Abstract
Purpose of Review
Studies have revealed a relation between birth weight (BW) and later risk of cardiovascular diseases (CVDs). This meta-analysis aimed to report the dose-response relationship between BW and risk of CVDs.
Recent Findings
The relation of BW to CVD subtypes was found to be U-shaped as BW below ~ 2500 g and above ~ 4000 g affected positively CVD risk (OR = 1.14 = 95%CI 1.03–1.27 and OR = 1.08; 95%CI 0.99–1.18, respectively). Regarding CVD subtypes, low BW was directly linked to greater risk of CHD (OR = 1.15; 95%CI 1.02–1.29) and stroke (OR = 1.28; 95% CI 1.05–1.55), while high BW was related to increased risk of arterial fibrillation in adulthood. A U-shaped nonlinear relationship was specifically demonstrated between BW and overall CVD and its subtypes.
Summary
There is a U-shaped association between BW and all CVD subtypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prenatal and postnatal life can have profound impacts on the programming of intracellular signals, cell-to-cell interactions, and metabolic pathways [1]. The “developmental origins of adult disease” hypothesis, known as “Barker hypothesis,” claims that adverse influences early in development, especially during intrauterine life, could lead to permanent changes in metabolism and physiology, which result in elevated susceptibility to adulthood chronic diseases [2]. A baby’s nourishment before birth and during infancy,” as manifest in patterns of fetal and infant growth, programs the development of risk factors involving in the pathophysiology of cardiovascular disease [3]. Strong body of evidences had confirmed that undernutrition and subsequent slow growth in utero can change body functions and metabolisms and enhance weight gain during childhood, which leads to an increased risk of CVD in later life [4, 5]. Although there is evidence that excessive energy supply to the fetus or infant, manifested as high birth weight (HBW), also has adverse health consequences [6], prior studies have proposed that birth weight (BW) had an inverse relationship with the risk of myocardial infarction (MI), [7] ischemic heart disease (IHD), [8] CHD, type 2 diabetes mellitus, [9] stroke [10], and coronary artery diseases (CAD) [11] in adulthood. Nonetheless, the findings of some studies revealed that BW is positively linked to relatively greater risk of CAD in males [12]. On the contrary, other reports presented that BW was not significantly associated to the later CHD or CVD mortality and morbidity [13, 14]. Besides, a cohort study on Danish men proposed that abnormal birth weight, whether less or more than normal ranges, contributed to increased risk of CHD [15]. Recently, a meta-analysis of prospective cohort studies reported that there was an remarkable association between risk of CHD and LBW, as 1 kg more birth weight can reduce 10–20% of CHD risk [16••].
As exposure to undernutrition or overnutrition during infancy, manifested by birth weight, results in the susceptibility to metabolic complication in adulthood, some studies have tried to reveal the relation of BW to CVD risk; however, previous studies have yielded inconsistent results. Moreover, the hypothesis of the possibility of nonlinear association between BW and CVD risk has not yet been described. Accordingly, this dose-response meta-analysis of all available observational studies was performed to assess the association between BW and risk of CVD incidence, including CHD, stroke, arterial fibrillation (AF), and myocardial infarction (MI).
Methods
Literature Search Strategy
This meta-analysis was performed and reported according to recommendations of the MOOSE (Meta-analysis of Observational Studies in Epidemiology) group. We undertook a systematic search of EMBASE (http://www.embase.com) and PubMed (http://www.ncbi.nlm.nih.gov/pubmed) through February 2018 for studies related to BW and CVD. The following search terms were entered in the database searches: birth weight, cardiovascular, coronary artery disease, coronary heart disease, ischemic heart disease, myocardial infarction, angina, atrial fibrillation, and stroke. In addition, Reference lists of all relevant articles and identified reviews were inspected to identify pertinent articles that could have been missed in the initial search. The PICOS (Participants, Intervention/exposure, Comparison, Outcomes, Study design) criteria used to define the research question are shown in Table 1.
