Opinion statement
Glioblastoma, an incurable, malignant, and highly vascular tumor, is a seemingly ideal target for anti-angiogenic therapies such as bevacizumab, an anti-vascular endothelial growth factor (VEGF) monoclonal antibody. Phase II trials in recurrent glioblastoma demonstrated bevacizumab was associated with clinical benefits, including decreases in brain edema and corticosteroids use resulting from reduced vascular permeability, as well as radiographic responses in 25 %–40 % of patients. In newly diagnosed disease, a phase III trial (AVAglio) showed adding bevacizumab to standard chemoradiotherapy improved progression free survival (PFS), with preservation of quality of life, and reduced corticosteroids use, but did not improve overall survival (OS). Another similar phase III trial (RTOG 0825) found similar PFS and OS trends, but suggested that the addition of bevacizumab resulted in more frequent cognitive decline compared with standard chemoradiotherapy. However, interpretation of those findings is limited by the fact that progressing patients were not evaluated, and patients remained longer on study in the bevacizumab arm. It is possible that the observed cognitive decline represented unrecognized tumor progression, rather than deleterious bevacizumab effects. Regardless, even if real, it is difficult to ascertain how improvements in PFS and quality of life compare with the associated economic costs and increased toxicities of bevacizumab, in the setting of no survival benefit. Further studies in recurrent disease are being conducted; preliminary results of a randomized trial showed favorable results with the combination with CCNU, and final results are awaited. Meanwhile, outside the realm of clinical trials, the current trend appears to be to reserve bevacizumab for use in recurrent disease, or for patients with moderate or severe neurologic symptoms, either in the newly diagnosed or recurrent setting. Further research efforts are needed to determine optimal candidates for this treatment from a molecular standpoint, as well as to develop imaging tools capable of accurately identifying response and progression, and to establish new drug combinations that could result in unquestionable clinical benefit and improved survival in these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite our growing knowledge of the biology and growth regulation of glioblastoma, treatment options are limited. With a multi-modal therapeutic approach including maximal surgical resection, concurrent radiation and temozolomide, and adjuvant temozolomide [1], prognosis is dismal with a median overall survival (OS) of 15–19 months [1, 2]. Two-year survival ranges from 25 %–30 % [1, 2], and progression free survival (PFS) is 6–7 months [1, 2].
When the Food and Drug Administration (FDA) approved the use of bevacizumab for recurrent glioblastoma in 2009, it marked the beginning of an exciting new era in the treatment of malignant brain tumors. Bevacizumab was the first new drug to gain approval for glioblastoma in a decade and the first to apply a novel mechanism of action, distinct from cytotoxic chemotherapy. However, in the 5 years since its widespread use, bevacizumab has yet to generate meaningful improvements in OS. Here, we discuss the role of angiogenesis in glioblastoma pathophysiology, review the clinical applications of bevacizumab and other anti-angiogenesis agents, and explore new uses of these agents.
Angiogenesis in glioblastoma
Glioblastoma is a highly aggressive, malignant, primary brain tumor with distinct pathologic features of necrosis and neovascular proliferation [3]. Angiogenesis, the formation of new blood vessels, is a necessary part of solid tumor growth and a hallmark of cancer. In general, when a tumor reaches a size of 1–2 millimeters, it can no longer subside on its existing blood supply. Decreased blood flow causes areas of hypoxia, which generate necrosis within the tumor. At this critical mass the tumor undergoes an “angiogenic switch”, triggering a cascade of molecular events leading to new blood vessel formation through predominance of pro-angiogenic factors over angiogenesis inhibitors. For many tumors, the turn of the angiogenic switch marks the beginning of the vascular phase of increased tumor growth. Glioblastoma is a highly vascular neoplasm that is thought to develop through two vascular phases: one in which the glioma cells coopt the normal cerebral blood vessels, followed by a second phase of neovascularization. In these tumors, angiogenesis seems to be triggered by expression of hypoxia-inducible factor (HIF-1) and vascular endothelial growth factor (VEGF) [4] in necrotic areas of the tumor. VEGF is a signal protein that stimulates angiogenesis and vasculogenesis. The normal function of VEGF is to induce new blood vessel formation during embryogenesis and after injury. Six secreted glycoproteins comprise the VEGF family of growth factors: VEGF-A, B, C, D, E, and placental growth factor [5]. VEGF concentrations correlate with glioma grade, such that higher VEGF concentrations are seen with higher grade tumors; thus, the highest VEGF concentrations are in glioblastoma [6]. Each VEGF isoform acts through a VEGF receptor tyrosine kinase, which include VEGFR-1/Flt-1, VEGFR2/Flk-1/KDR, and VEGFR-3. The VEGF receptor, though not normally expressed in brain epithelium, gets upregulated in glioblastoma endothelial cells [7]. VEGF expression can be upregulated by other growth factors, including platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and fibroblast growth factor (bFGF) [8, 9], however, the HIF-1 pathway seems to be the primary stimulus for VEGF expression. Integrin αυβ3 [10], interleukin 8 (IL-8) [11], angiopoietin 1 and 2 (Ang-1 and Ang-2) [12], and tumor necrosis factor α (TNFα) [13] are pro-angiogenic cytokines that also promote neovascularization. The endothelium of blood vessels expresses matrix metalloproteases, which are pro-angiogenic enzymes. While the tumor drives expression of these pro-angiogenic factors, the tumor micro-environment also harbors multiple inhibitors of angiogenesis. These include cytokines IL-4 [14], IL-12, interferons α, β, υ [15], endostatin [15], and tissue inhibitors of matrix metalloproteases [16], all of which counter-balance the activation of angiogenesis (Fig. 1).
