Abstract
Airborne fungal spores constitute a significant fraction of atmospheric bioparticles, and most of them are responsible for causing the respiratory allergy. The present study deals with the evaluation of fungal aerospora by microscopy-based and culture-based methods in one outdoor and six indoor microenvironments in Kolkata, India, from May 2014 to April 2017. The association of environmental parameters with spore concentrations was explored by Spearman’s rank correlation analysis and multiple regression analysis. The impact of spore concentrations on the local population was assessed through a questionnaire survey, linear regression analysis, and Skin Prick Test (SPT). The maximum spore concentration was found in the outdoor environment. Ascospores, Cladosporium spp., Aspergillus/Penicillium spp., and basidiospores were found as major taxa recorded by microscopy-based method, whereas in culture-based method, Aspergillus spp. were abundant. In the outdoor, particles with aerodynamic diameter < 10 µm (PM10) and in indoors, temperature, relative humidity, wind speed, average sun hour, PM10, and ambient nitrogen dioxide concentration (NO2) were identified as significant predictors. The linear regression analysis showed several positive associations of major taxa with respiratory diseases in the local inhabitant. SPT with several fungi was able to induce allergic inflammation in a selected atopic patient cohort. Analysis of spore concentrations and their relation with environmental parameters will give an insight into the air quality in Kolkata. The association with respiratory diseases will shed a light on the increasing burden of airway diseases in the urban megacity. Observations from this study will be useful for assessing the potential health impact on residents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Exposure to airborne bio-particulate matters, such as fungal spores and pollen grains, commonly termed as “bioaerosols” results in a variety of adverse health effects including allergic disorders like severe asthma, allergic rhinitis, anaphylaxis, allergic broncho-pulmonary diseases, and other respiratory diseases. Fungi, being ubiquitous, contribute approximately 4–11% of ambient bioaerosols in the urban and rural environment (Womiloju et al. 2003) and are known to cause major health risks by having a greater impact on human health as they are associated with a broad panel of diseases including IgE-mediated type I hypersensitivity, life-threatening primary and secondary infections in immunocompromised patients, allergic bronchopulmonary mycosis (ABPM), hypersensitivity pneumonitis, fungal sinusitis, and toxic pneumonia. Additionally, they can produce a variety of mycotoxins having neurotoxic, mutagenic, carcinogenic, and teratogenic effects and cause mycotoxicoses (Simon-Nobbe et al. 2008). They also produce proteases, enzymes (Kauffman et al. 2000), and volatile organic compounds (Fischer et al. 1999) which may affect airways (Simon-Nobbe et al. 2008). Moreover, they can colonize inside the human body. Approximately, 1–1.5 million fungal species exist across the world of which 80,000 have been identified so far (Teresa et al. 2015). Fungi are common in the ambient air, and their small spore size (average size: 2–10 µm; Simon-Nobbe et al. 2008) leads to the enhancement of their dispersion in the atmosphere and deposition in the human respiratory tract as well (Yamamoto et al. 2012). About 30% of the total allergic patients are sensitized by fungal aeroallergen–induced allergy, imposing a major public health concern (Kurup et al. 2000; Singh and Shahi 2008). Till now, 113 fungal allergens from 30 fungal genera have been officially recognized by the World Health Organization and International Union of Immunological Society (WHO/IUIS) Allergen Nomenclature Sub-committee (www.allergen.org). Approximately, 112 genera especially belonging to Ascomycota and Basidiomycota phylum are considered as the possible source of allergens to induce type I hypersensitivity reactions (Teresa et al. 2015).
The influence of climate change on the prevalence and temporal distribution of airborne fungi in various countries has been documented in recent studies (Fernández-Rodríguez et al. 2018). Atmospheric variables influence the fungal spectrum of a specific area. Various studies have demonstrated the effect of meteorological parameters and air pollutants on ambient fungal spore concentrations in different geographic regions (Kallawicha et al. 2017; Pyrri and Kapsanaki-Gotsi 2017). Both indoor and outdoor fungal aerospora can be able to induce sensitization in atopic patients (Green et al. 2006). Therefore, to evaluate the exposure of ambient spore load, it is important to explore the diversity of fungal spores and their seasonal variation in outdoor as well as indoor environments, as the outdoor environments directly influence indoor environments (D’Amato et al. 2015).
In the present study, three-year-long biomonitoring was conducted in outdoor and several indoor environments of the Kolkata megacity. The aim of this study was the quantification of the fungal aerospora by monitoring in the given environments to investigate their temporal distribution and association with environmental factors and to determine the impact of their allergenicity on the health of the local population regarding respiratory allergy. Previously, several studies have been performed only in the outdoor environment of an urban area (Dey et al. 2018) and several suburb areas in Kolkata (Chakrabarti et al. 2012; Chakraborty et al. 2003; Das and Gupta-Bhattacharya 2012). This study was the first attempt to evaluate fungal aerospora in the different indoor areas along with the outdoor atmosphere in the core of the urban area in Kolkata and to investigate the impact of atmospheric pollutants on the spore concentrations.
Materials and methods
Study area and sampling sites
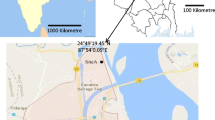
The aerial concentration of fungal spores was monitored from May 2014 to April 2017 in Kolkata. Kolkata (22.57° N and 88.37° E), situated on the Indo-Gangetic plain of West Bengal, is one of the largest urban megacities of India. It is the seventh-most populous city in India (Census India 2011) with a population density of 24,252/km2 and a male to female ratio of 1000:899. The study area is thickly populated and crowded with all civic amenities. Many institutional, commercial, and residential buildings along with the congested marketplace and garden are present in and around the area. The connecting roads have high traffic throughout the day. Several weeds, herbs, shrubs, and large trees including ornamental trees are present in the proximity of the sampling area. Seven microenvironments were selected as sampling sites in this study area. Among them, school, university, and library were chosen for the educational area, whereas a hospital, bank, and an institutional workshop were categorized under the occupational area. Along with these six indoor environments, a garden with an open field area was also selected as an outdoor environment. The geographic locations of selected sampling sites are presented in Fig. 1. The characteristic features of each site are described in Online Resource 1.
Sampling and identification of airborne fungal spores
Biomonitoring of fungal aerospora in different indoor and outdoor environments was executed by using Burkard personal volumetric sampler (Burkard Manufacturing Co. Ltd. Hertfordshire, UK) as well as Andersen two-stage sampler (Thermo Andersen, Smyrna, USA). The sampling was carried out at a height of 1.5 m above the ground level between 12 noon and 3 pm once every week of a month. Monthly average values of spore concentrations recorded by both samplers were considered for analysis.
Total fungal spores were trapped onto vaseline-coated glass slides placed inside the Burkard personal volumetric sampler (suction rate: 10 l/min). The sampling time was standardized for 15 min. Exposed slides were mounted with a coverslip and Dibutylphthalate Polystyrene Xylene (DPX). Trapped spores were observed by using Nikon high-resolution light microscope (Labophot 2, Nikon Corp., Japan) at 400x magnification. The spores were identified up to the genus level by using standard manuals (Ellis 1971; Smith et al. 1981) and counted according to the guidelines of The British Aerobiology Federation (1995). Spores that were difficult to identify were considered as “Others”. Finally, total spore counts were converted into a number per cubic meter of air (m3) by multiplying with the conversion factor of 6.67.
Ambient fungal spores were captured by using Andersen two-stage sampler (suction rate: 28.3 l/min) through the orifice at the top. Spores were trapped on petri plates containing potato dextrose agar (PDA) medium supplemented with chloramphenicol (34 µg ml−1) (SRL, India) for preventing bacterial growth. After 3 min of exposure, the petri plates were incubated at 27 ± 2 °C for 3–7 days until sporulation. The pure isolates were obtained through periodic subculturing and observed under the Nikon high-resolution light microscope (Labophot 2, Nikon Corp., Japan) at 400x magnification by using lactophenol blue stain (Sigma–Aldrich, USA). The fungal isolates were identified up to the genus level by following standard reference manuals (Onions et al. 1981; Subramanian 1971). Non-sporulating colonies or sterile mycelia were grouped as “Others.” Fungal colonies were counted and expressed as Colony Forming Units (CFU) per m3 of air by multiplying with the conversion factor of 11.77.
