Abstract
Purpose of Review
Atherogenesis, once thought to be a passive process, is now recognized as a dynamic, immune-driven process. The critical innate immune cells, including neutrophils, normal-density granulocytes, and their newly identified subset low-density granulocytes, are moving to the forefront of interest in cardiovascular medicine due to their abundance in atherosclerotic plaques and chronic inflammatory diseases associating with early cardiovascular disease (CVD) such as psoriasis. In this review, we discuss the emerging roles of neutrophils in CVD and how they play a potential role in early CVD observed in psoriasis patients. This review aims to describe the roles of neutrophils in both early atherosclerosis and psoriasis.
Recent Findings
Recent work has demonstrated mechanistic links between vascular inflammation and neutrophil frequency. Evolving mouse models and clinical trials targeting IL-17-associated pathways continue to elucidate contributions of neutrophils in both atherosclerosis and psoriasis.
Summary
Early animal, in vitro and human studies suggest an important emerging role of neutrophils in atherosclerosis and psoriasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with systemic inflammatory diseases are at a high risk of developing early-onset cardiovascular disease (CVD). Although the elevated CVD risk has been established, it is not known if the biological mechanisms associated with early-onset CVD in inflammatory patients differ from those observed in the general population. Psoriasis is a chronic inflammatory skin disorder affecting 2–4% of the population and represents a systemic inflammatory disease associated with an increased risk of atherosclerotic CV events [1,2,3]. Given the recent success of IL-17 biologic agents partially targeting neutrophils in treating psoriatic skin disease, neutrophils may be a potential link driving the initiation and progression of CVD in psoriasis patients. Neutrophils, a short-lived subset of granulocytes generated in the bone marrow, are the most abundant circulating white blood cell in mammals. Neutrophils are the first responders of the innate immune response, where they attach, role, and migrate through the endothelial barrier into various inflamed tissues upon recognition of pathogen invasion or tissue damage [4,5,6]. The primary defense mechanism of neutrophils is that they harbor granule proteins, such as myeloperoxidase, neutrophil elastase, and LL-37, that target microbes and digests tissues, promoting an inflammatory response. To this end, we summarize the current understanding of the role of neutrophils in CVD and their emerging mechanisms linking early atherogenesis in psoriasis.
Neutrophils in Early Atherogenesis
Atherosclerosis was initially described as a passive accumulation of lipids in the arterial wall at sites of disturbed laminar flow [7], but is now acknowledged as an immune-driven process leading to endothelial dysfunction [8]. Altered physiological states such as hyperlipidemia, increased circulating pro-inflammatory cytokines, and high shear stress all contribute to endothelial dysfunction which promotes atherogenesis [9]. These dysfunctional endothelial cells are marked by decreased barrier integrity, vasodilation, and expression of cell adhesion molecules, including intracellular adhesion molecule-1, E-selectin, and vascular cell adhesion molecule-1 [10]. Endothelial dysfunction is further exacerbated by oxidized lipids generated from reactive oxygen species that accumulate in the intima, resulting in leukocyte recruitment [9, 11]. If recruited leukocytes fail to clear the oxidized lipids, they undergo apoptosis, increasing cellular debri and further fueling the inflammatory response. Over time, the inflamed lesions lead to luminal narrowing and subsequent ischemia or superimposed thrombosis [12, 13].
It has become clear that both inflammation and hyperlipidemia are at the epicenter of atherosclerosis development [14]. While atherosclerotic murine models provide insight into certain mechanistic elements of atherogenesis, the full extrapolation of the biological mechanisms responsible is difficult due to differing immune systems between humans, mice, and rabbits [15, 16]. Despite this limitation, the innate and adaptive immune cells identified as key players in atherosclerosis, including T cells, monocytes, platelets, and dendritic cells, and their mechanisms have been extensively investigated [17,18,19]. However, the roles of neutrophils in early atherogenesis are understudied. Over the last decade, neutrophils are becoming recognized as important contributors to atherogenesis development and progression, despite their mechanisms of action remaining elusive.
The defense mechanisms of neutrophils are rather unique and specific to this short-lived innate immune cell. Neutrophils serve as important primary defenders in acute inflammatory responses, releasing reactive oxygen species and proteolytic enzymes to challenge foreign pathogens which can also contribute to tissue destruction [20]. Additionally, neutrophils possess important activator, regulator, and effector cell functions in innate immunity [21] including the synthesis and release of cytokines, chemokines, and growth factors upon stimulation. When interacting with damaged endothelium, neutrophils augment endothelial stress by releasing granule-based proteins that increase leukocyte recruitment to the endothelial cell layer and ultimately promote inflammation and foam cell development, a dysfunctional macrophage subset that drives atherosclerosis [22].
