Opinion statement
Mitral valve disease (MVD) related to mitral valve prolapse (MVP), coronary artery disease (CAD), and calcific mitral stenosis, is increasing in prevalence across the USA and Europe in the context of a longer life expectancy and aging population. In developing countries, rheumatic heart disease remains a major cause of MVD. Echocardiography represents the primary diagnostic modality for assessment of the mitral valve (MV). With the implementation of three-dimensional imaging, echocardiography has become an indispensable tool to evaluate the morphology, geometry, and function of the MV apparatus in the pre-operative setting. However, recognition of its limitations and advances in newer technologies have led to a growing interest in other imaging modalities such as cardiac magnetic resonance (CMR). Although still not widely available, CMR is an essential complement to echocardiography, especially when poor image quality, significant variability in flow diameter measurements, and geometric assumptions of flow orifice preclude accurate quantification of mitral regurgitation on echocardiographic images. In addition, CMR can reliably provide quantitative determination of ventricular volumes and function, hence facilitating surgical decision-making when serial linear echocardiographic measurements are discrepant. Finally, CMR assessment of fibrosis using late gadolinium enhancement allows better understanding of the interactions between MVD and the myocardium in both MVP and MVD related to CAD or other myopathy. In this review, we summarize the role of the available imaging modalities in understanding valvular pathology and determining severity of regurgitation or stenosis. Recently published valvular guidelines highlight the importance of monitoring MVD progression and the shift to intervention earlier in the course of disease. In this context, we also discuss the potential role of echocardiography and CMR in identifying early stages of MVD and/or pre-clinical markers of myocardial dysfunction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mitral valve disease (MVD) encompasses a number of specific valvular disorders that differ in etiology, natural history, diagnostic evaluation, and treatment. Over the last century, the primary cause of valvular dysfunction has shifted from rheumatic heart disease (RHD) to degenerative disease in industrialized nations, with an increasing prevalence of MVD across the USA and Europe in the context of a lengthening life expectancy and aging population. In developing countries, however, RHD remains a major cause of MVD. The differences in etiology and in expected natural history of MVD have important implications for diagnosis and management, particularly as options for interventional and surgical repair continue to develop. The major types of MVD are mitral regurgitation (MR) and mitral stenosis (MS), which have approximate prevalence in the USA of 1.7 and 0.1 %, respectively [1].
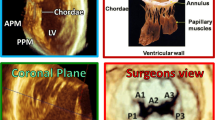
MR can either be due to a primary abnormality of the mitral valve (MV) apparatus or result from another cardiac disorder. The MV apparatus includes two leaflets, chordae tendinae that connect the leaflets to the papillary muscles, papillary muscles that tether the leaflets to the wall of the left ventricle (LV), and the annulus [2]. The most common cause of primary MR requiring surgery is mitral valve prolapse (MVP), which affects 2 to 3 % of the general population [3, 4]. MVP is characterized by myxomatous changes with systolic displacement of one or both MV leaflets of at least 2 mm into the left atrium (LA). Abnormalities in chordal structure can also be found in MVP [3]. Milder, non-diagnostic forms of MVP, first described in the familial context, are also present in the general population, can increase the risk of offspring MVP [5•, 6••]. Other causes of primary MR include RHD, congenital abnormalities, infective endocarditis, traumatic etiologies, and senile calcification of components of the valvular apparatus. The most common cause of secondary or functional MR is ischemic heart disease, which may lead to dilatation of the LV with associated distortion of MV and annular geometry [7]. Other causes of LV dilatation may also result in secondary MR, including other valvular diseases, or dilated cardiomyopathies of different etiologies.
MS is a result of deformation and subsequent immobility of the MV leaflets. Commonly seen in developing countries, rheumatic MS is characterized by fusion of the leaflet commissures, causing restricted mobility or immobility of the posterior leaflet and doming of the anterior leaflet (Videos 1 and 2). In industrialized nations, calcific MS is characterized by calcification of the mitral annulus that extends to the leaflets, leading to functional MS as a result of annular narrowing and impaired leaflet mobility. Commissural fusion is not observed in calcific MS. Rarely, MS can be due to congenital abnormalities of the MV apparatus, including malformation of the annulus, leaflets, papillary muscles, or chordae.
Clinical diagnosis of MVD is guided by reported symptoms and physical examination findings, but determination of severity and suitability for intervention requires further assessment with cardiac imaging. Echocardiography, either transthoracic (TTE) or transesophageal (TEE), represents the primary diagnostic modality for assessment of the MV. However, recognition of its limitations and advances in newer technologies have led to a growing interest in other imaging modalities such as cardiac magnetic resonance (CMR) and computed tomography (CT). In this review, we summarize the current state of knowledge regarding imaging techniques for the diagnostic assessment of MV disorders. We also highlight key areas in which there is ongoing development and discuss future research directions.
Diagnostic assessment
Mitral regurgitation
The role of imaging in MR is twofold: first, to determine the etiology of disease, and second, to quantitatively assess its severity and hemodynamic consequences. Differentiation between primary MR and secondary MR is critical for guiding treatment and management decisions. In primary MR, most commonly due to MVP, the abnormality lies in the MV itself, which is usually amenable to surgical correction. The diagnosis of MVP encompasses a wide spectrum of disease pathology, ranging from Barlow’s disease, which presents relatively early in life and is characterized by multiscallop prolapse, severe mitral annular enlargement, and elongated chordae, to fibroelastic deficiency, which presents later in life and is characterized by unisegmental involvement, only moderate mitral annular dilatation and thin, frequently ruptured chordae [8]. In secondary MR, the mitral leaflets are structurally normal, but spatial relationships between the MV and papillary muscles are altered secondary to LV remodeling [7]. Specifically, LV dilatation and/or isolated infarction of segments underlying the papillary muscles can lead to papillary muscle displacement, tethering of the MV leaflets, and incomplete MV leaflet closure. Since the LV is primarily involved in the pathophysiology, therapeutic decision-making for secondary MR is less clearly defined [7, 9••].
