Abstract
Acute coronary syndromes (ACS) frequently result from the rupture or erosion of a vulnerable coronary plaque, with associated intracoronary thrombosis. ACS also may occur in patients with angiographically normal coronary arteries. Some of these patients, however, still have angiographically silent underlying coronary artery disease. In this setting, subtle atherosclerotic changes frequently associated with unstable morphologic features or residual intracoronary thrombus may be detected with intracoronary imaging techniques. Nevertheless, other patients develop ACS as a result of nonatherosclerotic coronary artery disease (NA-CAD). ACS in patients with NA-CAD may be the consequence of coronary spasm or transient coronary embolic phenomena. In these patients, after the initial ischemic insult, late coronary angiography usually reveals normal epicardial coronary vessels. Kounis syndrome is a type of ACS generated by allergic reactions. Takotsubo cardiomyopathy is characterized by normal coronary arteries with a distinct pattern of transient left ventricular wall motion abnormalities. ACS also may occur in young patients following illicit drug use. Finally, spontaneous coronary artery dissection and intramural hematoma represent other etiologies of NA-CAD. In this review, we discuss current evidence regarding diagnostic and treatment strategies in patients presenting with ACS as a result of NA-CAD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A substantial portion of patients who have undergone coronary angiography because of acute coronary syndrome (ACS) do not have significant coronary artery disease (CAD). In clinical practice, these patients are considered to have normal coronary arteries, whereas the term nonsignificant coronary stenosis is used for an obstruction in lumen diameter <50 %. In this setting, nonobstructive CAD may range from completely normal arteries to minimal lumen irregularities or even moderate angiographic stenosis. The main underlying mechanism of nonsignificant coronary stenosis is incipient atherosclerosis. However, coronary vasospasm (CV) or spontaneous coronary artery dissection (SCAD) also may lead to significant coronary stenosis presenting later as mild to moderate lumen stenosis on coronary angiography. Frequently, clinicians must have a high level of suspicion and use additional diagnostic tools to unmask these entities or to confirm underlying atherosclerotic CAD or even unstable culprit plaques as the responsible mechanism. In this setting, the clinical presentation and patient characteristics may provide major diagnostic clues. Appropriate selection of additional tests, including intravascular imaging techniques, vasospasm tests, or even cardiac magnetic resonance (CMR), remains important in establishing the correct diagnosis.
It is known that secondary prevention medications are used less frequently in these patients than in those with obstructive CAD [1]. Moreover, these alternative mechanisms of ACS might benefit from specific management and treatment strategies. In some cases, pharmacologic therapy differs from that indicated in patients with atherosclerotic CAD. For example, vasodilators remain the first-line treatment in patients with CV. Alternatively, β-blockers should be avoided in this scenario and also in patients with ACS following cocaine abuse. Likewise, conservative management, with no coronary intervention, might be the best option for stable patients with SCAD.
Among patients presenting with myocardial infarction, the prevalence of nonsignificant coronary stenosis varies from 7 to 32 % in women and 6 to 12 % in men [2–5]. Taking into account only patients with angiographically normal coronary arteries, the prevalence falls to 1 to 5 % [6, 7]. Coronary angiograms in those without significant CAD are particularly prevalent in young patients, women, and black patients. The available data regarding the contribution of each “etiology” in patients with ACS and nonobstructive CAD are very scarce. Recently, a systematic study using intravascular ultrasound (IVUS) and CMR in women showed that 39 % of patients had angiographically silent plaque rupture, and 8 % of patients met the criteria for Takotsubo cardiomyopathy (TTC) [8••]. The estimated incidence of the different entities that may cause nonatherosclerotic CAD (NA-CAD) is summarized in Table 1.
From a different perspective, a pathologic assessment of hearts from patients who had sudden cardiac death (a potential manifestation of ACS) showed that 3 % were associated with NA-CAD. These patients were young and frequently male (62 %). In this scenario, the most common final diagnosis was an anomalous coronary artery (48 %), followed by SCAD (16 %), coronary artery vasculitis (12 %), and coronary artery spasm (12 %) [22]. Obviously, these results represent only the lethal face of NA-CAD and, therefore, cannot be used to estimate its incidence in routine clinical practice. Extrinsic compression of an otherwise healthy coronary vessel may result in myocardial ischemia or infarction. Extracardiac tumors and iatrogenic complications following thoracic or cardiac surgery also have been reported as rare causes of NA-CAD leading to ACS. The other end of the spectrum is represented by patients with angiographically normal vessels and so-called hemodynamic angina. In these patients, severe anemia, uncontrolled hypertension, and other noncardiac conditions may cause ischemia, often presenting as ACS. Finally, patients with “syndrome X” show evidence of ischemia despite angiographically normal coronary vessels. Some of these patients have impaired microvascular circulation or disturbed cardiac metabolism, but classically, they manifests with stable angina rather than as ACS.
Overall, the prognosis for patients with ACS and nonobstructive CAD is better than that for patients with obstructive CAD. The rate of death or reinfarction at 1 year consistently has been found to be 1 to 2 % [4, 23–25]. Table 1 summarizes the prognosis according to NA-CAD etiology.
Coronary Vasospasm
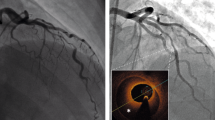
The term coronary vasospasm refers to a sudden and severe vasoconstriction of an epicardial coronary artery, leading to severe luminal stenosis or to total occlusion (Fig. 1). Although CV may contribute to other coronary syndromes, it is the primary cause of variant, or Prinzmetal, angina.
Ischemic episodes of variant angina show circadian variation and often occur at rest from midnight to early morning. Symptoms also may occur during mild exercise in the early morning. However, these episodes usually are not reproduced by strenuous exercise in the afternoon. Therefore, exercise capacity shows a circadian variation in most patients with variant angina [26–28]. Another important clinical feature of variant angina is the high frequency of asymptomatic ischemic episodes and its association with syncope during ischemic attacks [29, 30]. A large observational study revealed that 82 % of ischemic episodes (872 of 1,062) were completely asymptomatic, whereas 12 % of patients (30 of 240) had syncope as an associated symptom [31].
Transient ST-segment elevation with reciprocal changes, indicating transmural myocardial ischemia, constitutes typical electrocardiographic (ECG) changes in these patients. However, other, more subtle ECG changes, including a taller and broader R wave, the disappearance of the S wave, and a taller T wave compared with baseline findings, have been described during these episodes [26]. Arrhythmias, including ventricular tachycardia and high-degree atrioventricular block (occasionally leading to syncope), are detected relatively frequently during ischemic episodes [30, 31]. Prolonged transmural myocardial ischemia caused by a completely occlusive CV may lead to acute myocardial infarction or even sudden cardiac death [32].
