Abstract
There are compelling reasons to develop a tissue-engineered mitral valve, but this endeavor has not received the same attention as tissue engineering strategies for the semilunar valves. Challenges in regenerating a mitral valve include recapitulating the complex heterogeneity in terms of anatomy (differently sized leaflets, numerous chordae), extracellular matrix composition, biomechanical behavior, valvular interstitial cell and endothelial cell phenotypes, and interior vasculature and innervation. It will also be essential to restore the functional relationships between the native mitral valve and left ventricle. A growing amount of information relevant to tissue engineering a mitral valve has been recently collected through investigations of cell mechanobiology and collagen organization. It is hoped that the development of tissue-engineered mitral valves can build on knowledge derived from engineering semilunar valves, but the mitral valve will present its own unique challenges as investigators move toward a first-generation prototype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although the drive to generate tissue-engineered heart valves has been primarily focused on the semilunar valves (aortic and pulmonary), there are compelling reasons to develop tissue-engineered atrioventricular valves (mitral and tricuspid). The incidence of myxomatous mitral valve (MV) disease in industrialized nations such as the United States is estimated at 1–2% [1, 2]. Approximately 41,000 hospitalizations per year are due to surgical corrections of mitral valve regurgitation (MR) in the United States [1], yet this number represents only 44% of persons with severe MR who would benefit from surgical correction [2]. In addition, the initial durability of these repairs has been impressive, but there is an increasing recognition of the occurrence of late return of significant MR [2]. Furthermore, congenital heart defects in the United States occur in 9 of 1000 live births [1], the majority of which are associated with abnormal valves. There are very few options for treating congenital valve defects, such as valve repair or replacement with a tiny mechanical or bioprosthetic valve, and these corrections are only palliative and do not preclude the need for reoperation on the valve later in the patient’s life. The prognosis for children with congenital valve disease as well as adults with MV disease would be profoundly improved by the development of a living tissue-engineered mitral valve (TEMV).
The essential function of the MV is opening to allow blood flow from the left atrium to left ventricle and closing to prevent retrograde flow. This function is coordinated by the complicated structure and mechanical behavior of the MV, which in turn depend upon the inherent heterogeneity in extracellular matrix (ECM) and cell phenotype within these diverse anatomic structures. This review will address the different scales of tissue and cell mechanobiology found within the MV and explain how these present challenging design goals for a TEMV.
Anatomy
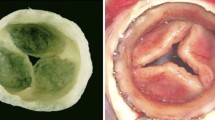
The MV consists of two primary leaflets and numerous chordae tendineae (Fig. 1a). The anterior leaflet (AL) of the MV begins as a distal continuation of the subaortic curtain below the left and noncoronary leaflets of the aortic valve. The posterior leaflet (PL) is attached to the lateral wall of the left ventricle just below the left atrium. Thus, the annulus (leaflet attachment region) is muscular for the PL and fibrous for the AL. Both leaflets extend into the mitral orifice, with the AL covering the majority of the orifice and the PL wrapping around the free edge of the AL to provide a sealed closure. The upper ends of the chordae tendineae attach either underneath the leaflet (basal, strut) or at the leaflet free edge (marginal), and the lower ends insert in the papillary muscles. Normal valve function involves coordinated motion of the leaflets and chordae as guided by blood flow, ventricular and atrial pressures, and contraction of the ventricle, papillary muscles, and annulus. The various components of the MV also play essential roles in ventricular function; the AL serves as a dynamic portion of the left ventricular outflow tract and helps to direct flow during ventricular ejection of blood through the aortic valve [2]. Additionally, it was recognized more than five decades ago that chordal tension makes critical contributions to ventricular health [2]. It will be important for a TEMV to recapitulate this fundamentally intertwined integration of MV and ventricular function.