Study Selection
Studies were included if they met the following criteria: (1) were observational studies, (2) reported diagnosis criteria for CVDs (including CHD, MI, AF, and stroke), and (3) reported relative risk (RR), odds ratio (OR), or hazards ratio (HR) estimates and 95% CIs (or data can be calculated) describing the relationship between BW and risk of CVDs. Incident CVD was primarily defined as a confirmed diagnosis of myocardial infarction, coronary heart disease, and stroke. Of note, atrial fibrillation was also included in the cardiovascular category because the number of studies with this condition was small and we aimed to comprehensively explore the relation of BW to cardiovascular-related outcomes (in case of availability of data) [17]. We excluded (1) no original data (editorials, reviews, meta-analyses), (2) studies that were on twins, (3) studies written in languages other than English, and (4) studies that investigated mortality instead of incidence. In the case of multiple reported papers from the same study, only those with the longest follow-up times or the highest number of cases were included in the meta-analysis.
Data Extraction and Quality Assessment
Two reviewers independently extracted data from the eligible studies using a standard form, which included the first author’s name, publication year, geographical location, sample size, mean or range of age, study design, source of cohort, duration of follow-up, source of BW data, cardiovascular outcomes, and covariates adjusted for in analyses. For information that was not reported in the published studies, the corresponding author was directly contacted to get the related data. Finally, we evaluated study quality by using the nine star Newcastle–Ottawa Scale (NOS) for observational studies [18]. It allows evaluating methodological quality in three main domains: selection, comparability, and exposure/outcome.
Statistical Analysis
For meta-analysis, risk estimates and 95 confidence intervals (CIs) for dichotomous outcomes from each study were pooled. Initially, we assessed the relation of LBW (BW < 2500 g vs. BW > 2500 g) and HBW (BW > 4000 g vs. BW < 4000) to the risk of CVDs and CVD subtypes. Then, we carried out a dose-response analysis using the nonlinear model. The mean or median BW in each category was assigned to the corresponding dose of the BW. The midpoint of the lower and upper bound was estimated as the dose of each category if the mean or median BW for each category was not provided. When the extreme categories were open-ended, the midpoint of these categories was calculated by assuming the length of the interval was the same as that of the adjacent interval. To assess a potential nonlinear dose-response relationship between BW and the risk of CVD, we used a restricted cubic spline regression analysis with 3 knots at 10%, 50%, and 90% percentiles of the distribution [19]. A P value for nonlinearity was obtained by testing against the null hypothesis that the coefficient of the second spline was equal to zero [20].
Heterogeneity among studies was evaluated using the Q statistic (considered significant at P < 0.10) and I2 metric to quantify the extent of statistical heterogeneity. We considered values of I2 of 50–75% as “moderate heterogeneity” and > 75% as “high heterogeneity” according to Higgins et al.’s proposal [21]. A fixed-effects approach was used to calculate the combined risk estimates in the absence of heterogeneity; otherwise, if there was evidence of significant heterogeneity among studies, a DerSimonian-Laird random effect model was used [22]. Small-study effects, such as publication bias, were evaluated using visual inspection of funnel plots and Egger’s and Begg’s tests. STATA version 12.0 software (College Station, TX, USA) was used for the analyses. All statistical tests were two sided with a significance level of 0.05.
Results
Studies Characteristics
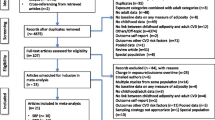
Figure 1 displays the process of selection of studies. A total of 7034 nonduplicate records were identified through previously mentioned literature search strategy. After excluding 6980 papers on the basis of titles and abstracts, 54 full-text articles were identified for detailed examination. Of the full texts, 30 were excluded, mainly because they were reviews, duplicated reports, or did not reported sufficient data for analysis; a total of 24 articles were finally included in the present meta-analysis. Sixteen studies reported BW only as a categorical variable and seven articles reported BW as both a continuous variable [11, 14, 23,24,25,26,27,28,29,30,31,32,33,34,35,36,37] and a categorical variable [38,39,40,41,42,43,44]. The studies were mainly derived from Europe (n = 16) [11, 23, 24, 26,27,28, 29••, 31, 32••, 37,38,39,40,41,42, 44••], followed by North America (n = 5) [25, 30••, 33, 34••, 43] and Asia (n = 3) [14, 35, 36••]. All included studies ascertained CVD by clinical examination and medical records. According to the Newcastle-Ottawa Quality Assessment Scale, twenty-two studies received scores of 6 or higher and were considered to be of high quality. The study characteristics are summarized in Table 2.
