Abstract
As a result of ambiguous results from several recent trials in diabetes, scrutiny has focused on the potential effects of insulin and its role in atherosclerosis. This article reviews the premise that anti-diabetes therapy (type 2 diabetes) with insulin causes vascular impairment that leads to atherothrombosis and compromises vascular integrity, which may further potentiates cardiovascular morbidity and mortality. Underlying mechanisms are discussed, including metabolic derangements (blood pressure, lipids, body weight, and glucose) and how these factors trigger insulin-like growth factor (IGF) receptors, leading to cancer. Cellular and molecular mechanisms are discussed, as well as whether the negative results seen in recent glucose trials support this premise. As with most drug therapy, aggressive therapies designed to reach glucose control targets trigger multiple and inter-related mechanisms that, in many cases, go far beyond the pre-determined physiologic targets. From a clinical perspective, physicians should always stress lifestyle modifications, including physical exercise and diet, to their patients who show the first signs of metabolic impairment. Yet even within this context, diet and exercise should be the cornerstone of good therapy when pharmacotherapy is necessary. Given the amount of evidence seen to date with existing agents and the amount of information we do not yet know, patient-centered approaches to modifying behavior before intensive drug therapy are needed should be stressed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to the overwhelming diabetes epidemic, major population-based research has focused on various mechanisms that either contribute or prevent disease. As more of the population develops type 2 diabetes (T2D) earlier than ever seen throughout history, and more individuals are prescribed and taking insulin therapy to control their disease, certain questions beg to be addressed regarding the effects of therapy designed to keep individuals euglycemic. Because diabetes seldom exists by itself, it stands to reason that scrutiny is necessary from a holistic approach to evaluate how metabolic treatments impact the overall cardiovascular system. Therefore, the goals of this review are to investigate whether there is a pathophysiologic link between exogenous insulin and atherosclerosis through understanding of the various components underlying vascular inflammation, evidence regarding an increase in cancer risk associated with insulin therapy, and clinical recommendations in this area. In addition, cellular and molecular mechanisms are discussed, as well as whether the negative results seen in recent glucose trials support this premise.
In the past few years, three large clinical trials (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation [ADVANCE], Action to Control Cardiovascular Risk in Diabetes [ACCORD], and Veterans Affairs Diabetes Trial [VADT]) have surprised the cardiovascular community with negative results on macrovascular and microvascular events with insulin therapy to achieve intensive glucose control targets. These three trials used insulin as the predominant agent for intensive glucose lowering to achieve glycated hemoglobin (A1C) < 7%. Results showed a lack of benefit on cardiovascular morbidity and an increase in cardiovascular mortality (ACCORD). The lack of cardiovascular benefits can be attributed to the deleterious consequences of severe recurrent hypoglycemia, adverse cardiovascular effects of weight gain due to aggressive blood glucose control using insulin as the predominant agent, and lack of benefit of intensive glucose control in late stages of cardiovascular disease [1•].
Aggressive glucose control with insulin promotes weight gain and worsens central obesity, variables that may very well explain the increased risk for cardiovascular disease. In the ACCORD trial, the increased use of hypoglycemic agents (primarily insulin) in the intensive-treatment arm led to increased mortality. The baseline A1C in the ACCORD trial was 8.1% and with insulin (35 units) it was reduced to 6.4%. Unfortunately, this was accompanied by a 7.7-pound weight gain and 16.2% incidence of hypoglycemia events. In the ADVANCE trial, the baseline A1C was 7.2%. Patients given 1.5 units of insulin in the intensive arm showed a decrease in A1C to 6.5%. The marked difference in weight gain was striking at 0.2 lb, with only 2.7% having hypoglycemia events. In contrast, patients in the VADT study started with an A1C of 9.4%, and after intensive treatment A1C was reduced to 6.5% with (52 units) insulin. Of note was that weight gain was impressive at 17 lb and associated hypoglycemia was reported in 24% of patients in the intensive arm [2•, 3].
Given these startling results, evidence from genomic studies holds promise that genomic risk stratification in patients with T2D may reduce the potential risk for adverse cardiovascular outcomes with exogenous insulin [3]. Given this premise, these results have led us to question whether insulin in patients with insulin-resistant T2D may be pro-atherogenic.
These trials bring into question the potential vasculotoxicity of exogenous insulin in patients who are insulin resistant. The addition of insulin to control blood sugar in patients who already have high circulating insulin levels may not only increase hunger and fluid retention, but may also elevate cardiovascular risk factors by increasing blood pressure and free fatty acid levels (Table 1). Therefore, a closer look at the potential amplification of cardiovascular risk from exogenous insulin is warranted.
