Abstract
The ERF-type transcription factors (TFs) play vital roles in plant secondary metabolism. ERF TFs simultaneously regulate the expression levels of key enzyme genes involved in the biosynthesis of secondary metabolites due to its “multi-point control” function. In this study, one gene of ERF TFs from Panax japonicus (PjERF1) was cloned. The open reading frame of PjERF1 was 801 bp and encoded 266 amino acids. Phylogenetic analysis showed that PjERF1 belonged to ERF subfamily with a typical conserved domain. Subcellular localization found that PjERF1 protein might be located in eukaryotic cell nucleus. Yeast one-hybrid assay demonstrated that PjERF1 could bind to the promoters of PjβAS, PjCAS, and PjSE specifically and regulate the expression levels of such key enzyme genes involved in the triterpene saponins biosynthesis. Therefore, in the PjERF1 overexpression cell lines, the expression levels of some key enzyme genes involved in the triterpenoid saponins biosynthesis were significantly increased compared with those in non-transgenic cell line. As a result of it, the biosynthesis of chikusetsusaponin IV and IVa, and other ginsenosides (Rd, Rb1, Re, and R0) were also promoted in the PjERF1 overexpression cell lines. This study indicated that PjERF1 could regulate the biosynthesis of saponins in P. japonicus through controlling the expression levels of key enzyme genes related to the biosynthesis of triterpenoid saponins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Panax japonicus is a kind of traditional Chinese herb used for hundreds of years. P. japonicus saponins (PJS) are the major bioactive components in P. japonicus. Up to now, more than 30 kinds of saponins have been isolated from the roots and leaves of P. japonicus, which were classified into dammarane-type saponins (ginsenosides Re, Rd, and Rb1) and oleanane-type saponins (ginsenoside R0, chikusetsusaponin IV, and chikusetsusaponin IVa) (Sz et al. 2020). Contrast to P. japonicus, the popular medicinal herbs, P. ginseng, P. quinquefolium, and P. notoginseng mainly contain dammarane-type saponins (Li et al. 2013; Yang et al. 2018). Such differences of saponins composition lead to the special pharmacological activities of P. japonicus (Leung and Wong 2010; Wei et al. 2014; Yang et al. 2014; Zhang et al. 2014).
PJS are triterpenoid saponins and synthesized via mevalonic acid (MVA) pathway (Tang et al. 2019). Mevalonic acid is catalyzed by geranyl phrophosphate synthase (GPS), farnesyl pyrophosphate synthase (FPS), squalene synthase (SS), and squalene epoxidase (SE) to produce 2,3-oxidosqualene (Zhang et al. 2015; Zhao and Li 2018). 2,3-oxidosqualene is an important precursor of triterpenoid saponins biosynthesis in Panax, which forms two kinds of saponin skeletons under the catalysis of dammarenediol-II synthase (DS) and β-amyrin synthase (β-AS) (Ghosh 2017), respectively. Cycloartenol synthase (CAS) is a key enzyme gene in the biosynthesis of phytosterols which share the same precursor, 2,3-oxidosqualene, with β-AS and DS (Jin et al. 2017). β-amyrin and dammarenediol could be modified by P450s and UGTs to form oleanane-type and dammarane-type saponins, respectively. Transcriptome sequencing showed that 18 UGTs might involve in the PJS biosynthesis but it is difficult to identify the exact function of such UGTs until yet (Zhang et al. 2015).
Transcription factors (TFs) widely involved in the process of plant growth and secondary metabolism, which would specifically bind to the promoters of genes to achieve the activation or repression of transcription. The artificial operation of TFs has become an effective strategy to regulate the biosynthesis of plant secondary metabolites through multi-point controlling the expression levels of key enzyme genes involved in the biosynthetic pathway. The transient expression of AabHLH in Artemisia annua increased the expression levels of ADS, CYP71AV1, and HMGR, which illustrated that the transcription factor AabHLH could positively control the biosynthesis of artemisinin (Ji et al. 2014). In P. quinquefolius, the transcription factor PqWRKY could regulate the transcription of related genes in triterpenoid saponins biosynthesis to improve the content of total saponins (Sun et al. 2013); The TSAR bHLH transcription factors have been reported to be involved in the regulation of saponin biosynthesis in Medicago truncatula (Mertens et al. 2016). Such studies proved that transcription factors could take part in the biosynthesis of secondary metabolism.
Among so many kinds of TFs, the ethylene-responsive factors (ERF) play important roles in both regulating the secondary metabolism and plant stress responses (Yang et al. 2012; Sun et al. 2014). ERF family includes a conserved domain consists of 60–70 amino acids, which is classified into five subfamilies according to the structure similarity and the number of conserved domains (Yao et al. 2020). It is known that ERF could specially bind to the GCC-box (AGCCGCC) and regulate the expression of genes (Sakuma et al. 2012). RNA interference of NtERF32 in tobacco decreased the expression of related key enzyme genes NtQPT2 and NtPMT1a to reduce the contents of nicotine and alkaloid (Sears et al. 2014). Jasmonate responsive ERF gene clusters control the biosynthesis of many important metabolites, such as nicotine, steroidal glycoalkaloids, artemisinin, vinblastine, and vincristine (Dai et al. 2009; Shoji and Yuan 2021). Furthermore, some studies have indicated that ERFs could regulate the biosynthesis of triterpenoids in medicinal plants, such as Gynostemma pentaphyllum and Artemisia annua (Xu et al. 2020; Ji et al. 2014). Base on such studies, we supposed that ERF TFs might also have the potential to affect biosynthesis of P. japonicus saponins (PJS) because PJS belonged to triterpenoids.