CV may occur at the site of an underlying atherosclerotic plaque (which may be only mildly obstructive) or in angiographically normal coronary arteries. The presentation as multivessel coronary spasm varies in frequency, ranging from 6 to 45 % according to the diagnostic method used [9, 33]. The incidence may be as high as 75 % when aggressive pharmacologic provocation techniques are used systematically during coronary angiography [34]. Various CV patterns have been described [33]: (i) migratory spasm affecting different sites on different occasions, (ii) sequential spasms successively affecting different sites, and (iii) simultaneous spasm occurring at different sites. Patients with sequential or simultaneous spasm at different sites appear to have the worst prognosis [35].
Diagnosis of CV may be difficult despite a typical presentation, given the transient occurrence of coronary stenosis. There is no global consensus regarding clear-cut diagnostic criteria, but the Japanese Circulation Society proposed some recommendations to establish the diagnosis. A typical presentation, with circadian variation of symptoms, ECG changes, and suppression of attacks with calcium channel blockers (but not with β-blockers), with induction of attacks by hyperventilation, is considered sufficient to make the clinical diagnosis of variant angina without a cardiac catheterization. If all the criteria are not met, cardiac catheterization with a drug-induced coronary spasm provocation test is needed. A positive finding for coronary spasm in a provocation test is defined as transient total or subtotal occlusion (>90 %) of a coronary artery associated with signs/symptoms of myocardial ischemia, which provides a definitive diagnosis of CV. If the test is negative but other clinical criteria are met, the diagnosis is “suspected CV” [36]. Notably, the latest clinical practice guidelines from the European and American societies of cardiology provide no clear recommendation regarding the indication of pharmacologic provocation testing.
The pharmacologic agents used most often in provocation testing for the diagnosis of CV are ergonovine and acetylcholine. Various testing protocols using intracoronary and intravenous ergonovine have been described (acetylcholine usually is given only via the intracoronary route), with good (>90 %) sensitivity and specificity, which are similar for both agents [37••]. It has been demonstrated that intracoronary ergonovine administration is safer than intravenous administration; intravenous ergonovine may produce multivessel spasm and hemodynamic instability. Additionally, intracoronary ergonovine has been shown to have a higher sensitivity (near 100 %) for identifying the culprit vasospastic vessel [38]. Acetylcholine also has a good safety profile, which was confirmed in a recent report of Caucasian patients with unobstructed coronary arteries and angina pectoris [39]. Another interesting finding of this study is that acetylcholine provocation testing revealed microvascular spasm in up to 25 % of patients (205 of 847 developed angina and typical ischemic ST-segment changes in the absence of significant [>75 %] epicardial coronary vessel constriction).
There is a relative paucity of published data on the safety of bedside ergonovine testing for the diagnosis of CV. Furthermore, the accuracy of this strategy in patients who have not undergone prior coronary angiography might be lower for diagnosing spasm, as ischemia also may result from undetected obstructive coronary disease [37••].
Intracoronary imaging techniques might be of value in selected patients with CV. Optical coherence tomography (OCT) of coronary arteries during drug provocation testing has shown a significant increase in mean intimal thickness with no significant change in intimal area during CV episodes. The intimal layer is compressed externally by the constricted medial layer and exhibits a characteristic “wavy” luminal border [40, 41]. Other OCT findings in ACS patients with nonobstructive CAD but positive provocation test results include intraluminal thrombi (33 %; 6 of 20 patients) and intimal erosion (10 %; 2 of 20 patients) [42].
Management of CV begins with lifestyle changes such as smoking cessation (smoking is a well-recognized trigger for CV) and avoidance of alcohol intake and fatigue or mental stress, as well as strict control of principal cardiovascular risk factors [36]. It is suggested that aerobic exercise in the afternoon may suppress the attacks as a result of improvements in endothelial function and oxidative stress [43].
Early treatment of variant angina is important to prevent complications such as acute myocardial infarction, fatal arrhythmias, and sudden death. Sublingual or intravenous administration of nitroglycerin is very effective in relieving CV attacks. Calcium channel blockers are extremely effective in preventing ischemic attacks. In the Japanese population, the efficacy rate of calcium channel blockers was >90 % and as high as 100 % when a combination of diltiazem and nifedipine was used. Long-acting nitrates are another classic drug for preventing ischemic attacks. Other proposed treatments are nicorandil, magnesium, antioxidants, and statins. Because the ischemic episodes show a circadian variation, drugs should be given taking this into account [29]. There is no consensus regarding the usefulness of acetylsalicylic acid in CV. Recent OCT findings of intracoronary thrombi in patients with CV presenting as ACS support the use of antiplatelet therapy [42].
The use of coronary stents has been suggested in very selected patients with severe recalcitrant CV in which the episodes occur in the same vessel segment. However, we strongly discourage the use of coronary interventions in these patients, because ischemic episodes eventually are controlled with adequate combined pharmacologic therapy and because CV location may be a moving target along the entire vessel.
The prognosis of patients with variant angina generally is favorable and appears to have improved in the 2000s [9, 44]. Several factors, such as smoking, underlying organic coronary stenosis, and multivessel spasm, are well-established prognostic factors. Recently, a novel scoring system was devised, based on the database of a large multicenter registry study by the Japanese Coronary Spasm Association. In this study, a total of 1,429 patients were analyzed, with a median follow-up of 32 months. After a multivariable analysis using a Cox model, seven predictors were selected and integrated into a risk scoring system. Predictive factors included smoking, angina at rest alone, history of out-hospital cardiac arrest, significant organic coronary stenosis, multivessel spasm, ST-segment elevation during angina attacks, and the use of β-blockers. Three risk strata were defined in accordance with the scoring system, and a change in stratum was associated with a two- to threefold increase in the risk of adverse clinical events [10•]. This scoring system has not yet been validated by independent external datasets; therefore, its clinical value should be confirmed in future studies.
Kounis Syndrome
Kounis syndrome (KS) was first described in 1991 as the simultaneous appearance of acute coronary events and anaphylactic or anaphylactoid allergic reactions [45]. During hypersensitivity, degranulation of mast cells occurs and a variety of stored and newly formed inflammatory mediators are released into the systemic circulation. Most of these mediators have important cardiovascular activity. For example, histamine induces coronary vasoconstriction and tissue factor expression and activates platelets [46•]. Currently, this syndrome is poorly understood, with most of the available information emerging from descriptions of isolated clinical cases.
Three variants of KS have been described so far [47, 48]. Type I includes normal or nearly normal coronary arteries with no risk factors for CAD. The allergic event induces coronary artery vasospasm following an acute release of inflammatory mediators. The cardiac enzymes may be normal or may reflect progression toward an acute myocardial infarction. The suggested explanations for this type of KS include endothelial dysfunction and microvascular angina. Type II includes culprit but quiescent preexisting atheromatous disease in which the acute release of inflammatory mediators induces either coronary artery vasospasm with or without plaque erosion or a plaque rupture manifesting as an acute myocardial infarction. Type III includes patients with coronary artery stent thrombosis in whom aspirated thrombus specimens reveal eosinophils (on hematoxylin and eosin staining) and mast cells (on Giemsa staining).