a Photograph of a mitral valve cut open and viewed from the ventricular side to demonstrate the anterior and posterior leaflets and the chordae tendineae. b Layered mitral valve microstructure shown in a radially oriented cross-section stained with Movat pentachrome, which colors elastic fibers black, hydrated proteoglycans and glycosaminoglycans blue, and collagen yellow. c Polarized light micrographs of mitral valve leaflet cross-section (in the “clear zone”) showing crimped nature of collagen in the fibrosa. The ventricularis is a thin layer of elastic fibers and loose collagen on the outflow surface of the mitral valve leaflet. A atrialis; F fibrosa; GAGs glycosaminoglycans; PGs proteoglycans; S spongiosa
Microstructure and ECM
The MV leaflets have a layered microstructure that is readily distinguishable using multicolor histologic stains and that can be examined to understand the mechanical behavior of the entire tissue. From top to bottom, the primary layers are the atrialis, spongiosa, and fibrosa. The fibrosa is a thick, heavily collagenous layer on the ventricular surface, the atrialis is a thin, predominantly elastic layer on the atrial surface, and the inner “spongiosa” layer contains proteoglycans (PGs) and the glycosaminoglycan (GAG) hyaluronan (Fig. 1b). These layers do not exclusively contain only one type of ECM; the atrialis and spongiosa both contain loosely organized collagen [3], the spongiosa also contains a network of elastic fibers [4], and the fibrosa and atrialis also contain certain types of PGs [4]. The total leaflet thickness and fibrosa layer thickness in the AL are greater than those of the PL [3]. Furthermore, the relative thicknesses of these layers varies within each leaflet from its attachment edge to its free edge [3]. This variation in layer thicknesses is particularly evident in the AL, where the proximal third of the leaflet is marked by a very thick fibrosa, rich in highly aligned collagen and small leucine-rich PGs, with only minor presence of the other layers. This region of the leaflet is also notable by an absence of underlying chordal insertions, and has been frequently denoted as the leaflet “clear zone.” The distal two thirds of the AL, in contrast, do have chordal insertions and the corresponding name for this region is “rough zone.” The PL has chordal insertions underneath its entire surface and thus lacks a clear zone. The rough zones (AL middle and free edge, PL) have much more substantial spongiosa layers and thus are rich in hydrated PGs and GAGs such as hyaluronan [4].
Collagen fibers within the MV leaflets show a primary alignment along the circumferential direction of leaflets, which is largely responsible for the observed anisotropy in leaflet mechanical behavior [3, 5, 6]. These collagen fibers are highly crimped with a wavy form that is readily observable using a polarized light microscope (Fig. 1c). The crimped collagen fiber structure allows the leaflet tissue to undergo substantial deformation under even low levels of stress, a behavior that promotes the coaptation of the two leaflets in the closed valve. The cross-linked state of the collagen in atrioventricular valves is different from that of semilunar valves; hydrothermal isometric tension data indicate that the atrioventricular valve collagen has fewer overall cross-links, which would allow more rapid remodeling than in the aortic and pulmonary valves [7•].
Chordae tendineae primarily contain collagen that is highly aligned along the chordal length. Chordal collagen fibril diameters range from 40 to 70 nm [8] and adjacent collagen fibrils interact via small leucine-rich PGs to assemble into parallel fiber bundles with a wavy crimp pattern (period, 10–20 μm; crimp angle, 20–40°) [8]. Liao et al. [9] recently showed that chordal collagen fibers are arranged in a skew-registered configuration and undulate in three dimensions, which better suits a rounded columnar structure than would a planar arrangement. As with the MV leaflets, the highly crimped nature of the chordal collagen fibers permits an initial tissue deformation under very low loading. Another important ultrastructural feature of chordal collagen is that the fibril size and packing pattern vary with chordal type [8, 9]. For example, marginal chordae have a smaller average fibril diameter, a higher fibril density, and a narrow fibril size distribution. In contrast, the basal and strut chordae exhibit a larger average fibril diameter, a lower fibril density, and a wider fibril size distribution.
Over the past several years, there has been increasing attention paid to the vascular networks and innervations of the MV tissues. Pediatric and young adult valves have a well-defined vascular network in their leaflets [10••] and chordae [11], although the vasculature is much less in the valves of older adults [12]. These vessels are believed to supply nutrients to cells within diffusion-limited regions of these young valve tissues; their absence in older valves may be associated with the quiescent state of the older valvular interstitial cells (VICs). Furthermore, heart valves have a well-defined network of nerve fibers that work together with the VICs to contract the valve tissue (recently reviewed by Borin et al. [13]). Both the vascular and innervation aspects of living valves represent challenges to be addressed in developing tissue-engineered atrioventricular valves, particularly for the pediatric population.
