Abstract
The physiological mechanical environment of the intact mitral valve is presumed to allow mechanotransductive, cell–cell and cell-extracellular matrix (ECM) signaling, which regulates cellular remodeling of the tissue ECM composition and structure. The goal of this work was to design an organ culture system to mimic the mechanical aspects of this environment, which in the future should allow for investigations to probe mitral valve biology and remodeling. This flow loop organ culture system uses an electronically pressure-controlled bladder pump to create a range of physiological pressure pulses in a ventricular chamber gated by inflow and outflow valves. The mitral valve attachment within the system is designed to maintain proper anatomical geometry and function. The entire system is filled with a culture medium and located within an environmental incubator to provide an appropriate environment for tissue viability. The system has been shown to accurately recreate a physiologic pressure waveform (up to 150 mmHg) and induce pulsatile flow (3 L/min), with a response time of 50 ms. In addition, the system can maintain sterility and has been used to culture mitral valves for up to 3 weeks. This system approximately recreates the physiological mechanical environment of a mitral valve. Future experiments will validate its ability to maintain the normal structure and composition of porcine mitral valves. After validation, this organ culture system can be used in longer term studies of heart valve responses to stimuli such as various biochemical agents or altered hemodynamics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the existence of successful and adequate techniques to address valve disorders, there is a dire need to investigate the pathogenesis of these conditions. Over twenty thousand Americans die of valvular disease each year, and these diseases contribute to over forty thousand additional deaths. While mitral valve disorders cause only one out of every eight valve-associated deaths, these disorders are responsible for approximately 43,000 valve disease-related hospitalizations per year.19 The searches for improved therapies for valvular disease and novel strategies for early diagnosis and prevention have spurred research into new technologies for investigating valve biology and disease progression and how they are influenced by valve structure, composition, and mechanics.
Mechanotransduction, the process through which tissue strains are transferred to valve cells through the cells’ attachment to the surrounding extracellular matrix, is believed to be a major factor promoting the maintenance and active remodeling of valve tissue. Numerous in vitro studies have shown that valve cells grown in 2D or 3D cultures respond to a range of mechanical stimuli by modulating their synthesis and turnover of DNA, proteins, collagen, and glycosaminoglycans (GAGs).9 , 16 , 23 , 24 Recent investigations have also shown the importance of the two major cell types of the mitral valve, valvular endothelial cells1 , 4 and valvular interstitial cells,21 which likely interact with each other through molecular and mechanical signals. Indeed, valvular endothelial and interstitial cells react differently to shear stresses when grown in co-culture than when cultured independently.3 The two cell types have been shown to independently respond to exogenous factors to modulate tissue stiffness,7 presumably through ECM composition. These cells influence the valvular ECM through synthesis and degradation and, in turn, receive signals through their ECM environment. Macroscopically, tissue strains affect cell phenotype and proliferation, ECM composition, and valvular structure4 , 8; the valve reciprocates through its control of cardiac flow characteristics. From the molecular scale to the macroscopic systemic level, the various tissue components of mitral valves take part in diverse forms of interactions. In order to gain a more complete understanding of valvular biology and responses to environmental variation, it is essential to study valves in a holistic setting that incorporates cells, ECM, tissue structure, and mechanics.
Among the various existing models for investigating valve biology, animal models present the only such holistic paradigm capable of reproducing all of these interactions, and even allow distal factors, such as circulating hormones originating in the liver, to exert subtle influences on valve behavior. Animal models, however, do not allow the flexibility and control of an ex vivo technique. In contrast, in vitro cell culture provides a paradigm for investigating cell-based interactions in an easily controlled environment. However, in this 2D, non-native environment, valve cells quickly undergo phenotypic modulation away from their native state.25 Recent methods involve cells seeded into 3D tissue constructs, but these constructs do not precisely mimic valvular laminar structure and anisotropic mechanics.2 Even native valve leaflets show reduced cell viability and tissue degradation when grown in static culture (without any mechanical stimulation).15 Furthermore, much more is known about effects of mechanical stimulation on valve cells than on intact valve tissues.22 For these reasons, dynamic organ culture is emerging as a technique that uniquely allows integration of the many levels of interaction (cells, tissue structure, mechanics, and biochemical influences) in healthy and diseased tissues, as well as flexibility and control of the culture environment.
