Abstract
Purpose of Review
Thoracic aortic calcium (TAC) has received some interest in recent studies as an important subclinical marker of atherosclerosis. Besides that, using computed tomography (CT) scans performed with cardiac or chest protocols, ECG-gated, or non-gated, TAC can be easily evaluated with no addition in radiation dose. This review discusses the particularities of the aortic wall calcium formation, as well as the differences between the aortic segments and summarizes the current status of TAC evaluation, mainly concerning the anatomical references used in the studies.
Recent Findings
The studies have evaluated TAC considering different anatomical references. It was identified two different study groups. In the first one, researchers have analyzed the aorta as the sum of calcium in the ascending aorta (ATAC), aortic arch (AAC), and descending thoracic aorta (DTAC). The second group has used cardiac CT scans to assess TAC; therefore, they did not include AAC; however, the aortic root calcium (ARC) was added in the analysis. So, caution is advisable when interpreting and comparing studies that used different TAC anatomical references.
Summary
The broad methodological variability, in addition to the variations in the population characteristics of the studies on TAC, may be in part contributing to the differences between results of different studies. Currently TAC does not have a role in clinical decisions, so it is necessary to create a standard protocol for the aortic calcium research as well as exists for the coronary artery calcium evaluation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis is a systemic, progressive, and chronic condition that can affect the entire vascular tree [1]. Calcium in the artery wall is considered a direct marker of atherosclerotic disease [1] and can be easily evaluated through computed tomography (CT) [2]. There are remarkable mass of robust data supporting the prime role of coronary artery calcium (CAC) in cardiovascular risk assessment of the intermediate-risk population, as well as specific subgroups, as patients with diabetes and family history of premature coronary heart disease (CHD) [3]. Several studies have shown that thoracic aortic calcium (TAC) is also a marker of subclinical atherosclerosis [4]. Distinct associations of TAC arouse interest in its particularities compared with CAC analysis. TAC also impacts the CV system, as aortic wall calcium worsen arterial stiffening [5], which is associated with several implications for end-organ damage [6]. CAC and TAC prevalence also seem to differ between men and women and race/skin color [7,8,9,10,11], though results are inconsistent. Moreover, unlike CAC, TAC has not been evaluated through standard CT protocol, mainly with regard to TAC anatomical extension [12,13,14] and the use of ECG synchrony during exam [15].
Because differences in TAC definition and acquisition might impair the evaluation of study’s results on the predictive value of TAC both at individual and population levels, our aim was to review recent studies about TAC, discussing the particularities of the aortic wall calcium formation and the differences between the aortic segments. And, finally, emphasize the anatomical references and the extension of the aorta included in the TAC studies.
Mechanisms Related to the Calcium Formation in the Thoracic Aorta Wall
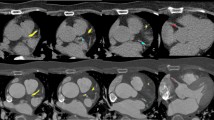
The distribution of calcium along the aorta is usually very heterogeneous. It is possible to identify coarse calcium in one segment, while there is no calcium in another segment from the same individual, as shown in Fig. 1. In the first case (images 1a and 1b), there was calcium in large amount in the arch and descending thoracic segments, while there was no calcium in the ascending aorta. In the second case (images 1c and 1d), the calcium concentration was much higher in the aortic arch compared with ascending and descending thoracic portions.
Heterogeneous distribution of calcium along the aorta. a, b CT reconstructions in the parasagittal plane. In this case, ascending aorta had no calcium (arrow in a), whereas in the arch and descending portions (arrow in b) there were circumferential plaques covering almost all aortic wall. c, d CT reconstructions in the axial plane. The most calcium concentration was in the aortic arch (arrow in c), while in ascending (superior arrow in d) and descending (inferior arrow in d) segments calcium were coarse, but sparse
The variation in the distribution of calcium across aorta segments may be in part associated with different embryonic origin of the vascular smooth muscle cells colonizing the aorta, which in the aortic arch derives from cardiac neural crest cells, whereas the calcium found in the descending aorta derives from the mesoderm [16]. The Leroux-Berger et al. study found correlation between the embryonic origin of vascular smooth muscle cells and the timing of the appearance of calcium [16]. Thus each aortic segment differs in their embryonic origin and is subject to different hemodynamic stress, which also appears to affect susceptibility to calcium [16], as the rate of calcium seems to differ among individuals [17]. Therefore, the calcium found in each aortic segment may be associated differently to cardiovascular risk factors [18] and probably has distinct predictive value for cardiovascular (CV) and non-CV morbidity and mortality, as suggested in some studies [12, 13, 19, 20•, 21•].