According to the 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease, decisions regarding surgical treatment are primarily based on grading of MR severity, symptoms, and the response of the LV to the MV lesion as depicted in a new four-stage progression classification [9••]. MR grading should be based on the integration of several quantitative parameters, including measurement of vena contracta, regurgitant volume, regurgitant fraction, and effective regurgitant orifice area; other parameters including measurement of the distal jet area and intensity of continuous-wave Doppler assessment should also be considered (Table 1) [9••]. In stage A, risk factors are present, but no significant MR is detected. Stage B or progressive MR is typically mild–moderate by echocardiography and occurs in the absence of symptoms. Stage D and C correspond to severe MR with and without symptoms, respectively. Echocardiographic data is usually obtained by two-dimensional (2D) TTE, but there are specific indications for which three-dimensional (3D) TTE, 2D and 3D TEE, or CMR might be recommended. CT has been shown to be as accurate as echocardiography and CMR in MR assessment [10, 11•, 12, 13] but is not routinely recommended due to its limitations of exposure to radiation and iodinated contrast.
Transthoracic echocardiography
-
Quantification of MR: Current 2D echocardiographic methods of MR quantification are based on complete visualization of the major components of the regurgitant jet: the proximal flow convergence area, the vena contracta, and the area of distal jet expansion in the LA [14].
-
Distal jet area and ratio: The distal jet area is measured using color Doppler flow and used to calculate the ratio of maximal MR jet area to total LA area. This method has excellent sensitivity and specificity, particularly for centrally directed jets, and correlates well with angiography grading of severity [15]. A distal jet area >40 % LA area generally reflects severe MR [9••]. Measurement of the distal jet area varies, however, with loading conditions, and the major limitation to this technique is the underestimation of the severity of eccentric jets.
-
Vena contracta (VC): The VC is the narrowest portion of the MR jet and estimates the diameter of the regurgitant orifice. It should be measured at or just downstream of the regurgitant orifice in the LA, in the parasternal long-axis view to provide adequate spatial resolution and avoid overestimation. This measurement accurately predicts severity of MR when compared to angiography, regardless of etiology or jet eccentricity [16]. A VC width ≥0.7 cm is associated with severe MR [7].
-
Proximal isovelocity surface area (PISA): The PISA method is based on the assumption that systolic mitral flow converging toward the regurgitant MV orifice forms concentric hemispheric shells of decreasing surface area and increasing velocity, such that the flow rate of each shell equals the flow rate across the regurgitant orifice. Effective regurgitant orifice area (EROA) is obtained by dividing the flow rate by the peak velocity of the regurgitant jet, according to the equation EROA = 2π × r 2 × V A / V MAX, where r is radius of the hemispheric convergence zone, V A is the aliasing velocity, and V MAX is the peak regurgitant velocity. The MR regurgitant volume (RVol) and regurgitant fraction (RF) can be further derived from the equations RVol = EROA × VTIMR and RF = RVol / mitral inflow stroke volume = EROA × VTIMR / (CSAMV × VTIMV), where VTIMR is the velocity time integral of the regurgitant jet, CSAMV is the cross-sectional area at the level of the mitral annulus, and VTIMV is the velocity time integral across the MV. Severe primary MR is typically characterized by EROA ≥0.40 cm2, RVol ≥60 mL, and RF ≥50 %. This method has been validated against jet area measurements and angiographic grading of MR severity in several studies but has also been shown to consistently underestimate the EROA [17, 18]. The PISA method relies on the geometric assumption of a hemispheric PISA region and most commonly underestimates the EROA in secondary MR as a result of the crescentic shape of the regurgitant orifice; to account for this difference, the criteria for severe secondary MR include lower cutoffs, specifically EROA ≥0.20 cm2 and RVol ≥30 mL [9••]. These cutoffs also take into account the observation that outcomes in secondary MR are worse at lower measurements of EROA compared to primary MR [19].
-
Volumetric method: Another method to determine RVol and RF uses pulsed-wave Doppler measurements and is based on the difference between stroke volume (SV) across the left-sided valves, according to the equation RVol = mitral inflow SV − aortic outflow SV. As above, mitral inflow SV = CSAMV × VTIMV, where measurements of cross-sectional area and VTI are both obtained at the level of the mitral annulus. Aortic outflow SV is calculated as CSALVOT × VTILVOT. This method assumes that no aortic regurgitation is present and has been validated against other methods of MR quantification [20].
-
-
Stress testing: In patients with chronic primary MR, exercise stress testing with Doppler echocardiography may be useful in asymptomatic patients with severe MR to establish symptom status and exercise capacity and in symptomatic patients where there is a discrepancy between symptoms and severity of MR at rest [9••]. Significantly reduced exercise tolerance, marked exercise-induced increase in MR severity, development of pulmonary hypertension, and exercise-induced RV dysfunction or lack of LV contractile reserve have all been shown to be associated with worse outcomes; these findings should prompt close follow-up and consideration of surgical repair [21–26]. In patients with chronic secondary MR, exercise stress testing is recommended to establish the etiology of disease and assess myocardial viability, specifically to identify patients with ischemic MR who may benefit from revascularization [9••]. Exercise-induced increase in MR severity and pulmonary hypertension are among the factors that have been associated with worse outcomes in secondary MR [27, 28]. In current AHA/ACC guidelines, however, there is no definite threshold for exercise-induced measurements according to which surgery or intervention is recommended in primary or secondary MR [9••].