Systems simulating human circulation by recreating physiological pressure and flow characteristics have been developed for various cardiovascular applications, including vasculature,11 tri-leaflet valves,10 , 15 and mitral valves.12 – 14 , 17 These systems have been used for tissue engineering,10 , 11 hemodynamic analysis12 – 14 and research into biological responses to various physiological, pathological, and other, conditions.15 One such physiologic, pulsatile, and sterile culture system, described by Hildebrand et al.,10 performed mechanical conditioning of tissue engineered tri-leaflet valve grafts, using a pressure regulator to create a physiological pressure waveform. That system also used a variable flow resistor to uncouple pressure and flow rates, and was able to maintain sterile conditions for at least 14 days. Another system was employed to analyze the effects of chordal, annular, and papillary muscle geometry and mechanical forces on mitral valve kinematics and hemodynamics.13 , 14 , 17 This non-sterile flow loop system featured moveable force-sensitive rods holding the chordae in place, which are able to measure papillary muscle loads, and clear windows for video and Doppler imaging. However, this latter system was designed for non-sterile hemodynamic studies, as opposed to organ culturing, and hence only needs to be run for a small number of simulated cardiac cycles. The same group has recently described the design of a flow loop organ culture system for porcine aortic valves.15 Using a piston pump to create sinusoidal cyclic pressure at physiological levels, this system was able to maintain biological characteristics through 48 h and sterile conditions over a period of 96 h.
Taken together, these previously reported systems demonstrate the feasibility and utility of recreating physiologic conditions ex vivo, as well as a need for systems that permit the longer-term organ culture of heart valves. Thus, the aim of this work was to design a bioreactor system that will provide physiological mechanical stimulation for sterile organ culture of a mitral valve, especially for longer term studies which would enable detection of slower remodeling changes in the valvular microstructure.
Methods
Design Criteria
Design criteria for the mitral valve organ culture system were categorized as functions, objectives, or constraints.6 The central function of this design was to maintain an organ cultured mitral valve in its native functional state by simulating its in vivo nutritional and mechanical environment. Valve cells in vivo are provided with nutrients and mechanical stimulation to remain viable, retain their phenotypic activity, and promote stability in ECM composition. Ex vivo, the valve must be immersed in a nutrient medium and housed in a system that recreates the normal valve annular size and shape as well as the position of the chordae tendineae. This geometry is essential to proper coaptation of the valve leaflets, thereby preventing regurgitant flow during systole, and to allow unrestricted forward flow during diastole12 , 13 (Fig. 1).
(a) Top view of mitral valve during systole, with the leaflets coapting to prevent regurgitant flow. (b) Top view of mitral valve during diastole, where the valve leaflets move aside to allow blood to fill the ventricle. The chordae tendineae and papillary muscles, which contract during systole to hold back the valve leaflets and prevent prolapse, are shown in the interior
To develop a system that most effectively performs these desired functions, several objectives were defined. The most important objective was to maximize the time period that mitral valves remain viable. Another objective was to maximize the ease of replacing the culture medium under sterile conditions, which must occur at least every 2 weeks.5 Reducing the complexity of medium exchange minimizes possible contamination and allows longer culture periods. In order to mimic in vivo conditions, a third objective was to create physiological flow rates (5.0 L/min) and pressures (peak of 120 mmHg). A related objective was to maximize flexibility and control of flow rates and pressure levels to match the variety of physiologic and pathophysiologic heart rates, blood pressures, and cardiac outputs and thus allow a system user to perform investigations related to alterations in cardiac mechanics.
The design was also dependent on several constraints. This system design was required to retain sterility throughout bioreactor assembly, valve dissection, valve insertion into the bioreactor, organ culture system operation and medium replenishment. As a second constraint, the system must not leak to prevent contamination or altered mechanics through loss of fluid volume. Third, the system size was also constrained to fit within an incubator to maintain temperature and gas composition.