Another important particularity refers to the molecular mechanism of plaque calcium in the aortic wall, which is mainly composed by two mechanisms:
- 1)
Intimal calcium: atherosclerosis, inflammatory response of tunica intima;
- 2)
Medial calcium: occurs independently of intimal calcium in the tunica media.
The intimal layer consists of endothelial cells that eventually form atheromatous plaques which can rupture and cause thromboembolic events, whereas the medial layer consists of smooth muscle cells and elastic fibers that are associated with blood flow and arterial pressure regulation [2]. Medial calcium is thought to cause arterial stiffening, reduce compliance, and limit distensibility [2]. Actually the way to distinguish intimal and medial calcium is through ex vivo histological analysis [22]. Then CT scans cannot define if the calcium is in the intimal or medial layer of aortic wall [2].
However, the patterns of calcium distribution observed in CT scans may suggest the predominance of intimal or medial calcium. Intimal calcium usually has a patchy distribution within atherosclerotic lesions and is most commonly amorphous without distinct architecture [23•]. On the other hand, vascular medial calcium is generally concentric, appears more circumferential, and has a diffuse distribution [23•]. Figure 2 shows schematically the patterns of calcium distribution in the tunica intima and media.
Frequently, medial calcium is associated with uremia, radiotherapy, or vascular inflammation which induces a phenotypic change of vascular smooth muscle cells into osteoblasts, a process of metabolite-induced (toxic) vascular changes in the absence of lipid deposits [24•]. However aging, chronic kidney disease, diabetes mellitus, and mediastinal radiation are also associated with accelerated intimal atherosclerosis [24•]. Therefore, the overlap of these two processes in the aortic wall might explain some differences in findings on cardiovascular risk factors associated with calcium in different vascular beds, and mainly between distinct aorta territories.
Differences in the Anatomical References Used at TAC Evaluation
The differences in TAC evaluation can impact both the identification of calcium as well as its quantification either in volume or using the Agatston score. So, caution is advisable when interpreting and comparing studies that used different TAC extensions. Table 1 shows selected studies published in the last 5 years evaluating calcium in the thoracic aorta. They were grouped based on the aortic segments included in the analysis. The first group evaluated calcium in three segments: ascending thoracic aorta (ATA), aortic arch, and descending thoracic aorta (DTA). The aortic root calcium was not included in this group. The second group represents the largest one, and evaluated TAC in the ATA, DTA, and in the aortic root, but not in the aortic arch. The third, fourth, and fifth groups included each a single study that used distinct anatomical references, respectively: the extended versions of ATA plus DTA, aortic arch, and aortic root. These studies shown in Table 1 also differ with respect on how they measured or analyzed the presence of calcium: yes/no [11], and/or Agatston [4, 12, 13, 25•,26,27,28,29,30,31,32] and/or volume [5, 33,34,35, 36••], and/or density [19, 20•, 21•], and/or semi-qualitative evaluation [14].
Does the Aortic Arch Add Relevant Information to TAC?
As shown by Craiem et al., the inclusion of aortic arch in combination with ATA and DTA doubled the TAC prevalence, mainly in middle-aged women [25•]. Besides the impact on the overall and sex-specific prevalence of TAC, the inclusion of the aortic arch in TAC evaluation might also relate to TAC predictive value on morbidity and mortality. Bos et al., for instance, analyzed only the aortic arch and found that the volume of calcium in this segment was related to increased CV and non-CV mortality, after adjustment for many CV risk factors including CAC, intracranial, and extracranial internal carotids calcium [33••]. A recent study of Cho et al. used the same TAC extension described by Craiem et al. and followed 702 patients without obstructive coronary artery disease (CAD) during 64 months and also found TAC as an independent predictor for outcomes, especially stroke [34]. Thus, taking account these latter results, it appears that calcium in the aortic arch might contribute to TAC prediction, but we cannot rule out that it may be also a marker of calcium in other thoracic aorta segments.