Burkard personal volumetric sampler trapped total fungal spores, i.e., both non-viable and viable fungi. In this method of sampling, we have identified and quantified the trapped fungal spores only by microscopy, due to being devoid of any growth medium. Therefore, in this study, this sampling method was considered as the microscopy-based method. On the other hand, Andersen two-stage sampler provided data on viable fungi cultured on the growth medium. Thus, this sampling method was denoted as the culture-based method.
Environmental parameters
Meteorological parameters were obtained from the Indian Meteorological Department, Kolkata. The concentrations of air pollutants were collected from the public database of the official website of The West Bengal Pollution Control Board (www.wbpcb.gov.in) for Kolkata. The environmental parameters used for this study were maximum, minimum, and average temperature (Tmax, Tmin, Tavg, respectively), relative humidity (r.h.), wind speed (W), total rainfall (R), average sun hour (Avg SH), suspended particulate matters (PM10 and particles with aerodynamic diameter < 2.5 µm, PM2.5), nitrogen dioxide (NO2), sulfur dioxide (SO2), carbon monoxide (CO), and ozone (O3). For statistical analysis, monthly average values of the environmental parameters were considered. The entire sampling period was divided into four distinguished seasons such as summer (March–May), monsoon (June–September), post-monsoon (October–November), and winter (December–February).
Collection of hospitalization data and questionnaire survey
Daily patient data were obtained by visiting the outpatient department of the nearest hospital for three consecutive years. 10,711 people (6574 males and 4137 females), from the local population, were taken into account for this survey. The total number of patients, with age groups ranging from 6–82 years, was divided into four categories, based on their diagnosed disease. Group A—“Asthma” (n = 1955); group B—“COPD” (chronic obstructive pulmonary disease) (n = 1673); group C—“PTB” (pulmonary tuberculosis) (n = 2141), and group D—“Others” (n = 4942), includes patients suffering from general respiratory and allergic symptoms like cough, cold, sneezing, wheezing, breathlessness, allergic rhinitis and some unspecific problems. The survey was executed with selected patients by using a set of questionnaires. The format was prepared by Bose Institute, Kolkata by following the format mentioned by Singh et al. (1999). The questionnaire consisted of questions on personal information of local people such as age, occupation, living environments, the impact of any respiratory symptoms and allergic disorders, and family history of atopic diseases.
Allergenicity assessment of fungi by SPT
For investigating the allergenicity of several fungal species obtained by the culture-based method, the SPT was performed against atopic patients (n = 145) with their prior consent according to the ethics of the hospital. From the local population, asthmatic patients (n = 52) from group A and patients with allergic rhinitis (n = 63) and other respiratory discomforts (n = 30) from the “Others” group (group D) were considered for this experiment. All selected subjects visited the hospital more than four times per year due to frequent sensitization. Smoking, pregnancy, and patients with severe asthma and other severe systemic diseases were excluded from the study. SPT was carried out with our supplied 20 µl of crude antigenic extract (1:10 w/v), prepared by extracting the crude total protein from spore mycelia of the selected species. It was placed on the surface of the arms and pricked with a sterile lancet. After 20 min, the wheel reaction was measured and graded from + 1 to + 4 (Stytis et al. 1982). Histamine diphosphate (1 mg ml−1) and phosphate buffer (0.01 M, pH 7.2) were used as the positive and negative control, respectively.
Statistical analysis
The data were analyzed in GraphPad Prism 6.0 (San Diego, CA, USA) and IBM SPSS version 24.0 (IBM, Armonk, NY, USA). The normality of the dataset was checked. The consistency in spore concentrations during the monitoring period was analyzed through one-way ANOVA followed by Tukey’s multiple comparison test. The temporal distribution of spore concentrations and environmental parameters across different seasons was analyzed through one-way ANOVA or Kruskal–Wallis test. The correlations of spore concentrations found by both sampling methods, with individual environmental parameters, were analyzed through Spearman’s rank correlation coefficient (r) (two-tailed), for each sampling site. Further univariate analysis was performed between spore concentrations and potential predictor environmental variables. Variables with a p-value < 0.2 were included in multiple regression analysis with aerial concentrations of total spores and major fungal taxa, obtained through both sampling techniques, for every sampling site, considering a linear relation between them. The stepwise linear regression analysis was performed, and variables with p-value < 0.01 were included in the final regression models. The seasonal changes of fungal concentrations were also considered as a categorical variable in multiple regression analysis. The spore concentrations were transformed to logarithmic base 10 for regression analysis for an approximation to normality. The linear relation between concentrations of total spores as well as major taxa of both methods and recorded respiratory disease cases were analyzed through regression analysis. The incidence of respiratory disease between males and females for every disease category was analyzed through an unpaired t-test.
Results
Spore concentrations in different microenvironments
In this study, the concentration of total fungal spores, sampled with the microscopy-based method, ranged from 9561.8 to 22,727.5 spores m−3 with mean ± SD: 13,176.11 ± 3795.618 spores m−3, and the total spore concentrations sampled by the culture-based method varied from 7111.8 to 14,741.28 CFU m−3 with mean ± SD: 10,748.89 ± 1698.957 CFU m−3. Total spore concentration was maximum in the open field area, i.e., outdoor environment for both sampling methods, whereas minimum concentration was found in the indoors such as the occupational area in terms of the microscopy-based method and the educational area for the culture-based method (Online Resource 2).
In the microscopy-based method, among seven sampling sites, the garden exhibited the highest spore concentration (mean ± SD: 4018.239 ± 2472.521 spores m−3), and the bank microenvironment showed the lowest concentration (mean ± SD: 607.4181 ± 250.7084 spores m−3) (Fig. 2a). The frequency distribution of fungal aerospora and their concentrations are presented in Table 1. Among them, ascospores, Cladosporium spp., Aspergillus/Penicillium spp., and basidiospores contributed more than 66% with mean concentrations of 2593.1, 2490.9, 2116.9, and 1580.8 spores m−3, respectively, hence considered as dominant taxa. However, the concentrations of major taxa varied in seven sampling sites (Fig. 2b). The distribution of fungi in seven microenvironments is also presented in Online Resource 3.
Temporal and spatial distributions of airborne fungi at all indoor and outdoor environments in Kolkata during May 2014–April 2017: a Total fungal spore counts from microscopy-based method with seasonal periodicity pattern. b Aerial concentrations of four major taxa obtained through the microscopy-based method. c Pattern of seasonal periodicity of total fungal spore concentrations of culture-based method. d Aerial concentrations of four major taxa found by using the culture-based method. Bars represent mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 using One-way ANOVA or Kruskal–Wallis test
On the other hand, for fungal isolates grown by culture-based method, school exhibited maximum spore concentration with mean ± SD: 1829.091 ± 384.8068 CFU m−3, whereas the lowest concentration was found in the bank (mean ± SD: 1014.451 ± 302.7418 CFU m−3) (Fig. 2c). Aspergillus spp., Penicillium spp., Rhizopus spp., and Curvularia spp. were observed as dominant with mean concentrations of 5682.3, 1013.7, 668.2, and 605.2 CFU m−3, respectively (Table 2). Being abundant in all studied microenvironments throughout the year, Aspergillus spp. was the most prevalent genus accounting for more than 52% of total spores of the culture-based method (Fig. 2d; Table 2). The concentrations of major fungal isolates in the individual microenvironment are shown in Fig. 2d. Additionally, we have observed different fungal spectra for different culture techniques, and the spore concentration obtained through the microscopy-based method is higher than the culture-based method. However, for each sampling method, mean concentrations of the total fungal spores and major taxa between consecutive years remain statistically insignificant, except for Aspergillus/Penicillium spp. as shown in Online Resource 4.