In this study, one gene of ERF TFs from Panax japonicus (PjERF1) was cloned and the interaction between PjERF1 and promoters of key enzyme genes involved in PJS biosynthesis was explored. Furthermore, PjERF1 overexpressed cell lines were constructed to ensure the function of PjERF1 in PJS biosynthesis.
Materials and methods
Plant materials and growth conditions
The callus was induced from the roots of P. japonicus and subcultured on Murashige and Skoog (MS) medium with 2.0 mg/L 2, 4-dichlorophenoxyacetic acid (2, 4-D) and 1.0 mg/L kinetin (KT) every 45 days. The standard compounds were derived from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). All other chemicals were purchased from Sangon Biotech Co. Ltd. (Shanghai, China).
Cloning and sequence analysis of PjERF1
According to the study of (Deng et al., 2017) the total RNA of P. japonicus was extracted through guanidine isothiocyanate method and the first-strand cDNA was prepared for RT-PCR. In this study, the degenerate primers were designed according to the conserved domain of the Catharanthus roseus ORCA family, and the core sequence of the PjERF1 transcription factor gene was amplified by homologous cloning. Then using the rapid amplification of cDNA ends (RACE) and taking the RACE-Ready cDNA as a template, the 5′ and 3′ end sequences of PjERF1 were cloned. Next, the cloned sequences above were used as the basis for designing the primers to amplify the full-length cDNA of PjERF1. The PCR products of PjERF1 full-length cDNA were selected by 1% agarose gel electrophoresis and cloned into the pGEM-T Easy vector (Promega, USA) for sequencing. Homology analysis of amino acid sequence was performed by DNAMAN. Phylogenetic tree of PjERF1 was built-in MEGA 6.0 with neighbor-joining methods. The homology modeling of PjERF1 was performed with the tool of SWISS-MODE (http://swissmodel.expasy.org/).
Yeast one-hybrid assay
To confirm whether PjERF1 could directly bind to the promoters of key enzyme genes involved in PJS biosynthesis, PjASP, PjCASP, PjSEP, and PjSSP were selected and investigated such interaction by yeast one-hybrid assays. According to the requirements of Universal Genome Walker 2.0 kit, promoters of the key enzyme genes were cloned. For the first time, the universal primer AP1 provided by the Universal Genome Walker 2.0 kit and the specific primer GSP1 was designed according to the instructions, which were used for PCR amplification of the key enzyme promoters. Since the amplified promoter fragments were based on Genome Walker Libraries, the sensitivity of the first PCR reaction is not enough to obtain specific promoter fragments. The primers used in the second PCR reaction were AP2 and GSP2, and the product of the first PCR was used as the template for the second PCR. The second PCR product all showed specific bands (Figure S1). The recovered specific bands were subjected to TA cloning, transformed into Escherichia coli; and the positive single clones were screened and sent for sequencing. Finally, the sequences of the promoters of PjAS, PjCAS, PjSE, and PjSS were obtained and the lengths of which were 2.52, 1.40, 1.54, and 0.88 kb, respectively.
Matchmaker™ Gold Yeast One-Hybrid Library Screening System (Clontech) was used for identifying promoters that bind to PjERF1. DNA was extracted and purified to construct the cDNA library and clone the promoters of key enzyme genes by Universal Genome Walker 2.0 (TaKaRa). Primers used in this assay are listed in Table S1. After sequencing, the promoters of related genes (PjASP, PjCASP, PjSEP, and PjSSP) were inserted into pAbAi vector and the full-length CDS of PjERF1 was inserted into the pGADT7 vector to get pGADT7-PjERF1 (Figure S2). The recombinant plasmids containing PjERF1 and promoter fragments were co-transformed into Y1HGold yeast strain to examine the interaction on medium lacking Leu with the optimal AbA screening concentration. The plasmid p53-AbAi could specifically bind to GAL4 to be the positive control. The empty vector pAbAi was used as the negative control.
Construction of PjERF1 overexpression vector
PjERF1 was amplified with specific primers (Table S2). The procedure of PCR was as follows: 95 °C for 5 min, 32 cycles of 94 °C for 30 s, 56 °C for 30 s and 72 °C for 50 s, a final cycle 10 min extension at 72 °C. After sequencing, PCR products digested by Sma I/Xba I were inserted into pCAMBIA2300S. The pCAMBIA2300S-PjERF1 overexpression vector was constructed. The recombined vector pCAMBIA2300S-PjERF1 was transformed into Agrobacterium tumefaciens EHA105 by the freeze–thaw method (Jin et al. 2017).