Leaflet Mechanics
Numerous studies have characterized the nonlinear anisotropic mechanical behavior of the MV leaflets [5, 6, 14–16]. The stress-strain curves of both leaflets can be described by three major regions: a relatively long, low slope “toe” region at the beginning, a nonlinear transition region following, and then a second linear region with a high slope (large tensile modulus) that functionally locks up further deformation. This long toe region is due to uncrimping of the collagen fibers, whereas the transitional region is due to collagen fiber recruitment, and the linear high slope region represents the behavior of the recruited, uncrimped collagen. Biaxial testing data also show that the behavior of leaflets in the circumferential and radial directions is coupled, implying that collagen fibers are interacting at the microstructural level [6]. Both the AL and PL are stiffer and less extensible in the circumferential direction than in the radial direction. Additionally, leaflet stiffness in both directions is significantly increased with advancing age [16]. Furthermore, the PL tends to exhibit greater extensibility and lower tensile stiffness; one proposed explanation for this difference between leaflets is that the PL has more chordal attachments that provide additional mechanical support and therefore it does not require such high strength [6]. Numerous in vivo and in vitro studies have reported that there is also heterogeneity of mechanical behavior throughout individual leaflets [17, 18]; for example, the clear zone of the AL has a circumferential tensile elastic modulus that is 30–200% higher than in the rough zone [14, 16]. This distinction between the clear zone and rough zone stiffness was confirmed in an interesting study of mitral AL heterogeneity using scanning acoustic microscopy [19]; this same study also found that the speed of sound was higher (and thus the stiffness was greater) in the collagenous fibrosa layer than in the other layers of the mitral AL.
Viscoelasticity of connective tissues is commonly measured by stress relaxation, creep, and strain-rate dependence tests. A recent study by Grashow et al. [5] showed that the stress-strain responses of MV ALs were remarkably independent of a wide range of strain rates, including physiologic strain rates. The MV leaflets did show a small degree of hysteresis (~ 12%) but that also did not vary with strain rate; this innate low level of hysteresis likely reduces energy dissipation during the cyclic opening and closing of the MV. Similar to other collagenous tissues, MV leaflets do exhibit significant stress relaxation [15, 20]; although interestingly they exhibit negligible creep when subjected to a constant physiologic level load over a 3-h period [20]. This combination of stress relaxation, creep, and strain rate results appears to be unique in the soft tissue literature [20]. Liao et al. [15] examined the molecular mechanism underlying this asymmetric viscoelasticity and found that the microstructural processes differ between creep and stress relaxation. When the leaflet samples were subjected to constant stress the collagen fibrils maintained a constant strain, but when subjected to constant strain the collagen fibril strain decreased over time, suggesting that some type of fibril-level “locking” mechanism exists to allow for stress release under constant strain conditions, but not under constant stress [15]. The above studies show that MV leaflets cannot be treated as classical viscoelastic materials. Rather, MV leaflets appear intrinsically designed at the fibril level to minimize creep and hysteresis, and behave more as “quasi-elastic” biological materials in a manner that favors valvular function [5, 15, 20]. Sacks et al. confirmed that MV leaflets do not exhibit creep under full systolic loading in vivo [18]. Whether engineered tissue constructs are able to imitate this unique “quasi-elastic” behavior might be an important factor that affects the long-term durability of an engineered MV leaflet.
Chordal Mechanics
The marginal, basal, and strut chordae attach to the MV leaflets in different ways and correspondingly perform distinct mechanical roles in the opening and closing of the MV [21]. The strut chordae are the two thickest chordae, the basal chordae are thinner than strut chordae, and the marginal chordae are the thinnest [8]. Lomholt et al. [22] reported that the strut and basal chordae bear higher systolic tension than do the marginal chordae. From a functional perspective, marginal chordae (also known as primary chordae) are more involved in MV competence, whereas the strut and basal chordae (also known as secondary chordae) participate more in governing the geometry and function of the left ventricle [22].
Many years ago, it was observed that chordae tendineae had an apparently paradoxical size-dependent mechanical behavior in that thinner chordae (i.e., marginal) were less extensible and more stiff than thick chordae (i.e., basal and strut) [14, 23]. Further investigation by Liao et al. [8] confirmed the trend of increasing extensibility with an increase in cross-sectional area, in the order of marginal, basal, and strut chordae, and additionally demonstrated the mechanism for this pattern by analysis of crimp periodicity. Thicker chordae had smaller crimp periods than thinner chordae (11.3 ± 1.4 vs 14.8 ± 3.0 μm), and thus their collagen was more highly crimped (both had similar crimp amplitudes). Thicker chordae could therefore undergo greater strain before the collagen was uncrimped (i.e., greater extensibility). Chordal mechanics are also affected by the size and packing pattern of collagen fibrils, as shown by transmission electron microscopy (TEM). Even though the area percentage of collagen fibrils in thinner and thicker chordae is equal, the tensile modulus of thinner chordae is greater than that of thicker chordae [8]. TEM also shows that thinner chordae have lower average fibril diameter than thicker chordae but greater average fibril density. Therefore, the greater modulus of thinner chordae can possibly be explained by the greater number of interfibrillar interactions via small leucine-rich PGs in these chordae; this explanation is supported both by quantitative measurements of GAG content and by the shear-lag theory of interfibrillar PGs [8, 24].