Neutrophil localization to atherosclerotic plaques has been evidenced by immunohistochemical and pathological murine and human studies. Using Ly6G, a murine neutrophil-specific antigen, and antibodies to myeloperoxidase (MPO), a primary neutrophil granule protein, neutrophils are identified in early lesions both in the intimal, subendothelial, and shoulder regions of atherosclerotic lesions in humans and murine models [23,24,25]. In high-fat diet rabbit models of atherosclerosis, valued because their lipoprotein metabolism is comparable to humans [16], atherosclerotic plaques showed strikingly similar characteristics to human plaque designations, including inflammation, cholesterol crystal development, diverse fibrous cap development, and increased macrophage and monocyte density, all subsequent phenomena of neutrophil infiltration [26, 27]. Additionally, activated neutrophils marked by Fpr2 and p22phox are observed in human atherosclerotic lesions [28]. Neutrophils expressing CD66b were found in high volume in rupture-prone human plaques characterized by large lipid cores, high macrophage counts, low smooth muscle counts, and low collagen counts [29]. Furthermore, neutrophils are found in occlusive thrombi and culprit lesions of acute coronary syndrome patients [30, 31] suggesting neutrophils play a role in atherosclerotic progression.
Neutrophil homeostasis is of primary importance in both acute inflammation and immune dysfunction. The frequency of circulating neutrophils at any given time is highly regulated from the bone marrow to the blood by granulocyte colony stimulating factor (G-CSF), which consequently is upregulated in atherosclerosis by pro-atherogenic cytokines including IL-17 and TNF-α [32, 33]. In humans, mechanisms elucidating the pathway of bone marrow derived neutrophil generation are unclear; however, chronic stress, via the sympathetic nervous system, increases circulating neutrophil counts in both humans and mice [34]. In the bone marrow, the CXCR4/CXCL12 and CXCR2/CXCL1-CXCL8 axes significantly impact the release of neutrophils into the blood stream. It has been shown that a disruption in the bone marrow CXCR4/CXCL12 interaction leads to neutrophilia and increased atherosclerosis [35]. Conversely, disruption in the neutrophil-mobilization CXCR2/CXCL1-CXCL8 interaction results in neutropenia and reduced atherosclerosis [36] suggesting that neutrophil homeostasis is critical in the development of atherosclerosis. Furthermore, neutrophil counts are significantly higher in patients with increased incidence of major adverse CV events, and their frequency associated with cardiovascular outcome, a relationship not observed with eosinophil, basophil, monocyte, or lymphocyte counts [37]. Finally, in patients lacking NADPH oxidase resulting in decreased reactive oxygen species production (chronic granulotomatous disease), the manifestation of atherosclerosis is not observed despite elevated CVD risk factors [38]. Taken together, neutrophil homeostasis and atherosclerosis share a potential biological mechanism which warrants further investigation in humans.
Upon entry into the endothelial intima, neutrophils and their granule proteins recruit and activate monocytes, facilitating atherogenic progression. In murine studies, the absence of neutrophils significantly decreased the number of macrophages and monocytes within the arterial walls of atherosclerosis-prone mice [23]. Additionally, the neutrophil lysate of patients suffering from neutrophil granule deficiency, specifically alpha defensins, human neutrophil peptides, and LL-37, lacked the chemotactic effect on monocytes [39]. Azurocidin, LL37, and cathepsin G, all prominent neutrophil granule proteins found in atherosclerotic plaques, activate FPRs leading to slowing and extravasation of monocytes into the intima of endothelial cells [40, 41]. Proteinase-3, another neutrophil granule protein, activates CCL2 expression on endothelial cells, providing another mechanism to recruit monocytes [41]. Indeed, neutrophil granule proteins provide and augment monocyte recruitment to endothelial cells.
In addition to monocyte recruitment, neutrophil contents also play a role in foam cell formation and macrophage activation, a mid-stage hallmark characteristic of atherosclerotic plaques [42]. Human neutrophil peptides generated oxidative stress in macrophages, a critical component in foam cell formation [43]. MPO promotes the formation of oxidized low-density lipoproteins through generated reactive nitrogen species [44] and is present in high concentration in the shoulder region of atherosclerotic plaques in both humans and mice [25, 45]. MPO is also expelled during neutrophil extracellular trap (NET) formation, a mechanism that is triggered by cholesterol crystals. These NETs in turn trigger pro-IL-1 in macrophages, activating the inflammasome and ultimately leading to plaque destabilization [46, 47••]. Consequently, MPO levels are associated with coronary artery disease risk and independently predict endothelial dysfunction, suggesting that neutrophils and their protein products affect early vascular disease development [48].
