Abstract
Purpose of Review
The purpose of this review is to discuss the pathophysiology of neurogenic SUI in the female patient, examine the evidence supporting surgical and non-surgical treatment options, and outline our recommendations for the care of this population.
Recent Findings
AFPVS appears to be more efficacious than MUS for this group; however, almost all patients will require self-catheterization after surgery. MUS have a higher probability of maintaining spontaneous voiding but also care the risk mesh complications and higher failure rates. Bladder neck AUS placement may also be considered, but most studies show high reoperation rates and have only a few female subjects. In severe refractory cases of SUI or in the setting of urethral erosion, bladder neck closure has been shown to have good continence outcomes.
Summary
SUI in the setting of neurogenic lower urinary tract dysfunction is often more severe and harder to address than non-neurogenic SUI, due in part to the high rates of ISD in this population. Patients should be screened for other causes of urinary incontinence with UDS prior to any invasive interventions. AFPVS is an appropriate first-line therapy for these patients, particularly in individuals who already perform self-catheterization. Finally, in the setting of moderate to severe urethral erosion, bladder neck closure or urinary diversion should be strongly considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stress urinary incontinence, the complaint of involuntary leakage of urine on effort or exertion, is a common and frequently bothersome genitourinary complaint [1••]. In a recent survey, prevalence of SUI in the general female population was found to be 23.7%, with peak incidence in the fifth decade of life [2]. Pregnancy, age, and prior hysterectomy are strong predictors of uncomplicated SUI [3]. Mid-urethral slings are effective, durable, and well-tolerated interventions for the majority of these patients [4•]. By contrast, neurogenic SUI, or stress incontinence in the setting of an underlying neurologic disorder, encompasses myriad etiologies and presentations, with no one intervention presenting an effective solution for all patients. In this review article, we will discuss the pathophysiology of neurogenic SUI in the female patient, examine the evidence supporting surgical and non-surgical treatment options, and outline our recommendations for the care of this population.
Pathophysiology
The female continence mechanism involves three distinct components: the internal urethral sphincter, the external urethral sphincter, and appropriate proximal urethral support [5]. All three aspects must be present to prevent SUI. The female urethra is on average, 3 cm in length, the proximal 20% of which is comprised of the internal sphincteric mechanism, consistent of a U-shaped band of smooth muscle at the level of the bladder neck. The internal sphincter is under sympathetic innervation. Stimulation of alpha-receptors in the bladder neck and proximal urethra via release of norepinephrine prompts smooth muscle contraction [6]. The distal 20–80% of the urethra comprises the external urethral sphincter, which, in women, is comprised of both smooth and striated muscle as well as vascular components, all of which contribute to urethral closing pressure [5]. The external sphincter is innervated by the somatic nervous system and is under voluntary control via the pudendal nerve. The third component of the continence mechanism, proximal urethra and bladder neck, consists of the arcus tendineus fasciae pelvis, levator ani muscles, and endopelvic fascia, which act as compressive mechanisms in the setting of increased abdominal pressure “Hammock Hypothesis” [7]. Somatic innervation from the sacral spinal cord is also responsible for pelvic floor muscle innervation, contributing to urinary continence [6].
Uncomplicated SUI is thought to arise predominantly from damage to the normal anatomic support of the urethra, via either obstetric or iatrogenic strain or trauma [8]. This belief underlies the theoretical basis for mid-urethral slings, as well as historical urethral suspension procedures. For women with underlying neurologic disorders, however, stress urinary incontinence may be multifactorial. Damage to lower motor neurons in the lumbar or sacral cord may lead to denervation of the urethral sphincter complexes, sphincteric incompetence, and subsequent SUI. This is commonly seen in patients with sacral spinal cord injuries, spina bifida, cauda equina syndrome, and sacral agenesis [9•]. Incontinence due to sphincteric dysfunction may be exacerbated by neurogenic detrusor over-activity or reduced bladder compliance. Furthermore, in the setting of long-standing indwelling catheter use, even patients with an initially intact continence mechanism may develop urethral erosion leading to severe stress incontinence [10]. It is important to keep in mind that the more common mechanisms of SUI found in the general population may also affect patients with neurologic conditions. A thoughtful diagnostic evaluation is therefore mandatory in this group.
Evaluation and Diagnosis
Clinical assessment of the female neurourology patient with urinary incontinence should include validated questionnaires to establish obstructive lower urinary tract symptoms, incontinence severity, and urologic quality of life. A detailed history of the presentation and chronicity of symptoms should be undertaken, as well as a 3-day voiding diary to define their present complaints [11]. History of prior medical and surgical interventions as well as obstetric history should be reviewed, and current medications evaluated for polypharmacy concerns (i.e., antipsychotic drugs or narcotics which may cause urinary retention and overflow incontinence). Physical exam should include a pelvic exam with examination of the urethra for erosion or lesions, cough test for stress urinary incontinence, assessment of pelvic organ prolapse, and evaluation of the levator muscles for injury, tenderness, and tone. Sacral cord mediated reflexes, such as the bulbocavernosus reflex and anal wink, may also be tested. A brief neurologic evaluation to assess degree of functional impairment should be performed as debility may predispose patients to functional incontinence and impact decision-making regarding treatment options. A urinalysis should be performed (with a urine culture if indicated), as worsening incontinence may be a symptom of urinary tract infection. If there is a concern for urinary retention or if invasive treatment is being considered, a post-void residual assessment may also be performed.