Establishment of PjERF1 transgenic cell lines
Agrobacterium tumefaciens EHA105 contained PjERF1 was cultured on MGL liquid medium until the OD600 reached 0.6 to 0.8. The wild-type (WT) cells of P. japonicus pre-cultured on MS medium with 40.0 mg/L acetosyringone for three days were suspended in A. tumefaciens liquid to obtain PjERF1 transgenic cell lines. After washing away the A. tumefaciens by sterile water containing 400.0 mg/L cefotaxime, the PjERF1 transgenic cells were incubated on the MS agar medium with 400.0 mg/L cefotaxime for 15 days. MS agar medium containing 50.0 mg/L kanamycin sulfate was used for selecting PjERF1 transgenic cell lines.
Genomic DNA of PjERF1 transgenic cells were extracted by CTAB method (Vzae et al. 2010). The kanamycin resistance gene (npt II) in T-DNA was detected to ensure the positive cell lines by PCR, and it had no effects on the expressions of other key enzyme genes or biosynthesis of secondary metabolites (Han et al. 2014; Jo et al. 2017). The PCR performed was as follows: 94 °C for 5 min; then 32 cycles of 94 °C for 30 s, 56 °C for 30 s, and 72 °C for 30 s; and a final 2-min extension at 72 °C. The PCR products were checked by 1% agarose electrophoresis.
Subcellular localization analysis
The pMD18-T-PjERF1 was digested by Nde I/Sma I and inserted into pBIN m-gfp5-ER vector. The specific primers are listed in Table S3. The recombined vector pBIN m-gfp5-ER-PjERF1 was expressed transiently in onion epidermal cells by A. tumefaciens infiltration (EHA105) using the same method as described above (Li et al. 2021). After being cultured in dark, different stages of onion epidermal cells were prepared for observing under confocal microscopy. The fluorescence was detected at 395 nm.
Expression analysis by qRT-PCR in PjERF1 transgenic cell lines
Total RNA was extracted and reverse-transcribed into cDNA in P. japonicus cells by the method described above. The 18S rRNA was selected as the reference gene. The qRT-PCR of GoTaq®2-Step RT-qPCR System (Promega, USA) was as follows: 95 °C, 2 min; next 40 cycles of 95 °C for 15 s, 57 °C for 30 s, and 72 °C for 30 s. Each sample was prepared in triplicate. The relative expression levels of key enzyme genes were analyzed by \(2^{{ - \Delta \Delta C_{{\text{T}}} }}\) method and all the primers are listed in Table S3 (Livak and Schmittgen 2001).
Determination of P. japonicus saponins content
All the sample fresh cells were dried to the constant weight at 55 °C and grinded into powder. Samples soaked in methanol overnight were ultrasonically extracted (60 W) for 2 h. Liquid supernatant was collected after centrifugation to get the solution of PJS. According to the coloring reagents by vanillin and perchloric acid at 60 °C, the concentration of total saponins was detected. The absorbance of the reaction products was determined at A550, and the content of total triterpene saponins was calculated based on standard curve.
HPLC analysis of monomer saponins
Monomer saponins of ginsenoside Rd, Rb1, Re, R0, and chikusetsusaponin IV, IVa were detected by Agilent 1260 (Santa Clara, CA, USA) with a Waters XTerra MS C18 column (5 µm, 250 mm × 4.6 mm). The mobile phase was water-phosphoric acid (A)/acetonitrile (B). The flow-rate was 1.0 mL min−1 and the column temperature was maintained at 30 °C. The gradient elution was as below: 0–20 min, 20% acetonitrile; 20–30 min, 20–35% acetonitrile; 30–40 min, 35% acetonitrile; 40–50 min, 35–40% acetonitrile; 50–60 min, 40% acetonitrile; 60–65 min, 40–100% acetonitrile; 65–75 min, 100% acetonitrile. The wavelength of detection was set at 203 nm. All standards were purchased from National Institutes for Food and Drug Control (China).
Statistical analysis
All analyses were performed with three replications. According to Student’s t test, the error bars indicate the standard deviations from the means of triplicates. The asterisk indicates a significant difference in measured parameters (*P < 0.05; **P < 0.01).
Result
Cloning and bioinformatics analysis of PjERF1 from P. japonicus
The transcription factor gene PjERF1 was obtained according to the ERF cDNA sequence in P. japonicus (GenBank Accession Number KP890784). The open reading frame (ORF) of PjERF1 was 801 bp encoding 266 amino acids with the molecular weight of 30.068 kDa and a PI of 5.40. NCBI-Protein BLAST predicted that PjERF1 covered a conserved domain which located in 74–134 amino acids. Based on the deduced amino acid sequence, multiple sequence alignment showed that PjERF1 had the same conserve domains that covered YRG and RAYD elements with the ERF TFs in other plants. Furthermore, the 37th amino acid (alanine) could bind to cis-acting element GCC-box which proved that PjERF1 might belong to ERF transcription factors (Figure S3).