Birth Weight and Cardiovascular Disease Risk
Because sufficient dichotomous data for BW and CVD were not available for 6 articles, these studies could not be included in the meta-analysis [32••, 34••, 38, 40,41,42]. The remaining 17 articles [11, 14, 23,24,25,26,27,28,29,30,31, 33, 35, 36••, 37, 39••, 43, 44••] were included in the meta-analysis. Sixteen studies [14, 23,24,25,26,27,28,29,30,31, 33, 35, 36••, 37, 39••, 43, 44••] analyzed the risk of CVD in subjects with LBW (BW < 2500 g) compared with that of subjects with BW > 2500 g. Results from this analysis indicated a positive association between LBW and total CVD (OR = 1.14; 95% CI 1.03–1.27, P = 0.02). Data from these studies were assessed using the random effects model (I2 = 59.9%, P < 0.01) (Fig. 2; Table 3). Pooled analysis of 11 studies [11, 23, 25, 26, 28, 29••, 30••, 31, 36, 39••, 44••] showed that HBW (BW > 4000 g), compared with BW < 4000 g, was marginally associated with increased risk of CVD (OR, 1.08; 95% CI 0.99–1.18, P = 0.08), and this effect was observed using the random effects model (I2 = 62.7%, P < 0.01) (Fig. 3; Table 3).
The relation between BW and total CVD events was found to be U-shaped with the use of a restricted cubic model (P nonlinearity < 0.001) (Fig. 4). At BW below ~ 2500 g, there was a higher risk of CVD compared with higher BW. BW between ~ 3000 and 4000 g conferred the lowest risk of CVD. By contrast, at BW above ~ 4000 g, a higher BW was progressively associated with a higher risk of CVD.
Visual inspection of funnel plots did not reveal substantial asymmetry and Egger’s (P = 0.216 for studies comparing BW < 2500 g vs. BW > 2500 g; P = 0.981 for studies comparing BW > 4000 g vs. BW < 4000) and Begg’s test (P = 0.51 for studies comparing BW < 2500 g vs. BW > 2500 g; P = 0.93 for studies comparing BW > 4000 g vs. BW < 4000) for publication bias were not statistically significant for studies investigating the relationship between high and low BW with CVD risk.
Birth Weight and Risk of Cardiovascular Disease Subtypes
Table 3 shows the meta-analyses of the association between BW and CVD subtypes, including CHD, AF, MI, and stroke. LBW (BW < 2500 g vs. BW > 2500 g) was associated with an increased risk of CHD (12 studies [14, 23, 24, 27, 28, 31, 33, 35, 37, 39, 43, 44]; OR = 1.15; 95% CI 1.02–1.29, P = 0.02) and stroke (2 studies [26, 43]; OR = 1.28; 95% CI 1.05–1.55, P = 0.01), but not with AF and MI. Furthermore, HBW (BW > 4000 g vs. BW < 4000) was associated with a higher risk of AF (2 studies [29••, 30••]; OR = 1.11; 95% CI 1.03–1.20, P = 0.008), while no significant association was observed with CHD, MI, and stroke (Table 3).
Also, there was a significant evidence of a U-shaped association between BW and all investigated CVD subtypes, including CHD (16 studies [11, 14, 23, 24, 27, 28, 31, 32••, 33, 35, 37, 38, 39••, 41,42,43]; P nonlinearity < 0.001), AF (3 studies [25, 29••, 30••]; P nonlinearity < 0.001), MI (3 studies [32••, 33, 44••]; P nonlinearity = 0.01), and stroke (3 studies [26, 42, 43]; P nonlinearity = 0.002) in nonlinear dose-response meta-analysis (Fig. 4).