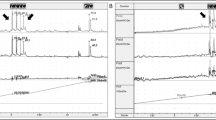
Urodynamics (UDS) are not necessary prior to proceeding with MUS placement in the setting of uncomplicated SUI [12]. For patients with neurogenic stress urinary incontinence, however, they provide necessary disambiguation since these patients have a high prevalence of poor bladder compliance and detrusor overactivity. They should be performed prior to any operative intervention, and may aid in decision making regarding more conservative therapies [13]. Bladder compliance, detrusor function, bladder outlet obstruction, and Valsalva leak point pressure should be evaluated via complex cystometrogram and pressure flow studies. Intrinsic sphincter deficiency (ISD), is typically characterized by a VLPP < 60 cm H20 and is predictive of more severe SUI [14]. Electromyography (EMG) should be performed along with multichannel UDS to characterize external sphincter function. If available, fluoroscopy has the added benefit of evaluating for an open bladder neck, which may affect surgical decision making (Fig. 1). If detrusor over-activity is identified on UDS or patient has symptomatic urge incontinence, it should be treated prior to addressing SUI, as these symptoms are exacerbated by many SUI procedures, and this is often the predominant type of incontinence in the neurogenic population.
Non-surgical Treatment Options
Little data exists regarding conservative treatment options for SUI in the female neurogenic patient. Pelvic floor physical therapy (PFPT) is a mainstay of uncomplicated stress urinary incontinence management and poses no risk to the patient. In a recent Cochrane Review, women with SUI who underwent pelvic floor physical therapy were eight times more likely than controls to report resolution of incontinence symptoms (46/82 (56.1%) versus 5/83 (6.0%), 95% CI 3.68 to 19.07) [15]. For patients with mild-moderate symptoms who have sufficient neurologic function to perform therapeutic exercises and are motivated to avoid more invasive procedures, PFPT should be considered. Vaginal inserts or continence pessaries are similarly low risk options for patient who wish to avoid, or are unable to undergo, surgery [16]. Upper extremity function should be considered prior to offering these options, and patients without sensation are carefully monitored for erosions.
Pharmacologic options for the treatment of SUI are limited. Imipramine, the best studied of these agents, is a tricyclic antidepressant which acts on the urinary tract through multiple mechanisms, including alpha-adrenergic stimulation of the internal sphincter [17]. Lin et al. reported a 60% improvement in 20-min pad weight tests as well as a statistically significantly increase in VLPP following 3 months of imipramine therapy in a small series of 24 women with uncomplicated SUI [18]. No studies regarding imipramine alone for neurogenic SUI have been performed. Double- or triple-drug therapy using imipramine in combination with anticholinergic medications and alpha-blocking agents has been shown to increase bladder compliance and reduce incontinence episodes in carefully selected patients with neurogenic bladder [19]. Imipramine is associated with rare but severe cardiac adverse events, and we do not recommend its use in the setting of female neurogenic SUI with normal bladder compliance.
Urethral bulking agents, typically performed in the office, are another option for patients with neurogenic SUI and ISD who desire conservative management. Unfortunately, data on their efficacy is poor. Only one study specific to the adult neurogenic population exists: a case series published by Bennet et al. following 11 patients with intrinsic sphincter deficiency SUI and myelomeningocele or spinal cord injury, which showed a 60% improved or cured rate following collagen injection, an average VLPP increase of 57 cm H20, and no significant adverse events over an average of 24 months [20]. In the pediatric neurogenic SUI population, short-term improvement rates with urethral bulking agents range from 20 to 50%, but appear to decline over time [21, 22]. Among women with non-neurogenic SUI, similar improvement/cure rates have been noted at 2 years (48%) with only 26% reporting sustained improvement at 5 years [23, 24]. While these studies all used bovine collagen, there does not appear to be a significant difference in outcomes with currently available agents [25]. Due to the short duration and limited efficacy of urethral bulking agents in this group, we would recommend their use as a primary treatment for stress incontinence only if a patient does not desire or is unable to undergo surgery. In the setting of persistent incontinence following prior sling, urethral bulking agents have been shown to be of at least moderate benefit to most patients and should be considered [26], and we have achieved treatment success with this approach at our center.
Surgical Treatment Options
Surgical treatment options for neurogenic stress urinary incontinence in the female patient can be divided into three main categories: sling placement (synthetic or fascial), artificial urinary sphincter implantation, and urinary tract reconstruction or diversion.
Synthetic Midurethral Slings
Synthetic midurethral slings (MUS) have become the new standard in the treatment of stress urinary incontinence in the index case woman [27]. Reasons for the widespread adoption of this approach include a straightforward short procedure, a well-defined safety profile, and rapid return to baseline physical activity when compared with open incision surgical procedures such as the autologous fascia pubovaginal sling (AFPVS). Mesh midurethral slings are the best studied surgical procedures for incontinence in women with robust long-term data on their effectiveness. Their mechanism of action is to correct urethral hypermobility.