The chordal insertion zone represents another unique structural and mechanical aspect of the MV. Padala et al. [25] reported a heterogeneous stretch distribution in the strut chordal insertion region due to the complex collagen architecture spanning into the leaflet. The strut chordal insertion region is also believed to stretch and bear load during both systolic and diastolic phases [25]. This important interface between chordae and leaflet might represent a challenge for MV tissue engineering in terms of structural continuity and material strength (comparable to the challenge of recreating the bone-tendon interface).
Cells
VICs have a well-recognized range of phenotypic characteristics. Although originally the phenotypic description of VICs had a primary emphasis upon their nearly uniform expression of fibroblast characteristics and less common but notable expression of smooth muscle features, over the past few years these phenotypic definitions have become more tailored. Recently, Gotlieb et al. [26] summarized their phenotypes as quiescent, activated, progenitor, and osteoblastic (in the case of aortic VICs). The activated VICs, in particular, express smooth muscle α-actin (SMαA) and are responsible for repair and remodeling of the valve tissues; Stephens and Grande-Allen [27] reported that activated VICs located deep within the leaflet interior were always colocalized with regions of active collagen synthesis and/or degrading enzymes. Furthermore, VICs within the MV have been shown to be functionally coupled with collagen fibers via α2β1 integrin connections to the actin cytoskeleton, and KCl-stimulated isometric force generation by the mitral VICs in situ can be blocked either by blocking the α2β1 integrins with antibodies or blocking actin polymerization via cytochalasin [28].
Although VICs from the aortic valve have been characterized to a much greater extent than those from the MV, mitral VICs have been shown to demonstrate some unique features. Merryman et al. [29] reported that aortic and mitral VICs demonstrated comparable cell membrane stiffness, expression of SMαA, and expression of a molecular chaperone involved in collagen synthesis. Additionally, both aortic and mitral VICs demonstrate a heterogeneous phenotype in vitro and in vivo [30–32]. However, mitral and aortic VICs respond differentially to cytokine stimulation: specifically, treatment with transforming growth factor-β (TGF-β) reduced proliferation by aortic VICs [33] but increased mitral VIC proliferation [34]. Similarly, actively migrating mitral VICs in an in vitro scratch wound model upregulate fibroblast growth factor-2 (FGF-2) and FGF growth factor receptor 1 [35] as well as greater expression of TGF-β and SMαA and phosphorylation of Smad2/3 [34]. This process appears to be different from aortic VICs in which FGF-2 inhibits TGF-β–mediated upregulation of SMαA expression [36].
The diverse anatomy and microstructural composition of the MV is also reflected in the cell phenotype within these regions. Blevins et al. [32] reported that VICs isolated from distinct anatomic regions of porcine MVs (specifically the AL clear zone, PL, and chordae) show heterogeneity in ECM synthesis, adhesion to substrates, and phenotype. For example, PL cells grew faster than chordal cells, but demonstrated less SMαA, vimentin, and internal complexity, suggesting that the PL cells were less differentiated than the chordal cells [32]. These cells reside within a leaflet rich in GAGs and PGs and experience more compressive loading than do the cells within the chordae and AL clear zone [32]. When PL cells were divided into subpopulations by differential adhesion, the two differentially adhesive cell groups did not demonstrate differential production of distinct GAG classes. This result was different from the results of the same method applied to VICs from the other two regions, in which the cells demonstrating stronger adhesion synthesized more sulfated GAGs. VICs from the AL clear zone exhibited the fastest metabolism but slowest growth, and also secreted less collagen into the culture medium but more 4-sulfated GAGs than other cells. This heterogeneity of phenotypic behavior of VICs across the valve structure is also shown by the valve remodeling in animal models of MR. In a sheep model of MR induced by tachycardia-induced cardiomyopathy, VICs from the middle regions of the mitral leaflets showed significant changes in activation and expression of matrix remodeling enzymes, whereas those VICs located more proximally to the annulus were less affected [37].