Transesophageal echocardiography
-
In most situations, 2D TTE can adequately assess the etiology and quantify the severity of MR. 2D TEE (Fig. 1a) is preferred when TTE is suboptimal or technically limited, or when complex, calcified or endocarditic lesions are being evaluated.
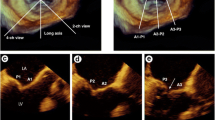
Fig. 1 Example of primary mitral regurgitation (MR) (a–d): Prolapse of the middle posterior mitral valve scallop (P2) shown in a long-axis view of (a) a two-dimensional (2D) transesophageal echocardiogram (TEE) with associated severe, eccentric, anteriorly directed MR [red arrows indicate the vena contracta (VC), yellow arrow indicates the proximal isovolumic surface area (PISA)] and (b) a three-dimensional (3D) TEE en face or surgical view. Echocardiographic multiplanar reconstruction of the VC area and cardiac magnetic resonance (CMR) steady-state free processing (SSFP) short-axis view of regurgitant orifice area are shown in panels c and d, respectively. Example of secondary MR (e–h): Leaflet tenting with significant MR is shown in a long-axis view of a (e) 2D TEE and en face view of a 3D TEE (f). Echocardiographic multiplanar reconstruction of the VC area (g) and CMR SSFP image of the regurgitant orifice (h) show the crescent shape of the orifice in secondary MR. Panels g and h were adapted from reference [38]. AO aorta, LA left atrium, LV left ventricle.
3D echocardiography
-
The 2D echocardiographic methods described above are complemented and enhanced by 3D echocardiography. 3D TTE-guided direct planimetry of the VC cross-sectional area, measurements using the PISA method, and volumetric assessment of EROA have all been compared to 2D echocardiography and shown to be accurate regardless of MR etiology or orifice shape [29–31]. In patients with MVP, 3D TTE provides reliable data regarding anatomic localization of prolapsing scallops with accuracy comparable to that provided by 2D TEE and overall allows better spatial localization of commissures and valvular pathology [32].
-
3D TEE provides a distinct advantage over 2D TEE because of its ability to visualize the entire MV apparatus with more detail. It has been shown to be superior to 2D TEE for accurate localization of pathology, including scallop-specific involvement based on Carpentier’s classification in three anterior segments (lateral A1, middle A2, medial A3) and three posterior segments (lateral P1, middle P2, medial P3) (Fig. 1b). Similarly to 3D TTE, quantification of MR severity is also improved using 3D TEE, which allows direct visualization and planimetry of the VC cross-sectional area by using multiplanar reconstruction software (Fig. 1c, g). This alternate measure of EROA is not affected by orifice shape or jet direction; this may be of particular importance in secondary MR, in which the crescentic regurgitant orifice might otherwise be underestimated by using linear VC (Fig. 1e, g) [33]. Assessment by 3D TEE has been shown to be both feasible and accurate as compared with CMR and may reclassify a significant proportion of patients to a greater MR severity when compared with 2D TEE results [34].
-
Current guidelines highlight the potential benefit of using 3D TTE and 3D TEE for evaluation of MR, particularly in the pre-operative setting, but neither is yet routinely recommended [9••].
Cardiac magnetic resonance
-
CMR represents a novel noninvasive imaging modality that provides detailed anatomic and quantitative information regarding cardiac structure and function. AHA/ACC guidelines recommend the use of CMR to assess LV and RV size and function, as well as severity of MR, in situations where TTE is technically limited [9••].
-
Though data are limited, several studies have indicated that CMR may be useful for establishing MV morphology and pathology. Comparison of CMR with TTE has shown that standard TTE diagnostic criteria for MVP, specifically systolic MV leaflet excursion into the LA of at least 2 mm, may be applied to CMR with high sensitivity and specificity [35]. In the same study, MVP patients with papillary muscle fibrosis by late gadolinium enhancement (LGE) were at higher risk of ventricular arrhythmia on Holter monitoring. In another study of patients with acute myocardial infarction, CMR identified useful correlations between LGE-based infarct distribution and papillary muscle involvement and severity of secondary MR [36•]. Planimetry of the anatomic regurgitant orifice area (AROA) using CMR has been validated against both invasive methods and calculation of EROA using the PISA method with TTE [37]. Assessment of AROA has also confirmed the asymmetric and crescentic shape of the regurgitant orifice in secondary MR, suggesting that it may be possible to distinguish etiologies of MR using parameters of MV geometric anatomy (Fig. 1d, h) [38].
-
The most commonly used CMR method to quantify isolated MR is a combination of velocity-encoded and cine steady-state free processing sequences [39]. Specifically, RVol is calculated according to the equation RVol = LV SV − aortic outflow SV. RF is then obtained as the ratio of RVol and LV SV. Severe MR is typically characterized by a RF >48 % (Table 1). This CMR method has been validated against TTE methods of jet area assessment and volumetric calculations using pulsed-wave Doppler, and similar calculation of RVol index has been shown to correlate well with invasively acquired data during catheterization [40–42]. Most recently, a comparison of CMR with 2D echocardiography, including both TTE and TEE, in the assessment of MR severity after MV surgery showed CMR to be significantly more accurate in quantification of MR severity [43••].
Mitral stenosis
Imaging of MS, like that of MR, is directed at establishing the etiology of disease and its hemodynamic severity, both essential components of determining appropriate medical therapy or suitability for intervention. The hemodynamic severity of MS is classified according to mitral valve area (MVA); the most recent criteria established in the 2014 AHA/ACC Guidelines for the Management of Patients with Valvular Heart Disease define severe MS as MVA ≤1.5 cm2 with and without symptoms (stages D and C, respectively) (Table 2) [9••]. MVA is usually measured non-invasively by 2D TTE, but the use of other imaging modalities such as 3D TTE, 2D and 3D TEE, CMR, and CT is growing. CT assessment of MVA is comparable to echocardiography and CMR [44–47] but is not routinely recommended due to the exposure to radiation and iodinated contrast. Other components of the assessment of MS severity by echocardiography include pressure half time (PHT), which may be considered an independent marker of severity or used to calculate MVA, transmitral mean pressure gradient, and consequences of MS such as changes in LA size and elevation of pulmonary artery systolic pressure.