There is some evidence, however, that mesh slings are less effective in women with intrinsic sphincter deficiency as they do not close the bladder neck [28]. However, as previously stated, some women with neurogenic lower urinary tract dysfunction (NGB) may also have urethral hypermobility from pelvic floor dysfunction or prior childbirth which are the same risk factors as neurologically intact women and their incontinence probably via the same mechanism [29]. Also worth noting is that many women with NGB perform clean intermittent catheterization which theoretically could increase the risk of urethral erosion of a mesh slings because of repeated urethral trauma.
Synthetic mid urethral slings are designed to be tension-free hence increasing the tightness of the sling to promote retention is not recommended whereas AFPVS are designed to allow for variable tensioning. Mid urethral slings cause less obstruction because of their tension-free design hence are a good choice for women who void volitionally. This effect has been studied in the female neurogenic bladder population. El-Azab and El-Nashar performed a non-randomized clinical trial evaluating the effectiveness of TVT (n = 20) or AFPVS (n = 20) in 40 women with spinal cord injury or pathology below the S2 spinal segment [9•]. These women with neurogenic SUI due to LMNL had low ALPP from 47 to 40 cm H2O. The women were given the choice of procedure and were counseled about the increased risk of de novo requiring CIC after AFPVS hence more women who voided volitionally chose the TVT over the AFPVS. In the TVT group, 25% performed CIC at baseline and 55% of the AFPVS performed CIC.
As predicted by the surgeons, 100% of patients had a PVR > 150 cc [3] after AFPVS and all required CIC (9 new) whereas in the TVT group, 12/20 required CIC after surgery (7 new). De novo urgency was higher in the TVT group (30% vs 10%). In patients with new UUI on post-surgical urodynamics, two patients had new low compliance and six had new DO. All were effectively treated with oral anticholinergics. One vaginal mesh erosion into the vagina was noted in the TVT group.
In terms of continence, 20% of the TVT group failed the 250 cc [3] cough stress test and were considered failures whereas 15% of the AFPVS group failed. However, with this test repeated at 2 years, 6% of the TVT patients failed and 0% of the AFPVS. In both groups, there were significant reductions in the UDI-6 and IIQ-7 scores. This study underscores that 42% of patient who voided pre-op maintained voiding after a TVT.
Several studies have assessed the outcome of synthetic mid urethral slings in women with neurogenic bladder. Unfortunately, many of these studies did not utilize objective outcomes or validated questionnaires to assess for cure making these results difficult to compare [30,31,32,33].
Losco et al. [34] assess 27 women with SCI who had a TOT for their SUI. All had urodynamic proven SUI, and six had concomitant NDO that was treated with botulinum toxin bladder injections pre-op to achieve control of the DO first. Among these women, 11 performed CIC, 4 had indwelling urethral catheters, 7 SP tubes and 5 Valsalva voided. No description of the incontinence severity was reported in the paper. The procedure was described as the tape being “tightened more snug underneath the urethra” than in a typical procedure.
Patient self-reported dry rate without the need for pads (no objective assessment or validated questionnaires) was 81.5% (n = 22) and one patient was improved. Two out of five patients who voided now require CIC, and two patients developed de novo OAB. Eleven percent had transient thigh pain resolving between 3 days and 6 months but no mesh erosions noted. The authors hypothesize that the sling configuration with a TOT is less obstructive and better for the voiding patient.
These results are similar to the success of TOT in a non-neurogenic population which is not expected given the more complex etiology. The authors explain this in that women with SCI are less active hence may not generate high intra-abdominal pressures, so success might be high regardless of the sling type.
In contrast, Pannek et al. found very different results [29]; they performed TOT on nine women who all had para or tetraplegia, and seven performed CIC, one voided, and one had an SP tube at baseline. Three out of nine (33%) were cured or very much improved. One patient developed a urethral erosion (vaginal and urethral fistula) which occurred during late follow-up. Five out of the six failures eventually went on to have an AUS or urinary diversion to control their incontinence. Preoperatively, five had an open bladder neck, and four were closed on fluoroscopy during urodynamics. Median LPP was very low at 28 cm H2O. The only patients with treatment success had closed bladder necks but this did not guarantee success since two patients with closed bladder necks did fail.
The authors explained their poor results were due to the TOT and the non-obstructing nature of mesh slings which should not be tightened excessively to avoid erosion. It has been previously shown that in a RCT of non-neurogenic women with ISD, TOT had a poorer cure rate than TVT. This is likely due to the different axis of the sling since TVT is more perpendicular creating greater circumferential pressure [28]. The authors have abandoned mesh slings for this population.
Lombardi et al. [35] in a study of several procedures for SUI presented the results of four women with spinal cord lesions and SUI who failed prior bulking agents. One was voiding preoperatively and is now in retention. Overall, the patients had a combined 90% reduction in SUI at 6 years of follow-up and two were completely dry.