The differential adhesion result noted above [32], together with other studies [38–40], has led to the speculation that the mitral chordal cells are initially more responsive to their mechanical environment than the mitral leaflet cells. Gupta et al. [38–40] have reported that both PL VICs and chordal VICs are influenced by stretch, in that the presence and magnitude of stretch applied to VICs cultured within a three-dimensional collagen gel influences the retention of GAGs and PGs within the gel as well as the secretion of these ECM components into the culture medium. Interestingly, chordal VICs subjected to constant stretch synthesized fewer GAGs than did PL VICs under the same culture conditions [39], which reflects the abundance of GAGs within actual chordal versus leaflet tissue [4]. Two other studies found that chordal VICs responded to changes in applied stretch by altering their ECM synthesis in a more rapid manner [40] and were more affected by stretch magnitude [38] than were PL VICs. However, over time the PL VICs did show the ability to change the type of ECM produced in response to altered stretching conditions [40], indicating that despite their phenotypic heterogeneities, mitral VICs are all mechanosensitive. This latter point was recently demonstrated in vivo in a sheep model of ventricular dysfunction, mitral regurgitation, and abnormal MV stresses caused by papillary muscle displacement and thus leaflet tethering [41•]. Within 60 days, an endothelial-mesenchymal transdifferentiation response by the valve cells resulted in active remodeling, growth, and thickening of the chordae and MV leaflets. Overall, these studies make it evident that improving our understanding of MV biology and active adaptive mechanisms will greatly benefit the development of a TEMV.
Valvular endothelial cells (VECs) are also garnering attention, although the mitral VECs have not been investigated to the same extent as have aortic VECs. In recent studies by Flanagan et al. [42], VECs were found to synthesize the basement membrane components laminin and type IV collagen, but not type I collagen and chondroitin sulfate. VECs were also found to synthesize endothelial nitric oxide synthase (NOS) both in vivo and in vitro, in contrast with VICs, for which only a minority of cells expressed neuronal NOS in vitro [42]. These same authors found that growing mitral VECs on collagen-chondroitin sulfate scaffolds promoted a more in vivo—like phenotype than did culture on collagen-only scaffolds [43]. It is also likely that mitral VECs will demonstrate a regionally specific (leaflets, chordae) and/or side-specific (atrial, ventricular) phenotype much like that of aortic VECs [44]. Overall, these characterizations of the in vitro and in vivo phenotypes of various MV cell populations can provide vital information for an TEMV, particularly for determining the ideal cell sources and effective scaffold designs to direct cell differentiation and function.
Towards a TEMV
Thus far, only a handful of publications describe actual efforts to generate a TEMV tissue. In an early stage effort to create a TEMV, Shi and Vesely [45] focused on fabricating a simple tendinous structure that mimicked chordae tendineae. These constructs were generated by mixing smooth muscle cells with soluble collagen and allowing the cells to contract the collagen gel in response to tension induced by anchors in the culture dish [45]. Over several weeks this method produced a highly compact collagen construct with well-aligned dense, crimped collagen fibrils, an elastic fiber sheath around the collagen core, and nonlinear stress-strain behavior resembling tendinous tissues [45]. The material strength of these constructs was improved by applying bioreactor-controlled mechanical stimuli and by optimizing the shape and material used in the construct anchors [46]. This approach was then extended to demonstrate more complex branching structures using either direct fabrication or culture-driven fusion of two single constructs [47].
Other publications demonstrate an active movement toward technology development to make TEMV endeavors possible. For example, investigators have developed sterile bioreactors that could be used for culturing tissue-engineered or organ-cultured MVs [38, 40, 48]. A new bioreactor system described by Lieber et al. [49••] is particularly interesting because it was designed for the culture of murine hearts at various ages (perinatal through adults). Although many of these culture systems are being currently used to investigate the biology and pathology of MV tissues and cells, these research efforts are nonetheless useful in developing design goals for a TEMV. Another promising direction for gaining insight into the development of TEMVs is to investigate the signaling pathways involved in embryonic development of the valves [50••]. As noted above, it is also important to determine how valvular cells will respond to a tissue-engineered heart valve scaffold; for example, valve cells can vary their phenotypes when they are cultured on substrates of different stiffness. Stephens et al. [51] recently reported that mitral VICs show age-dependent and valve region-dependent levels of expression of SMαA, prolyl-4-hydroxylase, and heat shock protein 47 when cultured atop polyethylene glycol diacrylate hydrogels of different stiffnesses.