Association of ambient fungal spore concentrations with environmental parameters
The distribution of environmental parameters across different seasons varied significantly (Table 3). The seasonal changes showed a significant effect on ambient spore concentrations in indoor and outdoor microenvironments for both sampling techniques (Fig. 2a. c). Maximum spore concentrations were observed during the post-monsoon season with mean ± SD: 17,840.41 ± 2623.446 spores m−3 (microscopy-based method) and 12,098.45 ± 2533.606 CFU m−3 (culture-based method) and minimum during the winter season (microscopy-based method: mean ± SD: 10,870.82 ± 2011.283 spores m−3; culture-based method: mean ± SD: 8587.931 ± 803.483 CFU m−3) (Fig. 3a, b).
For fungal spores recorded by microscopy-based method, the result revealed that Cladosporium spp. was the key contributor to the post-monsoon season showing the maximum peak. Ascospores were also identified as the major component of the subsidiary peak found in the summer season (Fig. 3a). Basidiospores were found to be higher in post-monsoon, whereas monthly concentrations of Aspergillus/Penicillium spp. revealed its abundance throughout the year and no such prominent seasonal dependency was displayed. Monthly concentrations of the major taxa of both microscopy-based and culture-based techniques were graphically represented in Online Resource 5 and Online Resource 6 respectively. In the outdoor area, only basidiospores showed significant seasonal variation, whereas it was prominent in total spore concentrations (Fig. 2a) and all major fungi in several indoor microenvironments (Online Resource 7).
In the culture-based method, Aspergillus spp. exhibited maximum concentration in the post-monsoon season, whereas, Penicillium spp., Rhizopus spp., and Curvularia spp. were found to be higher in the summer season (Fig. 3b). Concentrations of total spores (Fig. 2c), Aspergillus spp., Penicillium spp., and Curvularia spp. showed significant seasonal periodicity in the outdoor as well as in different indoor microenvironments (Online Resource 7). Contrarily, Rhizopus spp. did not show any significant seasonal variation.
To assess elaborately, the correlations between environmental parameters and the ambient fungal spore concentrations sampled by both methods, in different microenvironments, were determined through Spearman’s rank correlation coefficient (r) (Table 4). Furthermore, the stepwise multiple regression analysis identified the key atmospheric predictors of the total spores and dominant taxa in different microenvironments (Table 5).
Correlation of fungal spore concentrations with meteorological parameters
In this study, the ambient temperature showed a significant positive association with all major fungal taxa found by microscopy-based as well as culture-based methods in both outdoor and indoor environments except Cladosporium spp. (Table 4). A significant negative association between temperature and Cladosporium spp. was found in the garden. Besides, in the university, the average and minimum temperature were found to be significant predictors for Aspergillus/Penicillium spp. and Aspergillus spp., respectively, by multiple regression analysis (Table 5). The regression model also showed the average temperature as a significant predictor of total spores of the culture-based method in the hospital environment (Table 5).
A significant positive association between average sun hour and ascospores in outdoor as well as indoor environments (school and workshop) was observed. In several indoor areas, it also had a significant positive impact on Aspergillus/Penicillium spp., basidiospores, total spores of the culture-based method, Aspergillus spp., and Penicillium spp. (Table 4). Moreover, it was attributed as an important predictor of ascospores in the school along with total spores of the culture-based method in the university and the library through the multiple regression analysis (Table 5).
Rainfall presented negative correlations with Cladosporium spp. in outdoor and several indoor microenvironments (Table 4), whereas ascospores showed a significant positive relationship with it in the garden.
Relative humidity was positively associated with ascospores and basidiospores in the outdoor environment (Table 4). It showed a positive association with basidiospores in all indoor microenvironments and was identified as a significant predictor in the university and the library through the regression analysis (Table 5). Conversely, in the library, it was negatively correlated with Cladosporium spp. and detected as a key predictor as well (Table 5).
Wind speed showed a significant positive association with ascospores and basidiospores in the garden. A significant negative impact of wind speed was also observed on Cladosporium spp., Curvularia spp., and total spore concentrations of the culture-based method in the outdoor environment (Table 4). Among several positive associations observed indoors, it was identified as a key predictor for ascospores in the university, whereas in the library, it was associated negatively with total spore concentrations of the microscopy-based method and also detected as a significant predictor (Table 5).
Correlation of fungal spore concentrations with atmospheric pollutants
The concentrations of both PM2.5 and PM10 in the outdoor area have a significant positive correlation with Cladosporium spp., total spores of the culture-based method, and Curvularia spp. (Table 4). In the outdoor environment, PM10 was identified as the key predictor regulating aerial concentrations of Cladosporium spp. by the regression model (Table 5). Conversely, ascospores and basidiospores were negatively associated with PM2.5 and PM10 in the outdoor area (Table 4). On the other hand, in indoor areas, both PMs showed a negative impact on ascospores, Aspergillus/Penicillium spp., and basidiospores. The multiple regression analysis also detected PM10 as an important predictor variable having a negative effect on basidiospores in the bank as well as total spores of the culture-based method in the university (Table 5).
All gaseous pollutants (SO2, NO2, CO, and O3) mostly affect different major taxa negatively in both environments (Table 4). SO2 and NO2 were negatively associated with ascospores and basidiospores in outdoor and indoor areas. Cladosporium spp. also showed negative correlations with them in outdoor. Multiple regression analysis also revealed that NO2 could be a key predictor of Curvularia spp. by affecting negatively in the workshop (Table 5). Although SO2 and NO2 exhibited few significant positive associations with total spores of the culture-based method in the outdoor as well as with Cladosporium spp. in the university. NO2 also had negative impact on ascospores and Cladosporium spp. in the bank and library respectively.
CO showed several significant negative associations in indoor microenvironments, whereas in outdoor, Cladosporium spp. and basidiospores were negatively correlated with CO (Table 4). A significant positive impact of CO was only observed with total spores of the culture-based method in the garden, Cladosporium spp. in the hospital, and Curvularia spp. in the bank microenvironment. A negative association between O3 and Aspergillus/Penicillium spp. was also observed in the hospital microenvironment. Similarly, ascospores and total spores of the culture-based method exerted a negative association with it, whereas a positive correlation was also noticed with Rhizopus spp. (Table 4).
Assessment of the effect of ambient spore concentrations on respiratory health
To evaluate the effect of fungal spore concentrations on the local people, patients’ data were collected during the entire monitoring period. It revealed that among four disease categories, the “Others” group which includes cough, cold, sneezing, wheezing, breathlessness, allergic rhinitis, etc. was the most frequent, reported in a maximum number of patients (46.14%), followed by “PTB” (19.99%), “Asthma” (18.25%), and “COPD” (15.62%). It was also observed that patients visited the hospital most in the winter season and minimum in the post-monsoon season, contradicting our findings from the monitoring. However, upon analyzing the monthly patients’ visits to the hospital and monthly concentrations of total spores and major taxa of seven microenvironments (Fig. 4), as well as total spores of the outdoor environment and indoor environments (Online Resource 8) for both sampling methods, it seems that the exposure to fungal spores might have an important role in promoting sensitization to the patients with underlying lung conditions.
Relationship between monthly respiratory disease cases and concentrations of total spores as well as major taxa at both indoor and outdoor environments in Kolkata from May 2014 to April 2017: monthly patients’ visits with a total spores and Cladosporium spp.; b ascospores, Aspergillus/Penicillium spp., and basidiospores, recorded by the microscopy-based method; c total spores and Aspergillus spp.; d Penicillium spp., Rhizopus spp., and Curvularia spp., obtained by the culture-based method. Symbols represent mean ± SD
For further analysis, the relationship between diseases and total spore concentrations of both sampling methods along with the major taxa of both environments was analyzed by linear regression analysis. It showed several direct linear relationships, i.e., positive association. It reveals that the total number of hospitalized patients had a direct linear relationship with total spores, Aspergillus/Penicillium spp., and Cladosporium spp. found through the microscopy-based method as well as total spores and Penicillium spp. from the culture-based method (Online Resource 9).