What Do We Know About the Presence of Calcium in ATA and DTA?
The largest study group presented in Table 1 is the ones that assessed TAC using the same scan performed for CAC assessment. Thus, only the presence of calcium in the ascending thoracic aorta (ATAC) and descending thoracic aorta (DTAC) were evaluated. Eight studies are from Multi-ethnic Study of Atherosclerosis (MESA), and they measured calcium using Agatston, volume, and/or density [19, 20•, 21•, 27,28,29,30, 35]. The others are from Heinz Nixdorf Recall Study [12, 31], Framingham Heart Study [37], and EISNER [4], and all of them used Agatston to measure TAC. All these studies included the aortic root in the TAC and excluded the aortic arch. As demonstrated by Tesche et al., calcium in the aortic root is a stronger and independent predictor of CAC and of obstructive CAD [36••], suggesting that a similar process lead to calcium in these vascular beds. Thus, like the CAC [38], it is possible that calcium in aortic root reflects more localized than generalized atherosclerosis, differently from other thoracic aorta segments.
When taken together, results on ATAC plus DTAC associations and predictive value are controversial [27,28,29,30,31,32, 35]. However, when Thomas et al. and Kälsch et al. studied the ATAC and DTAC separately, they found that while greater ATAC volume predicted the incidence and progression of CHD and CVD [12, 21•], DTAC was associated with the occurrence of non-CV morbidity and mortality [20•]. These authors also showed that greater ATAC density, contrary to greater volume, was associated with lower risk of CAD [19, 21•], and explained such differences between aorta segments in terms of embryology, wall constitution and pathophysiologic mechanisms of calcium formation. It is thus, possible, that such differences in DTAC and ATAC also account for the controversial results reported by the other studies included in this group, as they are based on ATAC plus DTAC [27, 28, 30, 32]. In light of these recent findings, further research using the same anatomical references for each thoracic aorta segment should be stimulated.
Anatomical References for TAC Segmentation
Based on the current anatomical references used in some TAC studies, and understanding the possible value of studying each aortic segment separately, including the aortic arch, we created the video 1 to show each portion of the aorta slice-by-slice in axial CT images. Since aorta has an oblique path, some details are of importance. The following anatomical references were used for TAC segmentation:
- 1)
ATAC: from the sinutubular junction to the lower edge of pulmonary artery bifurcation (Some caution with the first slices, because of the initial curvature of ascending aorta above aortic root, where there are some slices that both appear in the same axial slice).
- 2)
Aortic arch calcium: from ascending to descending thoracic aorta at the same anatomical reference, which is the level of the lower edge of pulmonary artery bifurcation.
- 3)
DTAC: from the lower edge of pulmonary artery bifurcation to the apex of the heart.
What Is the Best Way to Measure TAC?
In addition to anatomical definitions, other methodological TAC parameters deserve to be considered. Agatston method has been widely used; however, the quantification of TAC can vary considerably between different CT systems once the acquisition of CAC scans, usually used to measure TAC, was not created for this application [39]. Mori et al. in 2015 described and validated a new volume-rendering approach to quantify TAC that demonstrated an excellent agreement of the pixel-based TAC score with volumetric TAC score and observed that volume-based score was less influenced by slice thickness as compared with pixel-based score [40••]. Agatston score depends nonlinearly on the measured Hounsfield Unit density of each pixel in the calcium, which changes with different x-ray energies, while the calcium volume is only slightly affected by scanning at different energies [41]. Since TAC is in the early development phase, perhaps now is the time to think about more accurate measures of quantifying the TAC [39].