Nine ERF amino acid sequences were selected from NCBI to construct a phylogenetic tree. PjERF1 showed high homology with other ERF transcription factors (Fig. 1). Phylogenetic analysis proved that PjERF1 belongs to ERF transcription factor family. To some extent, the function of the protein was determined by the tertiary structure. The 3D structure of PjERF1 was predicted with the SWISS-MODE algorithm to comprehend the structure–function relation-ship of plant AP2/ERF TFs. Using the crystal structure of AtERF096 (5wx9.1.A) as a template, the 3D model of PjERF1 was built and shared 72.88% identity with the AtERF096 (Fig. 2).
Phylogenetic tree analysis of PjERF1. The sequences used were PqERF1 (Panax quinquefolium ERF1), PgERF1(Panax ginseng ERF1), SiERF071 (Sesamum indicum, XP_011079624.1), JcERF073 (Jatropha curcas, XP_012082821.1), NtERF071 (Nicotiana tabacum, XP_016475776.1), CcERF071-like (Cynara cardunculus var. scolymus, XP_024966446.1), HaERF (Helianthus annuus, XP_022035166.1), and InERF (Ipomoea nil, XP_019163870.1). The neighbor-joining method phylogenetic tree was constructed using the bootstrap method of MEGA 6.0 with 1000 replications and the respective plant species of the above proteins were shown in the tree. The black circular symbols indicate the PjERF1 protein
PjERF1 is located to the cell nucleus
The subcellular localization of PjERF1 protein was determined by confocal microscope combined with GFP and propidium iodide (PI) markers. PI is a kind of staining reagent for nucleus. The PjERF1-GFP fusion gene was transiently expressed in onion epidermal cells transiently transformed by A. tumefaciens. The panels on the left of Fig. 3 show that the recombinant fusion green fluorescent protein was mainly concentrated in the nuclear region and thus confirmed that the PjERF1 transcription factor was located to the nucleus. The panels on the right of Fig. 3 indicate that the onion inner epidermal cells was transfected with GFP vector without PjERF1 gene; and the green fluorescence could not only be observed in the nucleus but also in the cytoplasm.
Subcellular localization of the PjERF1-GFP fusion protein. The PjERF1-GFP fusion protein was detected in the nucleus after the genetic transformation mediated by A. tumefaciens. GFP, fluorescent light; Bright, white light; PI, propidium iodide (for staining nuclear DNA); Merged, overlay of the GFP and PI images
ERF TFs are considered as important regulators to control plant secondary metabolism. We speculated that PjERF1 might be related to triterpenoid synthesis. Based on the “multi-point control” character of TFs, the interactions between PjERF1 and the promoters of key enzyme genes related to PJS biosynthesis were discussed next.
PjERF1 is a direct regulator of PjAS, PjCAS, and PjSE promoters
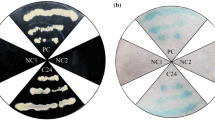
In the process of yeast one-hybrid assays, when PjERF was binding to the suitable site of the recombinant bait plasmid, the expression of downstream AUR1-C reporter gene was activated and Y1HGold could grow on the medium containing AbA. As shown in Fig. 4, Y1HGold could grow up normally on SD/-Leu/AbA solid medium and produced fusion protein GAL4-PjERF. Through the analysis of the promoter sequence, it was found that the promoters of PjAS, PjCAS and PjSE contained GC-box (GCCGCC) which was considered as a classical binding box of ERF TFs. Moreover, the results of yeast one-hybrid assay also indicated that PjERF1 could bind to the promoters of PjAS, PjCAS, and PjSE (Fig. 4A–C). No yeast strain existed in SD/-Leu/AbA medium of Fig. 4D showed that PjERF1 did not interact with the promoter of PjSS. It was known that the ERF transcription factors could specific bind to GC-box (GCCGCC) and regulated the expression of downstream genes (Sakuma et al. 2002). In our study, sequence analysis confirmed that the GC-box was included in the promoters of PjAS, PjCAS, and PjSE which was consistent with result of yeast one-hybrid assay. Furthermore, PjERF1 might multi-point control the expression levels of some key enzyme genes involved in biosynthesis of PJS and then influenced the biosynthesis of PJS indirectly and effectively.
PjERF1 increased the expression levels of key genes involved in saponin biosynthesis
The PjERF1 transgenic cell lines were produced by Agrobacterium tumefaciens-mediated genetic transformation. PjERF1 was overexpressed in P. japonicus cells to explore whether PjERF1 could regulate the expression levels of key enzyme genes in PJS biosynthesis pathway. The PjERF1 overexpressed cell lines were screened on the selective medium with kanamycin, and the npt II resistance gene was amplified to screen positive PjERF1 transgenic cell lines. The amplified npt II products were in the positive cells and there was no signal in the WT (Figure S4). Four positive PjERF1 transgenic cell lines (T1, T2, T3, and T4) were used for further studies.