The most intriguing defense mechanism of neutrophils recently described is the release of their cytosolic granule proteins bound to nuclear material to combat foreign pathogens through a biological process termed NETosis [49,50,51]. Three types of NETosis have been characterized: suicidal, vital, and mitochondrial. Definitive characteristics of NET classification and pathological relevancy to various diseases are debated in the scientific community [52]. Despite this, studies of NETs show promising links to atherogenesis; immunochemical stainings have identified the presence of NETs at sites of endothelial cell erosion and plaque rupture in human carotid plaque sections [53•]. NETs have been shown to potentiate atherosclerosis by macrophage priming and cytokine release to activate Th17 cells [47]. Additionally, in another chronic inflammatory disease associated with early-onset CVD, systemic erythematosus lupus (SLE), NETs are shown to induce endothelial cell dysfunction, stress, activation, and apoptosis [54,55,56]. In SLE, a subset of neutrophils termed low-density granulocytes that undergo spontaneous NETosis have been identified. Low-density granulocytes from SLE are currently characterized by high pro-inflammatory activity, altered phagocytic function, and elevated type I interferon production upregulating the inflammatory response and ensuing tissue damage [56,57,58]. While this specific neutrophil subset may show important atherogenic properties, further profiling of these cells and NET-specific action in atherogenesis and autoimmune disease is needed.
Psoriasis and Early Atherogenesis
Psoriasis is a complex immune-mediated chronic inflammatory disease that affects 2–3% of US adult population, with higher prevalence among persons of northern European descent [59]. Psoriasis prevalence has increased over time per retrospective cohort studies of adults and children [60, 61]. Development of psoriasis has strong genetic components [62,63,64,65] and much of this genetic susceptibility has been centered around the HLA-C locus [66]. Continued genetic analysis of psoriasis patients has helped elucidate potential association and susceptibility alleles [67]. Psoriasis causes hyperproliferation of epidermal cells and premature maturation of keratinocytes, subsequently manifesting as areas of red and scaly skin [59]. The severity of psoriatic skin disease is classified into mild (< 3% body surface area affected), moderate (3–10%), and severe (> 10% area affected) [68]. Psoriasis can also be quantified using the Psoriasis Area Severity Index (PASI) score, a score that combines severity of lesions and the area affected, mainly used in clinical trials (psoriasis.org/about-psoriasis) [68].
The inflammatory changes in the skin of patients with psoriasis can be divided into an infiltrate in the dermis—mainly activated keratinocytes, dendritic cells, macrophages, and T cells—and cells in the epidermis—neutrophils with some subtypes of T cells [59]. In addition to inflammatory cells, a host of innate immunity proteins, pro-inflammatory cytokines and chemokines form a cornerstone of the pathophysiology of this disease [69]. The most widely implicated culprits are pro-inflammatory cytokines from the IL-1 family, with significant contribution from the IL-36 sub-family, IL-17, and TNF-α among others [70]. The inflammatory cells and accompanying cytokines that are associated with skin disease in psoriasis have direct and indirect effects in other parts of the body including liver dysfunction, arthritis, metabolic syndrome, and psychiatric illness [71].
Epidemiological studies demonstrate that severe psoriasis has been linked to a 58% increased risk of major adverse cardiovascular events and 57% increased risk of cardiovascular death, suggesting implications with psoriasis in atherogenesis [72, 73]. Moreover, it has been proposed that severity of psoriasis may relate directly to the degree of systemic inflammation and extent of cardiovascular disease as measured in vivo beyond traditional risk factors [73, 74••]. The process of atherosclerosis resulting from a primary defect in the endothelial cells has been shown to be accelerated in chronic inflammatory states [75]. This hypothesis is strengthened by recent studies that have shown that reduction in distant skin inflammation reduced vascular inflammation and coronary plaque burden [76••, 77••].