Discussion
Existing empirical and meta-analysis studies have shown that birth weight influences the risk of CVD during adulthood. However, there is no study on the dose-response relationship between birth weight and risk of CVD. Thus, this study was aimed primarily to investigate the dose-response relationship between birth weight and CVD in general as well as its specific forms. We also provided summary estimates on the extent of association of BW with various forms of CVDs. We found that LBW is significantly associated with overall CVD, CHD, and stroke. There were increments of 14%, 15%, and 28% in the odds of overall CVD, CHD, and stroke, respectively, in individuals with a history of LBW, compared with individuals of no history of LBW. HBW was also associated with a higher risk of CVD and AF. There were increments of 8% and 11% in the odds of overall CVDs and AF, respectively. We also observed a nonlinear relationship between BW and overall CVDS. A U-shaped association was specifically demonstrated between BW and all of the conditions assessed, such that the risk of CVD increases as BW decreases further below 2500 g and also increases further above 4000 g. Individuals with BW 3000–4000 g demonstrated the least CVD risk. Our finding that LBW is associated with various CVD conditions is consistent with previous studies. In their meta-analysis, Wang et al. [16••] reported a higher risk of CHD in individuals born with BW of < 2500 g, compared with individuals born with BW > 2500 g. The same findings were also reported by various empirical studies [45, 46]. Our finding of a greater risk of CVD associated with HBW is in concordance with most of the existing evidence [45], though in disagreement with the report of the work by Wang et al. [16••]. They found a lower risk of CHD in HBW individuals, compared with both LBW and NBW individuals. Provided both undernutrition and overnutrition could result in deranged nutritional metabolism, including insulin resistance [47, 48•, 49], we believe our finding is of a plausible biological rationale. The adverse effects of LBW are not limited to cardiovascular outcomes, but have also been linked to a variety of other poor health and nutritional outcomes.
A number of potential mechanisms have been suggested to explain the link of BW with the risk of CVD in adults [48•]. The most widely mentioned and highly likely mechanism is related to the “developmental origins of adult disease” hypothesis which states adverse influences during early stages of life could result in irreversible metabolic, physiologic, and structural changes, some of which become disadvantageous by increasing the risk of negative health outcomes late in life [48•, 50,51,52,53]. Permanent alteration of postnatal metabolic and physiologic states of animals has been demonstrated by manipulating maternal diet during pregnancy. In these studies, exposure to global undernutrition or specific nutrient deficiency during early stages of life resulted in impaired glucose tolerance, high blood pressure, and LBW [48•, 54]. Nutritional stress during pregnancy has also been hypothesized to result in excess production of glucocorticoid; the exposure of it has been associated with a permanently reduced number of hypothalamic glucocorticoid receptors and subsequently impairment in hypothalamic–pituitary–adrenal hormonal feedback mechanism [48•]. Impairment in hypothalamic-hormonal feedback mechanism could lead to glucose intolerance as well as other adverse health conditions. Besides these fetal programming and glucocorticoids mechanism, nephrogenic, neurogenic [55], genetic, and epigenetic influences [46, 48•, 49] have also been implicated. Despite the evidence which is not clear on which of the proposed mechanism is largely responsible for the link of BW to CVD, it could, however, be presumed that those born with abnormal BW would also have impaired metabolism and glucose intolerance and hypertension. These conditions, in turn, will increase the risk of CVD events.
Policy and Research Implications
LBW remains a major public health problem, particularly in developing countries [56]. Evidence shows that children born with LBW have low survival and wellbeing. They have a higher risk for childhood morbidity and mortality and also poor cognitive and motor development [57]. Thus, the World Health Organization has already prioritized LBW as a central global agenda and aimed a 30% reduction in its prevalence over the period 2012–2025 [56]. While LBW by itself poses a significant burden on the health system, its association with a higher risk of CVD is more concerning. CVDs are among the top contributors to health impairment globally, in both developing and developed nations. Like reducing BW, combating CVDs is also a global public health agenda [58]. Thus, the promotion of optimal BW in general and the prevention of LBW, in particular, might have dual benefits, reducing the burden of poor health outcomes during childhood and cardiovascular diseases during adulthood. Thus, the existing LBW prevention programs might be viewed and emphasized not only to ensure wellbeing during childhood, but also to prevent cardiovascular incidents in adulthood and promote healthy aging.
Studies on the association of LBW with CVD are limited by geographic area. The existing estimates are based on studies conducted mainly in western countries where the prevalence of LBW is low, and child care is better, compared with some regions of Asia and Africa where the burden of LBW is high, and child care practices are still below the acceptable levels. Thus, presuming LBW increases the risk of CVD and other competing risk factors remain the same, it could be presumed that developing countries would bear an extract risk of CVDs. Thus, public health authorities and policymakers of nations with high LBW need to note the extra CVD risks and design mitigation efforts, including health-enhancing lifestyle interventions. Designing a system of early screening CVDs for individuals with a history of LBW would also stand worthy of consideration. However, there is no study on the effectiveness of screening programs in reducing the extra risk of chronic diseases in LBW adults. Thus, further studies are warranted to explore strategies to mitigate the extra CVD risk associated with LBW, including the feasibility of incorporating screening programs into the existing health system.