Transthoracic echocardiography
-
Mitral valve area: MVA can be assessed by direct planimetry, Doppler-derived PHT, the continuity equation, or the PISA method [48].
-
Direct 2D planimetry of MVA is performed in the parasternal short-axis view by tracing the MV orifice at the time of largest opening in diastole, at the level of the smallest orifice, which is usually found at the leaflet tips. Accurate measurement using this method requires proper localization of the true commissural edge and particular attention to instrument settings [49]. 3D TTE provides the ability to slice along any desired plane, thereby providing better localization of the smallest orifice to optimize accuracy and reproducibility of planimetry. Compared to 2D TTE, it has been shown to better correlate with invasive MVA measurements and to have significantly less interobserver variability, both before and after percutaneous intervention [50–54]. Color-defined 3D planimetry may be particularly useful in calcific MS, where the smallest orifice is often found near the annulus and 2D planimetry can be limited by acoustic shadowing [55].
-
The diastolic PHT method is based on the observation that the decrease in velocity of diastolic transmitral flow is inversely correlated with MVA as determined by catheterization data and is therefore directly correlated with the severity of MS [56, 57]. PHT is measured by tracing the deceleration slope of the E wave on a continuous-wave Doppler assessment of the transmitral inflow, and MVA is calculated from measured PHT according to the equation MVA = 220/PHT. This method is widely used due its simplicity and ease of measurement, yet it has important limitations. For example, atrial fibrillation and tachycardia, which decrease diastolic filling time, affect the accuracy and reliability of PHT measurements [58]. The use of PHT is further limited by factors that affect left-sided chamber pressure changes, such as aortic regurgitation, the presence of an atrial septal defect, or any changes in LA or LV compliance [59–62]. These limitations should be given particular consideration in calcific MS, which is more common in older patients, and more likely to be associated with hypertension, atrial fibrillation, diastolic dysfunction, and senile aortic stenosis, all of which can directly or indirectly affect PHT.
-
When direct planimetry is not possible and PHT is technically inadequate, the continuity equation can be used to determine MVA. The continuity method is based on the assumption that SV through the stenotic MV is equal to SV across the left ventricular outflow tract (LVOT). MVA is calculated as the ratio of aortic SV to the MV VTI, according the equation MVA = CSALVOT × VTILVOT / VTIMV, where CSA is cross-sectional area. Similar to the PHT method, the continuity equation should be averaged over several beats in atrial fibrillation to account for beat-to-beat variability in flow and SV. Additionally, differential flow across the MV and LVOT due to mitral or aortic regurgitation may affect the calculation of MVA.
-
The PISA method has been validated for the calculation of MVA in patients with MS [63–67] but is not widely used in clinical practice. In MS, it is based on the assumption that diastolic mitral flow converging toward the MV orifice forms concentric hemispheric shells of increasing velocity when assessed with color Doppler. MVA is calculated by dividing MV flow by the peak mitral inflow velocity, according to the equation MVA = (α / 180) × 2π × r 2 × V A/V MAX, where r is radius of the hemispheric convergence zone, V A is the aliasing velocity, V MAX is the peak mitral inflow velocity, and α is the opening angle of the MV leaflets. Use of 3D TTE may further improve accuracy of the PISA method and has shown better correlation with planimetry and PHT than 2D PISA, with good intra- and interobserver agreement [68••].
-
-
Mean transmitral pressure gradient: Echocardiographic assessment of transmitral pressure gradient using continuous-wave Doppler across the MV is well-correlated with invasively acquired measurements and has been shown to be more accurate than that obtained by conventional catheterization using pulmonary capillary wedge pressure [69]. A gradient of >5 to 10 mmHg generally corresponds to MVA ≤1.5 cm2. As mean transmitral gradient is dependent on transvalvular flow, it may vary with changes in cardiac output or heart rate and is influenced by LA and LV compliance. Mean transmitral gradient is particularly useful in patients with calcific MS, in whom its measurement by TTE may be more accurate than MVA assessment.
-
Stress testing: In patients with MS, dobutamine or exercise testing with Doppler echocardiography is recommended to evaluate the response of the mean mitral gradient and pulmonary artery pressure to exercise when there is a discrepancy between resting Doppler echocardiographic findings and clinical symptoms or signs [9••, 70–72]. The current guidelines do not propose any clear thresholds for exercise-induced measurements above which intervention is strongly recommended but state that in symptomatic patients with MVA >1.5 cm2 and mean transmitral pressure gradient >15 mmHg during exercise, percutaneous mitral balloon commissurotomy (PMBC) may be considered [9••].
-
Wilkins score: Suitability for PBMC is determined by MV morphology and quantification of concomitant MR. The Wilkins scoring system assigns a point value from 1 to 4 for each of the following echocardiographic variables: leaflet mobility, leaflet thickening, valve calcification, and involvement of the subvalvular apparatus [73]. The best outcomes for PBMC have been associated with a Wilkins score of <9 and less than moderate MR.
Transesophageal echocardiography
-
In the majority of patients, imaging by TTE is sufficient to accurately assess MV morphology and function and to quantitatively determine the severity of MS. When TTE is technically limited or suboptimal, TEE is able to provide better imaging of MV morphology. TEE is also recommended in pre-operative planning for MV surgery or in patients being considered for PMBC for two reasons. First, compared to TTE, it has greater sensitivity for detection of LA or LA appendage clot, which must be excluded given the potential risk of peri-procedural embolization with PMBC [74]. Second, TEE provides better assessment and quantification of MR, which is important because significant MR is generally a contraindication to PMBC.