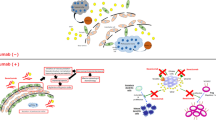
Mechanisms of action of anti-angiogenesis therapy. Angiogenesis occurs when the balance of pro-angiogenic factors outweighs the anti-angiogenic factors. Vascular endothelial growth factors (VEGF), platelet derived growth factor (PDGF), tumor necrosis factor-alpha (TNFα), plasminogen activators, matrix metalloproteases (MMPs), transforming growth factor alpha (TGFα), angiogenin, integrin ανβ3, and angiopoietin 1 and 2 (Ang1, Ang2) are all pro-angiogenic cytokines and proteins, many of which are now specific drug targets. Targeting the cancer stem cell is another mechanism that may halt tumor growth. The anti-angiogenic cytokines, including tissue inhibitors of metalloproteases (TIMPs), thrombospondin, interleukin-4 (IL-4), IL-12, IL-18, interferon (IFN), angiostatin, endostatin, troponin-1, and platelet factor-4, act as intrinsic brakes on angiogenesis.
Treatment
Effects and properties of anti-angiogenic therapy
Anti-angiogenic agents halt tumor growth through a variety of mechanisms [17]. The agents may have a direct effect on the tumor endothelium, leading to increased apoptosis of endothelial cells and tumor starvation. In addition, anti-angiogenic agents can induce normalization of tumor vasculature, decrease blood vessel diameter and permeability, and lead to decreased tumor interstitial pressure, increasing perfusion and oxygenation. Vascular normalization may increase the response to radiation therapy and delivery of chemotherapy. Anti-angiogenic therapy may interrupt pro-angiogenic signaling from bone-marrow derived myeloid cells, inhibiting new blood vessel formation. Finally, these agents may have some activity against glioma stem-like cells, which cluster in perivascular niches, and may be the epicenter of tumor regrowth. Interestingly, glioma stem-like cells have ability to differentiate into endothelial progenitors and may play a direct role in angiogenesis maintenance [18].
The mechanisms of action of anti-angiogenic therapy are distinct from conventional chemotherapy in many ways. Anti-angiogenic agents are cytostatic, not cytotoxic to tumor cells. The main target of anti-angiogenic agents is the endothelial cell, rather than the tumor cell itself. Thus, to have an appreciable effect on tumor growth, anti-angiogenic therapy generally needs to be administered for a prolonged period of time. Theoretically, anti-angiogenic therapy carries a decreased risk of drug resistance compared with chemotherapy because endothelial cells are more genetically stable than tumor cells and are less likely to acquire resistance mechanisms. Anti-angiogenic agents may be better tolerated than conventional chemotherapy since the effects are tumor-specific and have minimal effect on other dividing cells; the nonoverlapping toxicities with cytotoxic agents allow for relatively easy combination with these agents.
Clinical experience with bevacizumab
Bevacizumab, a humanized monoclonal antibody that inhibits VEGF-A, is currently approved by the FDA for the treatment of metastatic colorectal cancer, renal cell carcinoma, and nonsquamous non-small cell lung cancer, in addition to recurrent glioblastoma. While development in other cancer types was expedited, the development in glioblastomas was slow, reflecting concerns of increasing the risk of intracranial bleeding. The first anecdotal experience in recurrent glioblastoma was reported in 2005 in a series of patients who received a treatment that mirrored a colorectal regimen, combining bevacizumab and irinotecan in an off-label use [19]. That report suggested the treatment was safe and associated with tumor responses. Phase II trials were initiated, focusing on combined bevacizumab and irinotecan in recurrent disease. Interesting results were found, with a 6-month PFS of 46 %, 6-month OS of 77 %, and a safe toxicity profile that dispelled the initial fears of intracranial hemorrhage associated with anti-angiogenic treatments in brain tumors [20, 21].