Strength and Limitations
Strengths of this meta-analysis include the comprehensive analyses of different categories or ranges of BW in relation to various cardiovascular disease outcomes including overall CVD, CHD, AF, MI, and stroke; detailed subgroup analysis, nonlinear dose-response analysis; inclusion of large number of studies covering a large number of individual participants; and the high quality of the studies included. The study also has important limitations worthy of mentioning. First, there was a high level of between studies heterogeneity, most of which could not be avoided even after subgroup analysis. However, our use of random effect meta-analysis models could have reduced the problem of heterogeneity. Second, we compared the risk of HBW in reference to BW < 4000 g. This could be problematic as BW < 4000 includes both NBW and LBW. BW 2500–4000 as a reference of comparison would have provided a better estimate. Third, we cannot exclude that our meta-analysis was underpowered to detect a significant difference in MI risk by BW because of the small number of studies included in the BW-MI subgroup analysis. Fourth, in this study, observational studies were included. Thus, causal inference could not be made on the relation of BW with any of the CVD outcomes assessed.
Conclusion
This meta-analysis revealed that LBW is significantly related to increased risks of CVD, CHD, and stroke, whereas HBW is associated with a higher risk of CVD and AF in adulthood. In nonlinear dose-response analyses, a U-shaped association was identified between BW and CVD, CHD, MI, AF, and stroke, such that the risk increased as BW decreases further below 2500 g and also increases further above 4000 g. In order to reach a robust conclusion, further prospective cohort studies are required to assess the relation of BW to the risk of the incidence of MI, stroke, and AF in adulthood.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Dover GJ. The Barker hypothesis: how pediatricians will diagnose and prevent common adult- onset diseases. Trans Am Clin Climatol Assoc. 2009;120:199.
De Boo HA, Harding JE. The developmental origins of adult disease (Barker) hypothesis. Aust N Z J Obstet Gynaecol. 2006;46(1):4–14.
Paneth N, Susser M. Early origin of coronary heart disease (the “Barker hypothesis”): British Medical Journal Publishing Group; 1995.
Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2(8663):577–80.
Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353(17):1802–9.
Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3):e290–6.
Tanis BC, Kapiteijn K, Hage RM, Rosendaal FR, Helmerhorst FM. Dutch women with a low birth weight have an increased risk of myocardial infarction later in life: a case control study. Reprod Health. 2005;2:1.
Huxley R, Owen CG, Whincup PH, Cook DG, Rich-Edwards J, Smith GD, et al. Is birth weight a risk factor for ischemic heart disease in later life? Am J Clin Nutr. 2007;85(5):1244–50.
Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73.
Lawlor DA, Ronalds G, Clark H, Smith GD, Leon DA. Birth weight is inversely associated with incident coronary heart disease and stroke among individuals born in the 1950s: findings from the Aberdeen Children of the 1950s prospective cohort study. Circulation. 2005;112(10):1414–8.
Gunnarsdottir I, Birgisdottir BE, Thorsdottir I, Gudnason V, Benediktsson R. Size at birth and coronary artery disease in a population with high birth weight. Am J Clin Nutr. 2002;76(6):1290–4.
Banci M, Saccucci P, Dofcaci A, Sansoni I, Magrini A, Bottini E, et al. Birth weight and coronary artery disease. The effect of gender and diabetes. Int J Biol Sci. 2009;5(3):244–8.
Eriksson M, Wallander MA, Krakau I, Wedel H, Svardsudd K. The impact of birth weight on coronary heart disease morbidity and mortality in a birth cohort followed up for 85 years: a population-based study of men born in 1913. J Intern Med. 2004;256(6):472–81.
Fan Z, Zhang Z-X, Li Y, et al. Relationship between birth size and coronary heart disease in China. Ann Med. 2010;42(8):596–602.
Osler M, Lund R, Kriegbaum M, Andersen AM. The influence of birth weight and body mass in early adulthood on early coronary heart disease risk among Danish men born in 1953. Eur J Epidemiol. 2009;24(1):57–61.
•• Wang SF, Shu L, Sheng J, et al. Birth weight and risk of coronary heart disease in adults: a meta-analysis of prospective cohort studies. J Dev Orig Health Dis. 2014;5(6):408–19 This meta-analysis is among the first scomprehensive studies reporting a higher risk of coronary heart disease in individuals born with birth weight of <2500gm, compared with individuals with birth weight > 2500 gm.