Conclusions
The MV demonstrates heterogeneity and the capacity for rapid cell-driven remodeling, both of which impose challenges and opportunities for developing a TEMV. These mechanical and structural heterogeneities mandate a careful design to match the structural and functional roles of the target MV components, and to maintain their relationships with other cardiac structures. A disruption of the native structural and mechanical balance of the MV might cause unfavorable remolding in the leaflet or chordal tissues. Conversely, the capability of MV tissues or engineered components to adapt could indicate a self-adjusting mechanism, in which positive adaptation would favor proper MV function.
Although the development of TEMVs is in its infancy compared with other engineered tissues, this time lag offers the opportunity to reflect upon the successes and failures of tissue-engineered aortic valves. For example, are the polymer mesh scaffolds and acellular approaches that are so widely investigated for tissue-engineered aortic valves appropriate for TEMVs? Are newer scaffold approaches such as electrospinning, smart biofunctional materials, or small intestinal submucosa feasible options? What cell source should be used in a TEMV, and how will the TEMV be assembled to recapitulate the diverse cell phenotypes found across the native MV? How should vasculature and innervation be addressed? Finally, how will the TEMV be implanted in a manner that maintains or restores the essential functions of the MV and its integral relationship with the left ventricle? These and other questions are certain to be addressed as investigators move toward a first-generation prototype of a TEMV.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lloyd-Jones D, Adams RJ, Brown TM, et al.: Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation 2010, 121:e46–e215.
Fedak PW, McCarthy PM, Bonow RO: Evolving concepts and technologies in mitral valve repair. Circulation 2008, 117:963–974.
Kunzelman KS, Cochran RP, Murphree SS, et al.: Differential collagen distribution in the mitral valve and its influence on biomechanical behaviour. J Heart Valve Dis 1993, 2:236–244.
Stephens EH, Chu CK, Grande-Allen KJ: Valve proteoglycan content and glycosaminoglycan fine structure are unique to microstructure, mechanical load and age: Relevance to an age-specific tissue-engineered heart valve. Acta Biomater 2008, 4:1148–1160.
Grashow JS, Yoganathan AP, Sacks MS: Biaixal stress-stretch behavior of the mitral valve anterior leaflet at physiologic strain rates. Ann Biomed Eng 2006, 34:315–325.
May-Newman K, Yin FC: Biaxial mechanical behavior of excised porcine mitral valve leaflets. Am J Physiol 1995, 269:H1319–1327.
• Aldous IG, Veres SP, Jahangir A, Lee JM: Differences in collagen cross-linking between the four valves of the bovine heart: A possible role in adaptation to mechanical fatigue. Am J Physiol Heart Circ Physiol 2009, 296:H1898–1906. This work finds considerable differences in collagen cross-linking between the semilunar and atrioventricular valves, which may have implications for the fundamental distinctions between aortic valve disease (calcific) and MV disease (myxomatous).
Liao J, Vesely I: A structural basis for the size-related mechanical properties of mitral valve chordae tendineae. J Biomech 2003, 36:1125–1133.
Liao J, Priddy LB, Wang B, et al.: Ultrastructure of porcine mitral valve chordae tendineae. J Heart Valve Dis 2009, 18:292–299.
•• Swanson JC, Davis LR, Arata K, et al.: Characterization of mitral valve anterior leaflet perfusion patterns. J Heart Valve Dis 2009, 18:488–495. This study is the first in vivo demonstration of perfusion of the blood vessels within the MV leaflets and chordae.
Ritchie J, Warnock JN, Yoganathan AP: Structural characterization of the chordae tendineae in native porcine mitral valves. Ann Thorac Surg 2005, 80:189–197.
Duran CM, Gunning AJ: The vascularization of the heart valves: A comparative study. Cardiovasc Res 1968, 2:290–296.
Borin C, Vanhercke D, Weyns A: Innervation of the atrioventricular and semi-lunar heart valves: A review. Acta Cardiol 2006, 61:463–469.
