Abstract
Purpose of Review
This review aims to examine gender differences in both the epidemiology and pathophysiology of hypertension and to explore gender peculiarities on the effects of antihypertensive agents in decreasing BP and CV events.
Recent Findings
Men and women differ in prevalence, awareness, and control rate of hypertension in an age-dependent manner. Studies suggest that sex hormones changes play a pivotal role in the pathophysiology of hypertension in postmenopausal women. Estrogens influence the vascular system inducing vasodilatation, inhibiting vascular remodeling processes, and modulating the renin-angiotensin aldosterone system and the sympathetic system. This leads to a protective effect on arterial stiffness during reproductive age that is dramatically reversed after menopause.
Summary
Data on the efficacy of antihypertensive therapy between genders are conflicting, and the underrepresentation of aged women in large clinical trials could influence the results. Therefore, further clinical research is needed to uncover potential gender differences in hypertension to promote the development of a gender-oriented approach to antihypertensive treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertension is a prominent risk factor for global mortality and morbidity and has been broadly associated with cardiovascular (CV) diseases [1]. The therapeutic control of high blood pressure (BP) remains largely unsatisfactory. Gender is a further important determinant to be evaluated, likewise well-established factors such as age, race, and comorbidities. CV and renal diseases are more frequent in males than age-matched premenopausal females. However, BP and consequent CV events increase in postmenopausal women as well [2]. In this context, females showed a higher risk of age-related left ventricular hypertrophy [3] and symptomatic postinfarction heart failure vs. smaller infarctions or preserved left ventricular ejection fractions than similarly aged men [4, 5]. These differences are not exactly explained by classical risk factors but could be ascribed to differences by gender in terms of hemodynamic parameters, such as central and peripheral arterial BP. Hayward et al. [6] reported that men have a higher systolic BP until middle age, while women’s BP significantly increases with aging. Both sexes exhibit a direct age-dependent increase of the augmentation index, a specific marker of central aortic BP. Moreover, Redfield et al. [7] showed that aging and female gender were related to an increased vascular and myocardial stiffness even without known CV disease. Among the possible determinants, sex differences in hypertension could be considered as the consequence of biological and behavioral issues. The most important biological factors protective against high BP in females could include hormones, gene variability, and further biological gender variables, some of these dominant in youth and until the menopausal phase in women [8, 9]. Behavioral risk factors for hypertension include obesity, smoking, and low physical activity, as well as the response to antihypertensive therapy, the adherence, and to both pharmacological and non-pharmacological interventions. Gender differences have been investigated in several studies for both CV risk factors and events, but research findings are not conclusive. This review aims to examine gender differences in both the epidemiology and the pathophysiology of hypertension and to explore gender peculiarities on the effects of antihypertensive agents in decreasing BP and CV events.
Epidemiology
There is a continuous linkage between high levels of BP and CV morbidity and mortality [10]. Hypertension is associated with reduced life expectancy and early developing of CV disease [4]. Mean systolic BP in the overall population progressively increases with aging in both sexes. Although the prevalence of high BP does not present significant gender differences overall, certain discrepancies emerge between men and women in BP values during life. In particular, studies collecting data from the USA show that BP is higher in men than women during early adulthood [11, 12]. The National Health and Nutrition Examination Survey (NHANES) 2007–2012 shows a smaller gap in the prevalence of hypertension between women and men in younger groups in respect to previous survey. Thus, premenopausal women less develop hypertension compared with age-matched men [13]. However, this benefit gradually ends after menopause. The prevalence of hypertension is 68% in women between the ages of 65 and 74 years, and it is greater than men [13]. Accordingly, other studies confirm that hypertension becomes more prevalent in women among elderly individuals. Similar findings were observed in recent large population Canadian studies [14], likewise in researches conducted in developing countries (India, China, and Latin America) [15]. Contrarily, an Italian survey failed to find any gender differences in BP control [16]. Moreover, aging of world population and longer life expectancies in women than in men are the principal reasons of the increase in the prevalence of hypertension among females. Consequently, hypertension has been predicted more frequent in women than in men in the near future [17]. The data from the 2007–2010 NHANES survey reveal that awareness, treatment, and control rates are significantly better in women than men, being evident for all races. Although similar treatment, women had lower control of BP compared with men, especially in older subjects. Awareness of high BP was major in females than males but increased more in males [18, 19]. Data from this survey showed that treatment of hypertension increased in both sexes, and it was higher in females [18, 19]. The poorer outcome observed in women may be at least partially related to the wrong perception by both patients and physicians that women are at substantial lower risk than men [20, 21].