-
3D TEE may provide incremental benefit over standard 2D imaging. MVA planimetry using real-time 3D TEE has been shown to compare favorably to established 2D TTE methods, with less likelihood of MVA overestimation [75, 76]. Furthermore, 3D TEE provides superior anatomic imaging of commissural calcification and fusion (Video 2), which is particularly important in patients undergoing PMBC, as well as detection of post-intervention commissural splitting and leaflet tears [75, 77, 78].
Cardiac magnetic resonance
-
Velocity-encoded CMR can accurately determine severity of MS through measurement of mean transmitral gradient and shows good correlation with transmitral gradient measured by Doppler TTE [79]. Velocity-encoded CMR can also quantify MVA using the PHT method in a manner analogous to that used in TTE; this method is both reproducible and reliable when compared with TTE results [80]. Finally, CMR can be used to perform planimetry of the MV orifice and has been shown to be reliable, despite slightly overestimating MVA when compared to the echocardiographic PHT method and to invasive hemodynamics [81].
-
CMR may be particularly useful in patients undergoing PMBC. When evaluated for its ability to identify LA appendage thrombus in patients with atrial fibrillation, CMR results correlated well with TEE results; though not specifically studied in patients with MS, extrapolation of these results suggests that CMR may be useful for excluding LA and LA appendage clot in patients being considered for PMBC [82]. Additionally, planimetry of MVA by CMR post-intervention correlates well with results from echocardiographic PHT method and invasive hemodynamics and may be a sensitive and reliable method for noninvasive visualization of small changes in MVA, particularly after intervention [83].
-
Additional studies of CMR in MS are needed to validate these small case series and further define the best applications of this modality.
Future research directions
In this review, we summarize the current state of knowledge regarding imaging techniques for the diagnostic assessment of MV disorders, with a focus on the incremental role of 3D echocardiography and the ongoing development of CMR. Imaging techniques are described in the context of the new 2014 AHA/ACC guidelines, which highlight the importance of progression of MVD (stages A to D, Tables 1 and 2) and the shift to intervention earlier in the disease, prior to symptoms and prior to onset of LV remodeling (stage C1). Echocardiographic studies examining the role of earlier markers of myocardial dysfunction such as strain and strain-rate imaging or CMR assessment of diffuse myocardial fibrosis by T1 mapping may help identify individuals with primary MR at risk for progression to severe stages. Advances in the understanding of MVP pathophysiology may lead to new medical therapies aimed at preventing the progression of disease by targeting early, non-diagnostic MVP morphologies or mild diagnostic MVP without MR (stage A) [5•, 6••].
Conclusion
Echocardiography plays a critical role in the assessment and management of MVD. With the implementation of 3D imaging, echocardiography has become an indispensable tool to evaluate the morphology, geometry, and function of the MV apparatus in the pre-operative setting. Although still not widely available, CMR is an essential complement to echocardiography for MR quantification and more accurate assessment of LV volumes and function, both essential aspects of surgical decision-making.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–11.
Otto CM. Textbook of clinical echocardiography. 5th ed. Philadelphia: Elsevier/Saunders; 2013. Print. p 247.
Delling FN, Vasan RS. Epidemiology and pathophysiology of mitral valve prolapse: new insights into disease progression, genetics, and molecular basis. Circulation. 2014;129:2158–70.
Freed LA, Levy D, Levine RA, et al. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. 1999;341:1–7.
Delling FN, Gona P, Larson MG, et al. Mild expression of mitral valve prolapse in the Framingham offspring: expanding the phenotypic spectrum. J Am Soc Echocardiogr: Off Publ Am Soc Echocardiogr. 2014;27:17–23. This article discusses the prevalence and echocardiographic characteristics of early, non-diagnostic forms of mitral valve prolapse (MVP) in the general population.
Delling FN, Rong J, Larson MG, et al. Familial clustering of mitral valve prolapse in the community. Circulation. 2015;131:263–8. This manuscript discusses how non-diagnostic MVP morphologies in parents can increase the risk of offspring MVP, thereby demonstrating that these morphologies may have clinical importance.
Hung JW. Ischemic (functional) mitral regurgitation. Cardiol Clin. 2013;31:231–6.
Fornes P, Heudes D, Fuzellier JF, et al. Correlation between clinical and histologic patterns of degenerative mitral valve insufficiency: a histomorphometric study of 130 excised segments. Cardiovasc Pathol: Off J Soc Cardiovasc Pathol. 1999;8:81–92.
Nishimura RA, Otto CM, Bonow RO, et al. 2014 aha/acc guideline for the management of patients with valvular heart disease: a report of the american college of cardiology/american heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:e57–185. This report from AHA/ACC provides diagnostic and management algorithms for valvular heart disease, and includes updated criteria for the staging and grading of severity of mitral regurgitation (MR) and mitral stenosis (MS).
Delgado V, Tops LF, Schuijf JD, et al. Assessment of mitral valve anatomy and geometry with multislice computed tomography. J Am Coll Cardiol Img. 2009;2:556–65.
Beaudoin J, Thai WE, Wai B, et al. Assessment of mitral valve adaptation with gated cardiac computed tomography: validation with three-dimensional echocardiography and mechanistic insight to functional mitral regurgitation. Circ Cardiovasc Imaging. 2013;6:784–9. This investigation studies CT-based measurements of MV size in patients with functional or secondary MR, and finds the emerging technology of CT to be comparable to 3D echocardiography.
Alkadhi H, Wildermuth S, Bettex DA, et al. Mitral regurgitation: quantification with 16-detector row ct–initial experience. Radiology. 2006;238:454–63.