On May 5, 2009 the FDA approved bevacizumab as a single agent for patients with glioblastoma based on two subsequent phase II studies [22–24]. The first study (N = 157) was an industry-sponsored, noncomparative randomized phase II trial of bevacizumab (10 mg/kg every 2 weeks) and bevacizumab plus irinotecan [23]. Eighty-five patients with glioblastoma were treated in the bevacizumab arm, and achieved objective response rate (ORR) of 26 %, 6-month PFS of 36 %, and OS of 9 months. In the bevacizumab plus irinotecan arm, efficacy was similar, with ORR of 38 %, 6-month PFS of 50 %, and OS of 9 months, although greater toxicity was seen. Importantly, corticosteroid use decreased after initiation of treatment in both arms. The second study was a single institution, single arm phase II trial of 48 patients with recurrent glioblastoma [24]. Patients were treated with bevacizumab until further progression and then were given bevacizumab and irinotecan. The PFS was 16 weeks, with a 6-month PFS of 29 % and OS of 31 weeks. Of the 19 patients treated with the combination of bevacizumab and irinotecan at progression, there were no responses, and 18 of 19 patients experienced disease progression by the second cycle. Taken together, those studies showed bevacizumab is active in glioblastomas, but suggested irinotecan did not add to efficacy. However, the design of these studies had limitations that affect the interpretation of the clinical outcomes to support FDA approval. The randomized study had included bevacizumab in both arms and therefore, there was no control group, limiting the use of PFS and OS as evidence for treatment effect. Instead of survival, ORR was the agreed upon study endpoint. The FDA workshop discussants agreed that a response rate of “sufficient magnitude,” defined as >30 %, would likely be associated with clinical benefit. However, the use of radiographic criteria to determine response rate posed additional problems. Because of the infiltrative nature of glioblastoma, its irregular borders, and the combination of both enhancing and nonenhancing components, concordance rates of MRI measurements by neuroradiologists are only around 50 %. Furthermore, the action of bevacizumab, which stabilizes the blood brain barrier and decreases vascular permeability, leads to changes on the MRI including decreased tumor edema and decreased gadolinium enhancement that may not reflect an actual effect on tumor growth (Fig. 2) [25]. Traditional response criteria may misestimate the treatment’s effect. Despite the limitations of these studies, bevacizumab was approved as a single agent for recurrent glioblastoma with ten votes yes and zero votes no, with the condition that there would be close postmarketing analysis, and a randomized placebo-controlled study of bevacizumab in combination with radiochemotherapy for newly diagnosed glioblastoma.
Challenges in radiographic evaluation during bevacizumab. T1 postcontrast MRI (A) showing large heterogeneously enhancing glioblastoma prior to bevacizumab, and corresponding hyperintensity on MRI FLAIR sequence (B). Following treatment with bevacizumab, the T1 postcontrast sequence (C) shows near-resolution of contrast enhancement, suggesting a response to treatment. However, the corresponding FLAIR sequence (D) shows increasing hyperintensity, suggesting treatment failure and disease progression in the form of worsening nonenhancing tumor. Reproduced with permission from Glioblastoma: Molecular Mechanisms of Pathogenesis and Current Therapeutic Strategies. Springer Science, ISBN-10: 1441904093 [25].
Reports on phase II studies of bevacizumab in the up-front setting quickly followed the FDA approval for recurrent glioblastoma. A phase II trial of bevacizumab and irinotecan added to chemoradiotherapy with temozolomide in newly diagnosed glioblastoma showed median overall survival of 21.2 months [26]. A trial of bevacizumab added to standard chemoradiotherapy found median OS of 19.6 months [27], while bevacizumab combined with hypofractionated radiotherapy and temozolomide was associated with median OS of 19 months [28]. In early 2014, the results of two large phase III multicenter randomized control trials of bevacizumab with radiation and temozolomide for newly diagnosed glioblastoma were published simultaneously [29••, 30••].
The AVAglio study was a large, industry-sponsored phase III trial based primarily in Europe that enrolled 921 patients with newly diagnosed glioblastoma, with OS and PFS as co-primary endpoints [29••]. The experimental arm (N = 458) received standard radiotherapy with concurrent/adjuvant temozolomide and bevacizumab, given at 10 mg/kg every 2 weeks. The control arm (N = 463) received the same chemoradiotherapy regimen and placebo; 31 % of those patients received bevacizumab at recurrence, as salvage therapy. The OS was the same for both arms: 16.7 months for the control group and 16.8 months in the bevacizumab group (P = 0.1). The PFS was significantly improved in the bevacizumab group (median PFS 10.6 months, vs 6.2 months for controls, P < 0.001). Not surprisingly, there were more adverse events in the bevacizumab group (67 % vs 51 %) and more severe adverse events (33 % vs 16 % grade ≥ 3 toxicities). The companion study, RTOG 0825, was the North American version of this trial [30••]. A total of 978 patients were registered and 637 randomized. The experimental arm received bevacizumab added to concomitant radiotherapy with temozolomide and adjuvant temozolomide. The control group was treated with placebo added to concurrent radiochemotherapy followed by adjuvant temozolomide, and was offered bevacizumab at recurrence, in a cross-over design. The OS was not significantly different for the two arms: 16.1 months for the control group and 15.7 months for bevacizumab group. Though PFS was improved in the bevacizumab group (10.7 months vs 7.3 months for controls, P = 0.007), it did not meet the pre-specified target of improvement per trial design, based on a dual primary endpoint that called for statistical significance set at P = 0.004 for the PFS. Although relatively rare, there were more adverse events in the bevacizumab group, including hypertension, thromboembolic events, intestinal perforation, and neutropenia.