In PjERF1 transgenic cell lines, the expression level of PjERF1 was increased significantly compared with WT cell line. We measured the saponins of P. japonicus cells in WT and transferred with empty pCAMBIA2300 load, and found that there was no difference between them (Figure S5). Especially in the T2 cell line, the expression level of PjERF1 was approximately 9.5 times higher than that in control (Fig. 5A). In the T4 cell line, the expression level of PjAS was promoted even tenfold of that in control, which indicated that the content of oleanane-type saponins might be increased remarkably (Fig. 5H). The expression level of key enzyme gene PjDS related to dammarane-type saponins biosynthetic pathway was also enhanced (Fig. 5G); therefore, the biosynthesis of dammarane-type saponins should be influenced. Besides, the expression levels of other key enzyme genes such as PjSE, PjCAS were also increased to some extent (Yin et al. 2017). To sum up, PjERF1 overexpression in P. japonicus cells could regulate the expression levels of key enzyme genes involved in the biosynthesis of PJS.
Relative expression levels in four PjERF1 transgenic cell lines. A–H represented the expression levels of PjERF1, PjHMGR, PjFPS, PjSS, PjSE, PjDS, PjCAS, and PjAS, respectively. Standard deviation (SD) of means from three independent experiments was indicated by error bars. 18S rRNA was selected as the internal reference gene. \(2^{{ - \Delta \Delta C_{{\text{t}}} }}\) method was used to calculate the relative expression levels. Significant difference between the wild-type (WT) cell line and the transgenic cell line was indicated by asterisks (*P < 0.05; **P < 0.01)
Overexpression of PjERF1 enhanced the biosynthesis of PJS
Compared with non-transgenic cell line, the contents of total saponins in the four PjERF1 transgenic cell lines were significantly increased. In T2 transgenic cell line, the content of PJS was 1.53 times of that in control (Fig. 6). The result indicated that PjERF1 transcription factor played an important role in the regulation of PJS biosynthesis.
The major monomer saponins (ginsenoside Rd, Re, Rb1, R0, chikusetsusaponin IV, and IVa) were detected by HPLC (Figure S6). In PjERF1 transgenic cell lines, the contents of six monomer saponins were all enhanced (Fig. 7). The content of chikusetsusaponin IV and IVa were the highest in P. japonicus among six monomer saponins. In T2 transgenic cell lines, the content of chikusetsusaponin IV and IVa was 1.47 times and 1.32 times of those in control, respectively. In T4 transgenic cell line, ginsenoside Rd had the highest increase in content which was about 1.69 times of that in control; and in T2 cell line the content of ginsenoside Re was 1.55 times of that in WT cell line. Overexpression of PjERF1 could promote the biosynthesis of oleanane-type saponins and dammarane-type saponins in P. japonicus.
The contents of major monomer saponins in P. japonicus cell lines. Concentrations of six monomer saponins in PjERF1 transgenic cell lines and WT cell line. a-f stands for the Rd, Rc, Rb1, R0, IV, and IVa six monomer saponins. Significant difference between WT and transgenic cells was indicated by asterisks (*P < 0.05; **P < 0.01)
Discussion
Transcription factors have the advantage of “multi-point control” in the regulation of secondary metabolism. Up to now, ERF transcription factors were isolated from some kinds of plants; and the functions of them were explored comprehensively. Suppressing of OpERF2 resulted in the reduced expression levels of genes which related to the biosynthesis of secologanin and strictosidine in Ophiorrhiza pumila (Udomsom et al. 2016). When the GmERF3 from Glycine max was overexpressed in tobacco, the resistance to the pathogens such as Ralstonia solanacearum, Alternaria alternata and tobacco mosaic virus was also enhanced (Zhang et al. 2009). In Catharanthus roseus, ORCA3 (ERF TF) overexpression could regulate the biosynthesis of terpenoid indole alkaloid (Zhou et al. 2010). According to these studies, the regulation of secondary metabolism by ERF TFs might be a common phenomenon in plants. In this study, a new PjERF1 gene from P. japonicus was cloned. The multiple alignment and phylogenetic analysis demonstrated that PjERF1 belongs to ERF subfamily and the 37th amino acid residue (alanine) in PjERF1 conserved domain was able to bind to GCC-box cis-acting element.
ERF TFs control the secondary metabolism mainly through regulating the expression levels of key enzyme genes in the biosynthetic pathway. When AaERF1 and AaERF2 were overexpressed in Artemisia annua L, the expression levels of key enzyme genes ADS and CYP71AV1 in artemisinin biosynthetic pathway were increased; and the biosynthesis of artemisinin was promoted (Yu et al. 2012). Transcription factor CitERF71 could enhance the content of E-geraniol in sweet orange fruit by activating the terpene synthase gene CitTPS16 (Li et al. 2017). In this study, we demonstrated that PjERF1 could bind to the promoters of PjSE, PjAS, and PjCAS, and regulate the expression levels of such genes. Moreover, although the expression levels of some key enzyme genes, such as PjFPS, PjDS, and PjSS, were increased, there was no interaction between PjERF1 and the promoters of these genes. The possible mechanism of such result might be that when the main metabolic flux was increased, the expression levels of relative key enzyme genes involved in biosynthesis pathway of saponins should be increased passively to process more metabolites.