Design Overview
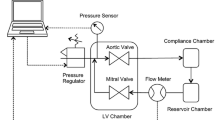
Based on these design criteria, a mitral valve organ culture system was designed around a mock ventricle that uses a pressure-controlled bladder pump to produce pulsatile flow (Fig. 2). Generally, fluid systems can be controlled through flow or through pressure differentials; by controlling pressure, this system allows closer control of the pressure differentials across the mitral valve, but makes flow rates difficult to adjust or predict. The mock ventricle/pumping chamber includes inflow and outflow ports that house the tissue mitral valve and mechanical aortic valve (Fig. 3). A slack, dome-shaped silicone rubber membrane separates a culture medium-filled compartment from an air-filled compartment. A computer-controlled pressure regulator increases the air pressure, pushing the membrane into the medium-filled side and propelling medium out of the mechanical aortic valve, until the ‘ventricular’ air pressure is decreased to a level lower than pressure in the inflow medium, the ‘atrial’ pressure. At this point, medium flows back into the ventricular chamber through the mitral valve, pushing the silicone membrane back into the air-filled side of the chamber. ‘Aortic’ and ‘atrial’ pressure heads are created by elevating a medium reservoir, open to the air through a venting filter. A compliance chamber between the ventricular chamber and medium reservoir mimics the compliant nature of the circulation.
This diagram depicts flow into and out of the ventricular chamber during a simulated cardiac cycle. A raised medium reservoir provides an atrial filling pressure, P at, which propels medium into the ventricular chamber through the mitral valve. The aortic valve prevents the aortic pressure, P ao, from producing retrograde flow into the ventricle. As ventricular pressure, P v, is increased pneumatically, it overcomes P ao and medium flows out the aortic valve, while the mitral valve closes. As P v is decreased, the aortic valve closes and the mitral valve opens again to allow flow into the ventricular chamber. The black arrow indicates movement of the silicone membrane separating culture medium from air. Dual wavy arrows indicate flow of medium. The single narrow blue arrow indicates air flow
Ventricular Chamber. Pulses of pressurized air act through a dome-shaped silicone membrane diaphragm to pump culture medium through a mechanical aortic valve. The mitral valve allows medium to flow back into the ventricular chamber. Thus, the valve cells receive mechanical stimulation from both the hydrostatic pressure pulses and the shear flow across the valve surfaces
The main determinants for material selection were sterilization and biocompatibility. All the organ culture system parts were fabricated with materials able to withstand repeated cycles of autoclaving without deformations or other adverse affects; these materials have also never been reported to cause toxicity or affect tissue viability. The primary materials contacting culture medium include polycarbonate, silicone rubber, polypropylene, polyurethane tubing, and polyurethane coated nylon fabric. Stainless steel was used in chordal attachments and aluminum was used for an external frame.
The electronic components of the system were coordinated through a LabView (National Instruments, Austin, TX) virtual instrument (VI). This custom VI used an arbitrary signal based on an archetypical ventricular pressure pulse as the basis for the pressure pulse sent to the proportional pressure regulator. The VI allowed real-time adjustment of the signal magnitude, displayed instantaneous pressure and flow rate signals, and computed the average flow rate. The VI communicated with a National Instruments Data Acquisition (DAQ) unit in the main electrical control box that routed the appropriate power and communication sources to the computer, flow meter, proportional pressure regulator, and pressure sensor. Containing all electrical wiring within the box with plugs for each instrument allowed extremely quick and easy setup of the electrical system.
Flow Control and Measurement
An Airfit Tecno Basic PRE-U proportional pressure regulator (Parker Origa USA, Glendale Heights, IL) was used to create pressure pulses for the system. Two filter/regulator units were used on a compressed air source at 5.5 bar to produce a clean and stable air supply at 1.5 bar. Supported by this pressure source, the proportional pressure regulator translated a voltage signal into a pressure waveform ranging 0–200 mbar (0–150 mmHg). Pressure in the air-side of the ventricular chamber was recorded by a pressure transducer (Model EW-68075-40, Cole Parmer, Vernon Hills, IL). Flow into the ventricular chamber was measured using an ultrasonic flow meter (Transonic Systems, Ithaca, NY) that detects mass flow through tubing without contacting the fluid. The specific model, 14 PXL, was chosen for its range of measurable flow rates, 0.025–50 L/min, and was factory calibrated for both water at 25 °C and culture medium M199 at 37 °C.