In the setting of an unresolved inflammatory milieu, cytokines released from damaged endothelial cells cause proliferation of smooth muscle cells in the wall of the artery, which can lead to fibrous cap development. Thinning of the fibrous cap and expansion of the necrotic core may lead to the formation of high-risk or “vulnerable” plaque (HRP) [78]. These plaques are more likely to rupture, exposing the lumen of the vessel to the prothrombotic contents of the plaque core. As compared to healthy volunteers, patients with psoriasis have increased prevalence of HRP [77••]. Furthermore, these patients have equivalent HRP as older patients with hyperlipidemia [77••]. Another likely correlation between these diseases may be the fact that patients with psoriasis have been shown to have a more atherogenic lipoprotein profile with impaired HDL efflux capacity [79]. In addition to this, it has been shown that psoriasis severity directly correlates to an increase in obesity, adipose tissue inflammation, and insulin resistance [80,81,82]. Indeed, psoriasis presents a variety of direct and indirect risk factors in the development of atherosclerosis. Larger studies are required to further elucidate these mechanisms.
Treatments for psoriasis range from topical therapy and phototherapy (for mild to moderate severity) to systemic and biologic therapies (moderate to severe severity). There are several immunotherapeutic treatments targeting TNF, IL-17, and IL-23 pathways that have shown promise in recent years [83].
IL-17 Pathway: Atherosclerosis, Psoriasis, and Treatment
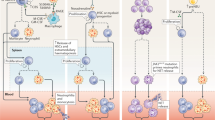
The neutrophil-activating effects of IL-17 and this concordant action in psoriasis and atherosclerosis implicate neutrophil activation as a potential link between the two diseases. The IL-17 pathway is an expansive pro-inflammatory pathway that may be implicated in both atherosclerosis and psoriasis (Fig. 1). Broadly defined, the IL-17 pathway is driven by interactions of IL-17, secreted by specific CD4+ T cells, neutrophils, monocytes, and NK cells, with other leukocytes fostering pro-inflammatory environments [83]. In the context of atherosclerosis, Th17 cells are present in early and developed human plaques as well as Apoe−/− mice aortic wall before and during atherogenesis [84,85,86]. The IL-17 family increases neutrophil proliferation, circulation, and recruitment via interactions with CXCL2, G-CSF, TNF-α, and CXCL8 [87]. Concordantly, multiple human studies summarized by [88] investigating serum IL-17 levels have found correlations to atherosclerosis development. Circulating IL-17 was also increased in patients who have suffered unstable angina [89]. In addition to clinical evidence of IL-17 implications in atherosclerosis, translational mouse models have augmented its importance in atherogenesis. With the blockade of IL-17 in APOE−/− mice, monocyte migration and ultimately atherosclerosis was reduced [90]. There is substantial evidence that IL-17 plays an important role in atherogenesis and contributes to neutrophil’s role as well.
The role of neutrophils in psoriasis and atherosclerosis may be operated in part through an IL-17 mechanism. The inflammatory microenvironment of a psoriatic lesion and early-stage atherosclerotic plaque share common elements in the interaction between IL-17 (green) and neutrophils (purple). Neutrophil net contents and released IL-17 activate keratinocytes in psoriatic lesions and activate endothelial cells in resting blood vessels. Shared inflammatory cytokines, and neutrophil and monocyte recruitment chemokines are released following activation, potentiating inflammation
Th17 cells are critical in the pathogenesis of psoriasis. Upon presentation of proliferation stimulants, Th17 cells, with stimulus from dendritic cells, become activated, generating a large pro-inflammatory environment of cytokines that drive inflammation and recruit other pro-inflammatory leukocytes to the psoriatic lesion [91,92,93,94]. While it is known that psoriasis patients exhibit elevated serum levels of IL-17 compared to healthy controls, the paradigm of the cellular source of IL-17 in psoriatic lesions is shifting [95]. A study by Bruce et al. reported in psoriatic lesions, IL-17 is released by neutrophils and mast cells, suggesting the minor source of IL-17 in these lesions is the Th17 T cell population. Immunohistochemical staining of biopsied lesional skin demonstrated the majority of IL-17 stain co-localized with MPO and multinuclear cells [96]. Furthermore, it was determined that the release of IL-17 from neutrophils is a NET-dependent process [96]. In murine models, the production of IL-17 from neutrophils was confirmed and shown to be a IL-6/IL-23-dependent mechanism. This finding was confirmed in human neutrophils by stimulating with recombinant IL-6 and IL-23 combined, therefore inducing IL-17 expression [97]. Despite the source of IL-17, this cytokine is independently implicated in both disease states, potentially providing association between psoriasis and accelerated atherosclerosis.
Limitations to verify these summations include both human and murine data. Currently, psoriasis-like murine models with atherosclerosis are not fully developed. Current studies investigating psoriasis-like skin inflammation and atherosclerosis have produced intriguing results [98•, 99]. A model with both psoriasis and atherosclerosis would be invaluable to elucidating and characterizing mechanistic features between the two.