Autologous Fascial Pubovaginal Sling
AFPVS was first introduced in 1907 by Von Giordano and re-popularized in 1978 by McGuire and Lytton [36,37,38]. AFPVS remains the most widely accepted method of managing the incompetent outlet for SUI in female patients with NGB due to its long-term durability, absence of risk of mesh-related urethral events, and ability to raise bladder outlet resistance. In the general population, the goal of the AFPVS is to restore continence by providing urethral compression during times of increased intra-abdominal pressure, while not obstructing the urethra during voluntary bladder-emptying [38, 39]. In general, however, patients with NGB undergoing AFPVS will subsequently require CIC due to the increased compression necessary to address sphincteric incompetence hence a tighter sling can be utilized [39, 40].
Autologous fascial slings are most commonly harvested from the rectus abdominis fascia or tensor fascia lata, which have been extensively studied and proven efficacious [41]. After harvesting an approximately 9-cm strip of fascia, the AFPVS procedure involves a small anterior vaginal incision. The sling is then passed through the retropubic space to create a suburethral hammock. The compression provided by the sling may be adjusted to accommodate patients with a widely open bladder neck and proximal urethra, and can be successful even in patients with extremely low VLPP (less than 10 cm H2O) [40]. In a modification known as the “crossover” procedure, sling sutures are crossed over one another, allowing the sling to surround the bladder neck and provide greater compression than that provided by traditional sling suspension [40]. Additional complications associated with this procedure, include bladder perforation, prolonged urinary retention, and de novo urge incontinence [42••, 43]. Patients are commonly discharged on the first post-operative day, once they have demonstrated the ability to perform self-catheterization.
Literature describing the use of autologous pubovaginal slings, although abundant for non-neurogenic patients, is very limited in the female neurogenic population. A 2012 study by Athanasopolos, et al. retrospectively reviewed outcomes of autologous fascia rectus treatment for women with neuropathic stress urinary incontinence [44]. Thirty-three female patients were included in the study of which 12 patients (36%) had spinal cord injury as the cause for their neuropathic bladder, while 21 (64%) had myelomeningocele. At a mean of 52 months (range 12–62), authors reported success in 30 of 33 (91%) women who ultimately continued to manage their bladders with CIC. Overall, five postoperative complications were reported (15%) including one urethral erosion, one vesicovaginal fistula, and one urethral stenosis (each requiring reoperation); and two patients developed OAB treated pharmacologically [44, 45]. Despite the need for additional research assessing its long-term efficacy and risk, autologous AFPVS placement is considered perhaps the most appropriate treatment available today for SUI in women with neurogenic bladder. This treatment in our practice is also effective when placed in an obstructing fashion in patients who have mild urethral erosion from catheters (see Fig. 2).
Artificial Urinary Sphincter
The artificial urinary sphincter (AUS) has been widely used for over four decades, with excellent long-term outcomes reported in its target population: patients with post-prostatectomy stress urinary incontinence [46, 47]. Its current form (AMS 800, Boston Scientific) remains essentially the same as its original incarnation and involves the cycling of fluid between a reservoir and urethral cuff via a patient-controlled pump. In neurologically intact men, the cuff is typically placed at the bulbar urethra. In women and neurologically impaired males, the device is typically placed at the bladder neck. While this largely stems from anatomic necessity in women, bladder neck placement has the advantage of using a larger cuff, which decreases the risk of erosion with urethral instrumentation or catheterization [48].
Like the other interventions discussed in this review, data on AUS use in the adult neurogenic population is relatively sparse, and we are again forced to extrapolate from the pediatric literature. We identified three studies with a significant number of female children included in their cohort. Periera et al. followed 35 children with a mean age of 14.4 for an average of 5.5 years following bladder neck AUS implantation [49]. Thirteen patients underwent concomitant enterocystoplasty. Seven patients required reoperation for mechanical failures; three devices were explanted for bladder neck erosion, and seven patients needed subsequent bladder augmentations for loss of compliance. Of the 32 patients who did not have their devices explanted, all were reported to be dry on follow-up. Similarly, Herndon and colleagues noted excellent outcomes in 142 pediatric patients (mean age 10 years) with a mean follow-up of 7.5 years [50]. Eighty-six percent of patients achieved continence, with failure reported in only 10%. Surgical revision rates for device malfunction ranged from 19 to 25% and were more common with older AUS models, and 30 patients required permanent device explant. This study also noted that 22% of patients continued to void spontaneously, which is frequently not achievable with more aggressive SUI interventions in this group. The longest follow-up in this population was reported by Hafez et al. The overall 10-year survival rate of the device was reported as 79%, with 90% of children achieving continence. Similar to previous reports, 20% of patients required removal due to erosion, a complication strongly correlated with a history of bladder exstrophy, and three patients went on to need bladder augmentation for upper tract deterioration [51].
