Abstract
Cellulose is a renewable and promising material. However, native cellulose has to face the challenge of the removal of heavy metals with low efficiency which limits its application. In this work, a cellulose derivative with EDTA-like chelating groups (EDTA-CL) is designed and prepared by the chemical grafting of cellulose. Cellulose is partially oxidized to dialdehyde cellulose which is treated with 20% excess of diethylenetriamine through a Schiff base reaction for the preparation of the aminated cellulose. The amine groups of the aminated cellulose are carboxymethylated by reacting with 20% excess of bromoacetic acid through a substitution reaction. The high-efficient adsorption of the both ions by the EDTA-CL with high adsorptive amounts (Pb2+: 438.3 mg g−1 and Cd2+: 287.2 mg g−1) can be accomplished by controlled parameters (pH of 4–6, contact time of 30 min and the dosage of 1 g L−1). The adsorptive processes of the both ions onto the EDTA-CL can be well fitted by pseudo-second-order and Langmuir equations. Thermodynamics data reveal that the adsorption of the both ions onto the EDTA-CL is a spontaneous and endothermic process. The loaded EDTA-CL can be regenerated five times with loss of adsorptive amounts (Cd2+: 14% and Pb2+: 17%).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pollution of heavy metals (such as lead (Pb2+) and cadmium (Cd2+)) is one of the main environmental problems in the world [1]. Non-biodegradable Pb2+ and Cd2+ ions as probable carcinogens can be accumulated in organisms, causing a serious threat for human health through food chain [2]. The effective removal of the Pb2+ and Cd2+ ions from wastewaters has attracted considerable attention [3]. The metal-bearing effluents with the high concentrations can be effectively treated by many methods [3, 4], whereas adsorption becomes a highly efficient treatment for the metal-bearing effluents containing the low concentrations at the level of mg L−1 [5]. Therefore, it is vital to develop the low cost, environmentally friendly, abundant reserves, biodegradable, and renewable sorbents for high-efficient removal of Pb2+ and Cd2+ [5].
In recent years, the adsorbents derived from the industrial/agricultural byproducts and the natural products with the advantages of high efficiency, low cost, abundant reserves, environmental friendliness, and biodegradability have attracted considerable attention for the removal of Pb2+ and Cd2+ ions [6, 7]. Cellulose derivatives are increasingly utilized as the sorbents in pollutants control. The native cellulose with poor efficiency of the metal removal is due to the shortage of active binding sites. Therefore, modification such as oxidation, sulfonation, acetylation, and grafting of functional groups can be used to improve the chemical activity of cellulose for the adsorptive removal of heavy metals [8]. Usually, the cellulose modified with some functional groups (such as carboxyl [9], amino [10], sulfonic acid [11], and sulfhydryl [12] groups) through surface chemical modification exhibits better efficiency for the metal removal than the native cellulose. Based on the rule of coordination chemistry, polydentate chelating ligands always exert the stronger affinity toward heavy metal ions than most monodentate ligands [13]. Thus, it is a good strategy to introduce some polydentate chelating ligands on the cellulose for enhancing the removal efficiency of heavy metals. Gurgel et al. [14] reported a succinylated mercerized cellulose modified with triethylenetetramine with adsorption capacities of 87.0 mg g−1 for Cd2+ and 192.3 mg g−1 for Pb2+. Ge et al. [15] reported a composite of cellulose/poly(ethylene imine) with adsorptive amount of 248.2 mg g−1 for Pb2+ due to poly(ethylene imine) with abundant N-donating atoms as coordinate sites for effectively capturing heavy metal ions. Nongbe et al. [16] reported that the cellulose grafted with spermine owned higher adsorption capacities for heavy metals than the cellulose grafted with ethylenediamine due to spermine with more N-donating atoms. Zhang et al. [17] prepared a sorbent through grafting polyethylenimine (PEI) onto carboxylated microcrystalline cellulose with the high adsorption capacities of 217.3 mg g−1 for Cd2+ and 357.1 mg g−1 for Pb2+ due to the sorbent with abundant amino and carboxyl groups. Hu et al. [18] prepared a cellulose grafted with EDTA-like groups which can coordinate with heavy metals and alkaline-earth metal and indicate good adsorption capacities (such as 80.3 mg g−1 for Cu2+ and 266.7 mg g−1 for Pb2+). The functional groups of the reactive cellulose derivatives usually were grafted through the direct substitution reaction on the cellulose units [19]. The functional groups can also be introduced by the Schiff base reaction between dialdehyde cellulose and the compounds containing the primary amine groups [20]. Most importantly, the Schiff base polydentate chelating ligand exhibits a better complexing ability with metal ions [21], and is expected to exhibit unique superiority in removal of heavy metals.