Multiple causes of KS have been described, including drugs, insect stings, foods, environmental exposure, and medical conditions, among others [49]. The diagnosis of KS eminently is clinical and is based on identification of signs and symptoms suggesting an acute allergic reaction coincident with the ACS clinical presentation. There is no diagnostic test pathognomonic for KS. In addition, it should be kept in mind that proinflammatory mediators similar to those found in KS (such as histamine, tryptase, arachidonic acid products, and interleukin-6) also may be found in some ACS patients with a nonallergic etiology [46•].
Treatment of KS is challenging because it must address both cardiac and allergic symptoms simultaneously. Furthermore, some drugs may worsen the allergy and/or aggravate the heart dysfunction [50]. In patients with type I KS, treatment of the allergic event alone may abolish the symptoms. The use of hydrocortisone and H1 and H2 antihistamines (diphenhydramine and ranitidine) appears adequate. Administration of vasodilators also may ameliorate hypersensitivity-induced vasospasm. In patients with type II KS, treatment should follow the general ACS treatment protocol and include corticosteroids and antihistaminic drugs. Epinephrine, the drug of choice in patients with anaphylaxis, may aggravate ischemia in patients with CAD. Finally, following urgent aspiration of the occlusive stent thrombus, management of patients with type III KS is similar to that of patients with type II [46•].
Available data regarding the prognosis of KS remain very scarce. Beyond the acute phase, the condition appears to be largely benign. Although no recurrences have been reported, repeat exposure to the allergen might trigger a new episode [49].
Coronary Artery Embolism
Coronary embolism is a rare cause of ACS, and its prevalence is unknown. Information about this etiology of NA-CAD is based on isolated case reports or short case series. This entity encompasses different mechanisms and sources of thrombus production. Coronary embolization eventually results in total occlusion or severe obstruction in an epicardial coronary artery. Therefore, although the initial treatment is similar to that in other patients with ACS (the main objective being to achieve urgent reperfusion of the occluded coronary artery), final management depends on the underlying condition promoting thrombus generation and embolization.
Coronary embolism has been associated with hypercoagulable states, valve prostheses [51], atrial fibrillation (Fig. 2b) [52], infective and noninfective endocarditis [53, 54], intracardiac thrombi [55], aortic sinus thrombosis (Fig. 2a) [56, 57], patent foramen ovale with paradoxic embolism [58] (including impending paradoxic embolism with a thrombus trapped during its passage from the right to left atrium through patent foramen ovale [59]), and even benign and malignant tumors [60, 61].
a Aortic sinus thrombosis. During primary coronary angioplasty for an ST-segment elevation myocardial infarction, a mass was seen occluding the left coronary artery ostium (note the round contrast-filling defect in the left sinus of Valsalva). After surgical extraction, pathologic examination of the mass revealed a massive red thrombus. No predisposing underlying conditions were found, and the aortic root showed no abnormalities. b Coronary artery embolism. Primary coronary angioplasty in a woman presenting with recent-onset atrial fibrillation and a large anterior acute myocardial infarction. b(1) Left anterior descending coronary artery occluded in the mid-segment. b(2) Left anterior descending coronary artery after thrombus aspiration; it shows TIMI 3 coronary flow and no residual lesion. b(3) Extracted red thrombus
The diagnosis of coronary embolism as a cause of ACS is based on clinical suspicion, in cases in which a high-risk associated condition accompanies angiographic findings highly suggestive of coronary embolization. The angiographic image may show an abrupt (cutoff) occlusion of a coronary artery (multiple occlusions raise the level of suspicion) with a generally normal appearance of the remaining coronary vessels. The lack of collateral vessels to the occluded artery has been suggested as an important sign to support the diagnosis of coronary embolism. The absence of compensatory collateral vessels may explain the severe regional myocardial damage seen in many of these patients. In some cases, the diagnosis of coronary artery embolism is not suspected until the coronary angiogram provides a clue. Sometimes, this occurs only after a large thrombus is extracted and minor irregularities, or even a completely normal coronary vessel, remain. In some cases, intravascular imaging (IVUS or OCT) may be instrumental in detecting or excluding underlying organic CAD [62]. In this scenario, transthoracic and transesophageal echocardiography are valuable tools to unravel the responsible underlying condition, such as prosthetic valve thrombosis, intracardiac thrombus, endocarditis, or patent foramen ovale.
Initial treatment depends on the cause and the image of the occluded vessel. In some cases, thrombus aspiration alone is sufficient to restore coronary flow and to obtain an excellent final angiographic result, with no need for further intervention. In other cases, however, balloon angioplasty or stent implantation is needed to achieve Thrombolysis in Myocardial Infarction (TIMI) grade 3 coronary flow and to ensure an adequate final result at the previously occluded coronary segment [51]. Potent intracoronary antiplatelet drugs (IIb/IIIa receptor platelet inhibitors) also are advocated frequently in patients with suboptimal results after thrombus aspiration.
Treatment of the underlying condition causing the embolic phenomenon should be individualized. Anticoagulation therapy at therapeutic levels is mandatory in cases of atrial fibrillation, prosthetic valve thrombosis, and intracardiac thrombi. On the other hand, surgical intervention has been proposed as the best option for rare cases associated with aortic sinus thrombosis, impending paradoxic embolism, endocarditis, or tumors, and, of course, in most cases of prosthetic valve thrombosis [53, 54, 56, 57, 59].
Takotsubo Cardiomyopathy
TTC, also known as broken-heart syndrome, apical ballooning syndrome, and stress-induced cardiomyopathy, was first described in 1990 in a Japanese medical text [63]. It was thought that the disorder affected only Asians, until the first study of TTC in Caucasians was presented in 2003 [64]. Nowadays, TTC is recognized as a widespread affliction of unknown etiology that accounts for up to 3 % of all ACS cases [12, 13]. Rates of TTC recently were suggested to be higher (up to 6 %) in postmenopausal women presenting with an ACS [14]. Moreover, this higher prevalence of TTC among women is concordant with the marked gender discrepancy observed in this entity: up to 90 % of patients with TTC are postmenopausal women [65].
The main characteristics of TTC are transient systolic ventricular dysfunction with regional wall motion abnormalities extending beyond a single vascular territory and in the absence of significant obstruction in the epicardial coronary vessels. Although currently there is no worldwide consensus on the precise definition, various diagnostic criteria have been proposed. The Mayo Clinical Criteria, published in 2004 and modified in 2008, currently are the most widely used and comprise the following:
-
1.
Transient hypokinesis, akinesis, or dyskinesis of the left ventricular mid-segments with or without apical involvement. The regional wall motion abnormalities extend beyond a single epicardial vascular distribution. A stressful trigger is often but not always present.
-
2.
Absence of obstructive CAD or angiographic evidence of acute plaque rupture.
-
3.
New electrocardiographic abnormalities (ST-segment elevation and/or T-wave inversion) or modest elevation in cardiac troponin.
-
4.
Absence of pheochromocytoma and myocarditis [66].
