Abstract
Purpose of Review
The aim of this study was to determine the effects of aerobic exercise on peak oxygen uptake (peak VO2), minute ventilation/carbon dioxide production (VE/VCO2 slope), and health-related quality of life (HRQoL) among patients with heart failure (HF) and preserved ejection fraction (HFpEF).
Recent Findings
We conducted a Cochrane Library, MEDLINE/PubMed, Physiotherapy Evidence Database, and SciELO search (from 1985 to May 2019) for randomized controlled trials that evaluated the effects of aerobic exercise in HFpEF patients. We calculated the mean differences (MD) and 95% confidence interval (CI). Ten intervention studies were included providing a total of 399 patients. Compared with control, aerobic exercise resulted in improvement in peak VO2 MD 1.9 mL kg−1 min−1 (95% CI 1.3 to 2.5; N = 314) and HRQoL measured by Minnesota Living with Heart Failure MD 5.4 (95% CI − 10.5 to − 0.2; N = 256). No significant difference in VE/VCO2 slope was found between participants in the aerobic exercise group and the control group. The quality of evidence for peak VO2 and HRQoL was assessed as being moderate.
Summary
Aerobic exercise moderately improves peak VO2 and HRQoL and should be considered a strategy of rehabilitation of HFpEF individuals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
About 50% or more of heart failure (HF) patients have preserved left ventricular ejection fraction (HFpEF). An important feature of HFpEF is reduced exercise tolerance, measured objectively as peak oxygen uptake (peak VO2) and decreased health-related quality of life (HRQoL) [1].
A hallmark of HFpEF is dyspnea upon exertion and a reduction in aerobic capacity secondary to impaired oxygen delivery and use by exercising skeletal muscle. Exercise training is an effective intervention to improve peak VO2 and quality of life in clinically stable HF patients [2, 3]. Exercise intolerance in HFpEF patients is also associated with poor prognosis and deserves special attention in clinical trials [4].
The strong association between exercise intolerance, physical inactivity, and risk of HFpEF argues for interventions aimed at improving aerobic capacity, in management of HFpEF [5]. However, effective therapeutic approaches for HFpEF are limited [6]. Pharmacological trials in HFpEF to improve outcomes and symptoms have been particularly disappointing [7]On the other side, exercise training is a well-established nonpharmacologic treatment for patients with HF [8, 9]. However, despite the exercise guidelines for rehabilitation of HF are well established, no consensus exercise training guidelines exist for management and safety of the exercise for HFpEF patients [8].
Dieberg et al. (2015) [10] reported results of a meta-analysis indicating that exercise is effective to improve cardiorespiratory fitness, diastolic function, and HRQoL of HFpEF patients. Thus, at present, one of the most promising evidenced-based strategies to improve exercise intolerance in HFpEF patients appears to be exercise training, but the optimal approach is still unknown. In addition, despite the positive results, a methodological limitation was the combination of results from studies that investigated several different exercise interventions (aerobic exercise, combined aerobic and resistance training, inspiratory muscle training, and functional electrical stimulation) to estimate associations with outcomes. In addition, we included ventilation/carbon dioxide production (VE/VCO2 slope) as outcome, because it has provided additional value for predicting outcomes in HFpEF [11] patients, beyond clinical characteristics and ejection fraction. Thus, this systematic review and meta-analysis aimed to analyze the published RCTs that investigated the effects of aerobic exercise on peak VO2, VE/VCO2 slope, and HRQoL among patients with HFpEF.
Methods
This systematic review was planned and conducted in accordance with Cochrane Collaboration recommendations and reported in accordance with PRISMA guidelines [12].
Eligibility Criteria
We included all RCTs that investigated the effects of aerobic exercise compared with control (no exercise) in HFpEF patients (defined as LVEF ≥ 50%) [4]. To be eligible, each RCT should have (a) included adult patients (aged ≥ 18 years) with HFpEF (≥ 50%) [4]; (b) a RCT design; and (c) aerobic exercise controlled by other exercise intervention or control (no exercise). We excluded studies that enrolled patients with other cardiac or respiratory diseases. The main outcomes of interest were peak VO2 measured during a cardiopulmonary exercise test (mL/kg/min), VE/VCO2 slope, and HRQoL measured by any standardized and validated scales or questionnaires.
Search Methods for Identification of Studies
Eligible studies were identified by searching in MEDLINE/PubMed, the Cochrane Library (CENTRAL Cochrane), Physiotherapy Evidence Database (PEDro), and Scientific Electronic Library Online (SciELO) up to May 2019 without language or publication status restrictions. We also performed hand-searches of relevant studies in Google Scholar. We used a standard protocol for this search and, whenever possible, a controlled vocabulary (Mesh term for MEDLINE and Cochrane). In search strategy, we used three groups of keywords and their synonymous: study design, participants, and interventions.