In this work, we propose a strategy of promoting the adsorptive capacity of heavy metals using the chemical grafting of cellulose by amino acetic acid functions through Schiff base reaction between dialdehyde cellulose and diethylenetriamine, and substitution reaction which occurs mainly on the amine groups of polyamines with bromoacetic acid. Amino and carboxyl groups are grafted onto the cellulose skeleton for obtaining the reactive cellulose derivatives modified with EDTA-like groups (EDTA-CL). The adsorptive performances, kinetic, and equilibrium features of Cd2+ and Pb2+ by the EDTA-CL have been investigated.
Experimental
Chemicals
All the chemicals are of analytical grade and were obtained from Sinopharm Chemical Reagent Co., Shanghai, China (Table S1). Stock solutions of Cd2+ or Pb2+ (1000.0 mg L–1) are prepared by dissolving the appropriate amounts of Pb(NO3)2 or CdCl2 in deionized water. Working solutions with the desired concentrations are diluted by stock solutions. The calibration curves of Cd2+ and Pb2+ have been displayed in Fig. S1. Dialysis bag (12, 000 MWCO, < 5 nm pore size) was purchased from Shanghai Yuanjv biological Technology Co., Ltd., Shanghai, China.
Preparation
The dialdehyde cellulose is prepared through an oxidation reaction of microcrystalline cellulose as reported previously and its process is described in Supporting Information. The aldehyde content of the dialdehyde cellulose is determined by hydroxylamine hydrochloride method [22]. 12 g of the dialdehyde cellulose with the content of the aldehyde groups of 2.8 mmol g–1 is obtained for the next step. The diethylenetriamine are grafted onto the dialdehyde cellulose surface though a Schiff base reaction. The dialdehyde cellulose (10 g) was dispersed in 200 mL diethylenetriamine solution with the molar ratio of aldehyde group to primary amino group at 1:1.2 at pH 3 using hydrothermal assisted method at 80 ℃ for 2 h. The suspension was transferred into dialysis bag for the removal of excess diethylenetriamine with changing the deionized water once every 12 h for 5 days, and then freeze-dried to get 9 g of the aminated cellulose. The N content of the aminated cellulose (8.2 mmol g–1) is determined by Kjeldahl method. 6 g of the aminated cellulose is dispersed in 100 mL N,N-dimethylformamide containing bromoacetic acid (13.9 g, 100 mmol) and sodium bicarbonate (8.4 g, 100 mmol) and was refluxed for 48 h by heating at 80 °C to form the target product. And then the mixture is filtered, and then washed with deionized water for several times and freeze-dried to obtain the EDTA-like cellulose derivatives functionalized with imine and carboxyl groups (marked as EDTA-CL). The content of carboxylic groups in EDTA-CL is determined by conductometric titration [23].
Batch Experiments
The batch adsorption experiments are performed with a constant dosage of 1 g L−1 for the sorbents in duplicate. The effects of different pH (3.0, 3.5, 4.0, 4.5, 5.0, 5.5, and 6.0), and the various contact time (5, 10, 15, 20, 25, 30, 40, 50, and 60 min) on the adsorption of Cd2+ or Pb2+ ion by the EDTA-CL are tested with the initial Cd2+ or Pb2+ concentration of 800 mg L−1. Effects of initial concentrations of the both metal ions from 100 and 1000 mg L−1 with an interval of 100 mg L−1 are investigated at pH 5 for 30 min at the changeable temperature (15, 30 and 45 ℃). After adsorption, the mixture is filtered, and then the residual concentrations of the both metal ions in the filtrate are determined by flame atomic absorption spectrometry (A6300c, Shimadzu Corporation, Japan). The adsorptive amounts of the both metal ions are calculated as the Eq.S1 described in Supporting Information. The average values of metal concentrations are reported with the measurement at least three times.