Flow Loop Components
The system components were connected with clear PVC or Tygon tubing. Original versions of the system used only 5/8″ ID Tygon tubing, but most sections of tubing were later replaced with 1/2″ ID PVC tubing to reduce the inertial mass of fluid in the system and allow greater flow in response to pressure pulses. Although narrower tubing does also increase flow resistance, the tubing diameter was still large enough so that these contributions to flow resistance were negligible. Approximately 1.5 L of cell culture medium acted as the flow medium for the system and provided nutrients; this medium was M199 with Earle’s salts and l-glutamine (Sigma, St. Louis, MO), supplemented with 10% bovine growth serum (Hyclone, South Logan, UT) and anti-microbials (200 IU/mL penicillin, 200 μg/mL streptomycin, and 500 ng/mL amphotericin B (Mediatech, Manassas, VA)).18 For a period of seven days, such a large volume of medium provides sufficient nutrients for a single valve and remains effective,6 so the culture medium was replaced once a week.
The ventricular chamber was 6.5 in. long and 4.5 in. in diameter (Fig. 3). The silicone rubber membrane separating the medium-filled and air-filled compartments was positioned at the midpoint of the chamber. This membrane was molded into a dome shape with a thickness of 0.015″, which allowed deformation during each pumping cycle without inducing significant resistance or tension within the membrane. At the ends of the chamber were caps with ports for the pressure transducer and the inflow and outflow of air and culture medium. A simple external frame was tightly clamped around the chamber with toggle latches to prevent leaking; additionally, many junctions in the flow circuit were fitted with silicone o-rings and tightened with hose clamps. A mechanical aortic valve (Carbomedics, Austin, TX) was housed in the outflow port of the ventricular chamber. The valve was held in place by a snugly fitting ring that slides into the outflow port around the valve (Fig. 4). The organ cultured mitral valve was held similarly in the inflow port of the ventricular chamber.
A primary function of this design was to hold the cultured mitral valve in a physiologic position. In the hemodynamic flow loop previously described,13 , 14 , 17 the mitral valve annulus was sewn to a swatch of Dacron and the papillary muscles were tied to a pair of metal posts, which was appropriate for the non-sterile mechanical studies performed. Our design adapted these concepts for an organ culture system that was intended to operate continuously for weeks instead of hours. The mitral valve was obtained from a pig heart obtained from a local abattoir within 3 h of death. The valve was dissected out of the heart inside a sterile biological safety cabinet with steam sterilized tools and gently washed in a ‘rinsing’ M199 culture medium, as described above, fortified with increased anti-microbials (1000 IU/mL penicillin, 1000 μg/mL streptomycin, and 2500 ng/mL amphotericin B). Before dissection, the valve annulus was sized using an annuloplasty sizing set (Duran Ancore, Medtronic, Minneapolis, MN). The valve annulus was sewn, using non-biodegradable 3–0 Ethilon sutures (Ethicon, Somerville, NJ), to an appropriately sized orifice on a circular swatch of sterilized polyurethane-coated nylon fabric (McMaster-Carr, Robbinsville, NJ) (Fig. 5). Care was taken during this process to ensure the valve annulus attained an anatomically proper “D” shape. The fabric swatch was then clamped within the inflow port. The chordae tendineae were also held in place relative to the inflow port to prevent valve prolapse. During dissection, the tips of the papillary muscles were detached from the left ventricular wall, retaining the connection with the chordae. The papillary tips held the chordae in place and were held in place by helical stainless steel wires wound around the papillary tips and interwoven between the chordae tendineae. These helices were in turn attached to the interior of the inflow port with additional stainless steel wires.
Mitral Valve Attachment. The valves are shown sewn to a circular swatch of polyurethane-coated nylon with 3–0 Ethilon sutures (Ethicon, Somerville, NJ). The papillary muscles are bound by steel helices, which hold them in place. (a) Top “atrial” view showing sutures securing valve annulus. (b) Bottom “ventricular” view showing steel helices wound around papillary muscles. (c) Bottom view in partial assembly of ventricular chamber showing steel helices held in place by straight steel wires. The mechanical aortic valve is seen at right in the outflow port
The remaining system components were the compliance chamber and atrial reservoir, both fabricated from polycarbonate bottles (Nalgene, Rochester, NY) modified with tubing ports. The compliance chamber had ports for medium inflow and outflow and contained approximately 400 mL of trapped air during operation. The atrial reservoir had ports for medium inflow and outflow as well as an air vent port, which connected to a sterile 0.2 μm venting filter to allow gas exchange and to provide venting to an atmospheric reference pressure.