In the present study, the content of PJS was increased due to the promoted expression levels of key enzyme genes involved in PJS biosynthetic pathway, but the content of diverse monomer saponins had different degrees of increase. We speculated that transcription factor PjERF1 may also regulate the expression levels of some genes of UDP-glycosyltransferases (UGTs) or P450s which related to the modification of triterpenoid skeleton. Up to now, several genes used for modifying the skeleton of saponins have been identified. Overexpressing of UGTPg71A29 could lead to the increased contents of Rg1 and Rb1 in P. ginseng (Lu et al. 2018). Similarly, overexpressing CYP716A52v2 could enhance the content of ginsenoside R0 in P. ginseng (Han et al. 2013). According to these studies, we considered that TFs might regulate the expression of UGTs and lead to the different increase of monomer saponins contents. As the UGTs and P450s are supergene families, most of them are difficult to be screened out and identified the exact functions. Such investigation will be carried out next.
Yeast one-hybrid technology could analyze the interaction between protein and DNA by detecting reporter genes (Wu et al. 2018). In this study, we performed yeast one-hybrid to explore whether PjERF1 could bind to the promoters of key enzyme genes related to the biosynthesis of PJS. As we have expected, PjERF1 bound to the promoters of PjCAS, PjAS and PjSE exactly. PjSE catalyzes squalene to form 2,3-oxidosqualene which is the precursor of phytosterols, dammarane-type, and oleanane-type saponins. Therefore, the biosynthesis of saponins would be affected in P. japonicus by regulating the expression of PjSE. Furthermore, PjAS is the first key enzyme gene in the branch of oleanane-type saponins biosynthesis pathway (Fig. 8). Overexpression of PjERF1 could enhance the biosynthesis of oleanane-type saponins by up-regulating the expression of PjAS and the contents of chikusetsusaponin IV and chikusetsusaponin IVa were increased significantly in transgenic cell lines. In the meantime, the biosynthesis of dammarane saponins was also promoted because the up-regulation of PjSE led to the enhancement of primary metabolic flux. In addition, because the PjERF1 could bind to the promoter of PjCAS and increase the expression level of PjCAS, so we considered that the synthesis of phytosterols would be enhanced. Further result showed that in the four transgenic cell lines, the content of phytosterols was higher than that control, which suggested that PjERF1 could promote the biosynthesis of phytosterols through the interaction with PjCAS (Fig. 9).
The biosynthetic pathway of PJS. The dotted line indicates that PjERF1 transcription factor can act on the promoters of PjAS, PjCAS, and PjSE. PjHMGR: 3-hydroxy-3-methylglutaryl CoA reductase, PjFPS farnesyl pyrophosphate synthase, PjAS amyrin synthase, PjSS squalene synthase, PjSE squalene epoxidase, PjDS dammarenediol-II synthase, PjCAS cycloartenol synthase
Furthermore, with the unique “multi-point control” advantage, the operation of TFs is more efficient than directly regulate the key enzyme genes involved in saponins pathway. So far, few transcription factors involved in the regulation of triterpenoid saponin synthesis have been identified and functionally characterized in P. japonicus. This study is an interesting exploration. As the promoter sequence information of the key enzyme genes involved in saponin synthesis was insufficient, which limited the functional investigation of transcription factors. In the further work, more details about transcription factors on the regulation of triterpenoid saponins biosynthesis will be delineated.
Conclusion
In this study, a transcription factor PjERF1 was cloned and analyzed from P. japonicus. PjERF1 could regulate the biosynthesis of saponins in P. japonica through controlling the expression levels of key enzyme genes related to the biosynthesis of triterpenoid saponins. The results might provide a theoretical reference for establishing efficient and stable regulation technology in the biosynthesis of triterpenoid saponins.