Reuse
The same sorbent is used in consecutive adsorption–desorption for testing the reusability of the EDTA-CL. The 0.2 mol L−1 HCl solution is used as the eluent to desorb the metal ions on the loaded sorbents for 60 min as reported previously [24]. The regenerative efficiency (RE%) of the regenerative EDTA-CL for the adsorptive amounts of the both metal ions is also calculated as Eq.S2.
Result and Discussion
Preparation of the EDTA-CL
As seen in Table S2, the content of N elements in the EDTA-CL by Kjeldahl method is found to be 8.1 mmol g–1, which is near to the N density of the aminated cellulose, indicating no loss of N elements during the substitution process. The density of carboxyl group in the EDTA-CL is found to be 11.5 mmol g–1. The preparation process of the EDTA-CL is illustrated in Scheme 1. The aminated cellulose is obtained through a Schiff base reaction between diethylenetriamine and the dialdehyde cellulose. The target compound is synthesized through a substitution reaction between the aminated cellulose and bromoacetic acid. Carboxyl substitution reaction is carried out mainly on the groups, primary and secondary amines of the aminated cellulose. These functional groups can play a leading role in the adsorptive removal of metal ions and coordinate with Cd2+ or Pb2+ ions to form several five-membered rings which is a stable structure for metal complexes [25].
Characterization
There is no change in the crystalline nature for the microcrystalline cellulose, dialdehyde cellulose, aminated cellulose, and EDTA-CL (Fig. 1). The diffraction peaks at 15.6°, 22.6°, and 34.8° are attributed to the (1–10)/(110), (200), and (004) crystal faces of cellulose, respectively [26]. The intensities for these peaks decrease due to the modification of cellulose. The similar phenomenon had been reported previously [27]. The dialdehyde cellulose, aminated cellulose, and EDTA-CL remain the characteristic of the fibrous structure in Fig. 1. After the oxidation of microcrystalline cellulose (Fig. 2a), the dialdehyde cellulose (Fig. 2b) looks peeling. The surface morphology of the aminated cellulose becomes rough with wrinkles due to the grafted diethylenetriamine on the dialdehyde cellulose (Fig. 2c). The EDTA-CL exhibits the rougher surface than the aminated cellulose (Fig. 2d), which is attributed to the substitution of amino groups by bromoacetic acid. The change trend in the BET surface area proved the results from the SEM (Table S2). The BET surface area is very important for the EDTA-CL [28, 29]. The BET surface area of the dialdehyde cellulose (7.8 m2g−1) decreases slightly compared with microcrystalline cellulose (10.1 m2g−1) due to the oxidation of sodium metaperiodate as reported previously [30]. The BET surface areas of the aminated cellulose (21.6 m2g−1) and EDTA-CL (28.8 m2g−1) increase gradually due to the introduction of more side chains.
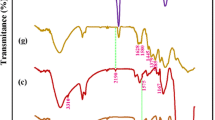
From Fig. 3, for all the cellulose derivatives, the absorption peaks at 3416 cm−1 (the O–H stretching vibration) [31], 2905 cm−1 (the C–H stretching vibration), 1634 cm−1 (the O–H bending vibration) [32], 1374 cm−1 (the C–H bending vibration), 1160 cm−1 (the C–O stretching vibration), 1060 cm−1 (the C–O–C stretching vibration), and 894 cm−1 from β-glycosidic linkages between the sugar units are associated with the characteristic of backbone [33]. From FT-IR spectra of the dialdehyde cellulose, the peak at 1733 cm−1 is due to the C=O stretching vibration of aldehyde group in the dialdehyde cellulose. From FT-IR spectra of the aminated cellulose, the peak at 1733 cm−1 disappears due to the grafting of diethylenetriamine on to the dialdehyde cellulose through a Schiff base reaction [34]. These illustrate that the diethylenetriamine has been grafted successfully onto the dialdehyde cellulose through the Schiff base reaction. From FT-IR spectra of the EDTA-CL, the characteristic peak of the C=O stretching vibration from carboxyl groups is re-emerged at 1727 cm−1 due to the substitution of bromoacetic acid on the amino groups [35]. A slight change in the wavenumbers of the C=O stretching vibration from 1733 to 1727 cm−1 is due to the change of functional groups from aldehyde group to carboxyl groups. These indicated that the EDTA-CL has been obtained.