Results
Mechanical Evaluation
Several mechanical evaluations were performed to determine whether the organ culture system could produce pressure and flow response waveforms mimicking a range of physiological conditions. The first of these evaluations was conducted at room temperature (25 °C) with water as the flow medium. The atrial reservoir and compliance chamber were raised 15 in. above the ventricular chamber and the system positioned exactly as it would be in an incubator; a mechanical heart valve was placed in the mitral position. Sinusoidal pressure control signals with a peak of 70 mmHg were used to determine the frequency response capabilities of the system (Fig. 6a). The measured pressure tracked the control signal within 10 mmHg at 1 Hz, and remained fairly accurate at a frequency of 3 Hz, reaching within 15 mmHg of the peak control signal. These indicators of the temporal response to a pressure control signal indicated that the system is capable of recreating a physiologic pressure pulse. Next, to mimic a systolic pressure pulse, the signal was modified to create 60 and 75 beat per minute (bpm) ‘physiological’ signals with pressure peaks ranging 75–150 mmHg (Fig. 6b). The pressure response tracked the control signal closely in these evaluations, over the full range of peak pressures. In each evaluation the measured pressure followed the control signal to its peak, within 5–10 mmHg, after a short lag of approximately 0.05 s. At the highest pressures, the atrial filling pressure was inadequate to fully replenish fluid in the ventricular chamber and the excess fluid breached the atrial reservoir air vent after a few minutes. The maximum peak pressure that could be sustained in the system without such a breach was 145 mmHg. These breaches were later prevented by modifying the silicone diaphragm to have a smaller range of displacement. The range of pressures that can be achieved by the current system is 0–150 mmHg, the approximate range of the proportional pressure regulator, and allows for the simulation of a range of heart pressures from hypotension to hypertension. This range also would allow the possibility of using this system to perform mechanical conditioning of engineered tissue constructs by applying gradually increasing pressures. Increases in peak pressure resulted in linear increases in average flow rate, while pulse frequency had little effect on flow rate (Fig. 7). The maximum average flow rate recorded in the system is nearly 3 L/min, which is lower than a typical porcine cardiac output of 5 L/min.20
Pressure and Flow Waveforms. Sinusoidal pressure control signals at (a) 1 Hz and (b) 3 Hz with peaks of 70 mmHg and a mechanical outflow valve. Physiologic pressure control signals at (c) 60 bpm and (d) 75 bpm with peaks of 120 mmHg and a mechanical outflow valve. Similar waveforms were recorded for control signals with peaks ranging from 75 to 150 mmHg
Similar system response characteristics were found when the system was evaluated with a porcine mitral valve in place. The system was filled with culture medium and placed in an incubator at 37 °C. The flow response was recorded within 3 h of assembling the system. The response included a spike of regurgitant (negative) flow during systole as the mitral valve leaflets were pushed up and then pressed together to close the orifice (Fig. 8). As diastole began, there was a surge of flow through the valve resulting from the pressure built up in the compliance chamber, decreasing until the next systolic period. At the highest peak pressure of 150 mmHg, the average flow rate reached 2.6 L/min. This evaluation demonstrated the ability of the organ culture system to house a properly functioning mitral valve and mimic its physiological flow environment. Although the data shown in Fig. 8 was performed using a single typical specimen, we have observed throughout the system’s development and evaluation that variations in valve size and geometry as well as inherent variation in the attachment procedure produce minor variations in flow response.
In repeated trials with porcine mitral valves (during the sterility testing described below), prolonged regurgitant flow was occasionally observed during systole when high pressures caused leaks to form along the valve annulus, and would increase in magnitude with time. Such leaks introduced non-physiological flow behavior and were reduced by reinforcing annular attachments with additional sutures.
Sterility
Sterility of this system was demonstrated through several progressively more complicated trials. First, the system was set up under zero-flow conditions with a mechanical valve in the mitral position and tested for the duration of a week. In this evaluation, each component was steam sterilized and then assembled and filled with M199, without antimicrobial additives, in a biological safety cabinet. After one week, multiple medium samples were collected from distant parts of the system. No sign of contamination was seen in the medium under microscope and after one day of culture of medium samples swabbed on agar plates, even when contamination was visible on positive control agar plates. In subsequent testing of the organ culture system, porcine mitral valves were subjected to physiological pulsatile flow with standard culture medium, as described previously. At this time, eleven valves have been tested in the system for a range of 3 days to 3 weeks. Every seven days, the system was removed from the incubator and placed in a biological safety cabinet where the culture medium was completely drained and refilled. The drained medium was found to be free of contamination, demonstrating no observable discoloration and turbidity. The pH of the drained medium was also frequently checked as a readout for oxygenation and accumulation of harmful metabolic products; in all cases the medium was found to retain the pH of the original medium. During these culture periods, the system was also observed to have no persistent leaks, which aided in preventing contamination.