Several studies with either cross-sectional or longitudinal design have been planned to assess the association between menopause and hypertension [22,23,24]. The effect of menopause on BP is controversial, and the observed increase of BP after menopause may not be evaluated independently of the potential confounding effects of aging, other CV risk factors, such as body weight and lipid levels, and comorbidities. It seems that aging and hormonal changes are responsible for the majority of effects traditionally attributed to menopause.
Moreover, pregnancy-associated hypertension is present in approximately 10% of all pregnancies, impacting on maternal and fetal mortality [25]. Hypertension in pregnancy emerged as a significant risk factor for future hypertension and CV events. Furthermore, preeclampsia is associated with an increased risk of chronic hypertension, type 2 diabetes, stroke, and coronary heart disease [25,26,27,28].
Overall, CV disease remains the main cause of death in women [29]. A wide range of evidence indicates that women are at a lower CV risk than men before menopause, while the risk for CV events increases significantly after menopause [29].
The importance of risk factors for the development of essential hypertension in women has been evaluated in a prospective cohort study of approximately 83,000 female subjects. Six modifiable lifestyle and dietary factors are independently associated with lower risk of developing hypertension: normal weight, physical exercise, correct dietary approach, modest alcohol intake, moderate use of analgesics, and intake of supplemental folic acid [30]. Other studies reported that females are more prone than males to be physically inactive at all ages, and physical inactivity worsens with age [31].
However, although increased salt sensitivity is strongly associated with all-cause mortality in men, no association is found in women [32]. Overall, an increased prevalence of concomitant risk factors, including central obesity, elevated total cholesterol and low high-density lipoprotein cholesterol (HDL) levels are likely to contribute to poor BP control in elderly women [33].
It has been predicted that the overall life expectancy for hypertensive adult female individuals is approximately 5 years shorter compared to normotensive individuals of the same age [34]. A large multicentric and international study estimates that stroke mortality was significantly higher among women compared to men [35]. Authors argue that it could be chiefly attributed to the longevity of women, being stroke rates higher in the elderly [35]. Similar findings were observed in stroke incidence rates in Netherlands [36]. Contrarily, a recent meta-analysis including more than 40,000 patients with a diagnosis of heart failure observed a lower mortality in women compared with men [37]. Overall, epidemiologic data suggest that hypertension has a consistent impact on CV morbidity and mortality, both in women and in men [37]. In particular, the study stated that women with heart failure are on average older and more prone to suffer from hypertension [37]. Pathophysiologic reasons of these findings will be explored in the next section.
Pathophysiology
Hormonal changes may represent the pathophysiologic mechanism underlying the age-dependent heterogeneity in hypertension between the two sexes, especially in women. Epidemiologic studies showed that menopause doubled the risk of high BP even if corrected for other known CV risk factors [38, 39]. The increase in BP among postmenopausal women is likely due to dramatic changes in sex hormones levels. It has been established that estrogen influences the vascular system inducing vasodilatation, increasing nitric oxide (NO) bioavailability, inhibiting vascular remodeling processes and vascular response to injury, and modulating the renin-angiotensin aldosterone system (RAAS) and the sympathetic system. [40,41,42,43]. On the same hand, progesterone has been shown to cause endothelium-dependent vascular dilation [40]. Estrogen influences the renin-angiotensin system in different ways. In normotensive postmenopausal women, plasma angiotensin II levels nearly double with oral administration of estrogen and medroxyprogesterone [41]. Estrogen affects the angiotensinogen gene expression and synthesis through modulation of regulatory elements in the gene promotor, thereby increasing circulating angiotensinogen concentrations in women [42]. Further, females have a lower expression of the angiotensin II type 1 receptor, angiotensin-converting enzyme, and plasma renin compared to males [43], and the fall in estrogen levels results in reduced NO bioavailability and improved angiotensin II activity which causes impaired renal sodium handling, oxidative stress, and high BP [44]. Among normotensive premenopausal women, salt-loading causes renal vasodilation and a reduced filtration fraction during the luteal phase of the menstrual cycle [45]. Conversely, in postmenopausal women, salt-loading induces a decrease in renal plasma flow and a rise in the filtration fraction [46]. According to these results, estrogens could play a pivotal role in renal response to salt loading, by enhancing NO bioavailability and modulating RAAS activity [46]. This is consistent with the observation that surgical menopause is linked to salt-sensitive hypertension [47].