Solid-state 13C-NMR spectra of the EDTA-CL are indicated in Fig. 4. The three derivatives exhibit the characteristic peaks of cellulose at 105.5 ppm (C1), 88.1 ppm (C4), 75.1 ppm (C2, C3, and C5), and 62.8 ppm (C6), which are associated with six carbon atoms of the glucose unit of cellulose [36]. A peak at 175 ppm is found due to the presence of carbonyl carbons of carboxyl groups in the EDTA-CL [37], which is consistent with the previous report [38], while the peaks at 61.6 and 42 ppm are due to the presence of two kinds of methylene carbon in diethylenetriamine and bromoacetic acid. The results of 13C-NMR spectra agree well with the results from IR spectra.
pH Effect
Figure 5a depicts the effect of solution pH on the adsorptive amounts of metal ions by the cellulose modified with EDTA-like groups (EDTA-CL). The adsorptive amounts of the both metal ions strongly depend on the solution pH. When solution increased from 3 to 4, a remarkable increase in the adsorptive amounts of the Cd2+ and Pb2+ ions is observed. At low pH, the N-donor atoms of EDTA-CL are protonated and the carboxylic groups of EDTA-CL are almost in an undissociated state [39], resulting in that the chelating groups lose their coordination ability with metal ions. With increasing pH, the protonation of chelating groups is weak. Oppositely, the adsorptive amounts adsorbed of the both metal ions increase at higher pH. In the pH range of 4–6, the adsorptive amounts of the both metal ions are kept constant, which is consistent with the same trend as reported previously [40]. The high stable adsorptive amounts of the both metal ions in this pH range rule out the importance of coordination of N, O-donor atoms of the EDTA-CL with metal ions, which plays a positive role in the enhanced adsorption, while it will be effectively weakened in solution by the competition effect of H+ ions with metal ions for the N, O-donor atoms of the EDTA-CL [41]. The precipitation of Cd(OH)2 or Pb(OH)2 formation will happen easily at pH > 6 [42, 43]. Thus, the operational condition of pH is controlled at pH 5.
a Effects of solution pH on the amounts adsorbed for the both ions: Concentration of ions = 800 mg L−1, time = 30 min, volume of solution = 20.0 mL, dosage of sorbent = 1 g L−1, temperature = 30 ℃; b effects of contact time on the adsorptive amounts for the both ions: Concentration of metals = 800 mg L−1, pH = 5, volume of solution = 20.0 mL, dosage of sorbent = 1 g L−1, temperature = 30 ℃; c amounts adsorbed of Cd2+ and Pb2+ by the EDTA-CL: Time = 30 min, pH = 5, volume of solution = 20.0 mL, dosage of sorbent = 1 g L−1, temperature = 30 ℃
Effect of Contact Time
Effect of contact time is characterized by an increase in the adsorptive amounts of the both metal ions with respect to time (Fig. 5b). A remarkable increase in the adsorptive amounts of the both metal ions is found within 25 min due to lots of available chelating groups with the strong coordination, and then the steady state of their adsorptive amounts is observed after 25 min due to the exhaustion of the available chelating groups, illustrating that the EDTA-CL is saturated at this level [44]. An optimum contact time of 30 min is sufficient for the adsorption of the both metal ions by the EDTA-CL for all the experiments.
Effect of Initial Concentrations
Figure 5c exhibits that the adsorptive amounts of the both metal ions are significantly dependent on their initial concentrations in feed solution. The increase in the initial concentrations of Cd2+ ions in the range of 100−500 mg L−1 results in its enhanced adsorptive amounts from 95.7 to 257.9 mg g−1, indicating that there are lots of the active chelating sites in the EDTA-CL. And then the adsorptive amounts of Cd2+ ions have no significant change with the increase of initial concentrations of Cd2+ ions from 500 to 1000 mg L−1, which is probably related to a saturation of the chelating sites. Meanwhile, the EDTA-CL toward the adsorption of Pb2+ ions exhibited a similar trend in the variation of adsorptive amounts on its initial concentrations (Fig. 5c). The maximum adsorptive amounts of Pb2+ and Cd2+ ions by the EDTA-CL at 30 °C are 438.3 and 287.2 mg g−1, respectively, which are higher than or comparable to those cellulose-based sorbents reported previously as indicated in Table 1 [14,15,16,17, 43,44,45,46,47,48,49,50,51,52,53,54]. There is a remarkable improvement in the adsorptive amounts through the chemical modification of cellulose due to the chelating groups on the rise. The same phenomena are reported previously [16, 17].