Current IL-17 antagonist therapeutics for psoriasis including secukinumab and ixekizumab have shown promising clinical efficacy, both targeting IL-17A chemokines. Secukinumab, having FDA approval, showed 77.1–81.6% response in PASI75 and 59% response in PASI90 patients. Ixekizumab has shown similar results with PASI75 patients as well as brodalumab, an IL-17 receptor antagonist [100, 101]. While these show promising results for psoriatic lesion reduction, effects on the vasculature of the patients with IL-17 antagonists remain unknown. Currently, a study is being conducted to test this hypothesis in psoriasis (Vascular Inflammation in Psoriasis-Secukinumab Trial (NCT02690701)). It is worthy to note that statin therapy, a common lipid lowering treatment for psoriasis and cardiovascular disease, has immunomodulatory effects on human immune cells that may be partially driven by IL-17 production [102]. Compounded with this, resolving factors have been shown to attenuate atherosclerosis with and without statin treatment, partially through downregulation of key IL-17 producing Th17 cells [103]. Given the success of biological therapy potentially reducing vascular inflammation of psoriatic patients [76••], it is reasonable to put forward that IL-17 antagonists could have similar effects.
Conclusions
Neutrophils play a critical role in the development of atherosclerosis. They contribute to endothelial dysfunction, monocyte recruitment, and foam cell formation. Evidence of neutrophils in atherosclerotic plaques of humans and murine models is abundant. Links to atherosclerosis and psoriasis have been recently suggested, as a variety of direct and indirect risk factors in the development of atherosclerosis are elevated in psoriatic patients. Neutrophils have been shown to play an important role in the pathogenesis of psoriasis as well. Mechanistic similarities between psoriasis and atherosclerosis through the IL-17 pathway give credence to the role of inflammation, specifically neutrophils, in both disease states. Further studies to better elucidate these relationships are highly needed and warranted.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512–6.
Prodanovich S, Kirsner RS, Kravetz JD, Ma F, Martinez L, Federman DG. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700–3.
Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the general practice research database. Eur Heart J. 2010;31:1000–6.
von Vietinghoff S, Ley K. Homeostatic regulation of blood neutrophil counts. J Immunol. 2008;181:5183–8.
Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–70.
Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89.
Steinberg D, Witztum JL. Lipoproteins and atherogenesis. Current concepts. JAMA. 1990;264:3047–52.
Gistera A, Hansson GK. The immunology of atherosclerosis. Nat Rev Nephrol. 2017;13:368–80.
Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74.
Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–75.
Skaggs BJ, Hahn BH, McMahon M. Accelerated atherosclerosis in patients with SLE—mechanisms and management. Nat Rev Rheumatol. 2012;8:214–23.
Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–22.
Anderson JL, Morrow DA. Acute myocardial infarction. N Engl J Med. 2017;376:2053–64.
Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–25.
Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–12.
Fan J, Kitajima S, Watanabe T, Xu J, Zhang J, Liu E, et al. Rabbit models for the study of human atherosclerosis: from pathophysiological mechanisms to translational medicine. Pharmacol Ther. 2015;146:104–19.
Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol. 2009;27:165–97.
Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–6.
Gotsman I, Sharpe AH, Lichtman AH. T-cell costimulation and coinhibition in atherosclerosis. Circ Res. 2008;103:1220–31.
Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–15.
Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–31.
Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circ Res. 2012;110:875–88.
Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122:1837–45.
van Leeuwen M, Gijbels MJ, Duijvestijn A, Smook M, van de Gaar MJ, Heeringa P, et al. Accumulation of myeloperoxidase-positive neutrophils in atherosclerotic lesions in ldlr-/- mice. Arterioscler Thromb Vasc Biol. 2008;28:84–9.
Rotzius P, Thams S, Soehnlein O, Kenne E, Tseng CN, Bjorkstrom NK, et al. Distinct infiltration of neutrophils in lesion shoulders in apoe-/- mice. Am J Pathol. 2010;177:493–500.
Phinikaridou A, Hallock KJ, Qiao Y, Hamilton JA. A robust rabbit model of human atherosclerosis and atherothrombosis. J Lipid Res. 2009;50:787–97.
Hyafil F, Cornily JC, Rudd JH, Machac J, Feldman LJ, Fayad ZA. Quantification of inflammation within rabbit atherosclerotic plaques using the macrophage-specific ct contrast agent n1177: a comparison with 18f-fdg pet/ct and histology. J Nucl Med. 2009;50:959–65.
Hosokawa T, Kumon Y, Kobayashi T, Enzan H, Nishioka Y, Yuri K, et al. Neutrophil infiltration and oxidant-production in human atherosclerotic carotid plaques. Histol Histopathol. 2011;26:1–11.