Among four categories of diseases, only Aspergillus/Penicillium spp. showed a positive linear relationship with the number of hospitalized patients for asthma exacerbation (Fig. 5a). COPD patients were positively associated with Cladosporium spp. (Fig. 5b), Penicillium spp. (Fig. 5c), and total spores of culture-based method (Online Resource 9). “PTB” also showed direct linear associations with Penicillium spp. (Fig. 5d), Aspergillus/Penicillium spp. (Fig. 5e), and total spores of the culture-based method (Online Resource 9). Besides, the “Others” group of patients had positive associations with total spores of both microscopy-based and culture-based methods (Online Resource 9), Penicillium spp. (Fig. 5f), Aspergillus/Penicillium spp. (Fig. 5g), and Cladosporium spp. (Fig. 5h). The rest of the major taxa showed an inverse linear relationship with diseases. In addition, several positive associations of total spore concentrations in indoor environments were also observed with the total number of hospitalized patients, “PTB” and “Others” groups by both sampling methods, whereas patients from the “COPD” group showed a positive association only with total fungal spore concentration observed through the culture-based method.
Linear regression analysis of four disease categories and their corresponding associated major taxa of both indoor and outdoor environments in Kolkata from May 2014 to April 2017 showing direct linear association: a asthma and Aspergillus/Penicillium spp. b COPD and Cladosporium spp. c COPD and Penicillium spp. d PTB and Penicillium spp. e PTB and Aspergillus/Penicillium spp. f Others and Penicillium spp. g Others and Aspergillus/Penicillium spp. h Others and Cladosporium spp.; Aspergillus/Penicillium spp., and Cladosporium spp. were found by the microscopy-based method, while Penicillium spp. was found by the culture-based method
For every disease category, males were more susceptible than females. The unpaired t-test revealed significant variations in the male to female ratio of all four categories and the total number of hospitalized patients (Fig. 6a). Patients aged 15–45 years suffered the most and visited the hospital more. Family history of allergenic respiratory symptoms was reported in 56.2% of the total number of hospitalized patients.
Determination of the male–female ratio of patients and assessment of the allergenic potential of fungal species obtained through the culture-based method: a Significant variations of male–female ratio of the total number of hospitalized patients and the patients of four groups of diseases such as asthma, COPD, PTB, and Others. *p < 0.05; ****p < 0.0001 using unpaired t-test; b results of Skin Prick Test performed by antigenic extracts of thirteen species of fungi grown by the culture-based method revealing the total percentage of positive responses
Finally, to assess the allergenic potential of the fungi recovered by culture-based sampling in the studied area, the skin prick test was performed. Thirteen antigenic extracts were prepared from thirteen different individual species based on their dominance and availability. The results of SPT demonstrated positive responses in 75.86% of the total subjects evaluated. The highest reactivity was observed from asthmatic patients (80.76%), whereas 77.77% of allergic rhinitis patients and 63.33% of patients with other respiratory discomforts showed allergenic sensitization. Among thirteen individual fungal extracts, Aspergillus fumigatus showed the maximum reactivity (50.9%), followed by Aspergillus flavus (40.0%), Aspergillus oryzae (37.27%), Aspergillus niger (36.36%), Aspergillus ochraceus (33.64%), and Aspergillus terreus (30.0%) (Fig. 6b). Extracts of these six species also showed + 1 to + 4 ranges of intensities of allergenic reaction. Apart from these species, Penicillium oxalicum (28.18%), Curvularia pallescens (22.72%), and Rhizopus oryzae (21.82%) also elicited higher sensitivity showing + 1 to + 3 intensity, whereas the other four species, Fusarium lateritium (14.55%), Candida albicans (13.64%), Trichoderma harzianum (11.82%), and Alternaria alternata (9.1%), reported < + 3 gradation.
Discussions
Spore concentration varies largely across the world depending on the geographical locations. In the air of an external space of the University of Costa Rica, a tropical country like India, the mean total spore concentration was 443.86 ± 201.68 spores m−3 (Brizuela-Hernández et al. 2019). However, in the rural agricultural areas of West Bengal, India, spore counts ranged from 82 to 2635 spores m−3 for total fungal spore concentration and 72–1769 CFU m−3 for viable fungi (Adhikari et al. 2004). It implies that the spore counts recorded in the present study were higher than the spore counts documented in the previous studies which might indicate the increase of risk factors of allergenic disorders for the local people of the studied area.
In this study, maximum spore concentration was observed in the open field area, an outdoor environment, rather than in the educational or occupational area for both sampling methods. A similar observation was also reported in earlier studies (Oberle et al. 2015; Sautour et al. 2009). The presence of the sources of fungal growth, like trees, weeds, grasses, and organic matter in the soil, and the effects of atmospheric parameters might be responsible for the prevalence of high airborne fungal spore load in the garden. This is in agreement with previously published findings. Ju et al. (2003) demonstrated that the abundance of multiple trees, shrubs, and herbaceous plants in the green area was responsible for higher fungal spore concentrations. The growth of several saprophytic and parasitic fungi on phylloplanes (leaf surface), reported by Picco and Rodolfi (2000), also enhanced the diversity and concentrations of fungi (Fang et al. 2019; Nageen et al. 2021). Fang et al. (2005) reported more diverse and significantly higher concentrations of culturable fungi in greener areas than in densely populated and polluted areas in Beijing. The strong influence of vegetation coverage on aerial concentrations of airborne fungi in outdoor environments was also observed in Hangzhou, China (Fang et al. 2019).
Among seven microenvironments, fungal spore concentration was also found to be maximum in the garden sampled with the microscopy-based method. On the contrary, for the culture-based method, indoor microenvironments were observed to be highly concentrated. These contrasting results might be reflections of the work purpose and infrastructures of studied indoor areas which provided favorable conditions for the growth of indoor fungi such as Aspergillus spp. and Penicillium spp. Large doors and windows in the airy classrooms of the school and the hospital might facilitate the influx of outdoor particles through air movements in indoor environments. Passive transport of spores by students, patients, and workers might be an important source of fungal growth inside almost all studied indoor microenvironments which was supported by the previous studies conducted in school (Oliveira et al. 2009) and hospital (Ekhaise et al. 2008). Additionally, the presence of cellulosic substrates like books, papers, and journals in the library might have served as a good source of cellulose-degrading fungi. Moreover, the moisture content of the wall, dampness, building materials, dust particles, and poor ventilation also influenced fungal colonization inside the hospital building, workshop, university, and library. Such kind of contamination of the indoor air was reported earlier (Górny 2004). Contrarily, the lowest spore concentration was observed in the bank microenvironment. The presence of air-conditioning, proper ventilation system, and thorough maintenance of human activity might be the reason for the less contamination of the air inside the bank which was reflected in the fungal spore concentrations.
During the monitoring period, both outdoor and indoor microenvironments showed a wide spectrum of various species of fungi. Most of the species belonged to phylum Ascomycota and Basidiomycota. According to Harley et al. (2009), early childhood wheezing was associated with high concentrations of ascospores and basidiospores. Chen et al. (2014) registered that approximately 1500 spores m−3 were the threshold concentration of airborne fungi for the reduction of lung function. In the present study, the mean concentrations of all major taxa recorded by the microscopy-based method such as ascospores, Cladosporium spp., Aspergillus/Penicillium spp., and basidiospores were found to be greater than the reported threshold value (Table 1). Apart from these taxa, an elevated concentration of Periconia spp. was observed in the outdoor as well as the indoor area (university and library). Besides, Ganoderma spp. and Rust spores from Basidiomycota phylum showed remarkably higher concentrations in workshop and library microenvironments, respectively (Online Resource 3). The constant exposure of these taxa to the local people might induce allergenic or respiratory disorders in sensitive patients. However, detailed epidemiological studies are needed to determine the threshold concentration of fungal spores to induce an allergic response to the susceptible population of the studied environments. In our investigation, Cladosporium spp. was found as one of the predominant fungi by the microscopy-based method. But, surprisingly, we did not recover any colonies of Cladosporium spp. through the culture-based method. It might be due to the high incubation temperature and short incubation period set in our study that did not favor the germination of the spores. Another possible reason might be the antagonistic activities of other fungal colonies, grown in the exposed petri plates, which could inhibit the growth of Cladosporium spp. (Barbosa et al. 2001).