Effect of Temperature
There is a mild increase in the adsorptive amounts of Pb2+ and Cd2+ ions with the increase in temperature from 15 to 45 ℃ (Fig. 6), which is attributed to the endothermic nature of the adsorption of the EDTA-CL for the both ions, illustrating that a higher temperature is favorable for the adsorption of the both ions by the EDTA-CL.
Isotherm
The Freundlich [55], Langmuir [56], Temkin [57] and Dubinin–Radushkevich (D–R) [58] isotherms are used to fit the adsorptive data. The parameters from the four isotherms are described as Eq.S3–S9 in Supporting Information. From Table 2, the adsorptive data of the both metal ions by the EDTA-CL are fitted satisfactorily with Langmuir model with high r2 values (> 0.99), whereas the r2 values of fitting curves using Freundlich, Temkin, and D–R models are relatively low. The highest KF values are obtained for Pb2+ followed by Cd2+, while all the values of 1/n fall in the range of 0–1 and are closer to 1, implying an effective adsorption with high strength [59]. The E values of metal ions from D-R model (16.7 kJ mol−1 for Pb2+, 13.1 kJ mol−1 for Cd2+) are higher than 8 kJ mol−1, illustrating the chemical adsorption of the EDTA-CL for the metal ions. The adsorptive amounts calculated from Langmuir model for Cd2+ and Pb2+ ions are 302.7 and 444.1 mg g−1, respectively, which have no significant difference with their experimental values. Therefore, the Langmuir model better fits the adsorption of the both metal ions by the EDTA-CL based on the above results. Similar adsorption behaviors are found as reported previously [60, 61].
Kinetic
The kinetic data of adsorption of the metal ions are fitted using pseudo-first-order model (PFOM) [62], pseudo-second-order model (PSOM) [63], Elovich model (EM) [64], and intraparticle diffusion model (IPDM) [65]. The linear equations of these models are described as Eq.S10–S13 in Supporting Information. In Table 3, the PFOM and EM are not suitable for the description of the adsorption of the both ions by the EDTA-CL due to the low values of r2 obtained from fitting curves of PFOM and EM. The values of r2 derived from fitting curves of PSOM for the both ions are close to unity, whereas the calculated values and experimental values of the both ions by the EDTA-CL are very close to each other, illustrating that the PSOM can describe well the adsorption of the EDTA-CL for the both ions [66]. These results illustrate that the adsorption of both ions onto the EDTA-CL is determined by chemisorption [66].
The IPDM was also used to fit the experimental date in Table 3; two straight lines are found: the first sharper line is attributed to the external transport, and the another line is associated with the gradual transport due to intraparticle diffusion. Two lines without passing through the origin suggest that the adsorption could be jointly controlled by the external, intraparticle, and boundary layer diffusion [67].
Thermodynamics
The calculated equations of Gibbs free energy change (ΔG0), enthalpy change (ΔH0), and entropy change (ΔS0) are calculated as reported previously [68] and described as Eq. S14 and S15 in Supporting Information. In Table 4, the values of lnb from Langmuir constant increase as the temperature increases from 15 to 45 ℃, which leads to the increase in the ΔG0 values in the negative direction. These confirm that the adsorption of the EDTA-CL for the both ions is more spontaneous at higher temperature. The values of ΔH0 and ΔS0 for the both ions are positive, illustrating that the adsorption is endothermic and the randomness at the solid/solution interface is increased during the adsorption. These result in more adsorption at higher temperature.