Kunzelman KS, Cochran RP: Stress/strain characteristics of porcine mitral valve tissue: Parallel versus perpendicular collagen orientation. J Card Surg 1992, 7:71–78.
Liao J, Yang L, Grashow J, Sacks MS: The relation between collagen fibril kinematics and mechanical properties in the mitral valve anterior leaflet. J Biomech Eng 2007, 129:78–87.
Stephens EH, de Jonge N, McNeill MP, et al.: Age-related changes in material behavior of porcine mitral and aortic valves and correlation to matrix composition. Tissue Eng Part A 2010, 16:867–878.
Chen L, McCulloch AD, May-Newman K: Nonhomogeneous deformation in the anterior leaflet of the mitral valve. Ann Biomed Eng 2004, 32:1599–1606.
Sacks MS, Enomoto Y, Graybill JR, et al.: In-vivo dynamic deformation of the mitral valve anterior leaflet. Ann Thorac Surg 2006, 82:1369–1377.
Jensen AS, Baandrup U, Hasenkam JM, et al.: Distribution of the microelastic properties within the human anterior mitral leaflet. Ultrasound Med Biol 2006, 32:1943–1948.
Grashow JS, Sacks MS, Liao J, Yoganathan AP: Planar biaxial creep and stress relaxation of the mitral valve anterior leaflet. Ann Biomed Eng 2006, 34:1509–1518.
Obadia JF, Casali C, Chassignolle JF, Janier M: Mitral subvalvular apparatus: Different functions of primary and secondary chordae. Circulation 1997, 96:3124–3128.
Lomholt M, Nielsen SL, Hansen SB, et al.: Differential tension between secondary and primary mitral chordae in an acute in-vivo porcine model. J Heart Valve Dis 2002, 11:337–345.
Lim KO, Boughner DR: Morphology and relationship to extensibility curves of human mitral valve chordae tendineae. Circ Res 1976, 39:580–585.
Liao J, Vesely I: Skewness angle of interfibrillar proteoglycans increases with applied load on mitral valve chordae tendineae. J Biomech 2007, 40:390–398.
Padala M, Sacks MS, Liou SW, et al.: Mechanics of the mitral valve strut chordae insertion region. J Biomech Eng 2010, 132:081004.
Liu AC, Joag VR, Gotlieb AI: The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol. 2007, 171:1407–1418.
Stephens EH, Grande-Allen KJ: Age-related changes in collagen synthesis and turnover in porcine heart valves. J Heart Valve Dis 2007, 16:672–682.
Stephens EH, Durst CA, Swanson JC, et al.: Functional coupling of valvular interstitial cells and collagen in the mitral leaflet. Cell Mol Bioeng 2010, 3:428–437.
Merryman WD, Youn I, Lukoff HD, et al.: Correlation between heart valve interstitial cell stiffness and transvalvular pressure: Implications for collagen biosynthesis. Am J Physiol Heart Circ Physiol 2006, 290:H224–231.
Rabkin E, Aikawa M, Stone JR, et al.: Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation 2001, 104:2525–2532.
Taylor PM, Batten P, Brand NJ, et al.: The cardiac valve interstitial cell. Int J Biochem Cell Biol 2003, 35:113–118.
Blevins TL, Peterson SB, Lee EL, et al.: Mitral valvular interstitial cells demonstrate regional, adhesional, and synthetic heterogeneity. Cells Tissues Organs 2008, 187:113–122.
Walker GA, Masters KS, Shah DN, et al.: Valvular myofibroblast activation by transforming growth factor-beta: Implications for pathological extracellular matrix remodeling in heart valve disease. Circ Res 2004, 95:253–260.
Liu AC, Gotlieb AI: Transforming growth factor-beta regulates in vitro heart valve repair by activated valve interstitial cells. Am J Pathol 2008, 173:1275–1285.
Gotlieb AI, Rosenthal A, Kazemian P: Fibroblast growth factor 2 regulation of mitral valve interstitial cell repair in vitro. J Thorac Cardiovasc Surg 2002, 124:591–597.
Cushing MC, Mariner PD, Liao JT, et al.: Fibroblast growth factor represses smad-mediated myofibroblast activation in aortic valvular interstitial cells. Faseb J 2008, 22:1769–1777.
Stephens EH, Timek TA, Daughters GT, et al.: Significant changes in mitral valve leaflet matrix composition and turnover with tachycardia-induced cardiomyopathy. Circulation 2009, 120:S112–119.