A recent meta-analysis by Farag et al. examined the data on AUS, sling, and urethral bulking agent use for SUI treatment in the neurogenic population. Both pediatric and adult studies were included in their analysis (average patient age 21, range 30–80) [52••]. AUS was performed in 399 patients (322 male, 77 female, 8 studies included), with the majority of sphincters implanted at the bladder neck. They reported high overall success rates (77 ± 16%) and low failure rates (10 ± 11%) with AUS placement, which were statistically superior to urethral bulking agents. No statistical difference in incontinence outcomes was shown when comparing AUS to slings. Reoperation rates were higher in the AUS group (51% ± 25%) than with the other two interventions; however, no difference was shown among complication rates between the three groups. Neither mid-urethral nor bladder neck sphincter placement was found to be superior in terms of incontinence or reoperation, and no difference in outcomes was noted based on gender composition. When taken together, these findings suggest that bladder neck AUS placement is a relatively durable and effective treatment for neurogenic SUI, although one which is likely to necessitate reoperation. Further research on this approach, specifically in women who are underrepresented in the existing data, is warranted.
Urinary Tract Reconstruction and Urinary Diversion
In addition to AUS and sling procedures, various forms of direct bladder neck reconstruction procedures have been described. These procedures, such as the Kropp, Young-Dees-Leadbetter, and Pippi Salle techniques, typically involve elongation of the urethra using bladder flaps [53,54,55]. They are rarely performed outside of the pediatric population, although the sequelae of these procedures, such as difficulty with self-catheterization, stricture, or recurrent incontinence, may be encountered in the adult neuro-urology patient.
In the setting of a destroyed urethra from prolonged indwelling catheter use or severe refractory SUI, bladder neck closure (BNC) or urinary diversion may be necessary to achieve continence (Fig. 3). In our practice, the decision to attempt bladder neck closure is typically made if the urethral defect is < 3 cm in size. Other criteria include no symphysis bone palpable on exam of the urethra. BNC may be accomplished via a vaginal or retropubic approach. Continence rates between these approaches have been shown to be comparable (85.7 vs 81.5%, p = 0.74); however, shorter mean operative time, hospital stay, and short-term complications were demonstrated in the vaginal group [56•]. BNC with suprapubic tube placement avoids an intestinal procedure and the strain a procedure of this magnitude may place on these frequently debilitated patients [57]. Alternatively, ileovesicostomy or continent catheterizeable channel formation may be performed in this setting. However, due to the morbid and technically challenging nature of these surgeries, urinary diversion ± simple cystectomy may be a preferable approach if a reconstruction of this magnitude is considered.
Prior to performing any procedure which increases bladder outlet resistance in a patient with neurogenic SUI, it is imperative that the patient be confirmed to have safe bladder physiology via UDS. If this is not demonstrated, concomitant augmentation cystoplasty (AC) and bladder outlet procedure, or, alternatively, urinary diversion, must be strongly considered. Even in the setting of normal preoperative compliance, iatrogenic bladder outlet obstruction may destabilize bladder function over time. In a cohort of 109 pediatric neuro-urology patients who underwent bladder neck reconstruction or sling placement without concomitant AC, the 10-year cumulative incidence of AC was 30%, with an alarming 50% risk of developing upper tract changes and 20% risk of chronic kidney disease [58]. While this study is limited to the pediatric population, it emphasizes the need for regular follow-up in the neurogenic SUI population following surgical intervention.
Conclusions
SUI in the patient with neurogenic bladder may be multifactorial and often coexists with other forms of urinary incontinence. It is important that these patients undergo a thoughtful clinical and urodynamic evaluation prior to any invasive procedure to ensure that they are being appropriately managed.
For voiding patients, approximately half will maintain voiding function after mesh sling, and almost all will need CIC after an AFPVS. However, AFPVS is somewhat safer with no mesh risk and is more effective particularly in the patient with low LPP. It is the preferred choice in patients with severe incontinence as it can be placed tightly at the bladder neck in a planned obstructing fashion. If a patient has concomitant detrusor overactivity, this will need to be treated first since DO and a closed outlet could lead to complications from higher storage pressures. Also patients without detrusor over-activity must be counseled that mesh slings do carry a risk of new onset DO that will require treatment.
In the setting of severe refractory stress incontinence or a destroyed urethra from chronic indwelling urethral catheter, bladder neck closure or urinary diversion should be strongly considered depending on the severity of the defect. While very mild cases may be salvaged with an AFPVS, more severe cases should be addressed with a bladder neck closure and cases where bone is palpable on urethral exam require urinary diversion. The appropriateness of a given intervention should be determined by patient’s dexterity, state of overall health, and life expectancy.
Finally, it is important that patients with neurogenic lower urinary tract dysfunction be followed carefully by a urologist following procedures which raise bladder outlet resistance. In the event of ominous changes to bladder physiology, prompt interventions should be undertaken to avoid upper tract deterioration.
References
Papers of particular interest, published recently, have been highlighted as: •Of importance ••Of major importance
•• Gajewski JB, Schurch B, Hamid R, et al. An International Continence Society (ICS) report on the terminology for adult neurogenic lower urinary tract dysfunction (ANLUTD). Neurourol Urodyn. 2017;(July):1–10. doi:https://doi.org/10.1002/nau.23397. Recent standardization of terminology for ANLUTD .