Discussion
Development of an organ culture system provides a new and powerful tool for studying mitral valve biology by recreating the approximate mechanical environment experienced by mitral valves in vivo. This system builds upon the existing paradigms of cell culture and animal models by making possible the ex vivo, controlled study of mitral valve tissue and cells experiencing physiologically relevant mechanical, cell–cell, and cell-ECM interactions. The novelty of this design lies in the applicability to mitral valves, as well as the pending demonstration of the extended culture period.
Mechanical evaluations have shown this system to successfully create a mechanical environment similar to that of the native mitral valve. The most notable discrepancy is the difference in average flow rate, although a flow rate of at least 2 L/min is expected to provide sufficient shear stress stimulation in conjunction with physiological hydrostatic pressure to maintain native valve characteristics. If desired, modifications to the shape and size of flow components and pressure control waveform may provide a more physiologic flow rate. For example, molding a silicone diaphragm shaped more like the interior of the left ventricle may encourage more physiological flow fields, displacing more medium. These mechanical performance characteristics are secondary to the primary goal of this design, maintaining valve cellular and microstructural viability; evaluating these characteristics will be a future step in this research effort. Ongoing tests of this system include mechanical and histological analyses of excised porcine mitral valves cultured for a period of three days to three weeks. Organ culture utilizing mechanical stimuli is expected to aid in maintaining the native cell viability and function and ECM composition and structure.
The system described above demonstrates the possibility of long-term culture of intact heart valves in a physiological mechanical environment. In addition to culturing mitral valves, this system could be readily modified to culture any of the other 3 cardiac valves or possibly other cardiovascular tissues. Overall, organ culture investigations are expected to provide a rich complement for previous and existing studies of valve cells, particularly to determine how cellular responses to altered mechanical and biochemical environments translate into changes in valve structure and function.
References
Balachandran, K., P. Sucosky, H. Jo, and A. P. Yoganathan. Elevated cyclic stretch alters matrix remodeling in aortic valve cusps: implications for degenerative aortic valve disease. Am. J. Physiol. Heart Circ. Physiol. 296(3):H756–H764, 2009.
Balguid, A., A. Mol, M. A. van Vlimmeren, F. P. Baaijens, and C. V. Bouten. Hypoxia induces near-native mechanical properties in engineered heart valve tissue. Circulation 119(2):290–297, 2009.
Butcher, J. T., and R. M. Nerem. Valvular endothelial cells regulate the phenotype of interstitial cells in co-culture: effects of steady shear stress. Tissue Eng. 12(4):905–915, 2006.
Butcher, J. T., and R. M. Nerem. Valvular endothelial cells and the mechanoregulation of valvular pathology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362:1445–1457, 2007.
Clerin, V., R. J. Gusic, J. O’Brien, P. M. Kirshbom, R. J. Myung, J. W. Gaynor, et al. Mechanical environment, donor age, and presence of endothelium interact to modulate porcine artery viability ex vivo. Ann. Biomed. Eng. 30(9):1117–1127, 2002.
Dym, C. L., and P. Little. Engineering Design: A Project-based Introduction. New York: John Wiley, 2004.
El-Hamamsy, I., K. Balachandran, M. H. Yacoub, L. M. Stevens, P. Sarathchandra, P. M. Taylor, et al. Endothelium-dependent regulation of the mechanical properties of aortic valve cusps. J. Am. Coll. Cardiol. 53(16):1448–1455, 2009.
Engelmayr, Jr., G. C., E. Rabkin, F. W. Sutherland, F. J. Schoen, J. E. Mayer, Jr., and M. S. Sacks. The independent role of cyclic flexure in the early in vitro development of an engineered heart valve tissue. Biomaterials 26(2):175–187, 2005.
Gupta, V., J. A. Werdenberg, J. S. Mendez, and K. J. Grande-Allen. Influence of strain on proteoglycan synthesis by valvular interstitial cells in three-dimensional culture. Acta Biomater. 4(1):88–96, 2008.