These criteria for TTC do not exclude patients with associated underlying CAD, and data exist showing significant coronary artery stenosis among patients with TTC. Many authors propose that obstructive CAD should not exclude the diagnosis of TTC, as these distinct diseases may occur concomitantly [67••]. However, we prefer to restrict the diagnosis of TTC to patients with normal epicardial coronary vessels, those with only minor luminal irregularities, and those with moderate lesions in “remote” vessels (unrelated to wall motion abnormalities). In our experience, though, the diagnosis of TTC eventually may be established in highly selected patients with associated severe CAD, but only if an exhaustive workup has determined that underlying CAD did not play a role in the pathophysiology of the current ACS presentation and if the complete clinical picture and the evolution of the left ventricular function are typical of this condition.
The clinical presentation of TTC often is identical to that of classic ACS. The most typical ECG abnormality is ST-segment elevation with evolutionary changes to diffuse and deep T-wave inversion, often with prolongation of the QT interval. Pathologic Q waves may be observed initially but rarely persist over time. Reciprocal changes are unusual and seen more commonly in ACS secondary to CAD [67••]. A modest elevation of cardiac biomarkers often is observed in TTC. Troponin levels, however, are much lower than those expected for ST elevation myocardial infarction with similar ECG findings and, characteristically, are out of proportion to the extensive wall motion abnormalities and hemodynamic compromise [12]. Cardiac brain natriuretic peptide (BNP) values often are notably increased (up to threefold) compared with those in patients with myocardial infarction [68].
A unique feature of TTC is preceding emotional stress or a physical trigger. Since the description of TTC, many precipitants have been reported, including anger and sadness as emotional triggers, and surgery, critical illness, neurologic disease, and exacerbations of asthma or chronic obstructive pulmonary disease as physical stressors [15, 67••]. Recently, we reported a case of TTC triggered by influenza A virus infection [69]. Interestingly, there is a gender disparity regarding what precipitates TTC. Physical triggers are observed more frequently in men, whereas women more often experience previous emotional stress [65]. Moreover, TTC episodes may occur without the typical presentation and may be discovered incidentally during hospitalization due to ECG abnormalities or elevated cardiac enzymes.
Systolic left ventricular dysfunction with evidence of regional wall motion abnormalities can be recognized readily at admission by echocardiography. However, coronary angiography to rule out significant CAD and left ventricular angiography depicting the typical left ventricular pattern remain the gold standard for confirming the diagnosis (Fig. 3). Four types of TTC have been identified: the classic form (with typical apical ballooning), midventricular TTC (classified as atypical), and the rarely reported basal and focal types [67••]. To rule out acute plaque rupture or the presence of thrombi as underlying conditions, additional intravascular imaging might help confirm the absence of any pathologic substrate at the vessel wall. OCT has shown structurally normal coronary arteries in both typical and atypical TTC cases [70, 71].
Echocardiography and left cardiac catheterization can detect and measure the degree of left ventricular outflow tract obstruction and the associated anterior motion of the mitral valve along with associated mitral regurgitation, which may have implications during acute management. On CMR, typical findings include the absence of delayed gadolinium hyperenhancement and the presence of myocardial edema at the site of the regional wall motion abnormality [72•].
No randomized controlled clinical trials have been performed to address the treatment of TTC. Initial treatment is based on the clinical diagnosis of ACS. If cardiogenic shock is present, an intra-aortic balloon pump may be inserted to support the hemodynamic state, but the use of catecholamines as inotropic agents should be considered very carefully, because this disease is thought to be catecholamine mediated and outflow tract obstruction may worsen [67••]. A recent prospective study in 13 patients with TTC and low ejection fraction showed that levosimendan, a calcium channel sensitizer, is a safe and feasible option [73]. Additional treatment strategies are based on clinical judgment: β-blockers are indicated in left ventricular outflow obstruction, especially considering the putative role of catecholamines in causing the disorder. If necessary, β-blockers may be combined with phenylephrine as a pressor agent if hypotension occurs. Because the main feature of TTC is severe systolic dysfunction of the left ventricle, the use of angiotensin-converting enzyme (ACE) inhibitors also has been proposed. If coronary atherosclerosis also is present, aspirin and statin administration should be considered [67••]. The left ventricle should recover within 2 to 8 weeks. The efficacy of chronic drug therapy in these patients is not well established. A recent meta-analysis based on available clinical TTC registries showed no evidence of clinical benefit for any long-term standard drug treatment [74].
Cocaine Abuse
Intake of cocaine, a widely used illegal drug, is a leading cause of emergency department visits among drug abusers [75, 76]. Its true incidence likely is underestimated because of patient underreporting, denial, and perhaps undertesting. In a systematic analysis of urine samples from consecutive patients presenting to the emergency department because of chest pain, cocaine was present in 17 % of the samples [77].
Cocaine has major direct effects on vessel wall physiology. The drug may elicit severe coronary vasoconstriction. Notably, greater vasoconstriction has been observed in vessels with underlying atherosclerotic plaques and in smokers [78, 79]. Cocaine abuse also is associated with accelerated coronary atherosclerosis [80]. In addition, cocaine has been found to cause alterations in platelet function and the coagulation cascade and to directly result in myocardial toxicity [81••].
Blood levels of cocaine are determined by the route of administration: intranasal cocaine is detected in 40 to 90 min and smoked cocaine in only 3 min. Drug metabolites may be detected several days after cocaine abuse. Cocaine effects are prolonged by concomitant alcohol intake as the result of an additional active metabolite: cocaethylene [81••].
The initial evaluation and management of patients with ACS associated with cocaine abuse must consider factors different from those of general practice in ACS patients. The specificity of creatine kinase MB in diagnosing myocardial necrosis may be reduced after cocaine abuse, but the specificity of troponin remains unaltered [82]. It is important to keep in mind that troponin elevation does not indicate the mechanism of myocardial injury, which may include plaque rupture, coronary vasospasm, demand ischemia in patients with underlying atherosclerosis, and, importantly, direct myocardial toxicity. Cardiac catheterization is indicated as a diagnostic tool in the setting of ACS. Intravascular imaging techniques may help reveal plaque rupture as the underlying cause.
Pharmacologic treatment is extrapolated largely from the recommendations established in the general ACS population; however, other considerations exist with regard to treating these patients. Benzodiazepines, because of their anxiolytic properties, may help decrease adrenergic stimulation and should be considered in patients in whom cocaine abuse is suspected. Nitroglycerin and calcium channel blockers are indicated, if no contraindication exists, as the initial treatment to relieve pain and reduce myocardial ischemia. β-Blockers, however, should be avoided; recent cocaine use is a formal contraindication for the use of these agents because they may precipitate vasoconstriction as a result of unopposed α-receptor stimulation [83]. The recommendations concerning β-blocker use in ACS patients after cocaine abuse are based on case reports, animal studies, retrospective case studies, and a few small human studies [81••]. Because of significant patient underreporting or denial of cocaine use at admission, these patients frequently receive β-blockers before the treating physician is aware of the cocaine abuse. Moreover, recent retrospective studies in cocaine-positive patients treated with β-blockers did not show a significant increase in adverse events during follow-up [84, 85]. Subsequently, a randomized trial of treatment with the α- and β-blocker labetalol versus diltiazem in ACS patients with active cocaine use found no differences in blood pressure or heart rate and revealed a similar clinical outcome [86]. Thus, although β-blocker use in patients who have used cocaine remains controversial, the α-/β-blocker combination agent labetalol appears a reasonable option, especially in patients with persistent hypertension and tachycardia after initial treatment with nitrates and calcium channel blockers.