Abbreviations
- HPLC:
-
High-performance liquid chromatography
- MVA:
-
Mevalonic acid
- 2, 4-D:
-
2,4-Dichlorophenoxyacetic acid
- Hyg:
-
Hygromycin B
- KT:
-
Kinetin
- CTAB:
-
Cetyltrimethylammonium bromide
References
Dai YL, Qin QL, Kong DL, Zha LS, Jin XJ (2009) Isolation and characterization of a novel cDNA encoding methyl jasmonate-responsive transcription factor TcAP2 from Taxus cuspidata. Biotechnol Lett 31:1801–1804
Deng B, Zhang P, Ge F, Liu DQ, Chen CY (2017) Enhancement of triterpenoid saponins biosynthesis in Panax notoginseng cells by co-overexpressions of 3-hydroxy-3-methylglutaryl CoA reductase and squalene synthase genes. Biochem Eng J 122:38–46
Ghosh S (2017) Triterpene structural diversification by plant cytochrome P450 enzymes. Front Plant Sci 8:1886
Han JY, Kim MJ, Ban YW, Hwang HS, Choi YE (2013) The involvement of beta-amyrin 28-oxidase (CYP716A52v2) in oleanane-type ginsenoside biosynthesis in Panax ginseng. Plant Cell Physiol 54(12):2034–2046
Han JL, Wang HZ, Lundgren A, Brodelius PE (2014) Effects of overexpression of AaWRKY1 on artemisinin biosynthesis in transgenic Artemisia annua plants. Phytochemistry 102:89–96
Ji Y, Xiao J, Shen Y, Ma D, Li Z, Pu G, Li X, Huang L, Liu B, Ye H (2014a) Cloning and characterization of AabHLH1, a bHLH transcription factor that positively regulates artemisinin biosynthesis in Artemisia annua. Plant Cell Physiol 55(9):1592–1604
Jin ML, Lee WM, Kim OT (2017) Two cycloartenol cynthases for phytosterol biosynthesis in Polygala tenuifolia willd. Int J Mol Sci 18(11):2–12
Jo HJ, Han JY, Hwang HS, Choi YE (2017) Beta-amyrin synthase (EsβAS) and beta-amyrin 28-oxidase (CYP716A244) in oleanane-type triterpene saponin biosynthesis in Eleutherococcus senticosus. Phytochemistry 135:53–63
Leung K, Wong A (2010) Pharmacology of ginsenosides: a literature review. Chin Med 5(1):20
Li C, Zhu Y, Xu G, Chao S, Chen S (2013) Transcriptome analysis reveals ginsenosides biosynthetic genes, microRNAs and simple sequence repeats in Panax ginseng C. A. Meyer. BMC Genomics 14:245–256
Li X, Xu Y, Shen S, Yin XR, Klee H, Zhang B, Chen KS, Hancock R (2017) Transcription factor CitERF71 activates the terpene synthase gene CitTPS16 involved in the synthesis of egeraniol in sweet orange fruit. J Exp Bot 68(17):4929–4938
Li S, Liu GZ, Pu LM, Liu XY, Liu DQ (2021) WRKY transcription factors actively respond to Fusarium oxysporum in Lilium regale Wilson. Phytopathology. https://doi.org/10.1094/PHYTO-10-20-0480-R
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 (−Delta Delta C(T)) method. Methods 25(4):402–408
Lu J, Lu Y, Li J, Liu S, Hu Y, Wang S, Liang W, Huang L, Dai Y, Wang J (2018) Characterization of UDP-glycosyltransferase involved in biosynthesis of ginsenosides Rg1 and Rb1 and identification of critical conserved amino acid residues for its function. J Agric Food Chem 66(36):9446–9455
Mertens J, Pollier J, Vanden Bossche RV, Lopez-Vidriero I, Franco-Zorrilla JM, Goossens A (2016) The bHLH transcription factors TSAR1 and TSAR2 regulate triterpene saponin biosynthesis in Medicago truncatula. Plant Physiol 170(1):194–210
Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-Shinozaki K (2002) DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem Biophys Res Commun 290(3):998–1009
Sears MT, Zhang HB, Rushton PJ, Wu M, Han SC, Spano AJ, Timko MP (2014) NtERF32: a non-NIC2 locus AP2/ERF transcription factor required in jasmonate-inducible nicotine biosynthesis in tobacco. Plant Mol Biol 84(1–2):49–66
Shoji T, Yuan L (2021) ERF gene clusters: working together to regulate metabolism. Trends Plant Sci 26(1):23–32
Sun YZ, Niu YY, Xu J, Li Y, Luo HM, Zhu YJ, Liu MZ, Wu Q, Song JY, Sun C, Chen SL (2013) Discovery of WRKY transcription factors through transcriptome analysis and characterization of a novel methyl jasmonate-inducible PqWRKY1 gene from Panax quinquefolius. Plant Cell Tissue Organ 114(2):269–277
Sun ZM, Zhou ML, Xiao XG, Tang YX, Wu YM (2014) Overexpression of a lotus corniculatus AP2/ERF transcription factor gene, LcERF080, enhances tolerance to salt stress in transgenic arabidopsis. Plant Biotechnol Rep 8(4):315–324
Sz A, Gw A, Tian ZA, Xz A, Ran XA, Wz A (2020) Comparative transcriptome analysis of rhizome nodes and internodes in Panax japonicus var. major reveals candidate genes involved in the biosynthesis of triterpenoid saponins. Genomics 112(2):1112–1119
Tang QY, Chen G, Song WL, Fan W, Wei KH, He SM, Zhang GH, Tang JR, Li Y, Lin Y, Yang SC (2019) Transcriptome analysis of Panax zingiberensis identifies genes encoding oleanolic acid glucuronosyltransferase involved in the biosynthesis of oleanane-type ginsenosides. Planta. 249:393–406