On the other hand, it has been described that androgens raise endothelin and catecholamines levels, increasing sodium renal reuptake and promoting vasoconstriction [40]. Similarly, testosterone lowers HDL and raises low-density lipoprotein cholesterol (LDL), increasing the synthesis of angiotensin II and homocysteine [40]. Overall, the abovementioned effects result in detrimental outcome in terms of hypertension and CV disease.
Postmenopausal women are more likely to develop metabolic syndrome [48]. Changes in weight and body fat distribution related to ovarian hormones deficiency lead to insulin resistance and hypertension [48]. Aside from hormone patterns, there are other differences in the CV system between genders. Even though diastolic pressure is lower in women in comparison to age-matched men, systolic and pulse pressures are higher in women older than 45 years [49]. On the same hand, isolated systolic hypertension is more common among aged females than among men [49]. It has been described that genders differ in terms of biomechanical properties of the artery wall that influence arterial stiffness. Women show an increased arterial stiffness compared to men during childhood years and a rise after menopause; on the contrary, stiffness increases linearly with aging within men, suggesting that female hormones modulate large artery stiffness during the reproductive phase [49].
Antihypertensive Therapy
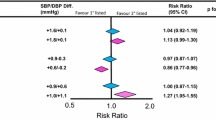
Many differences have been described about the pattern of antihypertensive drug prescription and use in hypertensive men vs. women. A large meta-analysis of 46 population-based studies in 22 countries, including 123,143 men and 164,858 women aged 20–59 years [50], showed that hypertensive women were 1.33-fold more likely to be treated with drugs and were more prescribed diuretics, while men more often used beta-blockers, ACE inhibitors, and calcium channel blockers. A further Dutch study [51] from the same group analyzed subjects from two cross-sectional population-based surveys of CV disease risk factors and reported a high prevalence of patients on monotherapy, 57 and 54% in treated men and women, respectively. Among patients on antihypertensive monotherapy, women were less likely to be taking beta-blockers, calcium channel blockers, or ACE inhibitors than diuretics compared to men. A possible explanation of these findings could be gender differences in side effects. Women are more prone to cough when treated with ACE inhibitors than men [52]. Women also seem to be more susceptible to vasodilation-related adverse symptoms by dihydropyridine calcium channel blockers [53]. However, women more frequently experience diuretic-induced adverse drug effects than men do. Women more likely suffer electrolytic disturbances and are less prone to diuretic-induced gout [54]. On the other hand, diuretics may reduce the risk of osteoporotic fractures, a possible advantage especially in older women [55]. Large randomized trials with BP lowering treatment have shown similar benefit in both sexes [56,57,58,59]. However, data from some small clinical studies suggest that gender differences in BP response might exist. For example, a prospective study comparing the effects of amlodipine monotherapy in men and women suggested that diastolic BP reduction was greater in women than in men, and BP control was achieved more in women than men [60]. Similarly, the angiotensin receptor blockers plus hydrochlorothiazide combination showed greater BP reductions in women [61, 62]. In the VALUE trial, CV morbidity and mortality was greater with valsartan compared to amlodipine in women but not in men [63]. Similarly, the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) showed a major effect against high BP values of calcium channel blockers compared with ACE inhibitors in women, related to a greater reduction in stroke rate [64]. In contrast, the Losartan Intervention For Endpoint Reduction (LIFE) study showed significantly safer effect of losartan compared to atenolol [65]. Despite comparable BP reduction, fewer events occurred in females than males [65]. The impact of gender on the benefits of antihypertensive therapy has been the subject of a large meta-analysis by the Blood Pressure Lowering Treatment Trialists’ Collaboration, which included 31 randomized trials with altogether about 100,000 men and 90,000 women with hypertension [66]. No significant differences in CV outcomes between men and women were observed. Moreover, all of the antihypertensive treatments analyzed conferred equal protection against CV events in both sexes. The investigators suggest that differences in CV risks between sexes are not associated with antihypertensive regimen but reflect other factors, such as awareness, detection, and management of high BP. However, in a recent meta-analysis of ten large randomized studies exploring the effects of antihypertensive agents on CV outcomes, we found that the occurrence of sudden death, stroke, myocardial infarction, heart failure, CV death, hospitalization for CV disease, and renal dysfunction was significantly higher in men compared with women (odds ratio [OR] 1.25, 95% confidence interval [CI] 1.17, 1.33, p < 0.001; I2 40.17%). This effect was robust in the sensitivity analysis, showing the independence of the pooled estimate from any single study [67•]. We believe that several reasons may explain the differences between this latter analysis and the present results. First, we included different studies, because of some differences in the inclusion criteria compared to the previous analysis. Second, we presented global results including all antihypertensive regimens, while Turnbull et al. [66] exhibited the results separately for each antihypertensive class. However, even though our findings are general and do not allow to discuss the effect of each pharmacological class, the results reached statistical significance, demonstrating sex differences in outcomes after antihypertensive treatment [67•].