Guo YK, Yang ZG, Ning G, et al. Isolated mitral regurgitation: quantitative assessment with 64-section multidetector ct–comparison with mr imaging and echocardiography. Radiology. 2009;252:369–76.
Choo WS, Steeds RP. Cardiac imaging in valvular heart disease. Br J Radiol. 2011;84(Spec No 3):S245–57.
Helmcke F, Nanda NC, Hsiung MC, et al. Color doppler assessment of mitral regurgitation with orthogonal planes. Circulation. 1987;75:175–83.
Fehske W, Omran H, Manz M, et al. Color-coded doppler imaging of the vena contracta as a basis for quantification of pure mitral regurgitation. Am J Cardiol. 1994;73:268–74.
Bargiggia GS, Tronconi L, Sahn DJ, et al. A new method for quantitation of mitral regurgitation based on color flow doppler imaging of flow convergence proximal to regurgitant orifice. Circulation. 1991;84:1481–9.
Rivera JM, Vandervoort PM, Thoreau DH, et al. Quantification of mitral regurgitation with the proximal flow convergence method: a clinical study. Am Heart J. 1992;124:1289–96.
Grigioni F, Enriquez-Sarano M, Zehr KJ, et al. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative doppler assessment. Circulation. 2001;103:1759–64.
Sun JP, Yang XS, Qin JX, et al. Quantification of mitral regurgitation by automated cardiac output measurement: experimental and clinical validation. J Am Coll Cardiol. 1998;32:1074–82.
Messika-Zeitoun D, Johnson BD, Nkomo V, et al. Cardiopulmonary exercise testing determination of functional capacity in mitral regurgitation: physiologic and outcome implications. J Am Coll Cardiol. 2006;47:2521–7.
Magne J, Lancellotti P, Pierard LA. Exercise-induced changes in degenerative mitral regurgitation. J Am Coll Cardiol. 2010;56:300–9.
Magne J, Lancellotti P, Pierard LA. Exercise pulmonary hypertension in asymptomatic degenerative mitral regurgitation. Circulation. 2010;122:33–41.
Kusunose K, Popovic ZB, Motoki H, Marwick TH. Prognostic significance of exercise-induced right ventricular dysfunction in asymptomatic degenerative mitral regurgitation. Circ Cardiovasc Imaging. 2013;6:167–76.
Lee R, Haluska B, Leung DY, et al. Functional and prognostic implications of left ventricular contractile reserve in patients with asymptomatic severe mitral regurgitation. Heart. 2005;91:1407–12.
Magne J, Mahjoub H, Dulgheru R, et al. Left ventricular contractile reserve in asymptomatic primary mitral regurgitation. Eur Heart J. 2014;35:1608–16.
Lancellotti P, Gerard PL, Pierard LA. Long-term outcome of patients with heart failure and dynamic functional mitral regurgitation. Eur Heart J. 2005;26:1528–32.
Lancellotti P, Magne J, Dulgheru R, et al. Clinical significance of exercise pulmonary hypertension in secondary mitral regurgitation. The American journal of cardiology. 2015;115:1454–61.
Yosefy C, Hung J, Chua S, et al. Direct measurement of vena contracta area by real-time 3-dimensional echocardiography for assessing severity of mitral regurgitation. Am J Cardiol. 2009;104:978–83.
Zeng X, Levine RA, Hua L, et al. Diagnostic value of vena contracta area in the quantification of mitral regurgitation severity by color doppler 3d echocardiography. Circ Cardiovasc Imaging. 2011;4:506–13.
de Agustin JA, Marcos-Alberca P, Fernandez-Golfin C, et al. Direct measurement of proximal isovelocity surface area by single-beat three-dimensional color doppler echocardiography in mitral regurgitation: a validation study. J Am Soc Echocardiogr: Off Publ Am Soc Echocardiogr. 2012;25:815–23.
Sharma R, Mann J, Drummond L, et al. The evaluation of real-time 3-dimensional transthoracic echocardiography for the preoperative functional assessment of patients with mitral valve prolapse: a comparison with 2-dimensional transesophageal echocardiography. J Am Soc Echocardiogr: Off Publ Am Soc Echocardiogr. 2007;20:934–40.
Altiok E, Hamada S, van Hall S, et al. Comparison of direct planimetry of mitral valve regurgitation orifice area by three-dimensional transesophageal echocardiography to effective regurgitant orifice area obtained by proximal flow convergence method and vena contracta area determined by color doppler echocardiography. Am J Cardiol. 2011;107:452–8.
Shanks M, Siebelink HM, Delgado V, et al. Quantitative assessment of mitral regurgitation: comparison between three-dimensional transesophageal echocardiography and magnetic resonance imaging. Circ Cardiovasc Imaging. 2010;3:694–700.
Han Y, Peters DC, Salton CJ, et al. Cardiovascular magnetic resonance characterization of mitral valve prolapse. J Am Coll Cardiol Img. 2008;1:294–303.
Chinitz JS, Chen D, Goyal P, et al. Mitral apparatus assessment by delayed enhancement cmr: relative impact of infarct distribution on mitral regurgitation. J Am Coll Cardiol Img. 2013;6:220–34. This study of post-myocardial infarction MR demonstrates that CMR is useful for identifying correlations between infarct distribution and severity of MR.
Buchner S, Debl K, Poschenrieder F, et al. Cardiovascular magnetic resonance for direct assessment of anatomic regurgitant orifice in mitral regurgitation. Circ Cardiovasc Imaging. 2008;1:148–55.
Buchner S, Poschenrieder F, Hamer OW, et al. Direct visualization of regurgitant orifice by cmr reveals differential asymmetry according to etiology of mitral regurgitation. J Am Coll Cardiol Img. 2011;4:1088–96.