Gupta V, Tseng H, Lawrence BD, Grande-Allen KJ: Effect of cyclic mechanical strain on glycosaminoglycan and proteoglycan synthesis by heart valve cells. Acta Biomater 2009, 5:531–540.
Gupta V, Werdenberg JA, Blevins TL, Grande-Allen KJ: Synthesis of glycosaminoglycans in differently loaded regions of collagen gels seeded with valvular interstitial cells. Tissue Eng 2007, 13:41–49.
Gupta V, Werdenberg JA, Lawrence BD, et al.: Reversible secretion of glycosaminoglycans and proteoglycans by cyclically stretched valvular cells in 3d culture. Ann Biomed Eng 2008, 36:1092–1103.
• Dal-Bianco JP, Aikawa E, Bischoff J, et al.: Active adaptation of the tethered mitral valve: insights into a compensatory mechanism for functional mitral regurgitation. Circulation 2009, 120:334–342. This investigation builds on previous studies of MV remodeling in functional mitral regurgitation by demonstrating that the valvular cells undergo a reactivation of embryonic remodeling pathways.
Flanagan TC, Black A, O’Brien M, et al.: Reference models for mitral valve tissue engineering based on valve cell phenotype and extracellular matrix analysis. Cells Tissues Organs 2006, 183:12–23.
Flanagan TC, Wilkins B, Black A, et al.: A collagen-glycosaminoglycan co-culture model for heart valve tissue engineering applications. Biomaterials 2006, 27:2233–2246.
Simmons CA, Grant GR, Manduchi E, Davies PF: Spatial heterogeneity of endothelial phenotypes correlates with side-specific vulnerability to calcification in normal porcine aortic valves. Circ Res 2005, 96:792–799.
Shi Y, Vesely I: Characterization of statically loaded tissue-engineered mitral valve chordae tendineae. J Biomed Mater Res A 2004, 69:26–39.
Vesely I, Shi Y, Dobkin D, et al.: Progress in developing a composite tissue-engineered aortic valve. Conf Proc IEEE Eng Med Biol Soc 2005, 7:7129–7130.
Shi Y, Rittman L, Vesely I: Novel geometries for tissue-engineered tendonous collagen constructs. Tissue Eng 2006, 12:2601–2609.
Gheewala N, Grande-Allen KJ: Design and mechanical evaluation of a physiological mitral valve organ culture system. Cardiovasc Eng Technol 2010, 1:123–131.
•• Lieber SC, Kruithof BP, Aubry N, et al.: Design of a miniature tissue culture system to culture mouse heart valves. Ann Biomed Eng 2010, 38:674–682. This new bioreactor device will allow the in vitro culture of a mouse heart. Using this system, scientists can leverage the enormous range of knockout and transgenic mouse models of cardiovascular and other diseases to understand the impact of these genes on the biology and pathology of heart valves and other cardiac tissues.
•• Chiu YN, Norris RA, Mahler G, et al.: Transforming growth factor beta, bone morphogenetic protein, and vascular endothelial growth factor mediate phenotype maturation and tissue remodeling by embryonic valve progenitor cells: Relevance for heart valve tissue engineering. Tissue Eng Part A 2010, 16:3375–3383. This work exemplifies the innovative combination of developmental biology and bioengineering strategies to understand the remodeling principles that are desired in the regeneration of replacement tissues. These approaches can also be used to investigate pathologic remodeling of heart valves, because it is believed that some valve disease mechanisms may invoke regression to embryonic phenotypes.
Stephens EH, Durst CA, West JL, Grande-Allen KJ: Mitral valvular interstitial cell responses to substrate stiffness depend on age and anatomic region. Acta Biomater 2011, 7:75–82.
Acknowledgment
K.J. Grande-Allen has received grant support from the National Institutes of Health and the National Science Foundation. J. Liao has received grant support from the NIH.
Disclosure
Conflicts of interest: K.J. Grande-Allen: has received honoraria from the Methodist Hospital CME program; and has received honoraria and travel expenses from the Georgia Tech/Hilton Head Conference on Valve Biology and Tissue Engineering; J. Liao: none.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grande-Allen, K.J., Liao, J. The Heterogeneous Biomechanics and Mechanobiology of the Mitral Valve: Implications for Tissue Engineering. Curr Cardiol Rep 13, 113–120 (2011). https://doi.org/10.1007/s11886-010-0161-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-010-0161-2