The optimally sensitive search strategy developed by Higgins and Green [13] was used for the identification of RCTs in MEDLINE/PubMed. The full search strategy for MEDLINE/PubMed can be found in Table E1 (Supplementary Material 1). To search the RCTs in other databases, we performed a search using similar descriptors. We checked the references of the studies included to identify other RCTs. For ongoing studies, or when the confirmation of any data or additional information was needed, authors were contacted by e-mail.
Data Collection and Analysis
Each title and abstract identified in the research was independently evaluated by 2 reviewers. If at least one of the reviewers considered a reference eligible, the study was obtained for analysis. Then, the full texts of the selected studies were independently assessed to verify if they met the eligibility criteria. Reference list was checked to identify other potentially eligible studies. Two reviewers independently extracted data from the published RCTs using standard forms adapted from Cochrane Collaboration [13]. Aspects of the study population as sex and mean age, disease characteristics, main outcomes measures, exercise intervention characteristics, follow-up period, and key findings were extracted.
Methodological Quality of RCTs
The methodological quality of RCTs included in this systematic review and meta-analysis was scored using the PEDro scale, which is based on important criteria, such as random allocation, concealed allocation, blinded, and intention-to-treat analysis [14]. These characteristics make the PEDro scale a useful tool for assessing the quality of rehabilitation RCTs [14,15,16].
Certainty in the Evidence and Summary of Findings Table
The quality of evidence was assessed using the GRADE (Grading of Recommendations Assessment, Development and Evaluation). We included the outcomes peak VO2, VE/VCO2 slope, and HRQoL in analysis. We used GRADEpro GDT 2015 to import data from Review Manager to create a “Summary of findings table”. The analysis included five items: risk of bias, imprecision, inconsistency, indirectness, and publication bias [13]. The quality of evidence was reported as high quality, moderate quality, low quality, or very low quality.
Statistical Assessment
Pooled-effect estimates were obtained by comparing the least square mean change from baseline to post-intervention for each group and were expressed as the MD between groups. For continuous outcomes, results were showed as the MD in the change in the variable between randomized groups. Conversion of median, range, and/or interquartile range data to means and standard deviation was based on recently established methods [17]. When the standard deviation of change was not available, but CI was available, we converted it to standard deviation as guided by Higgins and Green [13]. If the study was a multiple-arm RCT, all relevant groups (aerobic exercise versus control) had data extracted. In case of follow-up RCT with multiple endpoints, only data closest to the end of the intervention program were included. In cross-over RCT, data were only extracted at the first cross-over period.
One comparison was made: aerobic versus controls (no exercise) [MD] and 95% confidence interval (CI) was calculated. An α value < 0.05 was considered statistically significant. Heterogeneity of the treatment effect in meta-analysis was examined with Cochran’s Q and I2 statistic. Values of I2 greater than 40% were considered indicative of high heterogeneity [18] and in this case, random-effects model was chosen. Meta-analysis was conducted using Review Manager Software (Version 5.3) [19].
Results
Description of Selected Studies
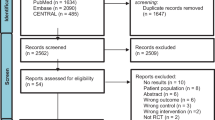
The initial search identified 3912 studies, from which 51 were considered potentially relevant. Ten studies [20•, 21•, 22•, 23•, 24•, 25•, 26•, 27•, 28•, 29•] were included. PRISMA flow diagram of studies in this review is shown in Supplementary Material 2 (Fig. 1). Table E2 (Supplementary Material 3) presents results of PEDro scores.
Study Characteristics
The number of participants in RCTs included ranged from 15 [22•] to 90 [21]. Mean age of participants ranged from 47.4 to 75.5 years old, and length of the intervention period from 4 to 26 weeks. General characteristics of the included studies are summarized in Table 1. The characteristics of aerobic exercise intervention in included RCTs are provided in Table E3 (Supplementary Material 4).
Effects of The Aerobic Exercise Versus Control
Peak VO2
Six studies [20•, 21•, 23•, 26, 27•, 28•] assessed peak VO2 as outcome. The total number of patients in the aerobic exercise group was 155, whereas 159 patients were included in the control group. The mean peak VO2 in the analyzed studies was 17.1 mL kg−1 min−1 at baseline, and it increased to 19.7 mL kg−1 min−1 at the end of the aerobic exercise intervention. The meta-analyses showed (Fig. 2) a significant improvement in peak VO2 of 1.9 mL kg−1 min−1 (95% CI 1.3, 2.5; N = 314) for patients in the aerobic exercise compared with the control group.