Insulin Therapy, Body Weight, Blood Pressure, and Lipids in Type 2 Diabetes
A number of mechanisms have been described to explain the link between weight gain with insulin therapy, such as caloric conservation, changes in metabolism, compensation for hypoglycemia, central effect on appetite and weight regulation, and unphysiologic insulin replacement.
Caloric Conservation
Improvements in glycemic control have been shown to induce caloric conservation and decrease energy expenditure. Thus, patients return to glycemic levels below the renal threshold. To illustrate this point, Carlson and Campbell [4] studied 6 patients with type 1 diabetes. Six adult patients with insulin-dependent diabetes mellitus (IDDM) were studied on conventional insulin therapy and after 2 months of intensive insulin therapy while maintaining constant caloric intake, and they were compared with a group of six matched nondiabetic volunteers. The daily blood glucose concentration was reduced from 14.8 to 7.7 mmol, with A1C reduced from 12.9% to 9.6% (both P < 0.01). This improved control resulted in an almost complete elimination of glycosuria [5]
Metabolic Mechanisms
Insulin produces anabolic mechanisms; as such, it inhibits hepatic glucose output, stimulates peripheral glucose uptake, inhibits lipolysis, and promotes lipogenesis and protein metabolism. Insulin does have the ability to inhibit protein catabolism, and has been used by athletes to augment performance and muscle building. Van den Berghe [6] and associates [5] studied patients in the intensive care unit and reported that insulin improved survival in this highly catabolic group of patients.
Hypoglycemia Compensation
Within this setting, carbohydrate and caloric intake in response to the perceived threat or experience of hypoglycemia is increased.
Central Effect on Appetite and Weight Regulation
Intact insulin and leptin signaling in the brain produce an anorectic response. Any single defect involving these signals could effectively cause “central insulin resistance,” resulting in the anabolic actions of circulating insulin no longer opposed by the catabolic and anorectic neuronal responses. This could trigger a vicious circle of chronic nutrient overload, weight gain, and insulin resistance [5].
Unphysiologic Insulin Replacement
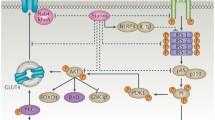
Weight gain associated with insulin therapy suggests that insulin replacement does not accurately reflect the normal physiologic balance between caloric intake and consumption. Intervals of oversupply potentiate hypoglycemic episodes. Subcutaneous insulin administration also has other physiologic effects that are not intrinsically balanced. In this scenario, the absorbed insulin first circulates systemically and has a disproportional influence on muscle and adipose tissue. The liver is “under-insulinized” and the periphery is “over-insulinized.” This cascade might explain the disproportionate increase in fat mass typically reported with insulin therapy. Unphysiologic under-insulinization of the liver due to systemic (subcutaneous) insulin delivery reduces hepatic insulin-like growth factor (IGF) production and therefore interferes with the maintenance of body composition and the balance of anabolism and catabolism.
These mechanisms have been shown in seminal trials, such as the Diabetes Control and Complications Trial (DCCT) [7]. Similarly, in the United Kingdom Prospective Diabetes Study (UKPDS), insulin-treated patients had the greatest weight gain, with a mean of 6.5 kg [8].
Effects of Insulin Therapy on Blood Pressure and Secondary Failure
Epidemiologic and experimental evidence have shown that insulin therapy is associated with significant elevation of both systolic and diastolic blood pressures (BP); however, its mechanism remains unclear and controversial. For example, an increase in arterial BP has been reported 6 months after initiation of insulin therapy in patients with secondary failure to oral hypoglycemic drugs (SFOH) [9].
In DCCT, a study designed to determine the effect of intensive diabetes therapy on the microvascular complications of type 1 diabetes, both systolic and diastolic BP were significantly higher only in the fourth quartile of weight gain compared with the first three quartiles (P < 0.05). These changes were similar to those seen in the insulin resistance syndrome [10]. Randeree et al. [9] assessed retrospectively a group of 80 diabetes patients with secondary failure to diet and maximum doses of oral hypoglycemic agents. Weight, blood glucose, and BP were recorded over a 3-month period before and after the initiation of insulin therapy. There was a significant rise in systolic (131.8 ± 1.7–148 ± 1.9 mm Hg; P < 0.05) and diastolic (80.9 ± 0.9–89.2 ± 1.0 mm Hg; P < 0.02) BP with insulin treatment [7, 9].