Is TAC Radiation Exposure Justified?
The last, but a very important consideration to be made, refers to the radiation dose involved in TAC extended exams (all segments). Although the increase in the radiation dose of extended CAC, necessary to include the aortic arch, is lower than that delivered for a bilateral mammogram [25•], its value remains uncertain. So far, there appear to be no doubt regarding the value of evaluating DTAC and ATAC on CAC scans, as CAC clinical indication is already established. Lung cancer screening trials [42] seem to offer some opportunity to evaluate the predictive value of all segments, especially the aortic arch.
Limitations
The current review of the literature is limited mostly due to the high variability across the studies included in the analysis. As previously detailed, there is no current standards to define which aortic segments to include or the most appropriate tool to quantify the presence and extent of TAC. Moreover, the outcomes included in each analysis are not similar. Collectively, those issues limit the comparison between studies and the potential to fully interpret those results in other populations or scenarios.
Future Directions
Future studies should focus on the standardization of image acquisition, areas of the thoracic aorta to be included and most appropriate tools to quantify TAC. Moreover, detailed investigation on the different role of each thoracic aorta segment for the prediction of different outcomes, including separate analysis for coronary artery disease events, cerebrovascular events, and incidence of acute aortic syndromes.
Additionally, more studies on the implications of such findings for clinical management are needed. Currently, TAC is understood to be atherosclerosis. However, the clinical management of asymptomatic individuals with atherosclerosis is currently based on the individual’s clinical risk profile with the potential use of other diagnostic tools, such as CAC scores in selected individuals. Yet, not clear role for TAC in selecting the most appropriate management strategy for those individuals exist.
Conclusions
TAC has been considered as subclinical marker of atherosclerosis; however, the lack of standard protocol regarding the anatomical segments included and measurement analytical unit have contributed to controversial results and studies comparability. The accumulated evidences indicate that each aorta segment should be evaluated separately, as they differ in terms of structural characteristics, embryologic origin, and pathophysiologic mechanisms of calcium formation along the aorta and predictive value.
Abbreviations
- AAC:
-
abdominal aortic calcium
- ATA:
-
ascending thoracic aorta
- ATAC:
-
ascending thoracic aorta calcium
- CAC:
-
coronary artery calcium
- CAD:
-
coronary artery disease
- CHD:
-
coronary heart disease
- CV:
-
cardiovascular
- CVD:
-
cardiovascular disease
- CT:
-
computed tomography
- DTA:
-
descending thoracic aorta
- DTAC:
-
descending thoracic aorta calcium
- MESA:
-
Multi-ethnic Study of Atherosclerosis
- TAC:
-
thoracic aortic calcium
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Rodríguez-Palomares JF, Evangelista MA. Aortic calcium score and vascular atherosclerosis in asymptomatic individuals: beyond the coronary arteries. Rev Española Cardiol (English Ed). 2016;69:813–6 Elsevier BV Available from: https://linkinghub.elsevier.com/retrieve/pii/S1885585716301347.
Wang Y, Osborne MT, Tung B, Li M, Li Y. Imaging cardiovascular calcification. J Am Heart Assoc. 2018;7:1–15.
Hecht HS. Coronary artery calcium scanning: past, present, and future. JACC Cardiovasc Imaging. 2015;8:579–96. https://doi.org/10.1016/j.jcmg.2015.02.006 Elsevier.
Brodov Y, Gransar H, Rozanski A, Hayes SW, Friedman JD, Thomson LEJ, et al. Extensive thoracic aortic calcification is an independent predictor of development of coronary artery calcium among individuals with coronary artery calcium score of zero. Atherosclerosis. 2015;238:4–8 Elsevier Ireland Ltd.
Cho I-J, Chang H-J, Park H-B, Heo R, Shin S, Shim CY, et al. Aortic calcification is associated with arterial stiffening, left ventricular hypertrophy, and diastolic dysfunction in elderly male patients with hypertension. J Hypertens. 2015;33:1633–41 Available from: https://insights.ovid.com/crossref?an = 00004872-201508000-00021.
Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol. 2008;105:1652–60.
Nasir K, Roguin A, Sarwar A, Rumberger J a, Blumenthal RS. Gender differences in coronary arteries and thoracic aorta calcification. Arterioscler Thromb Vasc Biol. 2007;27:1220–2.
Takasu J, Katz R, Nasir K, Carr JJ, Wong N, Detrano R, et al. Relationships of thoracic aortic wall calcification to cardiovascular risk factors: The Multi–Ethnic Study of Atherosclerosis (MESA). Am Heart J. 2008;155:765–71 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002870307009477.
Allison MA, Budoff MJ, Nasir K, Wong ND, Detrano R, Kronmal R, et al. Ethnic-Specific Risks for Atherosclerotic Calcification of the Thoracic and Abdominal Aorta (from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol. 2009;104:812–7. https://doi.org/10.1016/j.amjcard.2009.05.004 Elsevier Inc.
Kälsch H, Lehmann N, Möhlenkamp S, Hammer C, Mahabadi AA, Moebus S, et al. Prevalence of thoracic aortic calcification and its relationship to cardiovascular risk factors and coronary calcification in an unselected population-based cohort: the Heinz Nixdorf Recall Study. Int J Card Imaging. 2013;29:207–16.
Pedrosa JF, Ribeiro ALP, Santana PC, Araújo LF, Barreto SM. Relation of thoracic aortic and coronary artery calcium to cardiovascular risk factors (From The Brazilian Longitudinal Study of Adult Health [ELSA-Brasil]). Am J Cardiol. 2019; Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002914919309956.
Kälsch H, Lehmann N, Moebus S, Hoffmann B, Stang A, Jöckel K, et al. Aortic calcification onset and progression: association with the development of coronary atherosclerosis. J Am Heart Assoc. 2017;6:1–12 John Wiley and Sons Inc. Available from: https://www.ahajournals.org/doi/10.1161/JAHA.116.005093.
Dudink EAMP, Peeters FECM, Altintas S, Heckman LIB, Haest RJ, Kragten H, et al. Agatston score of the descending aorta is independently associated with coronary events in a low-risk population. Open Hear. 2018;5:1–8.
Rodríguez-Granillo GA, Reynoso E, Capuñay C, Antoniades C, Shaw LJ, Carrascosa P. Prognostic value of vascular calcifications and regional fat depots derived from conventional chest computed tomography. J Thorac Imaging. 2019;34:33–40.
Budoff MJ, Nasir K, Kinney GL, Hokanson JE, Barr RG, Steiner R, et al. Coronary artery and thoracic calcium on noncontrast thoracic CT scans: comparison of ungated and gated examinations in patients from the COPD Gene cohort. J Cardiovasc Comput Tomogr. 2011;5:113–8.
Leroux-Berger M, Queguiner I, MacIel TT, Ho A, Relaix F, Kempf H. Pathologic calcification of adult vascular smooth muscle cells differs on their crest or mesodermal embryonic origin. J Bone Miner Res. 2011;26:1543–53.
Wong ND, Gransar H, Shaw L, Polk D, Moon JH, Miranda-Peats R, et al. Thoracic aortic calcium versus coronary artery calcium for the prediction of coronary heart disease and cardiovascular disease events. JACC Cardiovasc Imaging. 2009;2:319–26. https://doi.org/10.1016/j.jcmg.2008.12.010 Elsevier Inc.
Churchill TW, Rasania SP, Rafeek H, Mulvey CK, Terembula K, Ferrari V, et al. Ascending and descending thoracic aorta calcification in type 2 diabetes mellitus. J Cardiovasc Comput Tomogr. 2015;9:373–81 Elsevier Inc.
Thomas IC, McClelland RL, Michos ED, Allison MA, Forbang NI, Longstreth WT, et al. Density of calcium in the ascending thoracic aorta and risk of incident cardiovascular disease events. Atherosclerosis. 2017;265:190–6. https://doi.org/10.1016/j.atherosclerosis.2017.09.009 Elsevier Ireland Ltd.