Since the initial approval of bevacizumab for recurrent glioblastoma was conditional, the FDA must reconvene in light of this phase III data to decide whether to continue to approve bevacizumab in this disease. Though the two phase III studies failed to show an OS benefit, both studies suggested gains in PFS, which in brain tumors may be associated with preservation of quality of life, as small tumor growths can be associated with devastating neurologic symptoms. Moreover, the rapid reduction in vascular permeability and edema, and decrease in use of corticosteroids are potentially helpful in the clinical management of these patients. Neither phase III study focused on quality of life as a primary endpoint. Analysis of AVAglio data suggested health-related quality of life and performance status were maintained longer in the bevacizumab group, with lower corticosteroids requirement [29••]. Conversely, neuropsychological evaluations performed in RTOG 0825 [30••] suggested that the addition of bevacizumab resulted in more frequent cognitive decline compared with standard chemoradiotherapy, although interpretation of such findings is limited by the fact that progressing patients were not evaluated, and patients remained longer on study in the bevacizumab arm, implying that the cognitive decline could represent unrecognized tumor progression, rather than direct bevacizumab effects. Regardless, even if real, it is difficult to ascertain how the improvements in quality of life associated with bevacizumab in newly diagnosed glioblastoma compare with the significant economic costs and increased toxicities, in the setting of no survival benefit. Further studies in recurrent disease are being conducted; preliminary results of a randomized phase II trial showed favorable results with the combination with CCNU [31], and the study has been expanded to a phase III design. Meanwhile, outside the realm of clinical trials, the current trend appears to be to reserve bevacizumab for use in recurrent disease or for patients with moderate or severe neurologic symptoms either in the newly diagnosed or recurrent setting. It must also be noted that unresectable patients were minimally represented in clinical trials, and those patients may derive more tangible benefits, as suggested in studies of neoadjuvant bevacizumab and irinotecan in unresectable tumors [32, 33], though such studies lacked appropriate evaluation of quality of life.
Biomarkers for selection of optimal candidates for anti-angiogenic therapies
In addition to the attempts to select patients based on clinical characteristics, tissue-based and neuroimaging biomarkers are being developed to aide in identifying the best candidates for anti-angiogenic treatments. A retrospective study in recurrent malignant gliomas suggested that high VEGF expression, as determined by immunohistochemistry, was associated with increased response to bevacizumab but did not predict survival, whereas expression of hypoxia-related factors such as CA9 was associated with poor survival outcome [34]. In a prospective phase II study in newly diagnosed disease [35], TCGA-based molecular classification of glioblastomas based on genome-wide gene expression analysis [36–39] was correlated with outcomes following bevacizumab combined with hypofractionated radiotherapy and temozolomide. The hypothesis was that the oncogenetic and angiogenic differences observed across different transcriptional signatures would translate into differential responses to anti-angiogenic treatments. It was expected, for example, that mesenchymal glioblastomas, which are enriched for the expression of angiogenesis-related genes, would derive the most benefit. Unexpectedly, results suggested that proneural glioblastomas, all of which were IDH-1 wild type, were associated with the worst outcomes among subclasses, which was surprising given that this signature is typically associated with better prognosis, raising concerns of a deleterious effect in this subclass. However, the lack of a control arm precluded establishing whether this was a prognostic or a predictive signature specific to angiogenic treatments. Further insights into this question were provided by a subsequent analysis of tissue collected in the AVAglio study [40], which utilized the same nanostring-based methodology. Preliminary results showed that proneural glioblastomas without IDH-1 mutation were associated with worse outcomes within the placebo arm, confirming that this signature is associated with a poor prognosis, independent of bevacizumab exposure. In fact, additional analysis suggested that adding bevacizumab may actually improve survival in these poor prognosis proneural tumors, a finding that requires further validation in larger, independent data sets.