Effects of Insulin Therapy on Lipids and Atherothrombosis
Cardiovascular risk related to insulin-resistant state comes from basic science research in animals completed by Matsumoto et al. [11], Unger [12], and Brown and Goldstein [13]. Matsumoto et al. [11] found that insulin stimulates phosphorylation of FoxO1, a transcription factor that stimulates glucose production. Insulin phosphorylates FoxO1, resulting in a reduction of genes required for glucose production. In the insulin-resistant state, FoxO1 becomes dysfunctional and glucose regulation goes unchecked. Brown and Goldstein [12] further amplified the concern by presenting research in mice with T2D. These mice express selective liver insulin resistance because insulin fails to suppress gluconeogenesis while at the same time continues to stimulate lipogenesis, producing a deadly combination of hyperglycemia and hypertriglyceridemia. These dual defects illustrate the reduction in FoxO1 function (loss of glucose control), and marked stimulation of the transcription factor SREBP-1c (increases genes involved in fatty acid/triglyceride biosynthesis) by insulin leads to increased potential lipotoxicity on the cardiovascular system.
Diabetes is a hypercoagulable state with predisposition for atherothrombosis that leads to increases in fibrinogen, plasminogen activator inhibitor-1 (PAI-1) levels, and platelet aggregability [14]. Using data from the randomized prospective Veterans Affairs Cooperative Study in Type II Diabetes Mellitus study, Emanuele et al. [15] reported the effects of intensive glycemic control therapy on plasma fibrinogen, serum triglycerides, total cholesterol, low-density lipoprotein (LDL), cholesterol, apolipoprotein B (Apo B), lipoprotein (a) (Lp[a]), high-density lipoprotein (HDL) cholesterol, and Apo A1. Subjects were male, aged 40–69 years, with T2D for 15 years or less and divided into intensive- and standard-treatment arms receiving maximum doses of sulfonylurea and/or any dose of insulin. Dyslipidemia was managed identically in both arms with diet and drugs. Fibrinogen levels and lipid fractions were measured in the full cohort; plasma fibrinogen levels rose significantly in the intensive treatment arm after 1 year (3.34 ± 0.12 g/L to 3.75 ± 0.15 g/L; P = 0.001). Plasma fibrinogen levels returned to levels that were not different from baseline after 2 years of intensive therapy (3.47 ± 0.12 g/L), and there was no change in plasma fibrinogen levels in the standard-treatment arm. Data were based on analyses of the full cohort. There was no correlation between change in insulin dose and change in fibrinogen level in either treatment arm or in the entire cohort. Likewise, there was no correlation between change in body mass index and change in fibrinogen level.
PAI-1 is considered to be an important regulator of fibrinolysis. PAI-1 levels are elevated in T2D and known to be associated with macro- and microvascular complications of diabetes due to increased risk of atherothrombosis. In addition, it is thought that there is a strong association between PAI-1 and the metabolic components of the insulin-resistance syndrome based on findings from prior clinical studies, suggesting that insulin resistance may regulate PAI-1. Visceral adipose tissue may be closely associated between android obesity and PAI-1, and adipose tissue PAI-1 production has been found to be elevated in obese human subjects, and particularly in visceral adipose tissue. High plasma PAI-1 levels are therefore thought to be related to android obesity and insulin resistance, but exact mechanisms remain to be elucidate [16]
Effects of Insulin Therapy on Cancer Risk
Recent literature has reported a link between insulin therapy and cancer (Table 2). Moreover, three of the five leading causes of cancer mortality in the United States (carcinoma of the colon, pancreas and breast) are associated with T2D, obesity, and insulin resistance [17, 18]. The estimated excess risk for each cancer is ~30% for colon [19], ~50% for pancreas [20], and ~20% breast cancer [21].
Several studies have evaluated the association between insulin and cancer risk in the setting of obesity, diabetes, and metabolic derangements and have consistently found that insulin is a major determining factor. Bowker et al. [22] evaluated the relationship between anti-diabetes therapy and mortality and found that cancer mortality was almost twice that among insulin users (hazard ratio [HR] of 1.9; 95% CI, 1.5–2.4; P < 0.0001) compared to patients receiving metformin.
In a population based study in Sweden by Jonassan et al. [23], 114,841 individuals on insulin were followed during 2006 and 2007. After adjusting for age and sex, patients taking insulin glargine alone compared with those who took other types of insulin had a relative risk of 1.99 (95% CI, 1.31–3.03) for breast cancer; 0.93 (95% CI, 0.61–1.40) for gastrointestinal cancer; 1.27 (95% CI, 0.89–1.82) for prostate cancer; and 1.07 (95% CI, 0.91–1.27) for any type of malignancy [23].