• Thomas IC, Thompson CA, Yang M, Allison MA, Forbang NI, Michos ED, et al. Thoracic aorta calcification and noncardiovascular disease-related mortality the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2018;38:1926–32 Recent and the only analysis of DTAC and noncardiovascular mortality.
• Thomas IC, McClelland RL, Allison MA, Ix JH, Michos ED, Forbang NI, et al. Progression of calcium density in the ascending thoracic aorta is inversely associated with incident cardiovascular disease events. Eur Heart J Cardiovasc Imaging. 2018;19:1343–50 Oxford University Press Recent and the only analysis of ATAC and cardiovascular events.
Lanzer P, Boehm M, Sorribas V, Thiriet M, Janzen J, Zeller T, et al. Medial vascular calcification revisited: review and perspectives. Eur Heart J. 2014;35:1515–25.
• Desai MY, Cremer PC, Schoenhagen P. Thoracic Aortic Calcification: Diagnostic, Prognostic, and Management Considerations. JACC Cardiovasc Imaging. 11:2018, 1012–26 Elsevier Inc. Recent review concerning thoracic aortic calcium.
• Abramowitz Y, Jilaihawi H, Chakravarty T, Mack MJ, Makkar RR. Porcelain aorta: a comprehensive review. Circulation. 2015;131:827–36 Recent and comprehensive review of porcelain aorta.
• Craiem D, Chironi G, Casciaro ME, Graf S, Simon A. Calcifications of the thoracic aorta on extended non-contrast-enhanced cardiac CT. Hendrikse J, editor. PLoS One. Public Library of Science; 2014;9:e109584. Available from: https://dx.plos.org/10.1371/journal.pone.0109584 The first study to propose an extended CAC scan to include aortic arch and measure the difference between TAC prevalences considering and not the aortic arch.
Craiem D, Chironi DG, Casciaro ME, Sirieix ME, Mousseaux E, Simon A. Association of thoracic aorta calcium and non-cardiac vascular events in cardiac disease-free individuals. Atherosclerosis. 2016;245:22–7 Elsevier Ireland Ltd.
Ong K-L, McClelland RL, Rye K-A, Cheung BMY, Post WS, Vaidya D, et al. The relationship between insulin resistance and vascular calcification in coronary arteries, and the thoracic and abdominal aorta: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2014;236:257–62. https://doi.org/10.1016/j.atherosclerosis.2014.07.015 Elsevier Ltd.
Yeboah J, Carr JJ, Terry JG, Ding J, Zeb I, Liu S, et al. Computed tomography-derived cardiovascular risk markers, incident cardiovascular events, and all-cause mortality in nondiabetics: The Multi-Ethnic Study of Atherosclerosis. Eur J Prev Cardiol. 2014;21:1233–41.
Youssef G, Guo M, McClelland RL, Shavelle DM, Nasir K, Rivera J, et al. Risk factors for the development and progression of thoracic aorta calcification: The Multi-Ethnic Study of Atherosclerosis. Acad Radiol Elsevier USA. 2015;22:1536–45.
Kim J, Budoff MJ, Nasir K, Wong ND, Yeboah J, Al-Mallah MH, et al. Thoracic aortic calcium, cardiovascular disease events, and all-cause mortality in asymptomatic individuals with zero coronary calcium: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2017;257:1–8 Elsevier Ireland Ltd Available from: https://linkinghub.elsevier.com/retrieve/pii/S0021915016315337.
Mahabadi AA, Lehmann N, Möhlenkamp S, Pundt N, Dykun I, Roggenbuck U, et al. Noncoronary measures enhance the predictive value of cardiac CT above traditional risk factors and CAC score in the general population. JACC Cardiovasc Imaging. 2016;9:1177–85 Elsevier Inc. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1936878X16304089.
Hoffmann U, Massaro JM, D’Agostino RB, Kathiresan S, Fox CS, O’Donnell CJ. Cardiovascular event prediction and risk reclassification by coronary, aortic, and valvular calcification in the Framingham Heart Study. J Am Heart Assoc. 2016;5:1–11. https://doi.org/10.1161/JAHA.115.003144.