Ionita MG, van den Borne P, Catanzariti LM, Moll FL, de Vries JP, Pasterkamp G, et al. High neutrophil numbers in human carotid atherosclerotic plaques are associated with characteristics of rupture-prone lesions. Arterioscler Thromb Vasc Biol. 2010;30:1842–8.
Naruko T, Ueda M, Haze K, van der Wal AC, van der Loos CM, Itoh A, et al. Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation. 2002;106:2894–900.
Kramer MC, Rittersma SZ, de Winter RJ, Ladich ER, Fowler DR, Liang YH, et al. Relationship of thrombus healing to underlying plaque morphology in sudden coronary death. J Am Coll Cardiol. 2010;55:122–32.
Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC. G-csf is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17:413–23.
Singh RB, Mengi SA, Xu YJ, Arneja AS, Dhalla NS. Pathogenesis of atherosclerosis: a multifactorial process. Exp Clin Cardiol. 2002;7:40–53.
Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20:754–8.
Zernecke A, Bot I, Djalali-Talab Y, Shagdarsuren E, Bidzhekov K, Meiler S, et al. Protective role of cxc receptor 4/cxc ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ Res. 2008;102:209–17.
Boisvert WA, Rose DM, Johnson KA, Fuentes ME, Lira SA, Curtiss LK, et al. Up-regulated expression of the cxcr2 ligand kc/gro-alpha in atherosclerotic lesions plays a central role in macrophage accumulation and lesion progression. Am J Pathol. 2006;168:1385–95.
Haumer M, Amighi J, Exner M, Mlekusch W, Sabeti S, Schlager O, et al. Association of neutrophils and future cardiovascular events in patients with peripheral artery disease. J Vasc Surg. 2005;41:610–7.
Sibley CT, Estwick T, Zavodni A, Huang CY, Kwan AC, Soule BP, et al. Assessment of atherosclerosis in chronic granulomatous disease. Circulation. 2014;130:2031–9.
Gallin JI, Fletcher MP, Seligmann BE, Hoffstein S, Cehrs K, Mounessa N. Human neutrophil-specific granule deficiency: a model to assess the role of neutrophil-specific granules in the evolution of the inflammatory response. Blood. 1982;59:1317–29.
Soehnlein O, Zernecke A, Eriksson EE, Rothfuchs AG, Pham CT, Herwald H, et al. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008;112:1461–71.
Lee TD, Gonzalez ML, Kumar P, Chary-Reddy S, Grammas P, Pereira HA. Cap37, a novel inflammatory mediator: its expression in endothelial cells and localization to atherosclerotic lesions. Am J Pathol. 2002;160:841–8.
Yu XH, Fu YC, Zhang DW, Yin K, Tang CK. Foam cells in atherosclerosis. Clin Chim Acta. 2013;424:245–52.
Quinn KL, Henriques M, Tabuchi A, Han B, Yang H, Cheng WE, et al. Human neutrophil peptides mediate endothelial-monocyte interaction, foam cell formation, and platelet activation. Arterioscler Thromb Vasc Biol. 2011;31:2070–9.
Podrez EA, Schmitt D, Hoff HF, Hazen SL. Myeloperoxidase-generated reactive nitrogen species convert ldl into an atherogenic form in vitro. J Clin Invest. 1999;103:1547–60.
Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94:437–44.
Nahrendorf M, Swirski FK. Immunology. Neutrophil-macrophage communication in inflammation and atherosclerosis. Science. 2015;349:237–8.
•• Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349:316–20. First manuscript to define cross-talk in atherosclerosis between neutrophils and macrophages.
Zhang R, Brennan ML, Fu X, Aviles RJ, Pearce GL, Penn MS, et al. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001;286:2136–42.
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5.
Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–41.
Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against candida albicans. PLoS Pathog. 2009;5:e1000639.
Doring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res. 2017;120:736–43.
• Quillard T, Araujo HA, Franck G, Shvartz E, Sukhova G, Libby P. Tlr2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur Heart J. 2015;36:1394–404. This manuscript defines a potential mechanism by which nuetrophils induce endothelial stress.
Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187:538–52.
Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S, et al. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to netosis-mediated cell death. FEBS Lett. 2010;584:3193–7.
Carmona-Rivera C, Zhao W, Yalavarthi S, Kaplan MJ. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis. 2015;74:1417–24.
Knight JS, Kaplan MJ. Lupus neutrophils: ‘Net’ gain in understanding lupus pathogenesis. Curr Opin Rheumatol. 2012;24:441–50.