Several studies have focused on advanced neuroimaging parameters that could predict clinical benefit from bevacizumab, as well as on identifying better parameters for response assessment, although, to date, results have been somewhat conflicting. As noted above, bevacizumab decreases vascular permeability, a parameter that has been historically used to map and measure malignant brain tumors, such as in contrast-enhanced MRI. While such parameter cannot be used as a reliable measure of tumor burden in the presence of bevacizumab, other effects of anti-angiogenesis treatments can be mapped and quantified by advanced neuroimaging techniques. In the phase II study of bevacizumab, temozolomide, and hypofractionated radiotherapy, perfusion MRI showed a nearly universal decrease in blood perfusion over time, demonstrating that the intended targeting of angiogenesis was accomplished. However, this effect did not hold any significance in terms of prognosis. Conversely, tumors with the lowest apparent diffusion coefficient (ADC) at baseline seemed to benefit most from treatment [41], a finding also reported in other studies [42], and that suggests diffusion-weighted MRI could be a more helpful prognostic biomarker. In another study, increases in blood perfusion observed after initiation of anti-VEGF therapy with cediranib was predictive of better outcomes, suggesting a link between normalization of blood vessels and efficacy. However, this information can only be determined after initiation of treatment, and is thus, of limited usefulness in patient selection [43]. A study of patients treated with irinotecan and bevacizumab utilizing 3’-deoxy-3’- [18F]-fluoro-L-thymidine PET (FLT-PET) suggested this technique is more accurate in predicting survival than MRI responses [44]. Further studies are needed to determine whether imaging techniques based on cell proliferation and metabolism such as MRI diffusion and PET, or perfusion and vasculature imaging are the best markers to guide patient selection for, and determine response to anti-angiogenic treatments. Studies investigating the role of blood or plasma based biomarkers in guiding anti-angiogenesis therapy, including plasma levels of angiogenesis related proteins, cytokines, and circulating endothelial progenitor cells have, so far, been inconclusive.
Bevacizumab for enhanced radiotherapy schedules
An emerging role for bevacizumab is concurrent use with radiotherapy in more aggressive schedules either for re-irradiation for recurrent disease or as an alternative schedule for upfront treatment. Bevacizumab has a potent effect in decreasing vascular permeability, as demonstrated by rapid reductions in contrast enhancement and peritumoral edema following treatment initiation [45]. This “corticosteroid-like” action allows for more aggressive hypofractionation, which otherwise is not typically used because of risks of symptomatic radionecrosis and brain edema. In a pilot trial of 25 patients with recurrent malignant glioma, 20 of whom had glioblastoma, bevacizumab was combined with hypofractionated re-irradiation with 30 Gy delivered in 5 fractions [46]. For the glioblastoma cohort, the overall response rate was 50 % and 6-month PFS was 65 %, with a safe toxicity profile and no symptomatic radionecrosis observed. Based on those findings, a phase II study was conducted in newly diagnosed disease, utilizing hypofractionated radiotherapy with temozolomide and bevacizumab, with 36 Gy to contrast-enhancement and 24 Gy to FLAIR hyperintensity with dose painting [28]. This regimen, delivered in 6 fractions, was biologically equivalent to a standard 60 Gy in 35 fractions. Results showed similar OS compared with historical controls (median OS 19 months), with a favorable safety profile and a more convenient schedule for patients. Based on these encouraging results, several studies are now investigating this approach further, in randomized or dose-escalation studies, aiming at improving efficacy. Bevacizumab has also been used to treat radionecrosis, and while a small randomized trial [47] suggested improvement in neurologic symptoms in radionecrosis in head and neck and low-grade CNS tumors patients, other authors have argued bevacizumab may actually exacerbate radionecrosis and induce neurologic deterioration over time in some patients, perhaps reflecting an “over-pruning” of blood vessels within the radiation field [48].
Clinical experience with other anti-angiogenic therapies
In addition to bevacizumab, treatment strategies that target VEGF pathways include small molecule VEGFR tyrosine kinase inhibitors (TKIs), TKIs of other growth factor pathways involved in VEGF regulation, and soluble decoy VEGFR. VEGFR TKIs offer the advantage of oral administration. Some of these TKIs target multiple receptors such as PDGFR and EGFR, but overall the results have been disappointing in glioblastomas [49]. In fact, VEGFR TKIs including sunitinib [50], vandetanib [51], and vatalanib [52] were not found to be associated with the same effects observed with bevacizumab in terms of decreased vascular permeability and tumor response. TKIs with greater specificity for VEGFR such as cediranib seem to achieve more potent modulation of this pathway, although perhaps still not as marked as bevacizumab. In a three-arm, randomized trial, 325 patients with recurrent glioblastoma were assigned to receive cediranib monotherapy, cediranib plus lomustine, or lomustine alone [53]. Cediranib was generally well tolerated, but the study failed to reach its primary endpoint of PFS, with median PFS of 92 days for cediranib alone, vs 125 days for cediranib plus lomustine, and 82 days for lomustine alone. In a phase I-II study of 46 patients with newly diagnosed glioblastoma received cediranib with temozolomide and radiation therapy, and the median PFS was 15.6 months and OS was 20 months. VEGF inhibition may also be accomplished with aflibercept (VEGF-Trap), an engineered soluble decoy VEGFR, which has been tested in clinical trials [54] that have suggested modest activity in recurrent glioblastoma (6-month PFS of 7.7 %), with a toxicity profile that seemed less favorable than bevacizumab. Overall, the development of VEGF pathway inhibitors other than bevacizumab has been hampered by uncertainty regarding the usefulness of bevacizumab itself.
Aside from anti-VEGF therapies, other approaches to target angiogenesis in glioblastoma have largely failed, including enzastaurin, a protein kinase C inhibitor, and thalidomide. Likewise, and in spite of promising preliminary studies, cilengitide, an αvβ3 and αvβ5 integrin inhibitor, has also failed to improve outcomes in either methylated or unmethylated MGMT glioblastomas [55].