In a retrospective cohort study in the United Kingdom by Currie et al. [24], a total of 62,809 T2D patients prescribed oral agents or insulin were divided into four groups: metformin alone, sulfonylurea alone, metformin plus sulfonylurea, or insulin. Insulin users were then divided by group based on whether they took insulin glargine, long-acting human insulin, biphasic analogue, or human biphasic insulin. Results showed that those in the metformin-monotherapy group had the lowest risk of cancer. In comparison, the adjusted HR was 1.08 (95% CI, 0.96–1.21) for metformin plus sulfonylurea, 1.36 (95% CI, 1.19–1.54) for sulfonylurea monotherapy, and 1.42 (95% CI, 1.27–1.60) for insulin-based regimens. Surprisingly, adding metformin to insulin reduced progression to cancer (HR = 0.54; 95% CI, 0.43–0.66). The risk for those on basal human insulin alone versus insulin glargine alone was 1.24 (95% CI, 0.90–1.70). Compared with metformin, insulin therapy increased the risk of colorectal (HR = 1.69; 95% CI, 1.23–2.33) or pancreatic cancer (HR = 4.63; 95% CI, 2.64–8.10), but did not influence the risk of breast or prostate cancer. Sulfonylurea use was associated with a similar pattern of risk as insulin. Thus the authors concluded that patients on insulin or insulin secretagogues were at a higher risk for the development of malignancy than those on metformin alone, and this excess malignancy risk was abolished with the addition of metformin to the treatment regimen. In addition, they also found that insulin analogues were not associated with increased cancer risk as compared with human insulin.
In a recent study by the Scottish Diabetes Research Network Epidemiology Group, Colhoun [25] used a nationwide diabetes clinical database that covered the majority of the Scottish population with diagnosed diabetes and examined patients with diabetes who were exposed to any insulin therapy between January 1, 2002 and December 31, 2005. Those receiving any insulin glargine (n = 3,959) had the same incidence rate for all cancers as those not receiving insulin glargine (HR = 1.02; 95% CI, 0.77–1.36; P = 0.9 in the fixed cohort). However, the subset of patients using insulin glargine alone (n = 447) had a significantly higher incidence of all cancers than those using other insulins only (n = 32,295) (HR = 1.55; 95% CI, 1.01–2.37; P = 0.045), and those using insulin glargine with other insulins (n = 3,512) had a slightly lower incidence (HR = 0.81; 95% CI, 0.55–1.18; P = 0.26). Overall, there was no increase in breast cancer rates associated with insulin glargine use (HR = 1.49; 95% CI, 0.79–2.83), although insulin glargine-only users had a higher rate than those using non-glargine insulin only (HR = 3.39; 95% CI, 1.46–7.85; P = 0.004). Overall, insulin glargine use was not associated with an increased risk of all cancers or site-specific cancers in Scotland over a 4-year time frame. Given the overall data, we consider the excess of cases of all cancers and breast cancer in the subgroup of insulin glargine-only users to more likely reflect allocation bias rather than an effect of insulin glargine itself [25].
At the molecular level, IGF is a peptide that regulates cell proliferation, differentiation, and apoptosis. IGF peptides occur in large concentrations in the circulation and have systemic, hormonal, and local paracrine effects on cell behavior. In the circulation, IGF-I binds mainly to the main IGF binding protein, IGFBP-3. IGF-I is mitogenic and antiapoptotic, whereas IGFBP-3, thought to inhibit growth, is anti-proliferative and pro-apoptotic. Results of early studies on risk of prostate, breast, colorectal, and lung cancer suggest that high circulating IGF-I concentrations are associated with an increased risk of cancer, whereas high IGFBP-3 concentrations are associated with a decreased risk.
In a recent systematic review and meta-regression analysis evaluating association between concentrations of IGF-I and IGFBP-3 and prostate, colorectal, premenopausal and postmenopausal breast, and lung cancer, Renehan et al. [26] identified 21 eligible studies (26 datasets), which included 3,609 cases and 7,137 controls. High concentrations of IGF-I were associated with an increased risk of prostate cancer (odds ratio comparing the 75th with the 25th percentile of 1.49 [95% CI, 1.14–1.95]) and premenopausal breast cancer (odds ratio of 1.65; 95% CI, 1.26–2.08), and high concentrations of IGFBP-3 were associated with increased risk of premenopausal breast cancer (odds ratio of 1.51; 95% CI, 1.01–2.27) [26].