VE/VCO2 Slope
Three studies [23•, 27•, 28•] assessed VE/VCO2 slope as outcome. The total number of patients in the combined aerobic and resistance training group was 66, whereas 71 patients were included in the control group. The meta-analyses showed (Fig. 3) a no significant difference (p = 0.55) in VE/VCO2 slope of 1.1 (95% CI − 4.5 to 2.4; N = 137) for patients in the aerobic exercise compared with the control group.
Health-Related Quality of Life
Five studies [21, 23•, 27•, 28•, 29•] assessed HRQoL using a disease-specific instrument as the Minnesota Living with Heart Failure Questionnaire (MLHF-Q). The total number of patients in the combined aerobic and resistance training group was 263, whereas 261 patients were included in the control group. The meta-analyses showed (Fig. 4) a significant difference in HRQoL of − 5.4 (95% CI − 10.5 to − 0.2; N = 256) for patients in the aerobic exercise compared with the control group.
GRADE Assessments
The quality of evidence is presented in Summary of Findings Table (Supplementary Material 5 Table E4). The quality of evidence for the outcomes peak VO2 and HRQoL was assessed as being moderate. The quality of evidence for the outcome VE/VCO2 slope was assessed as being low.
Discussion
Our meta-analysis showed that aerobic exercise resulted in improvement in peak VO2 and HRQoL. No significant difference between groups in VE/VCO2 slope was detected. We assessed the quality of evidence according to the GRADE system, which ranked four of them as moderate (peak VO2 and HRQoL) to low quality (VE/VCO2 slope).
Aerobic exercise is well established as an important treatment in people with HF with reduced ejection fraction, which is endorsed by different guidelines around the world [4, 9]. Thus, this systematic review with meta-analysis is important because it assesses aerobic exercises as a potential therapy strategy in the cardiac rehabilitation of patients with HFpEF. Moreover, we included peak VO2, VE/VCO2 slope, and HRQoL, important outcomes associated with prognosis and patients’ self-reported quality of life, living with heart failure in HF [11, 30, 31].
The magnitude of improvement with aerobic exercise (mean change + 2.2 mL kg−1 min−1) is superior to the difference observed following no exercise (mean change + 0.8 mL kg−1 min−1). This magnitude of improvement was higher than 15%. This is important because is known that an increase in peak VO2 > 10% after an exercise program is satisfactory and represents a good prognosis in patients with HF [32].
HRQoL is also a very important outcome in RCTs involving exercise for HF patients, as it is related to aerobic capacity and improves meaningfully when patients with HF are engaged in an exercise training [33]. Our meta-analysis demonstrated a magnitude of improvement with aerobic exercise of 5.4 points in MLHF-Q. The minimal clinically important difference for the MLFH-Q is 5 points [34].
Few studies used VE/VCO2 slope as an outcome [23•, 27•, 28•], although VE/VCO2 slope has provided incremental value for predicting outcomes in HFpEF [11].Patients with high VE/VCO2 slope (typically > 34) are at a greater risk of a cardiovascular event [34, 35]. However, the literature is not conclusive about the prognostic value of VE/VCO2 slope in HFpEF [35, 36]. To address this problem, future studies with HFpEF should analyze and report VE/VCO2 slope [37].
Despite the benefits and recommendations in favor of exercise training, there is a lack of utilization of exercise as treatment in HF patients. Studies indicate that between 40 and 91% of patients with heart failure do not engage in any regular exercise [38].
The quality of evidence for the analyzed outcomes was determined to be moderate to low, due to the inclusion studies without sample size calculation allocation concealment, or meta-analysis with high heterogeneity. In general, the studies presented moderate to low methodological quality. Subjects and experimenters were not blinded in most of the included studies. In addition, most included studies failed to report the method for concealed allocation and intention-to-treat analysis.
Given the small number of included studies in our meta-analysis, some caution is warranted when interpreting our results. This ultimately reflects the limited body of evidence regarding aerobic exercise and relevant outcomes for HFpEF patients. It is important to note that for patients with HFpEF, the body of evidence is limited because the studies are in the initial phase with specific and reduced samples.
Another notable limitation is the small sample size in most the RCTs. However, the presence of two independent authors (as reviewers), a wide search in different databases without time restrictions, and the use of specific methods for the analyses were carried out to minimize the biases involved in this study.