Reusability
0.2 mol L−1 HCl is used as the eluents to desorb the both ions from the loaded EDTA-CL with stirring for 1 h at room temperature and is shown 98.8% of Pb2+ and 97.9% of Cd2+ desorption. Figure 7 exhibits the adsorptive amounts of Cd2+ and Pb2+ ions by the regenerated EDTA-CL over five successive adsorption–desorption cycles. The adsorptive amounts of the both ions drop per cycle of reuse. But even it remains 86.5% for Cd2+ ions and 83.7% for Pb2+ ions of the virgin at the end of 5th cycle. The EDS mapping of the N elements in the regenerated EDTA-CL is exhibited in Fig. S2, indicating that the N elements are uniformly dispersed on the surface of cellulose. The N contents of the regenerated EDTA-CL are found to be in the range of 7.1–7.5 mmol g–1 (Table S3). A decrease in the N contents of the regenerated EDTA-CL with the increase of the cycle numbers is observed. Compared with the N contents of the virgin (8.1 mmol g–1), the loss of N elements is relatively low [69], which is consistent with the change in the values of RE%. These results illustrate that the EDTA-CL can be reused for several times without significant loss of the adsorptive amounts for the both ions.
Simple Application
An untreated industrial wastewater containing 38.9 mg L−1 of Pb2+ and 15.9 mg L−1 of Cd2+ with pH of 4.4 is used to test the adsorption efficiencies of the EDTA-CL for both ions with a dosage of 1 g L−1 under stirring. After adsorption of 30 min, the residual concentrations of Pb2+ and Cd2+ are found to be 0.7 mg L−1 and 0.2 mg L−1, respectively. The adsorption efficiencies of Pb2+ and Cd2+ by the EDTA-CL are 98.2% and 98.7%, respectively, illustrating that the EDTA-CL can effectively remove the both ions from wastewater.
Conclusion
EDTA-CL, an EDTA-like chelating material derived from cellulose, is obtained by the chemical grafting of amino acetic acid groups onto the cellulose through the Schiff base and substitution reaction. The EDTA-CL shows rapid chelation with Cd2+ and Pb2+, high adsorptive amounts (302.7 mg g−1 for Cd2+ and 444.1 mg g−1 for Pb2+), and good regeneration. The adsorptive behavior of the both ions by the EDTA-CL follows Langmuir model. The kinetic and thermodynamics fitting results display an endothermic and spontaneous chemisorption.
Availability of Data and Materials
The Supporting Information is available free of charge on the Springer Publications website at DOI:
References
D. Jiang, K. Sheng, G. Gui, H. Jiang, L. Wang, A novel smartphone-based electrochemical cell sensor for evaluating the toxicity of heavy metal ions Cd2+, Hg2+, and Pb2+ in rice. Anal. Bioanal. Chem. 413, 4277–4287 (2021)
X. Ye, X. Zheng, D. Zhang, X. Niu, S. Zhou, The efficient biomineralization and adsorption of cadmium (Cd2+) using secretory organo-biominerals (sobs) produced by screened alcaligenes faecalis k2. Environ. Res. 199, 111330 (2021)
F. Fu, Q. Wang, Removal of heavy metal ions from wastewaters: a review. J. Environ. Manage. 92, 407–418 (2011)
N.S. Ali, K.R. Kalash, A.N. Ahmed, T.M. Albayati, Performance of a solar photocatalysis reactor as pretreatment for wastewater via UV, UV/TiO2, and UV/H2O2 to control membrane fouling. Sci. Rep. 12, 16782 (2022)
H.J. Al-Jaafa, N.S. Alia, S.M. Alardhib, T.M. Albayati, Implementing eggplant peels as an efficient bio-adsorbent for treatment of oily domestic wastewater. Desalin. Water Treat. 245, 226–237 (2022)