Conclusions
The three negative large diabetes trials cause one to consider the current therapeutic options for diabetes, a condition that is largely preventable. The off-target (side effects) impact of drug therapy in this group of patients at high risk of subsequent cardiovascular disease requires further scrutiny. Fluid retention and elevations in BP in combination with increased lipogenesis risk associated with traditional diabetes therapy cause one to reconsider therapies that might be less atherogenic (those proven to reduce classic risk factors or that have neutral cardiovascular risk) first. It is important to remember these comments only relate to an insulin-resistant state, which is not common in type 1 diabetes. In light of concern with intensive glucose control using insulin therapy as a predominant agent in T2D, lifestyle modification with diet and exercise should always be first-line treatment options, and the use of agents other than insulin should be strongly considered earlier in the course of the therapy in order to minimize or prevent secondary morbidity and mortality.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
• Faramarz Ismail-Beigi MD, Timothy Craven MSPH, Mary Ann Banerji MD, Jan Basile MD. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. The Lancet, Volume 376, Issue 9739, Pages 419–30. This trial brings into question the protection of microvascular events with good glucose control. Thoughtful questions arise in this recent publication with comparison to older trials.
• Brown A, Reynolds LR, Bruemmer D. Intensive glycemic control and cardiovascular disease: an update. Nat Rev Cardiol. 2010 Jul;7(7):369–75. This is an excellent quick overview of the recent trials using intensive glycemic control. It has quick charts to read with important contrasts between trials.
Schernthaner G. Diabetes and Cardiovascular Disease: Is intensive glucose control beneficial or deadly? Lessons from ACCORD, ADVANCE, VADT, UKPDS, PROactive, and NICE-SUGAR. Wien Med Wochenschr. 2010 Jan;160(1–2):8–19.
Carlson MG, Campbell PJ. Intensive insulin therapy and weight gain in IDDM. Diabetes 1993;42:1700–07.
Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes—causes,effects and coping strategies. Diabetes Obes Metab. 2007 Nov;9(6):799–812. Review.
Greet Van den Berghe. J. Clin. Invest. 114:1187–95 (2004).
The DCCT Research Group. Weight gain associated with intensive therapy in the Diabetes Control and Complications Trial. Diabetes Care. 1988;11:567–73.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53.
Randeree H, Omar M, Motala A, Seedat M. Effect of insulin therapy of blood pressure NIDDM patients with secondary failure, Diabetes Care. 1992;15:1258–63.
Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT. Diabetes Control and Complications Trial. JAMA. 1998 Jul 8;280(2):140–6. Erratum in: JAMA 1998 Nov 4;280(17):1484.
Matsumoto M, Han S, Kitamura T, Accili D. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest. 2006 Sep;116(9):2464–72. Epub 2006 Aug 10.
Unger RH. How obesity causes diabetes in Zucker diabetic fatty rats. Trends Endocrinol Metab. 1997 Sep;8(7):276–82.
Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008 Feb;7(2):95–6. Review.
Thompson SG, Kienast J, Pyke SD, Haverkate F, van de Loo JC, for the European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. N Engl J Med. 1995;332:635–41.
Emanuele N. Veterans Affairs Cooperative Study in Type II Diabetes Mellitus. Arch Intern Med. 1998;158:2485-90.
Bastard JP, Piéroni L. Plasma plasminogen activator inhibitor 1, insulin resistance and android obesity. Biomed Pharmacother. 1999 Dec;53(10):455–61.
Coughlin SS et al. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159:1160–7.
Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate and pancreas. Gastroenterology. 2007;132:2208–25.
Larsson SC, Orsini N, Wolk A. Diabetes and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(1679–1687):3.
Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92(2076–2083):4.
Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121:856–62.
Bowker SL et al. Increased cancer-related mortality for patients with T2D who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–8.
Jonasson JM, Ljung R, Talbäck M, Haglund B, Gudbjörnsdòttir S, Steineck G. Insulin glargine use and short-term incidence of malignancies-a population-based follow-up study in Sweden. Diabetologia. 2009 Sep;52(9):1745–54. Epub 2009 Jul 9.
Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009 Sep;52(9):1766–77. Epub 2009 Jul 2.
Colhoun HM; SDRN Epidemiology Group. Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia. 2009 Sep;52(9):1755–65. Epub 2009 Jul 15.
Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004 Apr 24;363(9418):1346–53. Review.
Disclosure
Robert Chilton has received honoraria and speaking fees from Boston Scientific, Pfizer, MSD, Novo Nordisk, Amylin, Medtronics, Takada, and GlaxoSmithKline.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nandish, S., Bailon, O., Wyatt, J. et al. Vasculotoxic Effects of Insulin and Its Role in Atherosclerosis: What is the Evidence?. Curr Atheroscler Rep 13, 123–128 (2011). https://doi.org/10.1007/s11883-011-0165-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11883-011-0165-4