Minassian VA, Stewart WF, Wood GC. Urinary incontinence in women: variation in prevalence estimates and risk factors. Obstet Gynecol. 2008;111(2 Pt 1):324–31. https://doi.org/10.1097/01.AOG.0000267220.48987.17.
Peyrat L, Haillot O, Bruyere F, Boutin JM, Bertrand P, Lanson Y. Prevalence and risk factors of urinary incontinence in young and middle-aged women. BJU Int. 2002;89(1):61–6. http://www.ncbi.nlm.nih.gov/pubmed/11849162
• Schimpf MO, Rahn DD, Wheeler TL, Patel M, White AB, Orejuela FJ, et al. Sling surgery for stress urinary incontinence in women: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;211(1). doi:10.1016/j.ajog.2014.01.030) Excellent meta-analysis of sling outcomes:71.e1–71.e27.
DeLancey JO. Anatomy and physiology of urinary continence. Clin Obstet Gynecol. 1990;33(2):298–307. http://www.ncbi.nlm.nih.gov/pubmed/2190733
Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9(6):453–66. https://doi.org/10.1038/nrn2401.
DeLancey JO. Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol. 1994;170(6):1713-20-3. http://www.ncbi.nlm.nih.gov/pubmed/8203431–1723.
DeLancey JO. Stress urinary incontinence: where are we now, where should we go? Am J Obstet Gynecol. 1996;175(2):311–9. http://www.ncbi.nlm.nih.gov/pubmed/8765247
• El-Azab AS, El-Nashar SA. Midurethral slings versus the standard pubovaginal slings for women with neurogenic stress urinary incontinence. Int Urogynecol J. 2015;26(3):427–32. https://doi.org/10.1007/s00192-014-2521-8. Prospective pilot study of select women with SCI at S2 or below showing equivalent outcomes between TVT and AFPVS
Chancellor MB, Erhard MJ, Kiilholma PJ, Karasick S, Rivas DA. Functional urethral closure with pubovaginal sling for destroyed female urethra after long-term urethral catheterization. Urology. 1994;43(4):499–505. https://doi.org/10.1016/0090-4295(94)90241-0.
Panicker JN, Fowler CJ, Kessler TM. Lower urinary tract dysfunction in the neurological patient: clinical assessment and management. Lancet Neurol. 2015;14(7):720–32. https://doi.org/10.1016/S1474-4422(15)00070-8.
Nager CW, Brubaker L, Litman HJ, Zyczynski HM, Varner RE, Amundsen C, et al. A randomized trial of urodynamic testing before stress-incontinence surgery. N Engl J Med. 2012;366(21):1987–97. https://doi.org/10.1056/NEJMoa1113595.
Winters JC, Dmochowski RR, Goldman HB, et al. Adult Urodynamics: AUA/SUFU Guideline. Am Urol Assoc. 2012:1–30.
Pajoncini C, Costantini E, Guercini F, Porena M. Intrinsic sphincter deficiency: do the maximum urethral closure pressure and the Valsalva leak-point pressure identify different pathogenic mechanisms? Int Urogynecol J Pelvic Floor Dysfunct. 2002;13(1):30–5. http://www.ncbi.nlm.nih.gov/pubmed/11999202
Dumoulin C, Hay-Smith EJC, Mac Habée-Séguin G. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev. 2014;5:CD005654. https://doi.org/10.1002/14651858.CD005654.pub3.
Kobashi KC, Albo ME, Roger R, Ginsberg DA, Goldman HB. American Urological Association ( AUA )/SURGICAL TREATMENT OF FEMALE STRESS URINARY INCONTINENCE: AUA/SUFU GUIDELINE American Urological Association ( AUA )/Society of Urodynamics , Female Pelvic Medicine & Urogenital Reconstruction ( SUFU ) Stress. 2017;(March):1–33.
Khanna OP, Elkouss G, Heber D, Gonick P. Imipramine hydrochloride: pharmacodynamic effects on lower urinary tract of female dogs. Urology. 1975;6(1):49–51. http://www.ncbi.nlm.nih.gov/pubmed/238322
Lin HH, Sheu BC, Lo MC, Huang SC. Comparison of treatment outcomes for imipramine for female genuine stress incontinence. Br J Obstet Gynaecol. 1999;106(10):1089–92. http://www.ncbi.nlm.nih.gov/pubmed/10519437
Cameron AP, Clemens JQ, Latini JM, McGuire EJ. Combination drug therapy improves compliance of the neurogenic bladder. J Urol. 2009;182(3):1062–7. https://doi.org/10.1016/j.juro.2009.05.038.
Bennett JK, Green BG, Foote JE, Gray M. Collagen injections for intrinsic sphincter deficiency in the neuropathic urethra. Paraplegia. 1995;33(12):697–700. https://doi.org/10.1038/sc.1995.146.