New large-scale RCTs are needed to confirm the findings of this systematic review. Further investigations into the prescription of the aerobic exercise variables (e.g. volume, intensity, frequency, and duration of the intervention) are needed. Further investigations are required to explore how the positive effects of aerobic exercise can be sustained over time. We also need to determine the optimal prescription and to identify outcomes, to enhance our understanding of the effects of aerobic exercise in HFpEF patients.
Conclusion
Taking into account the available RCTs, the present meta-analysis showed that aerobic exercise should be considered a promising rehabilitation strategy of improving peak VO2 and HRQoL. This strategy should be further investigated as a component of rehabilitation for HFpEF patients.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Haykowsky M, Brubaker P, Kitzman D. Role of physical training in heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2012;9(2):101–6.
Tucker WJ, Lijauco CC, Hearon CM, et al. Mechanisms of the improvement in peak VO2 with exercise training in heart failure with reduced or preserved ejection fraction. Heart, Lung and Circulation. 2018;27:9–21.
Montero D, Diaz-Cañestro C. Determinants of exercise intolerance in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Int J Cardiol. 2018;254:224–9.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975.
Pandey A, Patel KV, Vaduganathan M, Sarma S, Haykowsky MJ, Berry JD, et al. Physical activity, fitness, and obesity in heart failure with preserved ejection fraction. JACC Heart Fail. 2018;6(12):975–82.
Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med. 2016;375(19):1868–77.
Upadhya B, Haykowsky MJ, Eggebeen J, Kitzman DW. Exercise intolerance in heart failure with preserved ejection fraction: more than a heart problem. JGeriatrCardiol. 2015;12(3):294–304. https://doi.org/10.11909/j.issn.1671-5411.2015.03.013.
Dickstein K, Cohen-Solal A, Filippatos G, JJ MM, Ponikowski P, Poole-Wilson PA, et al. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail. 2008;10:933–89.
Gomes Neto M, Durães AR, Conceição LSR, Saquetto MB, Ellingsen Ø, Carvalho VO. High intensity interval training versus moderate intensity continuous training on exercise capacity and quality of life in patients with heart failure with reduced ejection fraction: a systematic review and meta-analysis. Int J Cardiol. 2018;261:134–41.
Dieberg G, Ismail H, Giallauria F, Smart NA. Clinical outcomes and cardiovascular responses to exercise training in heart failure patients with preserved ejection fraction: a systematic review and meta-analysis. J Appl Physiol (1985). 2015;119(6):726–33.
Nadruz W Jr, West E, Sengeløv M, Santos M, Groarke JD, Forman DE, et al. Prognostic value of cardiopulmonary exercise testing in heart failure with reduced, midrange, and preserved ejection fraction. J Am Heart Assoc. 2017;6(11):e006000.
Moher D, Liberati A, Tetzlaff J, Altman DG, for the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Higgins JPT, Green S. The Cochrane library. Issue 4. Chichester: John Wiley & Sons; 2006. Cochrane handbook for Systematic Reviews of Interventions 4.2.6 [update September 2006
Olivo SA, Macedo LG, Gadotti IN, Fuentes J, Stanton T, Magee DJ. Scales to assess the quality of randomized controlled trials: a systematic review. PhysTher. 2008;88(2):156–75.
Verhagen AP, de Vet HCW, de Bie RA, Kessels AGH, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51(12):1235–41.
Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating of quality randomized controlled trials. Phys Ther. 2003;83(8):713–21.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. https://doi.org/10.1186/1471-2288-14-135.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Collaboration TC. Available at: www.cochrane.org. [Accessed 3 Feb 2008].
• Maldonado-Martín S, Brubaker PH, Eggebeen J, Stewart KP, Kitzman DW. Association between 6-minute walk test distance and objective variables of functional capacity after exercise training in elderly heart failure patients with preserved ejection fraction: a randomized exercise trial. Arch Phys Med Rehabil. 2017;98(3):600–3 This study demonstrated Peak VO2 improved in the AE.
Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2016;315(1):36–46.
• Angadi SS, Mookadam F, Lee CD, Tucker WJ, Haykowsky MJ, Gaesser GA. High-intensity interval training vs. moderate-intensity continuous exercise training in heart failure with preserved ejection fraction: a pilot study. J Appl Physiol (1985). 2015;119(6):753–8 This study demonstrated the HIIT improved VO 2 peak and left ventricular diastolic dysfunction grade.
• Kitzman DW, Brubaker PH, Herrington DM, Morgan TM, Stewart KP, Hundley WG, et al. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. J Am Coll Cardiol. 2013;62(7):584–92. https://doi.org/10.1016/j.jacc.2013.04.033 Exercise training increased peak VO 2 and quality of life.