More recently, the most appropriate targets for systolic BP to reduce CV disease morbidity and mortality among people without diabetes mellitus were analyzed in the Systolic Blood Pressure Intervention Trial (SPRINT) [68], a randomized controlled multicenter clinical study including about 9000 high risk non-diabetic patients, of whom 35% women. It showed a relation between a greater reduction of systolic BP (close to 120 mmHg) and lower rates of both fatal and non-fatal major CV events and death from any cause, although with notably higher rates of adverse events. Furthermore, the rate of the primary composite outcome in the intensive treatment vs. in the standard treatment group reached statistical significance in men (hazard ratio [HR] with intensive treatment, 0.72; 95% CI 0.59–0.88), but not in women (HR with intensive treatment, 0.84; 95% CI 0.62–1.14) despite that no interaction was observed between gender and degree of BP reduction. These differences among genders have been stressed in recent international guidelines [69••] on hypertension. The updated guidelines classify hypertension as BP reading of 130/80 mmHg or higher. They focus on the global evaluation of CV risk and identify new targets for BP reduction in patients with comorbidities. Although there is no recommendation for gender-oriented management of hypertension, guidelines take into account the evidence about more adverse effects of antihypertensive therapy in women and the need of new trials to assess the effect of BP reduction in women [69••].
Conclusion
Men and women differ in prevalence, awareness, and control rate of hypertension in an age-dependent manner. Studies suggest that sex hormones changes play a pivotal role in the pathophysiology of hypertension in postmenopausal women. Estrogens influence the vascular system inducing vasodilatation, inhibiting vascular remodeling processes, and modulating the RAAS and the sympathetic system. This leads to a protective effect on arterial stiffness during reproductive age that is dramatically reversed after menopause. Data on the efficacy of antihypertensive therapy between genders are conflicting, and the underrepresentation of aged women in large clinical trials could influence the results. Therefore, further clinical research is needed to uncover potential gender differences in hypertension to promote the development of a gender-oriented approach to antihypertensive treatment. Research should be specifically addressed to define the major clinical importance of difference in the complex expression of the CV risk and the effects of antihypertensive (pharmacological and non-pharmacological) treatment on BP reduction as well as CV morbidity and mortality.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
GBD 2013 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(10010):2287–323.
Burl VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, et al. Prevalence of hypertension in the US adult population: results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–13.
Marcus R, Krause L, Weder AB, Dominguez-Mejia, Schork NJ, Julius S. Sex-specific determinants of increased left ventricular mass in the Tecumseh Blood Pressure Study. Circulation. 1994;90:928–36.
Tofler GH, Stone PH, Muller JE, the MILIS Study Group, et al. Effects of gender and race on prognosis after myocardial infarction: adverse prognosis for women, especially black women. J Am Coll Cardiol. 1987;9(3):473–82. https://doi.org/10.1016/S0735-1097(87)80038-4.
Karlson BW, Herlitz J, Hartford M. Prognosis in myocardial infarction in relation to gender. Am Heart J. 1994;128:477–83.
Hayward CS, Kelly RP. Gender-related differences in the central arterial pressure waveform. J Am Coll Cardiol. 1997 Dec;30(7):1863–71. https://doi.org/10.1016/S0735-1097(97)00378-1.
Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112(15):2254–62. https://doi.org/10.1161/CIRCULATIONAHA.105.541078.
Everett B, Zajacova A. Gender differences in hypertension and hypertension awareness among young adults. Biodemography Soc Biol. 2015;61:1–17.
Doumas M, Papademetriou V, Faselis C, Kokkinos P. Gender differences in hypertension: myths and reality. Curr Hyperten Rep. 2013;15(4):321–30. https://doi.org/10.1007/s11906-013-0359-y.
Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med. 1993;153:598–615.
Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209.
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Circulation. 2013;127(1):143–52.
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322.
Robitaille C, Dai S, Waters C, Loukine L, Bancej C, Quach S, et al. Diagnosed hypertension in Canada: incidence, prevalence and associated mortality. CMAJ. 2012;184:E49–56.
Prince MJ, Ebrahim S, Acosta D, Ferri CP, Guerra M, Huang Y, et al. Hypertension prevalence, awareness, treatment and control among older people in Latin America, India and China: a 10/66 cross-sectional population-based survey. J Hypertens. 2012 Jan;30(1):177–87. https://doi.org/10.1097/HJH.0b013e32834d9eda.
Tocci G, Ferrucci A, Pontremoli R, Ferri C, Rosei EA, Morganti A, et al. Blood pressure levels and control in Italy: comprehensive analysis of clinical data from 2000–2005 and 2005–2011 hypertension surveys. J Hum Hypertens. 2015;29(11):696–701. https://doi.org/10.1038/jhh.2015.4.
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–23. https://doi.org/10.1016/S0140-6736(05)70151-3.
Lloyd-Jones DM, Evans JC, Levy D, et al. JAMA. 2005;294:466–72.
Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–50.
Townsend N, Nichols M, Scarborough P, Rayner M. Cardiovascular disease in Europe 2015: epidemiological update. Eur Heart J. 2015;36(40):2673–4.
Mosca L, Benjamin EJ, Berra K, Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. Circulation. 2011;123(11):1243–62. https://doi.org/10.1161/CIR.0b013e31820faaf8.
Casiglia E, Tikhonoff V, Caffi S, Bascelli A, Schiavon L, Guidotti F, et al. Menopause does not affect blood pressure and risk profile, and menopausal women do not become similar to men. J Hypertens. 2008;26(10):1983–92. https://doi.org/10.1097/HJH.0b013e32830bfdd9.
Zanchetti A, Facchetti R, Cesana GC, Modena MG, Pirrelli A, Sega R, et al. Menopause-related blood pressure increase and its relationship to age and body mass index: the SIMONA epidemiological study. J Hypertens. 2005;23(12):2269–76. https://doi.org/10.1097/01.hjh.0000194118.35098.43.
Cifkowa R, Pitha J, Lejskova M, Lanska V, Zecova S. Blood pressure around the menopause: a population study. J Hypertens. 2008;26(10):1976–82. https://doi.org/10.1097/HJH.0b013e32830b895c.
Mongraw-Chaffin M, Cirillo P, Cohn B. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension. 2010;56:166–71. 2011; 123:1243–62
Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53(6):944–51.
McDonald S, Malinowski A, Zhou Q, Yusuf S, Devereaux P. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156(5):918–30. https://doi.org/10.1016/j.ahj.2008.06.042.
Bellamy L, Casas J, Hingorani A, Williams D. Preeclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974–86. https://doi.org/10.1136/bmj.39335.385301.BE.
Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D’Agostino RB, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: the Framingham heart study. JAMA. 2002;287(8):1003–10.
Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA. 2009;302(4):401–11. https://doi.org/10.1001/jama.2009.1060.
Schiller J, Lucas J, Peregoy J. Summary health statistics for US adults: National Health Interview Survey 2010. Vital Health Stat. 2012;252:1–207.
Bursztyn M, Ben-Dov I. Sex differences in salt-sensitivity risk approximated from ambulatory blood pressure monitoring and mortality. J Hypertens. 2013;31(5):900–5. https://doi.org/10.1097/HJH.0b013e32835f29f4.
Ong KL, Tso AW, Lam KS, Cheung BM. Gender difference in blood pressure control and cardiovascular risk factors in Americans with diagnosed hypertension. Hypertension. 2008;51(4):1142–8. https://doi.org/10.1161/HYPERTENSIONAHA.107.105205.