Spontaneous Coronary Artery Dissection
SCAD, whose etiology remains unknown, is a separation of the layers of the arterial wall, with formation of two lumens: a true lumen and a false lumen. The dissection plane usually lies in the medial arterial layer. In fact, the resulting membrane usually comprises the intima and the inner two thirds of the medial layer. Sometimes, a tear is not detected and the false lumen is occupied entirely by blood or thrombus, and in these cases, the term intramural hematoma is preferred. The passage of blood into the false lumen and/or the development of a hematoma on the arterial wall may compromise the true lumen. Thus, obstruction of coronary blood flow causes myocardial ischemia with various presentations, with ACS one of the more frequent.
Since the first description in 1931 by Pretty in a postmortem study of a young woman who died suddenly after suffering chest pain [87], less than 700 cases have been reported in the medical literature. Most of them are isolated cases, but since the 1980s, some short series also have been reported, and more recently, some investigators presented a relatively large series with appropriate long-term clinical follow-up [19••, 21••].
The true prevalence of SCAD probably is underestimated because of difficulties in clinical diagnosis. Despite the use of coronary angiography, many cases may go unnoticed. In some cases, the diagnosis can be confirmed only after a thorough and critical review of the angiographic images and may even require further imaging with tomographic intracoronary techniques.
There is a higher incidence of SCAD among women. In the largest and most recent reports, the frequency of female gender in SCAD cases ranges between 58 and 82 %. We recently conducted a prospective and systematic registry of patients with suspected SCAD [19••]. In this study, patients were divided into two groups: those with SCAD “associated” with CAD (at least one coronary lesion, different from the image of coronary dissection, showing stenosis >50 %) and those with “isolated” SCAD (free of any CAD). Conspicuously, isolated SCAD was predominant in females (85 %), whereas the presentation associated with CAD appeared to be more frequent in males (only 17 % of patients with associated CAD were women).
Age of onset is between the fifth and sixth decades of life. However, in the differential diagnosis of ACS causes, it is important to keep in mind that this disease may affect very young patients despite the absence of classic risk factors or other significant comorbidity. If we analyze only the group of women younger than 50 years presenting with an ACS, the prevalence of SCAD reaches 9 %, but is as high as 11 % if the clinical presentation is ST-segment elevation myocardial infarction [20].
Perhaps the best-known pathophysiologic association of spontaneous coronary dissection is pregnancy and the postpartum period; however, the most recent large series found this association in only 4 to 18 % of women with SCAD [19••, 21••]. Although the association of SCAD with the peripartum period has been reported repeatedly, most young female patients with SCAD are not in the peripartum period. Alternatively, strenuous exercise is the main precipitating factor in males, constituting up to 44 % of SCAD cases in men. By contrast, this trigger is detected in only 2.8 % of women with SCAD [21••].
The fortuitous discovery (during femoral artery angiography to assess vascular access at coronary angiography) of “beading” in the wall of the external iliac artery (a pattern typical of fibromuscular dysplasia) in 8 of 16 patients with SCAD [21••] confirmed the previously reported association between SCAD and fibromuscular dysplasia [88]. In this regard, a retrospective analysis of 50 patients with isolated SCAD (98 % women) showed that 86 % had findings consistent with fibromuscular dysplasia in at least one vascular territory (58 % renal, 48 % iliac, and 46 % stroke) [89•]. In our opinion, it is highly unlikely that the high prevalence of fibromuscular dysplasia among patients with SCAD is just a coincidence, considering the infrequency of both diseases. However, further research in this direction is needed to unravel the etiologic and pathophysiologic implications of this intriguing association.
The diagnosis of SCAD is based on coronary angiography. The typical image of a coronary dissection is a thin, longitudinal, radiolucent line, which represents the intimal flap and the passage of contrast into the two lumens. The filling and emptying of dye in the false lumen may be delayed, leading to residual dye in the artery wall between coronary injections. Eventually, a diffuse narrowing of the vessel, resulting in an abnormally sized artery compared with other vessels, may correspond to the presence of an intramural hematoma causing diffuse luminal compression. A long stenosis of sudden onset with convex and smooth edges, especially in patients with otherwise smooth and healthy coronary arteries, is highly suggestive of SCAD (Fig. 4) [90].
Spontaneous coronary artery dissection. Coronary angiogram showing the left anterior descending coronary artery. Note the abrupt narrowing of its distal segment, with convex and smooth borders and a TIMI 0 coronary flow at the distal end. 1 and 2 indicate the corresponding OCT cross-sectional images. The three layers of the artery—the adventitia (a), media (b), and intima (c)—can be identified readily by the marked separation between the adventitia and the intima–media (arrow, diagonal side branch emerging from the true lumen; asterisk, wire artifact)
The use of tomographic intracoronary diagnostic techniques (IVUS and OCT) appears to be very valuable in selected SCAD cases. Imaging may confirm the presence of true and false lumens or an intramural hematoma at the vessel wall. Although often difficult, it is sometimes possible to identify the intimal tear. These anatomic data may be very useful in guiding an eventual percutaneous coronary intervention (PCI). Data exist to support the use of intravascular imaging techniques in these patients, especially if the diagnosis of SCAD is not evident on coronary angiography. In a study of 17 patients with suspected SCAD, the systematic use of OCT was considered a determinant in confirming the diagnosis and assisting in patient management. In this series, there were no complications associated with the use of OCT [91].
There are no general recommendations or clinical practice guidelines specifically addressing the treatment of SCAD. The paucity of cases limits the possibility of comparative studies evaluating the available treatment options. Initial medical treatment usually follows the general recommendation for ACS patients. However, once a SCAD diagnosis is established with coronary angiography, it is frequently debated whether continuing the medications already prescribed is appropriate. It seems reasonable to assume that ischemic myocardium secondary to a flow-limiting SCAD would benefit from treatment with β-blockers and ACE inhibitors. However, antithrombotic therapy, primarily aimed at preserving blood flow and limiting obstruction secondary to intracoronary thrombus or hematoma progression in the vessel wall, may be a double-edged sword. Theoretically, powerful antithrombotic therapy may help shrink the thrombus in the true lumen and prevent thrombus formation in the false lumen, thus improving true lumen flow. However, this treatment also might promote bleeding in the arterial wall and, therefore, compression of the true lumen [90].
Antithrombotic therapy, therefore, often is based on anticoagulation with low molecular weight heparin and on antiplatelet therapy, usually with acetylsalicylic acid and clopidogrel, with special care to avoid the use of fibrinolytics [90]. The potential value or risk associated with the novel, more potent, antiplatelet agents has not been studied.