V. Thakur, E. Sharma, A. Guleria, S. Sangar, K. Singh, Modification and management of lignocellulosic waste as an ecofriendly biosorbent for the application of heavy metal ions sorption. Materials Today: Proceedings 32, 608–619 (2020)
S.M. Alardhi, N.S. Ali, N.M.C. Saady, S. Zendehboudi, I.K. Salih, J.M. Alrubaye, T.M. Albayati, Separation techniques in different configurations of hybrid systems via synergetic adsorption and membrane processes for water treatment: A review. J. Ind. Eng. Chem. 130, 91–104 (2024)
L. Thompson, J. Azadmanjiri, M. Nikzad, I. Sbarski, J. Wang, A. Yu, Cellulose nanocrystals: production, functionalization and advanced applications. Rev. Adv. Mater. Sci. 58, 1–16 (2019)
D. Kundu, S.K. Mondal, T. Banerjee, Development of β-cyclodextrin-cellulose/hemicellulose-based hydrogels for the removal of Cd(II) and Ni(II): synthesis, kinetics, and adsorption aspects. J. Chem. Eng. Data 64, 2601–2617 (2019)
R.R. Navarro, K. Sumi, M. Matsumura, Improved metal affinity of chelating adsorbents through graft polymerization. Water Res. 33, 2037–2044 (1999)
G. Gülü, G. Gürda, S. Özgümü, Competitive removal of heavy metal ions by cellulose graft copolymers. J. Appl. Polym. Sci. 90, 2034–2039 (2003)
S. Movaghgharnezhad, A. Mirabi, M.R. Toosi, A.S. Rad, Synthesis of cellulose nanofibers functionalized by dithiooxamide for preconcentration and determination of trace amounts of cd(ii) ions in water samples. Cellulose 27, 8885–8898 (2020)
F. Tisato, F. Refosco, F. Ossola, C. Bolzati, G. Bandoli, Cheminform abstract: polydentate phosphinoamine ligands: a class of efficient chelating agents for the stabilization of various technetium(v) and rhenium(v) cores. Transition Met. Chem. 22, 606–607 (1997)
L.V.A. Gurgel, O.K. Júnior, R.P. de Freitas Gil, L.F. Gil, Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by cellulose and mercerized cellulose chemically modified with succinic anhydride. Bioresource Technol. 99, 3077–3083 (2008)
H. Ge, H. Huang, M. Xu, Q. Chen, Cellulose/poly(ethylene imine) composites as efficient and reusable adsorbents for heavy metal ions. Cellulose 23, 2527–2537 (2016)
M.C. Nongbe, G. Bretel, T. Ekou, L. Ekou, B.K. Yao, E.L. Grognec, F.-X. Felpin, Cellulose paper grafted with polyamines as powerful adsorbent for heavy metals. Cellulose. Cellulose 25, 4043–4055 (2018)
C. Zhang, J. Su, H. Zhu, J. Xiong, X. Liu, D. Li, Y. Chen, Y. Li, The removal of heavy metal ions from aqueous solutions by amine functionalized cellulose pretreated with microwave-H2O2. RSC Adv. 7, 34182–34191 (2017)
T. Hu, X. Hu, C. Tang, D. Liu, Adsorbent grafted on cellulose by in situ synthesis of EDTA-like groups and its properties of metal ion adsorption from aqueous solution. Cellulose 29, 941–952 (2021)
S. Hokkanen, A. Bhatnagar, M. Sillanpaä, A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 91, 156–173 (2016)
M. Guo, H. Wang, L. Sun, Y. Li, Synthesis, characterization and properties of cellulose-grafted glycine derivatives. J. Appl. Polym. Sci. 131, 40929 (2014)
H.-T. Fan, J.-X. Liu, D.-P. Sui, H. Yao, F. Yan, T. Sun, Use of polymer-bound Schiff base as a new liquid binding agent of diffusive gradients in thin-films for the measurement of labile Cu2+, Cd2+ and Pb2+. J. Hazard. Mater. 260, 762–769 (2013)
U.J. Kim, M. Wada, S. Kuga, Solubilization of dialdehydecellulose by hot water. Carbohyd. Polym. 56, 7–10 (2004)
L.F. Zemljič, Z. Peršin, P. Stenius, K.S. Kleinschek, Carboxyl groups in pre-treated regenerated cellulose fibres. Cellulose 15, 681–690 (2008)