Mechanisms of resistance
Glioblastoma has both intrinsic and evasive resistance to anti-angiogenic therapy [17], and mechanisms of resistance may differ from patient to patient. The tumor may evade anti-VEGF therapy by upregulation of alternate pro-angiogenic pathways, including PGF, PDGF, and bFGF. Increased tumor hypoxia from anti-angiogenic therapy leads to recruitment of bone marrow derived cells. These bone marrow derived cells include CD45+ cells, endothelial progenitor cells, and pericyte progenitor cells. An increase in hypoxia triggers release of pro-angiogenic cytokines, including HIF-1, stromal-derived factor 1α, and VEGF. As a temporary fix, glioblastoma tumor cells may reorganize tissues to patch the damaged existing vessels. Perhaps more worrisome, cancer stem cells can differentiate to tumor-derived endothelial cells, which may be more resistant to anti-angiogenic therapy. Further, the glioblastoma tumor itself may transition to a more invasive tumor phenotype, generally to a mesenchymal type, in response to the pressure of anti-angiogenic therapy; glioma cells may continue to migrate, invade and thrive in the brain microenvironment, even in the absence of angiogenesis.
From a clinical standpoint, radiographic tumor progression following bevacizumab exposure is associated with a poor prognosis, and it has been suggested that continuing bevacizumab is necessary to avoid rebound tumor progression and edema from increased vascular permeability [56, 57]. However, it is unclear whether this practice results in meaningful clinical benefits; clinical trials of alternative agents in patients failing bevacizumab did not seem associated with worse outcomes compared with trials including bevacizumab continuation [58], and, therefore, discontinuing bevacizumab for pursuing an alternative treatment remains a legitimate option.
Future directions
Several strategies are being developed to improve the efficacy of bevacizumab. Tables 1 and 2 lists the current anti-angiogenic clinical trials in glioblastoma (www.clinicaltrials.gov). These trials include bevacizumab combined with hypofractionated radiotherapy and with cytotoxic agents (eg, CCNU, irinotecan), cell signaling pathway inhibitors (everolimus, crizotinib, CTO), agents targeting alternative pro-angiogenic factors (anti CD105 monoclonal antibody TRC105 [59], anti Ang 1 and 2 antibody AMG 386, and others), targeting cancer stem cell (Notch inhibitors), other modulators of the brain microenvironment, and immunotherapy.
The tumor stability provided by bevacizumab and mostly non-overlapping toxicities provide a window of opportunity for the development of new agents in these rapidly growing tumors. While a large number of preclinical studies support new combinations, many basic questions remain unanswered. Although thought to improve drug delivery to tumors, it remains unknown if effects of bevacizumab in the brain could in fact result in decreased delivery of certain agents. Moreover, it remains unknown if the decreases in vascular permeability could decrease inflammatory reaction and development of immune responses, essential for emerging immunotherapies such as vaccines and immune checkpoint inhibitors. Finally, because responses are frequent, and radiographic evaluation is difficult during bevacizumab, the development of combination with new agents is slow, as trials require survival endpoints, and much larger sample sizes are needed to detect signals of efficacy.
Conclusions
Anti-angiogenic therapy is an important tool in the treatment of glioblastoma, and has several useful applications, although none seem to result in a survival benefit thus far. While withdrawing the FDA approval for glioblastoma would be an unfortunate event for patients with this disease, the development of this agent has been problematic from the beginning in this disease, and further research is clearly required. We need to continue to optimize the application of anti-angiogenic agents, and increase our understanding of when they should be administered, to which patients, and in combination with what other treatments, so that anti-angiogenic therapy may provide a meaningful and unquestionable clinical benefit to our patients.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96.
Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310:1842–50.
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol (Berl). 2007;114:97–109.
Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–8.
Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400.
Schmidt NO, Westphal M, Hagel C, Ergun S, Stavrou D, Rosen EM, et al. Levels of vascular endothelial growth factor, hepatocyte growth factor/scatter factor and basic fibroblast growth factor in human gliomas and their relation to angiogenesis. Int J Cancer. 1999;84:10–8.
Plate KH, Breier G, Farrell CL, Risau W. Platelet-derived growth factor receptor-beta is induced during tumor development and upregulated during tumor progression in endothelial cells in human gliomas. Lab Investig. 1992;67:529–34.
Tsai JC, Goldman CK, Gillespie GY. Vascular endothelial growth factor in human glioma cell lines: induced secretion by EGF, PDGF-BB, and bFGF. J Neurosurg. 1995;82:864–73.
Goldman CK, Kim J, Wong WL, King V, Brock T, Gillespie GY. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Mol Biol Cell. 1993;4:121–33.
Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–17.
Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005;7:122–33.
Stratmann A, Risau W, Plate KH. Cell type-specific expression of angiopoietin-1 and angiopoietin-2 suggests a role in glioblastoma angiogenesis. Am J Pathol. 1998;153:1459–66.