In the present study, maximum spore load was observed during the post-monsoon season, whereas minimum during the winter season for both sampling methods. A similar seasonal periodicity pattern has also been observed in the semi-rural and industrial townships in West Bengal (Karmakar et al. 2020; Roy and Gupta Bhattacharya 2020). Fang et al. (2019) also reported the lowest concentrations of culturable fungi during the winter at Hangzhou in southeastern China. However, our finding was different from some previous studies conducted in urban and suburban areas in Kolkata (Chakrabarti et al. 2012; Das and Gupta-Bhattacharya 2012; Dey et al. 2018) where they documented that monsoon season showed the highest spore concentrations. These variations might be occurred due to the yearly fluctuation of environmental variables, the nature of the sampling locations, and the selection of the sampler used for monitoring.
Spearman’s rank correlation between environmental parameters and fungal spore concentrations has revealed significant effects of meteorological parameters as well as air pollutants on fungal aerospora. Additionally, the stepwise multiple regression analysis has pointed out that these parameters were significant predictors for spore concentrations, especially in indoor environments.
In the present study, the ambient temperature was observed as one of the most important meteorological variables. We observed a significant negative association of temperature with Cladosporium spp. at outdoor, triggering an elevated concentration in post-monsoon, winter, and early summer (Fig. 3a). Previous studies have also reported the abundance of Cladosporium spp. in a cooler climate (Fang et al. 2019; Oliveira et al. 2010; Radon et al. 2002). A positive effect of temperature was also observed on ascospores and basidiospores in the open field area. High temperature (> 30 °C) along with low relative humidity (< 70%) is important for the “active release” of ascospores (Kwon-Chung and Sugui 2013) which could be a possible reason for the observed higher concentration during summer (Fig. 3a). Further analysis with the regression model also showed ambient temperature as an important predictor in different indoor environments.
Average sun hour was also detected as another important meteorological variable in our investigation. Kallawicha et al. (2017) stated that sunlight has influenced fungi by increasing air temperature and activating the growth and sporulation of some fungal taxa. They reported a weak and insignificant positive association of sunlight with ascospores. Contrarily, in the present study, we observed a significant positive association between average sun hour and ascospores. Moreover, it was found an important predictor of ascospores and total spores of the culture-based method in indoors.
Excessive rain usually washes the spores out of the ambient environment and tends to decrease spore concentrations (Pakpour et al. 2017). This kind of negative association of rainfall with Cladosporium spp. was demonstrated earlier that we found too (Oliveira et al. 2010). However, rainfall increases atmospheric moisture content which is required for spore release, especially ascospores (Magyar et al. 2009). Similarly, we observed a significant positive correlation with ascospores in the garden indicating the reason for the abundance of ascospores in the monsoon season (Fig. 3a).
In addition, relative humidity and wind speed also showed immense influence on fungal aerospora and were also detected as key predictors in several indoor microenvironments. Relative humidity can be positively associated with aeromycoflora by increasing moisture content that drives the production and release of spores, especially ascospores and basidiospores (Grinn-Gofroń and Bosiacka 2015). A similar result was also observed for ascospores and basidiospores in the present study. Besides, it showed a significant negative association with Cladosporium spp. as well. Increased humidity causes a reduction in the bouncy of suspended spores in the air due to the high spore sedimentation rate, which might be a possible reason for this inverse relation (Pyrri and Kapsanaki-Gotsi 2017).
Spore release and dispersal are enhanced by wind speed. Higher wind speed is responsible for the “passive release” of ascospores (Kwon-Chung and Sugui 2013). Such a positive effect with ascospores and basidiospores was observed in this study. In contrast, high wind speed may dilute the spore load in the air and affect it negatively. The negative impact of wind speed on total spores has also been reported by Almaguer et al. (2014). A similar association was also noticed in both environments in the present study (Table 4).
The increasing concentration of air pollutants itself imposes a serious effect on respiratory health. The high concentrations of atmospheric pollutants due to vehicular emissions and uncontrolled biomass burning have registered Kolkata as one of the most polluted cities in India. Various studies have confirmed the association between atmospheric pollutants and fungal spore concentrations (Kallawicha et al. 2017; Pyrri and Kapsanaki-Gotsi 2017). Cakmak et al. (2012) have reported that the effect of aeroallergens on asthma can be modified by air pollution. Gaseous pollutants like O3 and NO2 can chemically alter aeroallergens by producing reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Reinmuth-Selzle et al. 2017). Moreover, O3 can negatively affect the viability of culturable fungi (Wu et al. 2007). Sommer et al. (1981) have stated the effect of CO on fungi by suppressing growth. Particulate matters (PM) could change the biological and morphological characteristics of microorganisms by binding with them (Alghamdi et al. 2014).
In our investigation, the fungal aerosol in different microenvironments also showed significant associations with atmospheric pollutant concentrations. Moreover, PM10 was identified as the only predictor in the outdoor environment by the multiple regression analysis. Furthermore, PM10 and NO2 were also detected as significant key predictors in several indoor microenvironments. The concentrations of PM2.5 and PM10 had both positive as well as negative effects on fungal spore concentrations. Both PM2.5 and PM10 exhibited a significant positive association with Cladosporium spp. in the open field area. Some previous studies observed a similar association with Cladosporium spp. in the Taipei metropolis (Kallawicha et al. 2017) and Taiwan (Ho et al. 2005). Besides, Roy et al. (2017) reported a negative correlation between PM10 and basidiospores which we also found in the outdoor area. Fan et al. (2021) observed negative correlation of residential culturable fungi with PM2.5 and PM10 at indoor rooms in China. In our study, PM10 was also identified as a key predictor with a negative impact on total spores of the culture-based method in the university microenvironment.
Among gaseous pollutants, the negative association of NO2 and basidiospores was also observed earlier (Roy et al. 2017). Kallawicha et al. (2015, 2017) reported the negative effect of CO on basidiospores by inhibiting the growth of mushrooms. Similarly in our study, CO showed several significant negative associations in indoor environments. Conversely, in outdoor, we observed the positive impact of CO and NO2 on total spores of the culture-based method. A similar correlation with residential culturable fungi was also detected by Fan et al. (2021) but in indoors. Although Adhikari et al. (2006) found a positive association between O3 and Aspergillus/Penicillium spp. as a function of temperature, in our study, we observed a negative association in the hospital microenvironment.
The R2 values, indicating lower goodness of fit of the regression models, ranged from 0.191 to 0.744 (Table 5). The highest value (R2 = 0.744, p < 0.01) was found to be greater than the optimal value of 0.7, observed for the total spores of the culture-based method in the university microenvironment, indicating that the aerial concentration was highly regulated by environmental parameters. The R2 values of other fungal taxon models were comparable with previous studies (Kallawicha et al. 2017; Ponce-Caballero et al. 2013; Sousa et al. 2008). Among major taxa found by both microscopy-based and culture-based methods, basidiospores (R2 = 0.542) and Aspergillus spp. (R2 = 0.380), respectively, were mostly influenced by the environmental parameters in the university. Among all microenvironments, fungal aerospora in the university were immensely controlled by environmental parameters. Moreover, these regression models can predict the fungal spore concentrations by using these atmospheric variables which can be used for further investigation regarding health effects on local people, workers, and students and can help to minimize the exposure to fungal spores.
In the present study, biomonitoring clearly indicated the prevalence of allergenic fungi in the atmosphere in Kolkata. Thus, to evaluate the impact of these aeroallergens on the human health of the local population, a health survey was executed during the entire monitoring period. Our result showed that exposure to fungal spores might be one of the possible reasons to induce a delayed effect of the fungal aeroallergens on allergic respiratory symptoms. Besides, the exacerbation of asthma, COPD, or other allergenic diseases is not only dependent on one variable but also the combined interaction between aerial concentrations of fungal spores, air pollutants, meteorological factors, other bioaerosols, viral and bacterial infections, etc. Both PM and gaseous pollutants are harmful to human health. Table 3 also shows the elevated concentrations of air pollutants in the winter season. This fact might be responsible for increasing the rate of respiratory and allergenic disorders in the winter which was reflected in our findings. Moreover, during the survey, we noticed that there was a drifting of patients’ numbers in the October and November months, i.e., in the post-monsoon season. The unavailability of patients at the early stages of symptom development and air pollution due to the use of firecrackers in the festive seasons in October and November might contribute to an additional surge in patient numbers in December. However, exposure to ambient fungal concentration might be one of the key factors for promoting or worsening existing airway diseases.
