with mitral valve prolapse. Radiology. 2022;306:112–21. https://doi.org/10.1148/radiol.220454.
De Meester de Ravenstein C, Bouzin C, Lazam S, Boulif J, Amzulescu M, Melchior J, et al. Histological Validation of measurement of diffuse interstitial myocardial fibrosis by myocardial extravascular volume fraction from Modified Look-Locker imaging (MOLLI) T1 mapping at 3 T. J Cardiovasc Magn Reson. 2015;17:48. https://doi.org/10.1186/s12968-015-0150-0.
Fontana M, White SK, Banypersad SM, Sado DM, Maestrini V, Flett AS, et al. Comparison of T1 mapping techniques for ECV quantification. Histological validation and reproducibility of ShMOLLI versus multibreath-hold T1 quantification equilibrium contrast CMR. J Cardiovasc Magn Reson. 2012;14:88. https://doi.org/10.1186/1532-429X-14-88.
Kramer CM, Barkhausen J, Bucciarelli-Ducci C, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson. 2020;22:17. https://doi.org/10.1186/s12968-020-00607-1.
Treibel TA, Fontana M, Maestrini V, Castelletti S, Rosmini S, Simpson J, et al. Automatic measurement of the myocardial interstitium: synthetic extracellular volume quantification without hematocrit sampling. JACC Cardiovasc Imaging. 2016;9:54–63. https://doi.org/10.1016/j.jcmg.2015.11.008.
• Kitkungvan D, Yang EY, El Tallawi KC, Nagueh SF, Nabi F, Khan MA, et al. Extracellular volume in primary mitral regurgitation. JACC Cardiovasc Imaging. 2021;14:1146–60. https://doi.org/10.1016/j.jcmg.2020.10.010. Findings of this study showed that diffuse interstitial fibrosis as inferred by ECV was associated with MR severity, regardless of primary MR etiology and that ECV was independently associated with symptoms related to MR and clinical events.
Kitkungvan D, Yang EY, El Tallawi KC, Nagueh SF, Nabi F, Khan MA, et al. Prognostic implications of diffuse interstitial fibrosis in asymptomatic primary mitral regurgitation. Circulation. 2019;140:2122–4. https://doi.org/10.1161/CIRCULATIONAHA.119.043250.
Liu B, Neil DAH, Bhabra M, Patel R, Barker TA, Nikolaidis N, et al. Reverse myocardial remodeling following valve repair in patients with chronic severe primary degenerative mitral regurgitation. JACC Cardiovasc Imaging. 2022;15:224–36. https://doi.org/10.1016/j.jcmg.2021.07.007.
el Ibrahim SH. Myocardial tagging by cardiovascular magnetic resonance: evolution of techniques–pulse sequences, analysis algorithms, and applications. J Cardiovasc Magn Reson. 2011;13:36. https://doi.org/10.1186/1532-429x-13-36.
Williams LK, Forero JF, Popovic ZB, Phelan D, Delgado D, Rakowski H, Wintersperger BJ, Thavendiranathan P. Patterns of CMR measured longitudinal strain and its association with late gadolinium enhancement in patients with cardiac amyloidosis and its mimics. J Cardiovasc Magn Reson. 2017;19(1):61. https://doi.org/10.1186/s12968-017-0376-0.
Pedrizzetti G, Claus P, Kilner PJ, Nagel E. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J Cardiovasc Magn Reson. 2016;18:51. https://doi.org/10.1186/s12968-016-0269-7.
Schuster A, Hor KN, Kowallick JT, Beerbaum P, Kutty S. Cardiovascular magnetic resonance myocardial feature tracking: concepts and clinical applications. Circ Cardiovasc Imaging. 2016;9:e004077. https://doi.org/10.1161/circimaging.115.004077.
Padiyath A, Gribben P, Abraham JR, et al. Echocardiography and cardiac magnetic resonance-based feature tracking in the assessment of myocardial mechanics in tetralogy of Fallot: an intermodality comparison. Echocardiography. 2013;30:203–10. https://doi.org/10.1111/echo.12016.
Morton G, Schuster A, Jogiya R, Kutty S, Beerbaum P, Nagel E. Inter-study reproducibility of cardiovascular magnetic resonance myocardial feature tracking. J Cardiovasc Magn Reson. 2012;14:43. https://doi.org/10.1186/1532-429x-14-43.
Augustine D, Lewandowski AJ, Lazdam M, et al. Global and regional left ventricular myocardial deformation measures by magnetic resonance feature tracking in healthy volunteers: comparison with tagging and relevance of gender. J Cardiovasc Magn Reson. 2013;15:8. https://doi.org/10.1186/1532-429x-15-8.
Acknowledgements
Joanne Park is a scientific illustrator at Houston Methodist who received her master’s degree in medical illustration at the Rochester Institute of Technology and received her bachelor’s in biology from Boston University. As a medical illustrator and 3D generalist, she collaborates with researchers and allied health services to help illustrate their often dense and cutting-edge research or procedures.
Funding
None.
Author information
Authors and Affiliations
Contributions
A.D, A.B and M.S wrote the main manuscript. A.D and D.M prepared the figures and the table. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Darwish, A., Bersali, A., Saeed, M. et al. Assessing Regurgitation Severity, Adverse Remodeling, and Fibrosis with CMR in Primary Mitral Regurgitation. Curr Cardiol Rep 26, 705–715 (2024). https://doi.org/10.1007/s11886-024-02069-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-024-02069-8