Kon MW, Myerson SG, Moat NE, Pennell DJ. Quantification of regurgitant fraction in mitral regurgitation by cardiovascular magnetic resonance: comparison of techniques. J Heart Valve Dis. 2004;13:600–7.
Kizilbash AM, Hundley WG, Willett DL, et al. Comparison of quantitative doppler with magnetic resonance imaging for assessment of the severity of mitral regurgitation. Am J Cardiol. 1998;81:792–5.
Gelfand EV, Hughes S, Hauser TH, et al. Severity of mitral and aortic regurgitation as assessed by cardiovascular magnetic resonance: optimizing correlation with doppler echocardiography. J Cardiovasc Magn Reson: Off J Soc Cardiovasc Magn Reson. 2006;8:503–7.
Hundley WG, Li HF, Willard JE, et al. Magnetic resonance imaging assessment of the severity of mitral regurgitation. Comparison with invasive techniques. Circulation. 1995;92:1151–8.
Uretsky S, Gillam L, Lang R, et al. Discordance between echocardiography and mri in the assessment of mitral regurgitation severity: a prospective multicenter trial. J Am Coll Cardiol. 2015;65:1078–88. This study compares CMR imaging with 2D echocardiography and demonstrates the superiority of the newer imaging technique compared to both TTE and TEE for assessment of post-surgical MR severity.
Mahnken AH, Muhlenbruch G, Das M, et al. MDCT detection of mitral valve calcification: prevalence and clinical relevance compared with echocardiography. AJR Am J Roentgenol. 2007;188:1264–9.
Willmann JK, Kobza R, Roos JE, et al. ECG-gated multi-detector row CT for assessment of mitral valve disease: initial experience. Eur Radiol. 2002;12:2662–9.
LaBounty TM, Glasofer S, Devereux RB, et al. Comparison of cardiac computed tomographic angiography to transesophageal echocardiography for evaluation of patients with native valvular heart disease. Am J Cardiol. 2009;104:1421–8.
Messika-Zeitoun D, Serfaty JM, Laissy JP, et al. Assessment of the mitral valve area in patients with mitral stenosis by multislice computed tomography. J Am Coll Cardiol. 2006;48:411–3.
Zeng X, Tan TC, Dudzinski DM, Hung J. Echocardiography of the mitral valve. Prog Cardiovasc Dis. 2014;57:55–73.
Martin RP, Rakowski H, Kleiman JH, et al. Reliability and reproducibility of two dimensional echocardiograph measurement of the stenotic mitral valve orifice area. Am J Cardiol. 1979;43:560–8.
Sugeng L, Weinert L, Lammertin G, et al. Accuracy of mitral valve area measurements using transthoracic rapid freehand 3-dimensional scanning: comparison with noninvasive and invasive methods. J Am Soc Echocardiogr: Off Publ Am Soc Echocardiogr. 2003;16:1292–300.
Zamorano J, Cordeiro P, Sugeng L, et al. Real-time three-dimensional echocardiography for rheumatic mitral valve stenosis evaluation: an accurate and novel approach. J Am Coll Cardiol. 2004;43:2091–6.
Zamorano J, de Agustin JA. Three-dimensional echocardiography for assessment of mitral valve stenosis. Curr Opin Cardiol. 2009;24:415–9.
Binder TM, Rosenhek R, Porenta G, et al. Improved assessment of mitral valve stenosis by volumetric real-time three-dimensional echocardiography. J Am Coll Cardiol. 2000;36:1355–61.
Messika-Zeitoun D, Brochet E, Holmin C, et al. Three-dimensional evaluation of the mitral valve area and commissural opening before and after percutaneous mitral commissurotomy in patients with mitral stenosis. Eur Heart J. 2007;28:72–9.
Chu JW, Levine RA, Chua S, et al. Assessing mitral valve area and orifice geometry in calcific mitral stenosis: a new solution by real-time three-dimensional echocardiography. J Am Soc Echocardiogr: Off Publ Am Soc Echocardiogr. 2008;21:1006–9.
Libanoff AJ, Rodbard S. Atrioventricular pressure half-time. Measure of mitral valve orifice area. Circulation. 1968;38:144–50.
Hatle L, Angelsen B, Tromsdal A. Noninvasive assessment of atrioventricular pressure half-time by doppler ultrasound. Circulation. 1979;60:1096–104.
Voelker W, Regele B, Dittmann H, et al. Effect of heart rate on transmitral flow velocity profile and doppler measurements of mitral valve area in patients with mitral stenosis. Eur Heart J. 1992;13:152–9.
Nakatani S, Masuyama T, Kodama K, et al. Value and limitations of doppler echocardiography in the quantification of stenotic mitral valve area: comparison of the pressure half-time and the continuity equation methods. Circulation. 1988;77:78–85.
Flachskampf FA, Weyman AE, Gillam L, et al. Aortic regurgitation shortens doppler pressure half-time in mitral stenosis: clinical evidence, in vitro simulation and theoretic analysis. J Am Coll Cardiol. 1990;16:396–404.
Thomas JD, Wilkins GT, Choong CY, et al. Inaccuracy of mitral pressure half-time immediately after percutaneous mitral valvotomy. Dependence on transmitral gradient and left atrial and ventricular compliance. Circulation. 1988;78:980–93.
Karp K, Teien D, Bjerle P, Eriksson P. Reassessment of valve area determinations in mitral stenosis by the pressure half-time method: impact of left ventricular stiffness and peak diastolic pressure difference. J Am Coll Cardiol. 1989;13:594–9.
Kawahara T, Yamagishi M, Seo H, et al. Application of doppler color flow imaging to determine valve area in mitral stenosis. J Am Coll Cardiol. 1991;18:85–92.
Rodriguez L, Thomas JD, Monterroso V, et al. Validation of the proximal flow convergence method. Calculation of orifice area in patients with mitral stenosis. Circulation. 1993;88:1157–65.