Several reports confirm the success of coronary interventions with stents to tackle the dissection flap in select patients with SCAD. However, coronary revascularization, with either surgery or stent placement, remains very challenging in these patients, who already have a spontaneous rupture of the coronary vessel wall. In a systematic review by the Mayo Clinic [21••], of 87 patients identified with SCAD, 43 had been treated with PCI. The success rate of these interventions was only 65 % (much lower than the standard results of PCI in other settings). Stents may cause the dissection to progress to adjacent coronary segments. In addition, the inherent risk of surgery in patients with ongoing myocardial ischemia, associated with technical difficulties in performing anastomosis into a uniquely fragile vessel wall with the possibility of performing the anastomosis outside the true lumen, make coronary surgery an unattractive option for patients with stable SCAD or single-vessel disease. It also should be noted that in some previous series, the patency of bypass grafts at follow-up coronary angiography was very low [21••]. This observation may be a late demonstration of early graft failure but also may be related to the spontaneous sealing of the arterial wall, leading to competitive anterograde blood flow in the native vessel and eventually to graft failure.
We strongly believe that a conservative medical strategy is the best initial approach for most patients with stable SCAD once the acute ischemic insult is over. This strategy limits revascularization (stents or coronary surgery) to patients with persistent severe ischemia and/or clinical instability. Conservative initial management has been associated with an excellent clinical outcome [19••]. In a series of 45 consecutive patients with SCAD treated conservatively, the initial treatment was exclusively medical in 80 % of patients. Sixteen patients required revascularization during hospitalization, mainly with stent placement, and only one patient required coronary surgery. Except for one patient who died in the postoperative period after cardiac surgery, the clinical evolution at late follow-up was satisfactory. Our results support an initial conservative medical strategy in patients with stable SCAD, reserving coronary revascularization for patients with ongoing or recurrent ischemia. The observation that the vessel wall eventually heals spontaneously over time in most patients managed conservatively is yet another strong argument in favor of this approach [19••].
Conclusions
ACS frequently is related to atherosclerosis, with evidence of obstructive CAD. Many angiographically normal coronary arteries have silent underlying atherosclerosis; however, some nonatherosclerotic causes of ACS should be taken into account. An accurate diagnosis requires a high level of suspicion and/or the selection of appropriate diagnostic tests. Management and treatment also have special considerations depending on the specific etiology. Knowledge of these rare, but not exceptional, causes of ACS is important to ensure the best clinical outcomes.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Maddox TM, Ho PM, Roe M, et al. Utilization of secondary prevention therapies in patients with nonobstructive coronary artery disease identified during cardiac catheterization. Circ Cardiovasc Qual Outcomes. 2010;3:632–41.
Berger JS, Elliot L, Gallup D, et al. Sex differences in mortality following acute coronary syndromes. JAMA. 2009;302:874–82.
Hochman JS, Tamis JE, Thompson TD, et al. Sex, clinical presentation and outcome in patients with acute coronary syndromes. N Engl J Med. 1999;341:226–32.
Gehrie ER, Reynolds HR, Chen AY, et al. Characterization and outcomes of women and men with non-ST-segment elevation myocardial infarction and nonobstructive coronary artery disease: results of the CRUSADE quality improvement initiative. Am Heart J. 2009;158:688–94.
Chokshi NP, Iqbal SN, Berger RL, et al. Sex and race are associated with the absence of epicardial coronary artery obstructive disease at angiography in patients with acute coronary syndromes. Clin Cardiol. 2010;33:495–501.
Amman P, Marschall S, Kraus M, et al. Characteristics and prognosis of myocardial infarction in patients with normal coronary arteries. Chest. 2000;117:333–8.
Mazurkiewicz L, Bilinska ZT, Kruk M, et al. Baseline clinical characteristics and midterm prognosis of STE-ACS and NSTE-ACS patients with normal coronary arteries. Ann Noninvasive Electrocardiol. 2009;14:4–12.
Reynols HR, Srichai MB, Iqbal SN, et al. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation. 2011;124:1414–25. This prospective study evaluated IVUS and CMR findings in women with acute myocardial infarction and nonobstructive CAD.
Lanza GA, Sestito A, Sgueglia GA, et al. Current clinical features, diagnostic assessment and prognostic determinants of patients with variant angina. Int J Cardiol. 2007;118:41–7.
Takagi Y, Takahashi J, Yasuda S, et al. Prognostic stratification of patients with vasospastic angina. J Am Coll Cardiol. 2013;62:1144–53. The authors present a novel scoring system for the comprehensive risk assessment and prognostic stratification of patients with CV.
Manzano MC, Vilacosta I, San Roman JA, et al. Acute coronary syndrome in infective endocarditis. Rev Esp Cardiol. 2007;60:24–31.
Azzarelli S, Galassi AR, Amico F, et al. Clinical features of transient left ventricular apical ballooning. Am J Cardiol. 2006;98:1273–6.
Parodi G, Del Pace S, Carrabba N, et al. Incidence, clinical findings, and outcome of women with left ventricular apical ballooning syndrome. Am J Cardiol. 2007;99:182–5.
Sy F, Basraon J, Zheng H, et al. Frequency of Takotsubo cardiomyopathy in postmenopausal women presenting with an acute coronary syndrome. Am J Cardiol. 2013;112:479–82.
Sharkey SW, Windenburg DC, Lesser JR, et al. Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. J Am Coll Cardiol. 2010;55:333–41.
Elesber AA, Prasad A, Lennon RJ, et al. Four-year recurrence rate and prognosis of the apical ballooning syndrome. J Am Coll Cardiol. 2007;50:448–52.
Feldman JA, Fish SS, Beshnsky JR, et al. Acute cardiac ischemia in patients with cocaine-associated complaints: results of a multicenter trial. Ann Emerg Med. 2000;36:469–76.
Hollander JE, Hoffman RS, Gennis P, et al. Cocaine Associated Chest Pain (COCHPA) Study Group. Prospective multicenter evaluation of cocaine-associated chest pain. Acad Emerg Med. 1994;1:330–9.
Alfonso F, Paulo M, Lennie V, et al. Spontaneous coronary artery dissection. Long term follow-up of a large series of patients prospectively managed with a “conservative” therapeutic strategy. J Am Coll Cardiol Intv. 2012;5:1062–70. In this large prospective series of patients with SCAD, a conservative therapeutic strategy provided an excellent long-term prognosis.
Vanzetto G, Berger-Coz E, Barone-Rochette G, Chavanon O, Bouvaist H, Hacini R, et al. Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur J Cardiothorac Surg. 2009;35:250–4.
Tweet M, Hayes S, Pitta S, et al. Clinical features, management and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126:579–88. This is the largest series with long follow-up in patients with SCAD.
Hill SF, Sheppard MN. Non-atherosclerotic coronary artery disease associated with sudden cardiac death. Heart. 2010;96:1119–25.
Dey S, Flather MD, Devlin G, et al. Sex-related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: the Global Registry of Acute Coronary Events. Heart. 2009;95:20–6.