Nuyen J, Spreeuwenberg PM, Beekman AT, Groenewegen PP, van den Bos GA, Schellevis FG. Cerebrovascular risk factors and subsequent depression in older general practice patients. J Affect Disord. 2007;99(1–3):73–81.
Wells G, Shea B, O’connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. 2009. Epub Available from: URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm [cited 2009 Oct 19] 2013.
Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Stat Med. 2010;29(12):1282–97.
Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2011;175(1):66–73.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. British Med J. 2003;327(7414):557–60.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Andersen LG, Ängquist L, Eriksson JG, et al. Birth weight, childhood body mass index and risk of coronary heart disease in adults: combined historical cohort studies. PLoS One. 2010;5(11):e14126.
Banci M, Saccucci P, Dofcaci A, et al. Birth weight and coronary artery disease. The effect of gender and diabetes. Int J Biol Sci. 2009;5(3):244.
Conen D, Tedrow UB, Cook NR, Buring JE, Albert CM. Birth weight is a significant risk factor for incident atrial fibrillation. Circulation. 2010;122(8):764–70.
Eriksson J, Forsen T, Tuomilehto J, Osmond C, Barker D. Early growth, adult income, and risk of stroke. Stroke. 2000;31(4):869–74.
Fall C, Vijayakumar M, Barker D, Osmond C, Duggleby S. Weight in infancy and prevalence of coronary heart disease in adult life. Bmj. 1995;310(6971):17–20.
Kaijser M, Bonamy A-KE, Akre O, et al. Perinatal risk factors for ischemic heart disease: disentangling the roles of birth weight and preterm birth. Circulation. 2008;117(3):405–10.
•• Larsson SC, Drca N, Jensen-Urstad M, Wolk A. Incidence of atrial fibrillation in relation to birth weight and preterm birth. Int J Cardiol. 2015;178:149–52 In this cohort study, both high birth weight and low birth weight (in men), in particular in men born full-term, were associated with an increased risk of atrial fibrillation.
•• Lawani SO, Demerath EW, Lopez FL, et al. Birth weight and the risk of atrial fibrillation in whites and African Americans: the Atherosclerosis Risk In Communities (ARIC) study. BMC Cardiovasc Disord. 2014;14(1):69 This high quality cohort study revealed that low birth weight was associated with a higher risk of AF. This association was independent of known predictors of AF and is consistent with that observed for other cardiovascular diseases.
Osler M, Lund R, Kriegbaum M, Andersen A-MN. The influence of birth weight and body mass in early adulthood on early coronary heart disease risk among Danish men born in 1953. Eur J Epidemiol. 2009;24(1):57–61.
•• Rajaleid K, Janszky I, Hallqvist J. Small birth size, adult overweight, and risk of acute myocardial infraction. Epidemiology. 2011;22(2):138–47 This study revealed that low birth weight is associated with a 2-fold increased risk of myocardial infraction.
Rich-Edwards JW, Stampfer MJ, Manson JE, Rosner B, Hankinson SE, Colditz GA, et al. Birth weight and risk of cardiovascular disease in a cohort of women followed up since 1976. Bmj. 1997;315(7105):396–400.
•• Smith C, Ryckman K, Barnabei VM, et al. The impact of birth weight on cardiovascular disease risk in the Women’s Health Initiative. Nutr Metab Cardiovasc Dis. 2016;26(3):239–45 This high quality investigation found that low birth weight was significantly associated with all CVD outcomes.
Stein C, Fall C, Kumaran K, Osmond C, Barker D, Cox V. Fetal growth and coronary heart disease in South India. Lancet. 1996;348(9037):1269–73.
•• Tian J, Qiu M, Li Y, et al. Contribution of birth weight and adult waist circumference to cardiovascular disease risk in a longitudinal study. Sci Rep. 2017;7(1):9768 In this cohort, birth size and adiposity in adulthood interact to predict events of cardiovascular disease.
Yang L, Kuper H, Weiderpass E. Anthropometric characteristics as predictors of coronary heart disease in women. J Intern Med. 2008;264(1):39–49.
Eriksson M, Wallander MA, Krakau I, Wedel H, Svärdsudd K. The impact of birth weight on coronary heart disease morbidity and mortality in a birth cohort followed up for 85 years: a population-based study of men born in 1913. J Intern Med. 2004;256(6):472–81.