Kassouf W, Capolicchio G, Berardinucci G, Corcos J. Collagen injection for treatment of urinary incontinence in children. J Urol. 2001;165(5):1666–8. http://www.ncbi.nlm.nih.gov/pubmed/11342951
Chernoff A, Horowitz M, Combs A, Libretti D, Nitti V, Glassberg KI. Periurethral collagen injection for the treatment of urinary incontinence in children. J Urol. 1997;157(6):2303–5. http://www.ncbi.nlm.nih.gov/pubmed/9146659
Monga AK, Robinson D, Stanton SL. Periurethral collagen injections for genuine stress incontinence: a 2-year follow-up. Br J Urol. 1995;76(2):156–60. http://www.ncbi.nlm.nih.gov/pubmed/7663903
Gorton E, Stanton S, Monga A, Wiskind AK, Lentz GM, Bland DR. Periurethral collagen injection: a long-term follow-up study. BJU Int. 1999;84(9):966–71. http://www.ncbi.nlm.nih.gov/pubmed/10571621
Kirchin V, Page T, Keegan PE, Atiemo KOM, Cody JD, McClinton S, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD003881. https://doi.org/10.1002/14651858.CD003881.pub4.
Dray E, Cameron A, Hall M, Clemens JQ, Stoffel J. MP40-10 CAN URETHRAL BULKING AGENTS SALVAGE FAILED SLINGS? J Urol. 2017;197(4):e526–7. https://doi.org/10.1016/j.juro.2017.02.1257.
Kobashi KC, Albo ME, Dmochowski RR, Ginsberg DA, Goldman HB, Gomelsky A, et al. Surgical treatment of female stress urinary incontinence: AUA/SUFU Guideline. J Urol. 2017;198(4):875–83. https://doi.org/10.1016/j.juro.2017.06.061.
Schierlitz L, Dwyer PL, Rosamilia A, Murray C, Thomas E, de Souza A, et al. Effectiveness of tension-free vaginal tape compared with transobturator tape in women with stress urinary incontinence and intrinsic sphincter deficiency: a randomized controlled trial. Obstet Gynecol. 2008;112(6):1253–61. https://doi.org/10.1097/AOG.0b013e31818db391.
Pannek J, Bartel P, Gocking K. Clinical usefulness of the transobturator sub-urethral tape in the treatment of stress urinary incontinence in female patients with spinal cord lesion. J Spinal Cord Med. 2012;35(2):102–6. https://doi.org/10.1179/2045772312Y.0000000008.
Reuvers SHM, Groen J, Scheepe JR, et al. Heterogeneity in reporting on urinary outcome and cure after surgical interventions for stress urinary incontinence in adult neuro-urological patients: a systematic review. Neurourol Urodyn. 2017;(May). doi:https://doi.org/10.1002/nau.23364.
Abdul-Rahman A, Attar KH, Hamid R, Shah PJR. Long-term outcome of tension-free vaginal tape for treating stress incontinence in women with neuropathic bladders. BJU Int. 2010;106(6):827–30. https://doi.org/10.1111/j.1464-410X.2010.09203.x.
Hamid R, Khastgir J, Arya M, Patel HRH, Shah PJR. Experience of tension-free vaginal tape for the treatment of stress incontinence in females with neuropathic bladders. Spinal cord Off J Int Med Soc Paraplegia. 2003;41(2):118–21. https://doi.org/10.1038/sj.sc.3101399.
Patki P, Woodhouse JB, Patil K, Hamid R, Shah J. An effective day case treatment combination for refractory neurophatic mixed incontinence. Int Braz J Urol. 2008;34(1):63–72.
Losco GS, Burki JR, Omar YAI, Shah PJR, Hamid R. Long-term outcome of transobturator tape (TOT) for treatment of stress urinary incontinence in females with neuropathic bladders. Spinal Cord. 2015;53(7):544–6. https://doi.org/10.1038/sc.2015.70.
Lombardi G, Musco S, Celso M, Ierardi A, Nelli F, del Corso F, et al. A retrospective study on female urological surgeries over the 10 years following spinal cord lesion. Spinal Cord. 2013;51(9):688–93. https://doi.org/10.1038/sc.2013.64.
Jack C, Hou GEL. The role of fascial slings in the treatment of stress urinary incontinence in women: a 2013 update. Curr Urol Rep. 2013;14(3):247–52.
Bang SBM. Autologous pubovaginal slings: back to the future or a lost art? Res Reports Urol. 2016;8:11–20.
Gregory G, Bailly KVC. The pubovaginal sling: reintroducing an old friend. Can Urol Assoc J. 2017;11(6):S147+.
Arthi Satyanarayan Gary E, Lemack LG. Diagnosis and management of stress urinary incontinence in females with neurogenic disease. Curr Bl Dysfunct Rep. 2015;10:52–6.
Anne Pelletier Cameron Edward J. McGuire CL-G. Pubovaginal Fascial Slings. In: Sam D. Graham MD Thomas E. Keane MD J, ed. GLENN’S UROLOGIC SURGERY. Philadelphia, PA: LIPPIN COTT WILLIAMS & WILKIN S; 2010:271–277.
Karram MD, Zoorob Dani MD, Reynolds W, Stuart MD, Kaufman Melissa RMD, Dmochowski Roger MDM. Biologic bladder neck pubovaginal slings. In: Surgery for urinary incontinence. Philadelphia: Saunders, an imprint of Elsevier Inc. p. 2013.