The linear regression analysis revealed that four categories of diseases had direct linear relationships with several major taxa and total fungal spores obtained by both methods with a positive population regression coefficient (β1 or slop), indicating a positive association with them. Among the fungi from the microscopy-based method, total spores, Aspergillus/Penicillium spp., and Cladosporium spp. exhibited a direct linear relationship with symptoms. On the other hand, among the fungal isolates recovered by the culture-based method, total spores and Penicillium spp. were found to have a positive association with patients’ visits to the hospital. Our findings indicated that the number of hospitalized patients for asthma exacerbation was positively associated with only Aspergillus/Penicillium spp. There is vast evidence of the association between fungal exposure and asthma (Denning et al. 2014; Jones et al. 2011). Thus, further analysis of the monthly spore concentrations of Aspergillus/Penicillium spp., Aspergillus spp., and Penicillium spp. with asthmatic patients revealed that it might be due to the possibility of the delayed effect of Aspergillus spp. and Penicillium spp. (Online Resource 10). However, Aspergillus spp. did not exhibit a linear association with any diseases probably for this delayed effect.
For “COPD” patients, the coefficient of determination (R2 value), indicating the closeness of fit of the regression line, was found to be maximum for Cladosporium spp. followed by Penicillium spp. and total spores of the culture-based method. It indicated that among these positive associations, Cladosporium spp. was able to induce maximum sensitization in “COPD” patients. Co-infection of Aspergillus spp. with Mycobacterium tuberculosis in Pulmonary Tuberculosis (PTB) patients is well-established (Hosseini et al. 2020; Xerinda et al. 2014). In our study, we observed that Penicillium spp. was more positively associated with PTB than Aspergillus/Penicillium spp. and total spores of the culture-based method. Besides, the maximum number of major taxa and total fungal spores found by both techniques can be able to stimulate allergenicity and respiratory diseases by showing direct linear relationships with the “Others” group of patients. Among them, the highest sensitization was elicited by Penicillium spp. having maximum R2 value (Fig. 5f). In addition, it was also found that in the indoor environments, total spores of the microscopy-based method showed the maximum association with the “Others” group of patients, whereas total spores of the culture-based method showed a direct linear association with PTB (Online Resource 8).
The male to female ratio also showed the higher susceptibility of males to diseases, probably due to their nature of the occupation, regular transport in different outdoor and indoor areas, etc., leading to an increased level of fungal exposure. Maximum suffering of the patients of 15–45 years age group is also interpreted as the indication of the fungal exposure of students and workers belonging to the young and middle age population. Although studies have demonstrated that exposure to indoor fungal spores can worsen allergic responses (Adhikari et al. 2004; Levetin et al. 1995; Ye et al. 2021), outdoor exposure is more relevant for sensitization and disease expression. Generally, an average person spends most of the time his/her entire day in indoor environments; hence, both indoor and outdoor fungal concentrations were taken into account for analysis. However, the factors that may influence the spore concentration and species diversity in indoor environments should be taken into account. Besides, only one outdoor environment was selected for monitoring in our investigation. To overcome this limitation, more outdoor sampling sites should be considered for a better understanding of the fungal spectrum in the said environment.
The results of SPT exhibited the allergenic potentials of all the selected fungal species. Concerning inducing positive reactions and intensity of sensitization in the atopic patient cohort, Aspergillus fumigatus was found as the most reactive species. Similar findings were also reported in the previous studies (Chakrabarti et al. 2012; Dey et al. 2018). Besides, other dominant species of major taxa grown by the culture-based methods tested such as Penicillium oxalicum, Curvularia pallescens, and Rhizopus oryzae showed higher sensitization potential. Moreover, positive responses were also noticed to the extract of other fungal species as well. In our sampling area, we observed an abundance of these fungal species that are already reported as a potential source of aeroallergens, thus inferring that aeroallergens from these fungi can promote the worsening of respiratory health to the atopic patients in the population.
Therefore, the prevalence of aeroallergens in the air in Kolkata might fuel the burden of respiratory diseases and be a potential risk factor for human health. Thus, the regression equations, obtained from the regression analysis, can predict the value of the incidence of the diseases. Furthermore, the identification and characterization of allergens from the dominant fungi can facilitate a scope for clinicians for improved diagnostics in fungal allergy and can also design a sustainable model of allergen-specific immunotherapy for atopic patients.
Conclusions
In the present study, aerobiological monitoring has described the diversity of fungal aerospora in different indoor and outdoor environments in Kolkata, their association with environmental factors, and their impact on the local inhabitant. The outdoor environment showed a higher spore load than indoor environments, and the highest spore concentration was observed in the post-monsoon season. In the outdoor area, only the air pollutant PM10 was identified as a significant predictor through multiple regression analysis. In different indoor microenvironments, PM10, NO2, and all meteorological parameters except rainfall have a significant effect on aerial concentrations of fungi and can act as key predictor variables. These findings can help to understand the fungal spore distribution in different microenvironments in Kolkata. The regression models can be used as preliminary information for developing daily forecast models. The health survey revealed that the sensitization to these fungal allergens might promote a delayed response of allergic sensitization in patients with respiratory conditions. SPT also confirmed their allergenicity in the sensitized patients indicating the adverse effect of aeroallergens on human health. The entire study indicated that the prevalence of fungal aeroallergens in the atmosphere is significant enough to achieve as a risk factor for the local population of the studied area. The information obtained from the study will also be useful for clinicians in taking necessary safety measures and making awareness among the inhabitant in Kolkata of fungal spore exposure.
Data availability
The authors declare that all data generated or analysed during this study are available within the article and its supplementary information files.
Code availability
Not applicable.