Hildebrand, D. K., Z. J. Wu, J. E. Mayer, Jr., and M. S. Sacks. Design and hydrodynamic evaluation of a novel pulsatile bioreactor for biologically active heart valves. Ann. Biomed. Eng. 32(8):1039–1049, 2004.
Hoerstrup, S. P., G. Zund, R. Sodian, A. M. Schnell, J. Grunenfelder, and M. I. Turina. Tissue engineering of small caliber vascular grafts. Eur. J. Cardiothorac. Surg. 20(1):164–169, 2001.
Jimenez, J. H., D. D. Soerensen, Z. He, S. He, and A. P. Yoganathan. Effects of a saddle shaped annulus on mitral valve function and chordal force distribution: an in vitro study. Ann. Biomed. Eng. 31(10):1171–1181, 2003.
Jimenez, J. H., D. D. Soerensen, Z. He, J. Ritchie, and A. P. Yoganathan. Effects of papillary muscle position on chordal force distribution: an in vitro study. J. Heart Valve Dis. 14(3):295–302, 2005.
Jimenez, J. H., D. D. Soerensen, Z. He, J. Ritchie, and A. P. Yoganathan. Mitral valve function and chordal force distribution using a flexible annulus model: an in vitro study. Ann. Biomed. Eng. 33(5):557–566, 2005.
Konduri, S., Y. Xing, J. N. Warnock, Z. He, and A. P. Yoganathan. Normal physiological conditions maintain the biological characteristics of porcine aortic heart valves: an ex vivo organ culture study. Ann. Biomed. Eng. 33(9):1158–1166, 2005.
Ku, C. H., P. H. Johnson, P. Batten, P. Sarathchandra, R. C. Chambers, P. M. Taylor, et al. Collagen synthesis by mesenchymal stem cells and aortic valve interstitial cells in response to mechanical stretch. Cardiovasc. Res. 71(3):548–556, 2006.
Lefebvre, X. P., S. He, R. A. Levine, and A. P. Yoganathan. Systolic anterior motion of the mitral valve in hypertrophic cardiomyopathy: an in vitro pulsatile flow study. J. Heart Valve Dis. 4(4):422–438, 1995.
Lester, W., A. Rosenthal, B. Granton, and A. I. Gotlieb. Porcine mitral valve interstitial cells in culture. Lab. Invest. 59(5):710–719, 1988.
Lloyd-Jones, D., R. Adams, M. Carnethon, G. De Simone, T. B. Ferguson, K. Flegal, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 119(3):e21–e181, 2009.
Lucke, J. N. Determination of the cardiac output of anaesthetised pigs using a dye dilution method. Res. Vet. Sci. 21(3):364–365, 1976.
Mulholland, D. L., and A. I. Gotlieb. Cardiac valve interstitial cells: regulator of valve structure and function. Cardiovasc. Pathol. 6(3):167–174, 1997.
Sucosky, P., M. Padala, A. Elhammali, K. Balachandran, H. Jo, and A. P. Yoganathan. Design of an ex vivo culture system to investigate the effects of shear stress on cardiovascular tissue. J. Biomech. Eng. 130(3):035001, 2008.
Weston, M. W., and A. P. Yoganathan. Biosynthetic activity in heart valve leaflets in response to in vitro flow environments. Ann. Biomed. Eng. 29(9):752–763, 2001.
Xing, Y., J. N. Warnock, Z. He, S. L. Hilbert, and A. P. Yoganathan. Cyclic pressure affects the biological properties of porcine aortic valve leaflets in a magnitude and frequency dependent manner. Ann. Biomed. Eng. 32(11):1461–1470, 2004.
Yperman, J., G. De Visscher, P. Holvoet, and W. Flameng. Molecular and functional characterization of ovine cardiac valve-derived interstitial cells in primary isolates and cultures. Tissue Eng. 10(9–10):1368–1375, 2004.
Acknowledgments
This work was funded by a Pfizer Atorvastatin Research Award and the Oak Ridge Associated Universities Ralph E. Powe Junior Faculty Development Award. The authors thank Dr. R. E. Phillips at Carbomedics, Inc. for generously providing the mechanical heart valves.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ajit P Yoganathan oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Gheewala, N., Grande-Allen, K.J. Design and Mechanical Evaluation of a Physiological Mitral Valve Organ Culture System. Cardiovasc Eng Tech 1, 123–131 (2010). https://doi.org/10.1007/s13239-010-0012-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-010-0012-8