•• Morgan TO Jr, McGuire EJWOL. Pubovaginal sling: 4-year out-come analysis and quality of life assessment. J Urol. 2000;163:1845–8. Large prospective cohort study of patients undergoing AFPVS showing high efficacy and durability with low complication rates
Chaikin DC Blaivas JGRJ. Pubovaginal fascial sling for all types of stress urinary incontinence: long term analysis. J Urol. 1998;160:1312–6.
Athanasopoulos A, Gyftopoulos K, McGuire EJ. Treating stress urinary incontinence in female patients with neuropathic bladder: the value of the autologous fascia rectus sling. Int Urol Nephrol. 2012;44(5):1363–7. https://doi.org/10.1007/s11255-012-0247-4.
Crystal D, Sadik Ngoc-Bich P, Le DA. Evaluation and management of neurogenic stress urinary incontinence. Curr Bladder Dysfunct Rep. 2014;9(2):108–13.
Scott FB, Bradley WE, Timm GW. Treatment of urinary incontinence by an implantable prosthetic urinary sphincter. J Urol. 1974;112(1):75–80. http://www.ncbi.nlm.nih.gov/pubmed/4600662
Kim SP, Sarmast Z, Daignault S, Faerber GJ, McGuire EJ, Latini JM. Long-term durability and functional outcomes among patients with artificial urinary sphincters: a 10-year retrospective review from the University of Michigan. J Urol. 2008;179(5):1912–6. https://doi.org/10.1016/j.juro.2008.01.048.
Myers JB, Mayer EN, Lenherr S, Neurogenic Bladder Research Group (NBRG.org). Management options for sphincteric deficiency in adults with neurogenic bladder. Transl Androl Urol. 2016;5(1):145–57. https://doi.org/10.3978/j.issn.2223-4683.2015.12.11.
López Pereira P, Somoza Ariba I, Martínez Urrutia MJ, Lobato Romero R, Jaureguizar Monroe E. Artificial urinary sphincter: 11-year experience in adolescents with congenital neuropathic bladder. Eur Urol. 2006;50(5):1096–101; discussion 1101. https://doi.org/10.1016/j.eururo.2006.02.037.
Herndon CDA, Rink RC, Shaw MBK, et al. The Indiana experience with artificial urinary sphincters in children and young adults. J Urol. 2003;169(2):650–4; discussion 654. https://doi.org/10.1097/01.ju.0000047320.28201.9d.
Hafez AT, McLorie G, Bägli D, Khoury A. A single-centre long-term outcome analysis of artificial urinary sphincter placement in children. BJU Int. 2002;89(1):82–5. http://www.ncbi.nlm.nih.gov/pubmed/11849167
•• Farag F, Koens M, Sievert K-D, De Ridder D, Feitz W, Heesakkers J. Surgical treatment of neurogenic stress urinary incontinence: a systematic review of quality assessment and surgical outcomes. Neurourol Urodyn. 2016;35(1):21–5. https://doi.org/10.1002/nau.22682. Well-designed meta-analysis of existing data regarding neurogenic stress urinary incontinence
Kropp KA, Angwafo FF. Urethral lengthening and reimplantation for neurogenic incontinence in children. J Urol. 1986;135(3):533–6. http://www.ncbi.nlm.nih.gov/pubmed/3944902
Hohenfellner R. The Kropp-onlay procedure (Pippe Salle procedure). Br J Urol. 1995;76(4):525–6. http://www.ncbi.nlm.nih.gov/pubmed/7551908
Donnahoo KK, Rink RC, Cain MP, Casale AJ. The Young-Dees-Leadbetter bladder neck repair for neurogenic incontinence. J Urol. 1999;161(6):1946–9. http://www.ncbi.nlm.nih.gov/pubmed/10332478
• Willis H, Safiano NA, Lloyd LK. Comparison of transvaginal and retropubic bladder neck closure with suprapubic catheter in women. J Urol. 2015;193(1):196–202. https://doi.org/10.1016/j.juro.2014.07.091. Large retrospective review of bladder neck closure techniques, showing no difference in efficacy and reduced short-term complications with vaginal approach
Zimmern PE, Hadley HR, Leach GE, Raz S. Transvaginal closure of the bladder neck and placement of a suprapubic catheter for destroyed urethra after long-term indwelling catheterization. J Urol. 1985;134(3):554–7. http://www.ncbi.nlm.nih.gov/pubmed/4040980
Grimsby GM, Menon V, Schlomer BJ, Baker LA, Adams R, Gargollo PC, et al. Long-term outcomes of bladder neck reconstruction without augmentation cystoplasty in children. J Urol. 2016;195(1):155–61. https://doi.org/10.1016/j.juro.2015.06.103.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Elizabeth V. Dray, Anne P. Cameron, and Rachel Bergman declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Stress Incontinence and Prolapse
Rights and permissions
About this article
Cite this article
Dray, E.V., Cameron, A.P. & Bergman, R. Stress Urinary Incontinence in Women with Neurogenic Lower Urinary Tract Dysfunction. Curr Bladder Dysfunct Rep 13, 75–83 (2018). https://doi.org/10.1007/s11884-018-0471-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11884-018-0471-6