References
Adhikari A, Sen MM, Gupta-Bhattacharya S, Chanda S (2004) Airborne viable, non-viable, and allergenic fungi in a rural agricultural area of India: a 2-year study at five outdoor sampling stations. Sci Total Environ 326(1):123–141. https://doi.org/10.1016/j.scitotenv.2003.12.007
Adhikari A, Reponen T, Grinshpun SA, Martuzevicius D, LeMasters G (2006) Correlation of ambient inhalable bioaerosols with particulate matter and ozone: a two-year study. Environ Pollut 140:16–28. https://doi.org/10.1016/j.envpol.2005.07.004
Alghamdi M, Shamy M, Redal MA, Khoder M, Awad AH, Elserougy S (2014) Microorganisms associated particulate matter: a preliminary study. Sci Total Environ 479–480:109–16. https://doi.org/10.1016/j.scitotenv.2014.02.006
Almaguer MA, Aira MJ, Rodríguez-Rajo FJ, Rojas TI (2014) Temporal dynamics of airborne fungi in Havana (Cuba) during dry and rainy seasons: influence of meteorological parameters. Int J Biometeorol 58:1459–1470. https://doi.org/10.1007/s00484-013-0748-6
Barbosa MAG, Rehn KG, Menezes M, Mariano RdLR (2001) Antagonism of Trichoderma species on Cladosporium herbarum and their enzymatic characterization. Braz J Microbiol 32:98–104. https://doi.org/10.1590/S1517-83822001000200005
British Aerobiology Federation (1995) Pollens and spores – a guide to trapping and counting. Kimberley Clark Ltd., Lakefield, Aylesford UK
Brizuela-Hernández M, Jaikel-Víquez D, Riggioni-Cordero O, Escalante-Martínez AP, Gross NT (2019) Analysis of the seasonal variations of the concentration of air functional spores in the external space of the School of Microbiology of the University of Costa Rica. Acta Sci Microbiol 2(10):34–42. https://doi.org/10.31080/ASMI.2019.02.0369
Cakmak S, Dales RE, Coates F (2012) Does air pollution increase the effect of aeroallergens on hospitalization for asthma? J Allergy Clin Immunol 129:228–231. https://doi.org/10.1016/j.jaci.2011.09.025
Census India (2011) A-01: number of villages, towns, households, population and area (India, states/uts, districts and sub-districts) PC11_A01
Chakrabarti HS, Das S, Gupta-Bhattacharya S (2012) Outdoor airborne fungal spora load in a suburb of Kolkata, India: its variation, meteorological determinants and health impact. Int J Environ Health Res 22(1):37–50. https://doi.org/10.1080/09603123.2011.588323
Chakraborty P, Gupta-Bhattacharya S, Chanda S (2003) Aeromycoflora of an agricultural farm in West Bengal, India: a five-year study (1994–1999). Grana 42(4):248–254. https://doi.org/10.1080/00173130310016941
Chen BY, Chao HJ, Wu CF, Kim H, Honda Y, Guo YL (2014) High ambient Cladosporium spores were associated with reduced lung function in schoolchildren in a longitudinal study. Sci Total Environ 481:370–376. https://doi.org/10.1016/j.scitotenv.2014.01.078
D’Amato G, Holgate ST, Pawankar R et al (2015) Meteorological conditions, climate change, new emerging factors, and asthma and related allergic disorders. A statement of the World Allergy Organization. World Allergy Organ J 8(1):25. https://doi.org/10.1186/s40413-015-0073-0
Das S, Gupta-Bhattacharya S (2012) Monitoring and assessment of airborne fungi in Kolkata, India, by viable and non-viable air sampling methods. Environ Monit Asses 184(8):4671–4684. https://doi.org/10.1007/s10661-011-2294-1
Denning DW, Pashley C, Hartl D, Wardlaw A, Godet C, Giacco SD, Delhaes L, Sergejeva S (2014) Fungal allergy in asthma–state of the art and research needs. Clin Transl Allergy 4:14. https://doi.org/10.1186/2045-7022-4-14
Dey D, Ghoshal K, Gupta Bhattacharya S (2018) Aerial fungal spectrum of Kolkata, India, along with their allergenic impact on the public health: a quantitative and qualitative evaluation. Aerobiologia 35(1):15–25. https://doi.org/10.1007/s10453-018-9534-6
Ekhaise FO, Ighosewe OU, Ajakpovi OD (2008) Hospital indoor airborne microflora in private and government owned hospitals in Benin city. Nigeria World J Med Sci 3(1):19–23
Ellis MB (1971) Dematiaceous hyphomycetes. Commonwealth Mycological Institute, Kew, Surrey, England
Fan L, Wang J, Yang Y, Yang W, Zhu Y, Zhang Y, Li L, Li X, Yan X, Yao X, Wang L, Wang X (2021) Residential airborne culturable fungi under general living scenario: on-site investigation in 12 typical cities, China. Environ Int 155:106669. https://doi.org/10.1016/j.envint.2021.106669
Fang Z, Ouyang Z, Hu L, Wang X, Zheng H, Lin X (2005) Culturable airborne fungi in outdoor environments in Beijing. China Sci Total Environ 350(1–3):47–58. https://doi.org/10.1016/j.scitotenv.2005.01.032
Fang Z, Zhang J, Guo W, Lou X (2019) Assemblages of culturable airborne fungi in a typical urban, tourism-driven center of Southeast China. Aerosol Air Qual Res 19:820–831. https://doi.org/10.4209/aaqr.2018.02.0042
Fernández-Rodríguez S, Tormo-Molina R, Lemonis N, Clot B, O’Connor DJ, Sodeau JR (2018) Comparison of fungal spores concentrations measured with wideband integrated bioaerosol sensor and Hirst methodology. Atmos Environ 175:1–14. https://doi.org/10.1016/j.atmosenv.2017.11.038
Fischer G, Schwalbe R, Möller M, Ostrowski R, Dott W (1999) Species-specific production of microbial volatile organic compounds (MVOC) by airborne fungi from a compost facility. Chemosphere 39(5):795–810. https://doi.org/10.1016/s0045-6535(99)00015-6
Górny RL (2004) Filamentous microorganisms and their fragments in indoor air—a review. Ann Agric Environ Med 11(2):185–197
Green BJ, Tovey ER, Sercombe JK, Blachere FM, Beezhold DH, Schmechel D (2006) Airborne fungal fragments and allergenicity. Med Mycol 44:S245–S255. https://doi.org/10.1080/13693780600776308
Grinn-Gofroń A, Bosiacka B (2015) Effects of meteorological factors on the composition of selected fungal spores in the air. Aerobiologia (bologna) 31(1):63–72. https://doi.org/10.1007/s10453-014-9347-1
Harley KG, Macher JM, Lipsett M, Duramad P, Holland NT, Prager SS, Ferber J, Bradman A, Eskenazi B, Tager IB (2009) Fungi and pollen exposure in the first months of life and risk of early childhood wheezing. Thorax 64(4):353–358. https://doi.org/10.1136/thx.2007.090241
Ho HM, Rao CY, Hsu HH, Chiu YH, Liu CM, Chao HJ (2005) Characteristics and determinants of ambient fungal spores in Hualien, Taiwan. Atmos Environ 39(32):5839–5850. https://doi.org/10.1016/j.atmosenv.2005.06.034
Hosseini M, Shakerimoghaddam A, Ghazalibina M, Khaledi A (2020) Aspergillus coinfection among patients with pulmonary tuberculosis in Asia and Africa countries; a systematic review and meta-analysis of cross-sectional studies. Microb Pathog 141:104018. https://doi.org/10.1016/j.micpath.2020.104018
Jones R, Recer GM, Hwang SA, Lin S (2011) Association between indoor mold and asthma among children in Buffalo. New York Indoor Air 21(2):156–164. https://doi.org/10.1111/j.1600-0668.2010.00692
Ju TZ, Suo AN, Tian YJ, Feng KK (2003) Analysis on aerobiologia in Lanzhou. Ind Safety Environ Pollut 29:17–19
Kallawicha K, Chen YC, Chao HJ, Shen WC, Chen BY, Chuang YC, Guo YL (2017) Ambient fungal spore concentration in a subtropical metropolis: temporal distributions and meteorological determinants. Aerosol Air Qual Res 17:2051–2063. https://doi.org/10.4209/aaqr.2016.10.0450
Kallawicha K, Tsai YJ, Chuang YC, Lung SCC, Wu CD, Chen TH, Chen PC, Chompuchan C, Chao HJ (2015) The spatiotemporal distributions and determinants of ambient fungal spores in the Greater Taipei area. Environ Pollut 204:173–180. https://doi.org/10.1016/j.envpol.2015.04.020
Karmakar B, SenGupta K, Kaur A, Roy A, Gupta Bhattacharya S (2020) Fungal bio-aerosol in multiple micro-environments from eastern India: source, distribution, and health hazards. SN Appl Sci 2:565. https://doi.org/10.1007/s42452-020-2323-1
Kauffman HF, Tomee JF, Van de Riet MA, Timmerman AJ, Borger P (2000) Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J Allergy Clin Immunol 105:1185–1193. https://doi.org/10.1067/mai.2000.106210
Kurup VP, Shen HD, Banerjee B (2000) Respiratory fungal allergy. Microbes Infect 2(9):1101–1110. https://doi.org/10.1016/s1286-4579(00)01264-8