Kahlenberg JM, Carmona-Rivera C, Smith CK, Kaplan MJ. Neutrophil extracellular trap-associated protein activation of the nlrp3 inflammasome is enhanced in lupus macrophages. J Immunol. 2013;190:1217–26.
Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509.
Tollefson MM, Crowson CS, McEvoy MT, Maradit Kremers H. Incidence of psoriasis in children: a population-based study. J Am Acad Dermatol. 2010;62:979–87.
Icen M, Crowson CS, McEvoy MT, Dann FJ, Gabriel SE, Maradit Kremers H. Trends in incidence of adult-onset psoriasis over three decades: a population-based study. J Am Acad Dermatol. 2009;60:394–401.
Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007;25:535–46.
Mahil SK, Capon F, Barker JN. Genetics of psoriasis. Dermatol Clin. 2015;33:1–11.
Rahman P, Elder JT. Genetic epidemiology of psoriasis and psoriatic arthritis. Ann Rheum Dis. 2005;64(Suppl 2):ii37–9. discussion ii40-1
Lonnberg AS, Skov L, Skytthe A, Kyvik KO, Pedersen OB, Thomsen SF. Heritability of psoriasis in a large twin sample. Br J Dermatol. 2013;169:412–6.
Tsoi LC, Spain SL, Knight J, Ellinghaus E, Stuart PE, Capon F, et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat Genet. 2012;44:1341–8.
Risch N, Merikangas K. The future of genetic studies of complex human diseases. Science. 1996;273:1516–7.
Langley RG, Ellis CN. Evaluating psoriasis with psoriasis area and severity index, psoriasis global assessment, and lattice system physician’s global assessment. J Am Acad Dermatol. 2004;51:563–9.
Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–9.
Baliwag J, Barnes DH, Johnston A. Cytokines in psoriasis. Cytokine. 2015;73:342–50.
Kim N, Thrash B, Menter A. Comorbidities in psoriasis patients. Semin Cutan Med Surg. 2010;29:10–5.
Mehta NN, Yu Y, Pinnelas R, Krishnamoorthy P, Shin DB, Troxel AB, et al. Attributable risk estimate of severe psoriasis on major cardiovascular events. Am J Med. 2011;124:775. e1-6
Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–41.
•• Naik HB, Natarajan B, Stansky E, Ahlman MA, Teague H, Salahuddin T, et al. Severity of psoriasis associates with aortic vascular inflammation detected by fdg pet/ct and neutrophil activation in a prospective observational study. Arterioscler Thromb Vasc Biol. 2015;35:2667–76. Manuscript demonstrates that vascular inflammation is associated with psoriasis severity and that circulating neutrophil frequecies and their associated proteins are elevated in psoriasis patients.
Gonzalez-Juanatey C, Llorca J, Amigo-Diaz E, Dierssen T, Martin J, Gonzalez-Gay MA. High prevalence of subclinical atherosclerosis in psoriatic arthritis patients without clinically evident cardiovascular disease or classic atherosclerosis risk factors. Arthritis Rheum. 2007;57:1074–80.
•• Dey AK, Joshi AA, Chaturvedi A, Lerman JB, Aberra TM, Rodante JA, et al. Association between skin and aortic vascular inflammation in patients with psoriasis: a case-cohort study using positron emission tomography/computed tomography. JAMA Cardiol. 2017;2(9):1013–8. This manuscript shows treatment of psoriasis skin disease leads to improvement in vascular inflammation.
•• Lerman JB, Joshi AA, Chaturvedi A, Aberra TM, Dey AK, Rodante JA, et al. Coronary plaque characterization in psoriasis reveals high risk features which improve following treatment in a prospective observational study. Circulation. 2017;36(3):263–76. This manuscript shows treatment of psoriasis skin disease improves non-calcified coronary plaque.
Linton MF, Yancey PG, Davies SS, Jerome WGJ, Linton EF, Vickers KC. The role of lipids and lipoproteins in atherosclerosis. Science. 2000;111(2877):166–71.
Mehta NN, Li R, Krishnamoorthy P, Yu Y, Farver W, Rodrigues A, et al. Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis. 2012;224:218–21.
Fleming P, Kraft J, Gulliver WP, Lynde C. The relationship of obesity with the severity of psoriasis: a systematic review. J Cutan Med Surg. 2015;19:450–6.
Gyldenlove M, Storgaard H, Holst JJ, Vilsboll T, Knop FK, Skov L. Patients with psoriasis are insulin resistant. J Am Acad Dermatol. 2015;72:599–605.