Rifkin RD, Harper K, Tighe D. Comparison of proximal isovelocity surface area method with pressure half-time and planimetry in evaluation of mitral stenosis. J Am Coll Cardiol. 1995;26:458–65.
Bennis A, Drighil A, Tribouilloy C, et al. Clinical application in routine practice of the proximal flow convergence method to calculate the mitral surface area in mitral valve stenosis. Int J Cardiovasc Imaging. 2002;18:443–51.
Lee TY, Tseng CJ, Chiao CD, et al. Clinical applicability for the assessment of the valvular mitral stenosis severity with doppler echocardiography and the proximal isovelocity surface area (PISA) method. Echocardiography. 2004;21:1–6.
de Agustin JA, Mejia H, Viliani D, et al. Proximal flow convergence method by three-dimensional color doppler echocardiography for mitral valve area assessment in rheumatic mitral stenosis. J Am Soc Echocardiogr: Off Publ Am Soc Echocardiogr. 2014;27:838–45. This study highlights the incremental diagnostic benefit of 3D TTE over 2D TTE for application of the PISA method to mitral valve area calculations, which may allow it to be used more often for clinical assessment of MS.
Nishimura RA, Rihal CS, Tajik AJ, Holmes Jr DR. Accurate measurement of the transmitral gradient in patients with mitral stenosis: a simultaneous catheterization and doppler echocardiographic study. J Am Coll Cardiol. 1994;24:152–8.
Reis G, Motta MS, Barbosa MM, et al. Dobutamine stress echocardiography for noninvasive assessment and risk stratification of patients with rheumatic mitral stenosis. J Am Coll Cardiol. 2004;43:393–401.
Lev EI, Sagie A, Vaturi M, et al. Value of exercise echocardiography in rheumatic mitral stenosis with and without significant mitral regurgitation. Am J Cardiol. 2004;93:1060–3.
Picano E, Pibarot P, Lancellotti P, et al. The emerging role of exercise testing and stress echocardiography in valvular heart disease. J Am Coll Cardiol. 2009;54:2251–60.
Wilkins GT, Weyman AE, Abascal VM, et al. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J. 1988;60:299–308.
Kronzon I, Tunick PA, Glassman E, et al. Transesophageal echocardiography to detect atrial clots in candidates for percutaneous transseptal mitral balloon valvuloplasty. J Am Coll Cardiol. 1990;16:1320–2.
Schlosshan D, Aggarwal G, Mathur G, et al. Real-time 3d transesophageal echocardiography for the evaluation of rheumatic mitral stenosis. J Am Coll Cardiol Img. 2011;4:580–8.
Min SY, Song JM, Kim YJ, et al. Discrepancy between mitral valve areas measured by two-dimensional planimetry and three-dimensional transoesophageal echocardiography in patients with mitral stenosis. Heart. 2013;99:253–8.
Langerveld J, Valocik G, Plokker HW, et al. Additional value of three-dimensional transesophageal echocardiography for patients with mitral valve stenosis undergoing balloon valvuloplasty. J Am Soc Echocardiogr: Off Publ Am Soc Echocardiogr. 2003;16:841–9.
Applebaum RM, Kasliwal RR, Kanojia A, et al. Utility of three-dimensional echocardiography during balloon mitral valvuloplasty. J Am Coll Cardiol. 1998;32:1405–9.
Heidenreich PA, Steffens J, Fujita N, et al. Evaluation of mitral stenosis with velocity-encoded cine-magnetic resonance imaging. Am J Cardiol. 1995;75:365–9.
Lin SJ, Brown PA, Watkins MP, et al. Quantification of stenotic mitral valve area with magnetic resonance imaging and comparison with doppler ultrasound. J Am Coll Cardiol. 2004;44:133–7.
Djavidani B, Debl K, Lenhart M, et al. Planimetry of mitral valve stenosis by magnetic resonance imaging. J Am Coll Cardiol. 2005;45:2048–53.
Ohyama H, Hosomi N, Takahashi T, et al. Comparison of magnetic resonance imaging and transesophageal echocardiography in detection of thrombus in the left atrial appendage. Stroke; J Cereb Circ. 2003;34:2436–9.
Djavidani B, Debl K, Buchner S, et al. Mri planimetry for diagnosis and follow-up of valve area in mitral stenosis treated with valvuloplasty. RoFo: Fortschr Geb Rontgenstr Nuklearmed. 2006;178:781–6.
Acknowledgments
We thank Dr. Feroze Mahmood for the intra-operative 3D TEE images.
Compliance with Ethics Guidelines
Conflict of Interest
Francesca N. Delling is funded by National Heart, Lung and Blood Institute K23HL116652.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Imaging
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Video 1
Example of rheumatic mitral stenosis shown in a 2D TTE parasternal long-axis view. Both mitral leaflets are thickened and deformed, with a characteristic “hockey stick” appearance of the anterior leaflet as a result of diastolic leaflet doming, and poor excursion of the posterior leaflet. The narrowest orifice occurs at the leaflet tips, while the mitral valve annulus is not affected. 2D = 2-dimensional; TTE = transthoracic echocardiogram. (AVI 8,492 kb)
Video 2
A 3D TEE en face view of the mitral valve from the left atrial perspective, demonstrating prominent fusion of the lateral commissure and preserved A3 and P3 mobility. 3D = 3-dimensional; TEE = transesophageal echocardiogram. (MOV 329 kb)
Rights and permissions
About this article
Cite this article
Motiwala, S.R., Delling, F.N. Assessment of Mitral Valve Disease: A Review of Imaging Modalities. Curr Treat Options Cardio Med 17, 30 (2015). https://doi.org/10.1007/s11936-015-0390-1
Published:
DOI: https://doi.org/10.1007/s11936-015-0390-1