Franco O, Peeters A, Bonneux L, de Laet C. Blood pressure in adulthood and life expectancy with cardiovascular disease in men and women: life course analysis. Hypertension. 2005;46(2):280–6. https://doi.org/10.1161/01.HYP.0000173433.67426.9b.
Redon J, Olsen M, Cooper R, et al. Stroke mortality and trends from 1990 to 2006 in 39 countries from Europe and Central Asia: implications for control of high blood pressure. Eur Heart J. 2011;32(11):1424–31. https://doi.org/10.1093/eurheartj/ehr045.
Wieberdink R, Ikram M, Hofman A, Koudstaal P, Breteler M. Trends in stroke incidence rates and stroke risk factors in Rotterdam, the Netherlands from 1990 to 2008. Eur J Epidemiol. 2012;27(4):287–95. https://doi.org/10.1007/s10654-012-9673-y.
Martinez-Selles M, Doughty R, Poppe K, et al. Gender and survival in patients with heart failure: interactions with diabetes and aetiology. Results from the MAGGIC individual patient metaanalysis. Eur J Heart Fail. 2012;14:473–9.
Staessen J, Bulpitt CJ, Fagard R, Lijnen P, Amery A. The influence of menopause on blood pressure. J Hum Hypertens. 1989;3(6):427–33.
Amigoni S, Morelli P, Parazzini F, Chatenoud L. Determinants of elevated blood pressure in women around menopause: results from a cross-sectional study in Italy. Maturitas. 2000;34(1):25–32. https://doi.org/10.1016/S0378-5122(99)00089-4.
Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res. 2002;53:688–708.
Komukai K, Mochizuki S, Yoshimura M. Gender and the renin-angiotensin-aldosterone system. Fundam Clin Pharmacol. 2010;24(6):687–98. https://doi.org/10.1111/j.1472-8206.2010.00854.x.
Fischer M, Baessler A, Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res. 2002;53(3):672–7. https://doi.org/10.1016/S0008-6363(01)00479-5.
Sullivan JC. Sex and the renin-angiotensin system: inequality between the sexes in response to RAS stimulation and inhibition. Am J Physiol Regul, Integr Comp Physiol. 2008;294(4):R1220–6. https://doi.org/10.1152/ajpregu.00864.2007.
Hernandez Schulman I, Raij L. Salt sensitivity and hypertension after menopause: role of nitric oxide and angiotensin II. Am J Nephrol. 2006;26(2):170–80. https://doi.org/10.1159/000092984.
Pechere-Bertschi A, Burnier M. Female sex hormones, salt, and blood pressure regulation. Am J Hypertens. 2004;1(7):994–1001.
Schulman IH, Aranda P, Raij L, Veronesi M, Aranda FJ, Martin R. Surgical menopause increases salt sensitivity of blood pressure. Hypertension. 2006;47(6):1168–74. https://doi.org/10.1161/01.HYP.0000218857.67880.75.
Barton M, Meyer MR. Postmenopausal hypertension: mechanisms and therapy. Hypertension. 2009;54(1):11–8. https://doi.org/10.1161/HYPERTENSIONAHA.108.120022.
Nuzzo A, Rossi R, Modena MG. Hypertension alone or related to the metabolic syndrome in postmenopausal women. Expert Rev Cardiovasc Ther. 2010;8:1541Y1548.
Rossi P, Frances Y, Kingwell BA, et al. Gender differences in artery wall biomechanical properties throughout life. J Hypertens. 2011;29:1023–33.
Klungel OH, de Boer A, Paes AH, Seidell JC, Bakker A. Sex differences in the pharmacological treatment of hypertension: a review of population-based studies. J Hypertens. 1997;15(6):591–600. https://doi.org/10.1097/00004872-199715060-00004.
Klungel OH, de Boer A, Paes AH, Seidell JC, Bakker A. Sex differences in antihypertensive drug use: determinants of the choice of medication for hypertension. J Hypertens. 1998;16(10):1545–53. https://doi.org/10.1097/00004872-199816100-00021.
Os I, Bratland B, Dahlof B, Gisholt K, Syvertsen JO, Tretli S. Female preponderance for lisinopril-induced cough in hypertension. Am J Hypertens. 1994;7(11):1012–5. https://doi.org/10.1093/ajh/7.11.1012.