•• Heshmati A, Koupil I. Placental weight and foetal growth rate as predictors of ischaemic heart disease in a Swedish cohort. J Dev Orig Health Dis. 2014;5(3):164–70 This cohort study reported that placental weight and birth weight were negatively associated with ischaemic heart disease.
Hyppönen E, Leon D, Kenward M, Lithell H. Prenatal growth and risk of occlusive and haemorrhagic stroke in Swedish men and women born 1915-29: historical cohort study. Bmj. 2001;323(7320):1033–4.
Lawlor D, Smith GD, Ebrahim S. Birth weight is inversely associated with coronary heart disease in post-menopausal women: findings from the British women’s heart and health study. J Epidemiol Community Health. 2004;58(2):120–5.
Lawlor DA, Ronalds G, Clark H, Smith GD, Leon DA. Birth weight is inversely associated with incident coronary heart disease and stroke among individuals born in the 1950s: findings from the Aberdeen Children of the 1950s prospective cohort study. Circulation. 2005;112(10):1414–8.
Rich-Edwards JW, Kleinman K, Michels KB, Stampfer MJ, Manson JE, Rexrode KM, et al. Longitudinal study of birth weight and adult body mass index in predicting risk of coronary heart disease and stroke in women. Bmj. 2005;330(7500):1115.
•• Zöller B, Sundquist J, Sundquist K, Crump C. Perinatal risk factors for premature ischaemic heart disease in a Swedish national cohort. BMJ Open. 2015;5(6):e007308 In this large national cohort, low fetal growth was strongly associated with ischaemic heart disease (IHD) and myocardial infarction in young adulthood, independently of gestational age at birth, sociodemographic factors, comorbidities and family history of IHD.
OSler. The influence of birth weight and body mass in early adulthood on early coronary heart disease risk among Danish men born in 1953.
Jensen RB, Chellakooty M, Vielwerth S, et al. Intrauterine growth retardation and consequences for endocrine and cardiovascular diseases in adult life: does insulin-like growth factor-I play a role? Horm Res. 2003;60(Suppl 3):136–48.
Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261(5):412–7.
• de Boo HA, Harding JE. The developmental origins of adult disease (Barker) hypothesis. Aust N Z J Obstet Gynaecol. 2006;46(1):4–14 This study reported the ‘developmental origins of adult disease’ hypothesis, as the main mechanism justifing the relation of birth wieght to cardiovascular disease, which states adverse influences during early stages of life could result in irreversible metabolic, physiologic and structural changes, some of which become disadvantageous increasing the risk of negative health outcomes late in life.
Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutr. 2001;4(2b):611–24.
Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol Metab. 2002;13(9):364–8.
Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23(6 Suppl):588s–95s.
Barker DJ. The developmental origins of insulin resistance. Horm Res. 2005;64(Suppl 3):2–7.
Barker DJ. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. 2006;49(2):270–83.
Khan OA, Chau R, Bertram C, Hanson MA, Ohri SK. Fetal origins of coronary heart disease-implications for cardiothoracic surgery? Eur J Cardiothorac Surg. 2005;27(6):1036–42.
Amann K, Plank C, Dotsch J. Low nephron number—a new cardiovascular risk factor in children? Pediatric nephrol. 2004;19(12):1319–23.
de Onis M, Dewey KG, Borghi E, et al. The World Health Organization’s global target for reducing childhood stunting by 2025: rationale and proposed actions. Matern Child Nutr. 2013;9(Suppl 2):6–26.
Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371(9608):243–60.
Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1–25.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Reza Mohseni, Shimels Hussien Mohammed, Maryam Safabakhsh, Fatemeh Mohseni, Zahra Sajedi Monfared, Javad Seyyedi, Zahra Noorani Mejareh, and Shahab Alizadeh each declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Evidence-Based Medicine, Clinical Trials and Their Interpretations
Rights and permissions
About this article
Cite this article
Mohseni, R., Mohammed, S.H., Safabakhsh, M. et al. Birth Weight and Risk of Cardiovascular Disease Incidence in Adulthood: a Dose-Response Meta-analysis. Curr Atheroscler Rep 22, 12 (2020). https://doi.org/10.1007/s11883-020-0829-z
Published:
DOI: https://doi.org/10.1007/s11883-020-0829-z