Reynes G, Vila V, Martin M, Parada A, Fleitas T, Reganon E, et al. Circulating markers of angiogenesis, inflammation, and coagulation in patients with glioblastoma. J Neurooncol. 2011;102:35–41.
Hemmerle T, Neri D. The antibody-based targeted delivery of interleukin-4 and 12 to the tumor neovasculature eradicates tumors in three mouse models of cancer. Int J Cancer. 2014;134:467–77.
De Bouard S, Guillamo JS, Christov C, Lefevre N, Brugieres P, Gola E, et al. Antiangiogenic therapy against experimental glioblastoma using genetically engineered cells producing interferon-alpha, angiostatin, or endostatin. Hum Gene Ther. 2003;14:883–95.
Crocker M, Ashley S, Giddings I, Petrik V, Hardcastle A, Aherne W, et al. Serum angiogenic profile of patients with glioblastoma identifies distinct tumor subtypes and shows that TIMP-1 is a prognostic factor. Neuro Oncol. 2011;13:99–108.
Weathers SP, de Groot J. Resistance to antiangiogenic therapy. Curr Neurol Neurosci Rep. 2014;14:443.
Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–33.
Stark-Vance V. Bevacizumab and CPT-11 in the treatment of relapsed malignant glioma. Neurooncology. 2005;7(3):369. Meeting Abstracts.
Vredenburgh JJ, Desjardins A, Herndon II JE, Marcello J, Reardon DA, Quinn JA, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–9.
Vredenburgh JJ, Desjardins A, Herndon II JE, Dowell JM, Reardon D, Quinn J, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant gliomas. Clin Cancer Res. 2007;13:1253–9.
Cohen MH, Shen YL, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14:1131–8.
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–40.
Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–5.
Bazzoli E, Omuro AM. Antiangiogenic strategies for the treatment of gliomas. In: Ray SK, editor. Glioblastoma: molecular mechanisms of pathogenesis and current therapeutic strategies. New York: Springer; 2010. p. 243–64.
Vredenburgh JJ, Desjardins A, Reardon DA, Peters KB, Herndon II JE, Marcello J, et al. The addition of bevacizumab to standard radiation therapy and temozolomide followed by bevacizumab, temozolomide, and irinotecan for newly diagnosed glioblastoma. Clin Cancer Res. 2011;17:4119–24.
Lai A, Tran A, Nghiemphu PL, Pope WB, Solis OE, Selch M, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2011;29:142–8.
Omuro A, Beal K, Karimi S, Chan T, Panageas K, Nayak L, et al. Phase II study of bevacizumab (BEV), temozolomide (TMZ), and hypofractionated stereotactic radiotherapy (HFSRT) for newly diagnosed glioblastoma (GBM). J Clin Oncol. 2010;28(15 Suppl):2036. Meeting Abstracts.
Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–22. Landmark trial investigating bevacizumab for newly diagnosed glioblastoma.
Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. Companion article to Chinot et al above. Also a landmark trial for bevacizumab in newly diagnosed glioblastoma.
Taal W, Oosterkamp HM, Walenkamp AME, Beerepoot LV, Hanse M, Buter J, et al. A randomized phase II study of bevacizumab vs bevacizumab plus lomustine vs lomustine single agent in recurrent glioblastoma: the Dutch BELOB study. J Clin Oncol. 2013;31(Suppl): [Abstract 2001].
Chauffert B, Feuvret L, Bonnetain F, Taillandier L, Frappaz D, Taillia H, et al. Randomized phase II trial of irinotecan and bevacizumab as neo-adjuvant and adjuvant to temozolomide-based chemoradiation compared with temozolomide-chemoradiation for unresectable glioblastoma: final results of the TEMAVIR study from ANOCEFdagger. Ann Oncol. 2014;25:1442–7.
Lou E, Peters KB, Sumrall AL, Desjardins A, Reardon DA, Lipp ES, et al. Phase II trial of upfront bevacizumab and temozolomide for unresectable or multifocal glioblastoma. Cancer Med. 2013;2:185–95.
Sathornsumetee S, Cao Y, Marcello JE, Herndon II JE, McLendon RE, Desjardins A, et al. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol. 2008;26:271–8.
Omuro A, Beal K, Gutin P, Karimi S, Correa DD, Kaley TJ, DeAngelis LM, Chan TA, et al. Phase II study of bevacizumab, temozolomide and hypofractionated stereotactic radiotherapy for newly diagnosed glioblastoma. Clinical Cancer Research. 2014. doi: 10.1158/1078-0432
Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–73.
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110.
Brennan C, Momota H, Hambardzumyan D, Ozawa T, Tandon A, Pedraza A, et al. Glioblastoma subclasses can be defined by activity among signal transduction pathways and associated genomic alterations. PLoS One. 2009;4:e7752.
Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–77.