•• Bos D, Leening MJG, Kavousi M, Hofman A, Franco OH, Van Der Lugt A, et al. Comparison of Atherosclerotic Calcification in Major Vessel Beds on the Risk of All-Cause and Cause-Specific Mortality: The Rotterdam Study. Circ Cardiovasc Imaging. 2015;8:1–9 The only analysis of the presence of calcium in the aortic arch and its predictive value for mortality.
Cho IJ, Chang HJ, Cho I, Heo R, Lee SE, Shim CY, et al. Association of thoracic aorta calcium score with exercise blood pressure response and clinical outcomes in elderly individuals: differential impact of aorta calcification compared with coronary artery calcification. J Am Heart Assoc John Wiley and Sons Inc 2016;5
Katz R, Budoff MJ, O’Brien KD, Wong ND, Nasir K. The metabolic syndrome and diabetes mellitus as predictors of thoracic aortic calcification as detected by non-contrast computed tomography in the Multi-Ethnic Study of Atherosclerosis. Diabet Med. 2016;33:912–9.
•• Tesche C, De Cecco CN, Stubenrauch A, Jacobs BE, Varga-Szemes A, Litwin SE, et al. Correlation and predictive value of aortic root calcification markers with coronary artery calcification and obstructive coronary artery disease. Radiol Med. 2017;122:113–20 Springer Milan The only analysis of the presence of calcium in the aortic root and its predictive value for CAC and obstructive CAD.
Hoffmann U, Massaro JM, D’Agostino RB, Kathiresan S, Fox CS, O’Donnell CJ. Cardiovascular Event Prediction and Risk Reclassification by Coronary, Aortic, and Valvular Calcification in the Framingham Heart Study. J Am Heart Assoc [Internet]. 2016;5:1–11. Available from: https://www.ahajournals.org/doi/10.1161/JAHA.115.003144.
Elias-Smale SE, Odink AE, Wieberdink RG, Hofman A, Hunink MGM, Krestin GP, et al. Carotid, aortic arch and coronary calcification are related to history of stroke: the Rotterdam study. Atherosclerosis. 2010;212:656–60 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0021915010004934.
Saboo SS, Abbara S, Rybicki FJ, Chatzizisis YS. Quantification of aortic calcification – How and why should we do it? Atherosclerosis. 2015;240:469–71 Elsevier Ireland Ltd.
•• Mori S, Takaya T, Kinugasa M, Ito T, Takamine S, Fujiwara S, et al. Three-dimensional quantification and visualization of aortic calcification by multidetector-row computed tomography: a simple approach using a volume-rendering method. Atherosclerosis. Elsevier; 2015 [cited 2019 Jul 2];239:622–8. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0021915014016633 Focus on advanced techniques to measure TAC by CT.
Liyanage L, Lee NJ, Cook T, Herrmann HC, Jagasia D, Litt H, et al. The impact of gender on cardiovascular system calcification in very elderly patients with severe aortic stenosis. Int J Card Imaging. 2016;32:173–9 Springer Netherlands.
Heuvelmans MA, Vonder M, Rook M, Groen HJM, De Bock GH, Xie X, et al. Screening for early lung cancer, chronic obstructive pulmonary disease, and cardiovascular disease (the big-3) using low-dose chest computed tomography: Current evidence and technical considerations. J Thorac Imaging. 2019:160–9 Lippincott Williams and Wilkins.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Video 1
Anatomical references for TAC segmentation presented slice-by-slice in axial computed tomography images (WMV 23666 kb)
Rights and permissions
About this article
Cite this article
Pedrosa, J.F., Barreto, S.M., Bittencourt, M.S. et al. Anatomical References to Evaluate Thoracic Aorta Calcium by Computed Tomography. Curr Atheroscler Rep 21, 51 (2019). https://doi.org/10.1007/s11883-019-0811-9
Published:
DOI: https://doi.org/10.1007/s11883-019-0811-9