Harrington CL, Dey AK, Yunus R, Joshi AA, Mehta NN. Psoriasis as a human model of disease to study inflammatory atherogenesis. Am J Physiol Heart Circ Physiol. 2017;312:H867–H73.
Kim J, Krueger JG. Highly effective new treatments for psoriasis target the il-23/type 17 t cell autoimmune axis. Annu Rev Med. 2017;68:255–69.
Millonig G, Malcom GT, Wick G. Early inflammatory-immunological lesions in juvenile atherosclerosis from the pathobiological determinants of atherosclerosis in youth (pday)-study. Atherosclerosis. 2002;160:441–8.
Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of t cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6:131–8.
Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially l-selectin dependent. J Exp Med. 2006;203:1273–82.
Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–76.
Gong F, Liu Z, Liu J, Zhou P, Liu Y, Lu X. The paradoxical role of il-17 in atherosclerosis. Cell Immunol. 2015;297:33–9.
Cheng X, Yu X, Ding YJ, Fu QQ, Xie JJ, Tang TT, et al. The th17/treg imbalance in patients with acute coronary syndrome. Clin Immunol. 2008;127:89–97.
Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, et al. Blockade of interleukin-17a results in reduced atherosclerosis in apolipoprotein e-deficient mice. Circulation. 2010;121:1746–55.
Ley K, Smith E, Stark MA. Il-17a-producing neutrophil-regulatory tn lymphocytes. Immunol Res. 2006;34:229–42.
Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, Nograles KE, Tian S, Cardinale I, et al. Integrative responses to il-17 and tnf-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol. 2011;131:677–87.
Ramirez-Carrozzi V, Sambandam A, Luis E, Lin Z, Jeet S, Lesch J, et al. Il-17c regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol. 2011;12:1159–66.
Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suarez-Farinas M, Cardinale I, et al. Th17 cytokines interleukin (il)-17 and il-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159:1092–102.
Keijsers RR, Joosten I, van Erp PE, Koenen HJ, van de Kerkhof PC. Cellular sources of il-17 in psoriasis: a paradigm shift? Exp Dermatol. 2014;23:799–803.
Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, et al. Mast cells and neutrophils release il-17 through extracellular trap formation in psoriasis. J Immunol. 2011;187:490–500.
Taylor PR, Roy S, Leal SM Jr, Sun Y, Howell SJ, Cobb BA, et al. Activation of neutrophils by autocrine il-17a-il-17rc interactions during fungal infection is regulated by il-6, il-23, rorgammat and dectin-2. Nat Immunol. 2014;15:143–51.
• Wang Y, Gao H, Loyd CM, Fu W, Diaconu D, Liu S, et al. Chronic skin-specific inflammation promotes vascular inflammation and thrombosis. J Invest Dermatol. 2012;132:2067–75. Manuscript demonstrates that chronic skin inflammation in a murine model leads to increased vascular inflammation and thrombosis.
Madsen M, Hansen PR, Nielsen LB, Hartvigsen K, Pedersen AE, Christensen JP, et al. Effect of 12-o-tetradecanoylphorbol-13-acetate-induced psoriasis-like skin lesions on systemic inflammation and atherosclerosis in hypercholesterolaemic apolipoprotein e deficient mice. BMC Dermatol. 2016;16:9.
Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326–38.
Griffiths CE, Reich K, Lebwohl M, van de Kerkhof P, Paul C, Menter A, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (uncover-2 and uncover-3): results from two phase 3 randomised trials. Lancet. 2015;386:541–51.
Jameel A, Ooi KG, Jeffs NR, Galatowicz G, Lightman SL, Calder VL. Statin modulation of human t-cell proliferation, il-1beta and il-17 production, and ifn-gamma t cell expression: synergy with conventional immunosuppressive agents. Int J Inflam. 2013;2013:434586.
Salic K, Morrison MC, Verschuren L, Wielinga PY, Wu L, Kleemann R, et al. Resolvin e1 attenuates atherosclerosis in absence of cholesterol-lowering effects and on top of atorvastatin. Atherosclerosis. 2016;250:158–65.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Gregory Sanda, Agastya Belur, Heather Teague, and Nehal Mehta declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Vascular Biology
Rights and permissions
About this article
Cite this article
Sanda, G.E., Belur, A.D., Teague, H.L. et al. Emerging Associations Between Neutrophils, Atherosclerosis, and Psoriasis. Curr Atheroscler Rep 19, 53 (2017). https://doi.org/10.1007/s11883-017-0692-8
Published:
DOI: https://doi.org/10.1007/s11883-017-0692-8