Kajiwara A, Saruwatari J, Kita A, Oniki K, Yamamura M, Murase M, et al. Younger females are at greater risk of vasodilation-related adverse symptoms caused by dihydropyridine calcium channel blockers: results of a study of 11,918 Japanese patients. Clin Drug Investig. 2014;34(6):431–5. https://doi.org/10.1007/s40261-014-0191-4.
Pemu PI, Ofili E. Hypertension in women—part I. J Clin Hypertens (Greenwich). 2008;10:406–10.
Schoofs MW, van der Klift M, Hofman A, et al. Thiazide diuretics and the risk for hip fracture. Ann Intern Med. 2003;139:476–82.
Turnbull F, Woodward M, Anna V. Effectiveness of blood pressure lowering: evidence-based comparisons between men and women. Expert Rev Cardiovasc Ther. 2010;8(2):199–209. https://doi.org/10.1586/erc.09.155.
Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995–1003. https://doi.org/10.1016/S0140-6736(02)08089-3.
Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363(9426):2022–31. https://doi.org/10.1016/S0140-6736(04)16451-9.
Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian cardiac outcomes trial-blood pressure lowering arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366(9489):895–906. https://doi.org/10.1016/S0140-6736(05)67185-1.
Kloner R, Sowers J, DiBona G, et al. Sex- and age-related antihypertensive effects of amlodipine. The Amlodipine Cardiovascular Community Trial Study Group. Am J Cardiol. 1996;77:713–22.
Saunders E, Cable G, Neutel J. Predictors of blood pressure response to angiotensin receptor blocker/diuretic combination therapy: a secondary analysis of the irbesartan/hydrochlorothiazide blood pressure reductions in diverse patient populations (INCLUSIVE) study. J Clin Hypertens (Greenwich). 2008;10(1):27–33. https://doi.org/10.1111/j.1524-6175.2007.07195.x.
Everett BM, Glynn RJ, Danielson E, Ridker PM. Combination therapy versus monotherapy as initial treatment for stage 2 hypertension: a prespecified subgroup analysis of a community-based, randomized, open-label trial. Clin Ther. 2008;30(4):661–72. https://doi.org/10.1016/j.clinthera.2008.04.013.
Zanchetti A, Julius S, Kjeldsen S, McInnes GT, Hua T, Weber M, et al. Outcomes in subgroups of hypertensive patients treated with regimens based on valsartan and amlodipine: an analysis of findings from the VALUE trial. J Hypertens. 2006;24(11):2163–8. https://doi.org/10.1097/01.hjh.0000249692.96488.46.
ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981–97. https://doi.org/10.1001/jama.288.23.2981.
Os I, Franco V, Kjeldsen SE, Manhem K, Devereux RB, Gerdts E, et al. Effects of losartan in women with hypertension and left ventricular hypertrophy: results from the Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension. 2008;51(4):1103–8. https://doi.org/10.1161/HYPERTENSIONAHA.107.105296.
Turnbull F, Woodward M, Neal B, Barzi F, Ninomiya T, Chalmers J, et al. Do men and women respond differently to blood pressure-lowering treatment? Results of prospectively designed overviews of randomized trials. Eur Heart J. 2008;29(21):2669–80. https://doi.org/10.1093/eurheartj/ehn427.
• Giorgini P, Sahebkar A, Stamerra CA, Raparelli V, Petrarca M, Grassi D, et al. Comparison of clinical outcomes between genders following antihypertensive therapy: a meta-analysis. Curr Med Chem. 2017;24(24):2639–49. This paper represents the most recent meta-analysis on gender differences in clinical outcomes following antihypertensive therapy.
SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–16.
•• Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017. Recently updated guidelines for management of hypertension.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Paolo Di Giosia, Paolo Giorgini, Cosimo Andrea Stamerra, Marco Petrarca, Claudio Ferri, and Amirhossein Sahebkar declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Women and Ischemic Heart Disease
Rights and permissions
About this article
Cite this article
Di Giosia, P., Giorgini, P., Stamerra, C.A. et al. Gender Differences in Epidemiology, Pathophysiology, and Treatment of Hypertension. Curr Atheroscler Rep 20, 13 (2018). https://doi.org/10.1007/s11883-018-0716-z
Published:
DOI: https://doi.org/10.1007/s11883-018-0716-z