Phillips H, Sandmann T, Li C, Cloughesy TF, Chinot OL, Wick W, et al. Correlation of molecular subtypes with survival in AVAglio (bevacizumab [Bv] and radiotherapy [RT] and temozolomide [T] for newly diagnosed glioblastoma [GB]). ASCO Meet Abstr. 2014;32(15 Suppl):2001.
Daras M, Abrey L, Sanchez J, Beal K, Gutin P, Kaley T, et al. RA-013. Diffusion and perfusion magnetic resonance imaging in newly diagnosed glioblastoma following treatment with bevacizumab, temozolomide and hypo-fractionated stereotactic radiotherapy. Neurooncology. 2013;15 Suppl 3:iii191–205.
Pope WB, Lai A, Mehta R, Kim HJ, Qiao J, Young JR, et al. Apparent diffusion coefficient histogram analysis stratifies progression-free survival in newly diagnosed bevacizumab-treated glioblastoma. Am J Neuroradiol. 2011;32:882–9.
Batchelor TT, Gerstner ER, Emblem KE, Duda DG, Kalpathy-Cramer J, Snuderl M, et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci U S A. 2013;110:19059–64.
Chen W, Delaloye S, Silverman DH, Geist C, Czernin J, Sayre J, et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18 F] fluorothymidine positron emission tomography: a pilot study. J Clin Oncol. 2007;25:4714–21.
Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95.
Gutin PH, Iwamoto FM, Beal K, Mohile NA, Karimi S, Hou BL, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009;75:156–63.
Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79:1487–95.
Jeyaretna DS, Curry Jr WT, Batchelor TT, Stemmer-Rachamimov A, Plotkin SR. Exacerbation of cerebral radiation necrosis by bevacizumab. J Clin Oncol. 2011;29:e159–62.
Thomas AA, BC, DeAngelis LM, Omuro AM. Emerging therapies for glioblastoma. JAMA Neurology. 2014:[Pub pending].
Hutterer M, Nowosielski M, Haybaeck J, Embacher S, Stockhammer F, Gotwald T, et al. A single-arm phase II Austrian/German multicenter trial on continuous daily sunitinib in primary glioblastoma at first recurrence (SURGE 01-07). Neurooncology. 2014;16:92–102.
Kreisl TN, McNeill KA, Sul J, Iwamoto FM, Shih J, Fine HA. A phase I/II trial of vandetanib for patients with recurrent malignant glioma. Neurooncology. 2012;14:1519–26.
Brandes AA, Stupp R, Hau P, Lacombe D, Gorlia T, Tosoni A, et al. EORTC study 26041-22041: phase I/II study on concomitant and adjuvant temozolomide (TMZ) and radiotherapy (RT) with PTK787/ZK222584 (PTK/ZK) in newly diagnosed glioblastoma. Eur J Cancer. 2010;46:348–54.
Batchelor TT, Mulholland P, Neyns B, Nabors LB, Campone M, Wick A, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, vs lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31:3212–8.
de Groot JF, Lamborn KR, Chang SM, Gilbert MR, Cloughesy TF, Aldape K, et al. Phase II study of aflibercept in recurrent malignant glioma: a North American Brain Tumor Consortium study. J Clin Oncol. 2011;29:2689–95.
Stupp R HM, Gorlia T, Erridge S, Grujicic D, Steinbach JP, Wick W, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma and methylated O6-methylguanine-DNA methyltransferase (MGMT) gene promoter: key results of the multicenter, randomized, open-label, controlled, phase III CENTRIC study. J Clin Oncol. 2013;31(Suppl l):LBA2009.
Zuniga RM, Torcuator R, Jain R, Anderson J, Doyle T, Schultz L, et al. Rebound tumour progression after the cessation of bevacizumab therapy in patients with recurrent high-grade glioma. J Neurooncol. 2010;99:237–42.
Reardon DA, Herndon II JE, Peters KB, Desjardins A, Coan A, Lou E, et al. Bevacizumab continuation beyond initial bevacizumab progression among recurrent glioblastoma patients. Br J Cancer. 2012;107:1481–7.
Omuro A, Chan TA, Abrey LE, Khasraw M, Reiner AS, Kaley TJ, et al. Phase II trial of continuous low-dose temozolomide for patients with recurrent malignant glioma. Neurooncology. 2013;15:242–50.
Takahashi N, Haba A, Matsuno F, Seon BK. Antiangiogenic therapy of established tumors in human skin/severe combined immunodeficiency mouse chimeras by anti-endoglin (CD105) monoclonal antibodies, and synergy between anti-endoglin antibody and cyclophosphamide. Cancer Res. 2001;61:7846–54.
Compliance with Ethics Guidelines
Conflict of Interest
Antonio Omuro serves on the advisory boards for Roche Schering-Plough/Merck, Novocure, CarThera. Alissa A. Thomas declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thomas, A.A., Omuro, A. Current Role of Anti-Angiogenic Strategies for Glioblastoma. Curr. Treat. Options in Oncol. 15, 551–566 (2014). https://doi.org/10.1007/s11864-014-0308-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-014-0308-2