Yeh GY, Wood MJ, Wayne PM, Quilty MT, Stevenson LW, Davis RB, et al. Tai chi in patients with heart failure with preserved ejection fraction. Congest Heart Fail. 2013;19(2):77–84.
• Alves AJ, Ribeiro F, Goldhammer E, Rivlin Y, Rosenschein U, Viana JL, et al. Exercise training improves diastolic function in heart failure patients. Med Sci Sports Exerc. 2012;44(5):776–85 Exercise training increased the mean ratio of early to late mitral inflow velocities (E/A ratio) and decreased deceleration time (DT) of early filling in patients with mild and preserved LVEF.
Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60(2):120–8.
• Smart NA, Haluska B, Jeffriess L, Leung D. Exercise training in heart failure with preserved systolic function: a randomized controlled trial of the effects on cardiac function and functional capacity. Congest Heart Fail. 2012;18(6):295–301 After exercise training, the increment in peak VO 2.
Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail. 2010;3(6):659–67.
• Gary RA, Sueta CA, Dougherty M, Rosenberg B, Cheek D, Preisser J, etal. Home-based exercise improves functional performance and quality of life in women with diastolic heart failure. Heart Lung 2004;33(4):210–218. Exercise improved in the 6-min walk test.
Agostoni P, Corrà U, Cattadori G, Veglia F, La Gioia R, Scardovi AB, et al. Metabolic exercise test data combined with cardiac and kidney indexes, the MECKI score: a multi parametric approach to heart failure prognosis. Int J Cardiol. 2013;167:2710–8.
Faller H, Störk S, Schowalter M, Steinbüchel T, Wollner V, Ertl G, et al. Is health-related quality of life an independent predictor of survival in patients with chronic heart failure? J Psychosom Res. 2007;63(5):533–8.
Frankenstein L, Nelles M, Hallerbach M, Dukic D, Fluegel A, Schellberg D, et al. Prognostic impact of peak VO2-changes in stable CHF on chronic beta-blocker treatment. Int J Cardiol. 2007;122(2):125–30.
Ades PA, Keteyian SJ, Balady GJ, et al. Cardiac rehabilitation exercise and self-care for chronic heart failure. JACC Heart Fail. 2013;1(6):540–7.
Arnold M, Rajda M, Ignaszewski A, Howlett J, Leblanc M-H. Changes in the Minnesota living with heart failure questionnaire score and clinical outcomes in a large contemporary population of ambulatory heart failure patients in the Canadian heart failure network. J Card Fail. 2012;18(8 Supplement):S79.
Poggio R, Arazi HC, Giorgi M, Miriuka SG. Prediction of severe cardiovascular events by VE/VCO2 slope versus peak VO2 in systolic heart failure: a meta-analysis of the published literature. Am Heart J. 2010;160(6):1004–14.
Sato T, Yoshihisa A, Kanno Y, Suzuki S, Yamaki T, Sugimoto K, et al. Cardiopulmonary exercise testing as prognostic indicators: comparisons among heart failure patients with reduced, mid-range and preserved ejection fraction. Eur J Prev Cardiol. 2017;24(18):1979–87. https://doi.org/10.1177/2047487317739079.
Ingle L. Prognostic value and diagnostic potential of cardiopulmonary exercise testing in patients with chronic heart failure. Euro J Heart Fail. 2008;10:112–8.
Pozehl BJ, Duncan K, Hertzog M, McGuire R, Norman JF, Artinian NT, et al. Study of adherence to exercise in heart failure: the HEART camp trial protocol. BMC Cardiovasc Disord. 2014;14:172.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Mansueto Gomes Neto, André Rodrigues Durães, Lino Sergio Rocha Conceição, Leonardo Roever, Tong Liu7 Gary Tse, Giuseppe Biondi-Zoccai, Ana Lucia Barbosa Goes, Lura Gonzalez Nogueira Alves, Øyvind Ellingsen, and Vitor Oliveira Carvalho declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Evidence-Based Medicine, Clinical Trials and Their Interpretations
Rights and permissions
About this article
Cite this article
Gomes-Neto, M., Durães, A.R., Conceição, L.S.R. et al. Effect of Aerobic Exercise on Peak Oxygen Consumption, VE/VCO2 Slope, and Health-Related Quality of Life in Patients with Heart Failure with Preserved Left Ventricular Ejection Fraction: a Systematic Review and Meta-Analysis. Curr Atheroscler Rep 21, 45 (2019). https://doi.org/10.1007/s11883-019-0806-6
Published:
DOI: https://doi.org/10.1007/s11883-019-0806-6