Roe MT, Harrington RA, Prosper DM, et al. Clinical and therapeutic profile of patients presenting with acute coronary syndromes who do not have significant coronary artery disease. The Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy (PURSUIT) Trial Investigators. Circulation. 2000;102:1101–6.
Alfredsson J, Lindback J, Wallentin L, et al. Similar outcome with an invasive strategy in men and women with non-ST elevation acute coronary syndromes: from Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART). Eur Heart J. 2011;32:3128–36.
Prinzmetal M, Kennamer R, Merliss R, et al. Angina pectoris I: a variant form of angina pectoris; preliminary report. Am J Med. 1959;27:375–88.
Yasue H, Kugiyama K. Coronary spasm: clinical features and pathogenesis. Intern Med. 1997;36:760–5.
Yasue H, Nakagawa H, Itoh T, et al. Coronary artery spasm—clinical features, diagnosis, pathogenesis, and treatment. J Cardiol. 2008;51:2–17.
Kusama Y, Kodani E, Nakagomi A, et al. Variant angina and coronary artery spasm: the clinical spectrum, pathophysiology and management. J Nippon Med Sch. 2011;78:4–12.
Bastante-Valiente T, Gonzalez-Mansilla A, Parra-Fuertes JJ, et al. Sequential coronary spasm in Prinzmetal’s angina presenting as syncope. Rev Esp Cardiol. 2008;61:332–3.
Kishida H, Tada Y, Fukuma N, et al. Significant characteristics of variant angina patients with associated syncope. Jpn Heart J. 1996;37:317–26.
Romagnoli E, Lanza GA. Acute myocardial infarction with normal coronary arteries: role of coronary artery spasm and arrhythmic complications. Int J Cardiol. 2007;117:3–5.
Onaka H, Hirota Y, Shimada S, et al. Clinical observation of spontaneous angina attacks and multivessel spasm in variant angina pectoris with normal coronary arteries: evaluation by 24-hour 12-lead electrocardiography with computer analysis. J Am Coll Cardiol. 1996;27:38–44.
Okumura K, Yasue H, Horio Y, et al. Multivessel coronary spasm in patients with variant angina: a study with intracoronary injection of acetylcholine. Circulation. 1988;77:535–42.
Onaka H, Hirota Y, Shimada S, et al. Prognostic significance of the pattern of multivessel spasm in patients with variant angina. Jpn Circ J. 1999;63:509–13.
Akasaka T, Okumura K, Kawashima S, et al. Guidelines for the diagnosis and treatment of patients with vasospastic angina (coronary spastic angina) (JCS 2008). Circ J. 2008;72:1239–52.
Zaya M, Mehta PK, Merz BM. Provocative testing for coronary reactivity and spasm. J Am Coll Cardiol. 2014;63:103–8. This review of provocative testing for the diagnosis of CV summarizes various dosing protocols.
Hackett D, Larkin S, Chierchia S, et al. Induction of coronary artery spasm by a direct local action of ergonovine. Circulation. 1987;75:577–82.
Ong P, Athanasiadis A, Borgulya G, et al. Clinical usefulness, angiographic characteristics and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive Caucasian patients with unobstructed coronary arteries. Circulation. 2014;129:1723–30.
Morikawa Y, Uemura S, Ishigami K, et al. Morphological features of coronary arteries in patients with coronary spastic angina: assessment with intracoronary optical coherence tomography. Int J Cardiol. 2011;146:334–40.
Tanaka A, Shimada K, Tearney GJ, et al. Conformational change in coronary artery structure assessed by optical coherence tomography in patients with vasospastic angina. J Am Coll Cardiol. 2011;58:1608–13.
Park HC, Choi SI, Lee JU, et al. Morphological findings in typical variant angina presenting as acute coronary syndrome using optical coherence tomography. J Interv Cardiol. 2013;26:491–500.
Morikawa Y, Mizuno Y, Harada E, et al. Aerobic interval exercise training in the afternoon reduces attacks of coronary spastic angina in conjunction with improvement in endothelial function, oxidative stress, and inflammation. Coron Artery Dis. 2013;24:177–82.
Nishigaki K, Inoue Y, Yamanouchi Y, et al. Prognostic effects of calcium channel blockers in patients with vasospastic angina—a meta analysis. Circ J. 2010;74:1943–50.
Kounis NG, Zavras GM. Histamine-induced coronary artery spasm: the concept of allergic angina. Br J Clin Pract. 1991;45:121–8.
Kounis NG. Coronary hypersensitivity disorder: the Kounis syndrome. Clin Ther. 2013;35:563–71. This recent review of KS was written by the author who first described the association between ACS and allergic reactions.
Kounis NG, Mazarakis A, Tsigkas G, et al. Kounis syndrome: a new twist on an old disease. Futur Cardiol. 2011;7:805–24.
Chen JP, Hou D, Pendyala L, et al. Drug-eluting stent thrombosis: the Kounis hypersensitivity-associated acute coronary syndrome revisited. JACC Cardiovasc Interv. 2009;2:583–93.
Rico-Cepeda P, Palencia-Herrejon E, Rodriguez Aguirregabiria MM. Kounis syndrome. Med Intensiv. 2012;36:358–64.
Cevik C, Nugent K, Shome GP, et al. Treatment of Kounis syndrome. Int J Cardiol. 2010;143:223–6.
Tang L, Hu XQ, Zhou SH. Coronary artery embolism causing acute myocardial infarction in patients with mechanical heart valve prosthesis: which is the optimal treatment? Heart Lung Circ. 2013;23(5):422–7.
López-Lluva MT, Sánchez-Pérez I, Fernández-Vallejo V, et al. Non-atherosclerotic acute myocardial infarction: coronary artery embolism. Med Intensiv. 2013;31:209–11.
Czarina JR, Weekes AJ. Acute myocardial infarction caused by coronary embolism from infective endocarditis. J Emerg Med. 2011;40:509–14.
Bathina JD, Daher IN, Plana JC, et al. Acute myocardial infarction associated with nonbacterial thrombotic endocarditis. Tex Heart Inst J. 2010;37:208–12.
Angulo-Llanos R, Sanz-Ruiz R, Solis J, et al. Acute myocardial infarction: an uncommon complication of takotsubo cardiomyopathy. Catheter Cardiovasc Interv. 2013;82:909–13.
Han DC, Kim JS, Lee SK, et al. Native aortic valve thrombosis: an unusual cause of acute ST-elevation myocardial infarction. Cardiovasc Pathol. 2013;22:e23–6.
Hisatomi K, Yamada T, Odate T, et al. Intermittent coronary artery occlusion caused by a floating thrombus in the left coronary sinus of Valsalva of a patient with a normal aorta and protein C deficiency. Ann Thorac Surg. 2011;92:1508–10.
Ferreira AR, Freitas A, Magno P, et al. Acute coronary syndrome of paradoxical origin. Rev Port Cardiol. 2013;32:817–21.
Myers PO, Bounameaux H, Panos A, et al. Impending paradoxical embolism. Chest. 2010;137:164–70.