Kwon-Chung KJ, Sugui JA (2013) Aspergillus fumigatus—what makes the species a ubiquitous human fungal pathogen? PLoS Pathog 9(12). https://doi.org/10.1371/journal.ppat.1003743
Levetin E, Shaughnessy R, Fisher E, Ligman B, Harrison J, Brennan T (1995) Indoor air quality in schools: exposure to fungal allergens. Aerobiologia 11:27–34. https://doi.org/10.1007/BF02136141
Magyar D, Frenguelli G, Bricchi E, Tedeschini E, Csontos P, Li D-W, Bobvos J (2009) The biodiversity of air spora in an Italian vineyard. Aerobiologia 25(2):99–109. https://doi.org/10.1007/s10453-009-9115-9
Nageen Y, Asemoloye MD, Põlme S, Wang X, Xu S, Ramteke PW, Pecoraro L (2021) Analysis of culturable airborne fungi in outdoor environments in Tianjin, China. BMC Microbiol 21:134–144. https://doi.org/10.1186/s12866-021-02205-2
Oberle M, Reichmuth M, Laffer R, Ottiger C, Fankhauser H, Bregenzer T (2015) Non-seasonal variation of airborne Aspergillus spore concentration in a hospital building. Int J Environ Res Public Health 12:13730–13738. https://doi.org/10.3390/ijerph121113730
Oliveira M, Ribeiro H, Delgado JL, Abreu I (2009) Aeromycological profile of indoor and outdoor environments. J Environ Monit 11:1360–1367. https://doi.org/10.1039/b820736d
Oliveira M, Ribeiro H, Delgado L, Fonseca J, Castel-Branco MG, Abreu I (2010) Outdoor allergenic fungal spores: comparison between an urban and a rural area in Northern Portugal. J Investig Allergol Clin Immunol 20(2):117–128
Onions AHS, Eggins HOW, Smith G, Allsopp D (1981) Smith’s introduction to industrial mycology. Edward Arnold, London
Pakpour S, Li D-W, Klironomos J (2017) Relationships of fungal spore concentrations in the air and meteorological factors [2015]. Fungal Ecol 13:130–134. https://doi.org/10.1016/j.funeco.2014.09.008
Picco AM, Rodolfi M (2000) Airborne fungi as biocontaminants at two Milan underground stations. Inte Biodeterioration Biodegradation 45:43–47. https://doi.org/10.1016/S0964-8305(00)00047-0
Ponce-Caballero C, Gamboa-Marrufo M, López-Pacheco M, Cerón-Palma I, Quintal-Franco C, Giácoman-Vallejos G, Loría-Arcila JH (2013) Seasonal variation of airborne fungal propagules indoor and outdoor of domestic environments in Mérida. Mexico Atmósfera 26(3):369–377. https://doi.org/10.1016/S0187-6236(13)71083-X
Pyrri I, Kapsanaki-Gotsi E (2017) Functional relations of airborne fungi to meteorological and pollution factors in a Mediterranean urban environment. Fungal Ecol 30:48–54. https://doi.org/10.1016/j.funeco.2017.08.007
Radon K, Danuser B, Iversen M, Monsó E, Weber C, Hartung J, Donham K, Palmgren U, Nowak D (2002) Air contaminants in different European farming environments. Ann Agric Environ Med 9(1):41–48
Reinmuth-SelZle K, Kampf CJ, Lucas K et al (2017) Air pollution and climate change effects on allergies in the Anthropocene: abundance, interaction, and modification of allergens and adjuvants. Environ Sci Technol 51(8):4119–4141. https://doi.org/10.1021/acs.est.6b04908
Roy S, Chakraborty A, Maitra S, Bhattacharya K (2017) Monitoring of airborne fungal spore load in relation to meteorological factors, air pollutants and allergic symptoms in Farakka, an unexplored biozone of eastern India. Environ Monit Assess 189(8):370. https://doi.org/10.1007/s10661-017-6044-x
Roy S, Gupta Bhattacharya S (2020) Airborne fungal spore concentration in an industrial township: distribution and relation with meteorological parameters. Aerobiologia 36(1):575–587. https://doi.org/10.1007/s10453-020-09653-9
Sautour M, Sixt N, Dalle F et al (2009) Profiles and seasonal distribution of airborne fungi in indoor and outdoor environments at a French hospital. Sci Total Environ 407:3766–3771. https://doi.org/10.1016/j.scitotenv.2009.02.024
Simon-Nobbe B, Denk U, Pöll V, Rid R, Breitenbach M (2008) The spectrum of fungal allergy. Int Arch Allergy Immunol 145:58–86. https://doi.org/10.1159/000107578
Singh AB, Shahi S (2008) Aeroallergens in clinical practice of allergy in India- ARIA Asia Pacific Work-shop report. Asian Pac J Allergy Immunol 26(4):245–256
Singh AB, Singh A, Pandit T (1999) Respiratory diseases among agricultural industry workers in India: a cross-sectional epidemiological study. Ann Agric Environ Med 6(2):115–126
Smith G, Onions AHS, Eggins HOW, Allsopp D (1981) Smith’s introduction to industrial mycology. Edward Arnold, London
Sommer NF, Fortlage RJ, Buchanan JR, Kader AA (1981) Effect of oxygen on carbon monoxide suppression of postharvest pathogens of fruits. Plant Dis 65:347–349. https://doi.org/10.1094/PD-65-347
Sousa SIV, Martins FG, Pereira MC, Alvim-Ferraz MCM, Ribeiro H, Oliveira H, Abreu I (2008) Use of multiple linear regressions to evaluate the influence of O3 and PM10 on biological pollutants. Int J Environ Eng 2(8):105–110
Stytis DP, Stobo JD, Fudenberg HH, Wells JV (1982) Basic and clinical immunology, fourth ed. Lange Medical Publication, Maruzen Asia (Pvt.) Ltd.
Subramanian CV (1971) Hyphomycetes; an account of Indian species, except Cercosporae. Indian Council of Agricultural Research, New Delhi
Teresa ET, Curin M, Valenta R, Swoboda I (2015) Mold allergens in respiratory allergy: from structure to therapy. Allergy Asthma Immunol Res 7:205–220. https://doi.org/10.4168/aair.2015.7.3.205
Womiloju TO, Miller JD, Mayer PM, Brook JR (2003) Methods to determine the biological composition of particulate matter collected from outdoor air. Atmos Environ 37(31):4335–4344. https://doi.org/10.1016/S1352-2310(03)00577-6
Wu YH, Chan CC, Rao CY, Lee CT, Hsu HH, Chiu YH, Chao HJ (2007) Characteristics, determinants, and spatial variations of ambient fungal levels in the subtropical Taipei metropolis. Atmos Environ 41:2501–2509. https://doi.org/10.1016/j.atmosenv.2006.11.035
Xerinda S, Neves N, Santos L, Sarmento A (2014) Endotracheal tuberculosis and Aspergillosis co-Infection manifested as acute respiratory failure: a case report. J Mycobac Dis 4(4):160. https://doi.org/10.4172/2161-1068.1000160
Yamamoto N, Bibby K, Qian J, Hospodsky D, Rismani-Yazdi H, Nazaroff WW, Peccia J (2012) Particle-size distributions and seasonal diversity of allergenic and pathogenic fungi in outdoor air. ISME J 6(10):1801–1811. https://doi.org/10.1038/ismej.2012.30
Ye J, Qian H, Zhang JJ, Sun F, Zhuge Y, Zhuge X, Cao G (2021) Concentrations and size-resolved I/O ratios of household airborne bacteria and fungi in Nanjing, southeast China. Sci Total Environ 774(5):145559. https://doi.org/10.1016/j.scitotenv.2021.145559
Acknowledgements
The authors are grateful to the director, Bose Institute, Kolkata, for providing the infrastructural facilities, especially for allowing us to conduct our research work in the library, workshop, and garden. The authors sincerely acknowledge B. R. Singh Hospital, Kolkata for providing all hospital-related facilities; Raja Bazar Science College, University of Calcutta; State Bank of India, Maniktala Branch, and Kindergarten, Raja Bazar for permitting us to pursue the monitoring part of our research. Sincere thanks are due to Mr. Chanchal Chakraborty, Mr. Soumyo Subhro Gupta, and Mr. Asish Bera for their technical support.
Funding
This study was funded by the Department of Biotechnology, Government of India (Grant number: BT/512/NE/TBP/2013).
Author information
Authors and Affiliations
Contributions
Conceptualization and supervision: Swati Gupta Bhattacharya; investigation: Koyel SenGupta and Bijoya Karmakar; formal analysis: Koyel SenGupta and Sangeeta Roy; writing—original draft preparation: Koyel SenGupta; writing—reviewing and editing: Sangeeta Roy and Amarjeet Kaur.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the hospital.
Consent to participate
Consents were collected from the human participants according to the ethics of the hospital.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
SenGupta, K., Karmakar, B., Roy, S. et al. Analyzing airborne fungal concentration in Kolkata, India: temporal distribution, the effect of atmospheric parameters and health impact. Air Qual Atmos Health 16, 963–984 (2023). https://doi.org/10.1007/s11869-023-01316-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-023-01316-1