Udomsom N, Rai A, Suzuki H, Okuyama J, Imai R, Mori T, Nakabayashi R, Saito K, Yamazaki M (2016) Function of AP2/ERF transcription factors involved in the regulation of specialized metabolism in ophiorrhiza pumila revealed by transcriptomics and metabolomics. Front Plant Sci 7:1861
Vzae A, Nerkar G, Pagariya M, Devarumath RM, Theertha PD (2010) Isolationed PCR amplification of genomic DNA from dry leaf samples of sugarcane. Int J Pharma Bio Sci 1(2):1–3
Wei N, Zhang C, He H, Wang T, Liu Z, Liu G (2014) Protective effect of saponins extract from Panax japonicus on myocardial infarction: involvement of nf-kappab, sirt1 and mitogen-activated protein kinase signalling pathways and inhibition of inflammation. J Pharm Pharmacol 66(11):1641–1651
Wu R, Duan L, Pruneda-Paz JL, Oh DH, Pound MP, Kay SA, Dinneny JR (2018) The 6xABRE synthetic promoter enables the spatiotemporal analysis of ABA-mediated transcriptional regulation. Plant Physiol 177(4):1650–1665
Xu S, Yao S, Huang S, Tan Y, Huang D (2020) Transcriptome-wide analysis of the AP2/ERF transcription factor gene family involved in the regulation of gypenoside biosynthesis in Gynostemma pentaphyllum. Plant Physiol Biochem 154:238–247
Yang CQ, Fang X, Wu XM, Mao YB, Wang LJ, Chen XY (2012) Transcriptional regulation of plant secondary metabolism. J Integr Plant Biol 54(10):703–712
Yang X, Wang R, Zhang S, Zhu W, Tang J, Liu J (2014) Polysaccharides from Panax japonicus C.A. Meyer and their antioxidant activities. Carbohydr Polym 101:86–91
Yang JL, Hu ZF, Zhang TT, Gu AD, Gong T (2018) Progress on the studies of the key enzymes of ginsenoside biosynthesis. Molecules 23(3):589
Yao W, An TY, Xu ZC, Zhang LB, Gao H, Sun W, Liao BS, Jiang CH, Liu ZH, Liu ZQ, Duan LX, Ji AJ (2020) Genomic-wide identification and expression analysis of AP2/ERF transcription factors related to andrographolide biosynthesis in Andrographis paniculata. Industrial Crops and Products 157:112878
Yin J, Zhang D, Zhuang J, Huang Y, Mu Y, Lv SW (2017) Study on the correlation between gene expression and enzyme activity of seven key enzymes and ginsenoside content in ginseng in over time in Ji’an, China. Int J Mol Sci 18(12):2–12
Yu ZX, Li JX, Yang CQ, Hu WL, Wang LJ, Chen XY (2012) The jasmonate-responsive AP2/ERF transcription factors AaERF1 and AaERF2 positively regulate artemisinin biosynthesis in Artemisia annua L. Mol Plant 5(2):353–365
Zhang GY, Chen M, Li LC, Xu ZS, Chen XP, Guo JM, Ma YZ (2009) Overexpression of the soybean GmERF3 gene, an AP2/ERF type transcription factor for increased tolerances to salt, drought, and diseases in transgenic tobacco. J Exp Bot 60(13):3781–3796
Zhang H, Wang HF, Liu Y, Huang LJ, Wang ZF, Li Y (2014) The haematopoietic effect of Panax japonicus on blood deficiency model mice. J Ethnopharmacol 154(3):818–824
Zhang SP, Wu YY, Jin J, Hu BX, Zeng WY, Zhu WJ, Zheng YL, Chen P (2015) De novo characterization of Panax japonicus C. A. Mey transcriptome and genes related to triterpenoid saponin biosynthesis. Biochem Biophys Res Commun 466(3):450–455
Zhao YJ, Li C (2018) Biosynthesis of plant triterpenoid saponins in microbial cell factories. J Agric Food Chem 66(46):12155–12165
Zhou ML, Zhu XM, Shao JR, Wu YM, Tang YX (2010) Transcriptional response of the catharanthine biosynthesis pathway to methyl jasmonate/nitric oxide elicitation in Catharanthus roseus hairy root culture. Appl Microbiol Biotechnol 88:737–750
Funding
This work was supported by the National Natural Science Foundation of China (82060692); Yunnan Key Research and Development Program, China (2019ZF011-2); Key Projects of Basic Research Plan of Yunnan Province, China (202101AS070024).
Author information
Authors and Affiliations
Contributions
FG: conceived and designed the experiments. QC and YY: performed the experiments. JZ and DL: provided technical guidance and support. QC, YY, and XZ: contributed to the data analysis. XC: Provided the experimental materials. QC, YY, and RZ: wrote this paper. QC and YY: contributed to the work equally and were regarded as co-first authors. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Q., Yu, Y., Zhang, X. et al. The transcription factor PjERF1 enhances the biosynthesis of triterpenoid saponins in Panax japonicus. Plant Biotechnol Rep 15, 597–607 (2021). https://doi.org/10.1007/s11816-021-00698-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-021-00698-x