Brito JD, Almeida MS, Ribeiras R, et al. Recurrent myocardial infarction in a patient with papillary fibroelastoma. Arq Bras Cardiol. 2012;98:e7–10.
Konstanty-Kalndyk J, Wierzbicki K, Bartus K, et al. Acute myocardial infarction due to coronary embolisation as the first manifestation of left atrial myxoma. Kardiol Pol. 2013;71:403–5.
Protasiewick M, Rojek A, Gajek J, et al. Cardiac arrest due to left circumflex coronary artery embolism as a complication of subtherapeutic oral anticoagulation in a patient with mitral and aortic mechanical valve prostheses. Postep Kardiol Int. 2013;9:97–100.
Sato HTH, Tateishi H, Uchido T, et al. Takotsubo type cardiomyopathy due to multivessel spasm. In: Kodama K, Haze K, Hon M, editors. Clinical aspect of myocardial injury: from ischemia to heart failure (in Japanese). Tokyo: Kagakuhyouronsya Co.; 1990. p. 56–64.
Desmet WJ, Adriaenssens BF, Dens JA. Apical ballooning of the left ventricle: first series in white patients. Heart. 2003;89:1027–31.
Schneider B, Athanasiadis A, Stölberger C, et al. Gender differences in the manifestation of tako-tsubo cardiomyopathy. Int J Cardiol. 2013;166:584–8.
Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408–17.
Ghadri JR, Ruschitzka F, Lüscher TF, Templin C. Takotsubo cardiomyopathy: still much more to learn. Heart. 2014. doi:10.1136/heartjnl-2013-304691. The authors present an up-to-date review on TTC.
Nguyen TH, Neil CJ, Sverdlov AL, et al. N-terminal pro-brain natriuretic protein levels in takotsubo cardiomyopathy. Am J Cardiol. 2011;108:1316–21.
Lozano A, Bastante T, Salamanca J, et al. Tako-Tsubo cardiomyopathy triggered by influenza A virus infection. Int J Cardiol. 2014. doi:10.1016/j.ijcard.2014.04.033.
Alfonso F, Núñez-Gil IJ, Hernández R. Optical coherence tomography findings in Tako-Tsubo cardiomyopathy. Circulation. 2012;126:1663–4.
Alfonso F, Cárdenas A, Ibáñez B, et al. Mid-ventricular tako-tsubo cardiomyopathy with structurally normal coronary arteries confirmed by optical coherence tomography. J Invasive Cardiol. 2013;25:e214–5.
Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA. 2011;306:277–86. In this prospective multicenter registry of patients with TTC, CMR was used systematically at initial presentation and follow-up.
Santoro F, Ieva R, Ferraretti A, et al. Safety and feasibility of levosimendan administration in takotsubo cardiomyopathy: a case series. Cardiovas Ther. 2013;31:e133–7.
Santoro F, Ieva R, Musaico F, et al. Lack of efficacy of drug therapy in preventing takotsubo cardiomyopathy recurrence: a meta-analysis. Clin Cardiol. 2014;37(7):434–9.
US Department of Health and Human Services (DHS); Office of Applied Studies. Results from the 2008 National Survey on Drugs Use and Health: national findings. http://oas.samshsa.gov/nsduh/2k8nsduh/2k8Results.cfm.
US Department of Health and Human Services (DHS), Substance Abuse and Mental Health Service Administration, Office of Applied Studies. Drug Abuse Warning Network, 2007: national estimates of drug-related emergency department visits. Rockville, MD: 2010. https://dawninfo.smhsa.gov/files/ED2007/DAWN2k7ED.pdf
Burillo-Putze G, Borreguero León JM, García Dopico JA, et al. Incidence and impact of undisclosed cocaine use in emergency department chest pain and trauma patients. Int J Emerg Med. 2008;1:169–72.
Brogan III WC, Lange RA, Kim AS, et al. Alleviation of cocaine-induced coronary vasoconstriction by nitroglycerin. J Am Coll Cardiol. 1991;18:581–6.
Moliterno DJ, Willard JE, Lange RA, et al. Coronary-artery vasoconstriction induced by cocaine, cigarette smoking, or both. N Engl J Med. 1994;330:454–9.
Dressler FA, Malekzadeh S, Roberts WC. Quantitative analysis of amounts of coronary arterial narrowing in cocaine addicts. Am J Cardiol. 1990;65:303–8.
Finkel JB, Marhefka GD. Rethinking cocaine-associated chest pain and acute coronary syndromes. Mayo Clin Proc. 2011;86:1198–207. This review focuses on the relationship between cocaine abuse and ACS.
Hollander JE, Levitt MA, Young GP, et al. Effect of recent cocaine use on the specificity of cardiac markers for diagnosis of acute myocardial infarction. Am Heart J. 1998;135:245–52.
McCord J, Jneid H, Hollander JE, et al. Management of cocaine-associated chest pain and myocardial infarction: a scientific statement from the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology. Circulation. 2008;117:1897–907.
Dattilo PB, Hailperm SM, Fearon K, et al. Beta-blockers are associated with reduced risk of myocardial infarction after cocaine use. Ann Emerg Med. 2008;51:117–25.
Rangel C, Shu RG, Lazar LD, et al. Beta-blockers for chest pain associated with recent cocaine use. Arch Intern Med. 2010;170:874–9.
Hoskins MH, Leleiko RM, Ramos JJ, et al. Effects of labetalol on hemodynamic parameters and soluble biomarkers of inflammation in acute coronary syndrome in patients with active cocaine use. J Cardiovasc Pharmacol Ther. 2010;15:47–52.
Pretty H. Dissecting aneurysms of coronary artery in woman aged 42: rupture. BMJ. 1931;1:667.
Alfonso F. Spontaneous coronary artery dissection. New insights from the tip of the iceberg? Circulation. 2012;126:667–70.
Saw J, Ricci D, Starovoytov A, et al. Spontaneous coronary artery dissection. Prevalence and predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. J Am Coll Cardiol Intv. 2013;6:44–52. Patients with SCAD were prospectively and retrospectively screened for fibromuscular dysplasia. The results show a high prevalence of concomitant fibromuscular dysplasia in these patients.
Vrints C. Spontaneous coronary artery dissection. Heart. 2010;96:801–8.
Alfonso F, Paulo M, Gonzalo N, et al. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. J Am Coll Cardiol. 2012;59:1073–9.
Compliance with Ethics Guidelines
Conflict of Interest
Amparo Benedicto, Teresa Bastante, Jorge Restrepo, Javier Cuesta, Fernando Rivero, and Fernando Alfonso have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Management of Acute Coronary Syndromes
Rights and permissions
About this article
Cite this article
Bastante, T., Rivero, F., Cuesta, J. et al. Nonatherosclerotic Causes of Acute Coronary Syndrome: Recognition and Management. Curr Cardiol Rep 16, 543 (2014). https://doi.org/10.1007/s11886-014-0543-y
Published:
DOI: https://doi.org/10.1007/s11886-014-0543-y