Y. Zhou, X. Wang, M. Zhang, Q. Jin, B. Gao, T. Ma, Removal of Pb(II) and malachite green from aqueous solution by modified cellulose. Cellulose 21, 2797–2809 (2014)
N. Bicak, B.F. Senkal, D. Melekaslan, Poly (styrene sulfonamides) with EDTA-like chelating groups for removal of transition metal ions. J. Appl. Polym. Sci. 77, 2749–2755 (2000)
R.H. Khudhur, N.S. Ali, E.H. Khader, N.S. Abbood, I.K. Salih, T.M. Albayati, Adsorption of anionic azo dye from aqueous wastewater using zeolite NaX as an efficient adsorbents. Desalin. Water Treat. 306, 245–252 (2023)
T.M. Albayati, A.M. Doyle, Encapsulated heterogeneous base catalysts onto SBA-15 nanoporous material as highly active catalysts in the transesterification of sunflower oil to biodiesel. J. Nanopart. Res. 17, 109 (2015)
H. Yu, L. Zheng, T. Zhang, J. Ren, W. Cheng, L. Zhang, P. Meng, Adsorption behavior of Cd (II) on TEMPO-oxidized cellulose in inorganic/organic complex systems. Environ. Res. 195, 110848 (2021)
A.D. French, Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21, 885–896 (2014)
R. Kumar, R.K. Sharma, Synthesis and characterization of cellulose based adsorbents for removal of Ni(II), Cu(II) and Pb(II) ions from aqueous solutions. React. Funct. Polym. 140, 82–92 (2019)
T.M.N. Albayati, S.E. Wilkinson, A.A. Garforth, A.M. Doyle, Heterogeneous alkane reactions over nanoporous catalysts. Transp. Porous Media 104, 315–333 (2014)
T.M. Albayati, A.M. Doyle, SBA-15 supported bimetallic catalysts for enhancement isomers production during n-heptane decomposition. Int. J. Chem. Reactor Eng. 12, 345–354 (2014)
Y. Wang, Q. Lu, Dendrimer functionalized nanocrystalline cellulose for Cu(II) removal. Cellulose 27, 2173–2187 (2020)
S.M. Alardhi, H.G. Salih, N.S. Ali, A.H. Khalbas, I.K. Salih, N.M.C. Saady, S. Zendehboudi, T.M. Albayati, H.N. Harharah, Olive stone as an eco-friendly bio-adsorbent for elimination of methylene blue dye from industrial wastewater. Sci. Rep. 13, 21063 (2023)
Y. Liu, R. Liu, M. Li, F. Yu, C. He, Removal of pharmaceuticals by novel magnetic genipin-crosslinked chitosan/graphene oxide-SO3H composite. Carbohyd. Polym. 220, 141–148 (2019)
H. Kono, S. Yunoki, T. Shikano, M. Fujiwara, M. Takai, CP/MAS 13CNMR study of cellulose and cellulose derivatives. 1. Complete assignment of the CP/MAS spectrum of the native cellulose. J. Am. Chem. Soc. 124, 7506–7511 (2002)
H. Kono, T. Erata, M. Taka, CP/MAS 13C NMR study of cellulose and cellulose derivatives. 2. Complete assignment of the 13C resonance for the ring carbons of cellulose triacetate polymorphs. J. Am. Chem. Soc. 24, 7512–7518 (2002)
A.J. Varma, V.B. Chavan, P.R. Rajmohanan, S. Ganapathy, Some observations on the high-resolution solid-state CP-MAS carbon-13 NMR spectra of periodate-oxidized cellulose. Polym. Degrad. Stab. 58, 257–260 (1997)
N.S. Ali, H.N. Harharah, I.K. Salih, N.M.C. Saady, S. Zendehboudi, T.M. Albayati, Applying MCM-48 mesoporous material, equilibrium, isotherm, and mechanism for the effective adsorption of 4-nitroaniline from wastewater. Sci. Rep. 13, 9837 (2023)
M.R. Berber, Surface-functionalization of activated carbon with polyglucosamine polymer for efficient removal of cadmium ions. Polym. Compos. 41, 3074–3086 (2020)
R. Leyva-Ramos, J.R. Rangel-Mendez, J. Mendoza-Barron, L. Fuentes-Rubio, R.M. Guerrero-Coronado, Adsorption of cadmium(II) from aqueous solution onto activated carbon. Water Sci. Technol. 35, 205–211 (1997)
W. Wei, S. Kim, M.H. Song, J.K. Bediako, Y.S. Yun, Carboxymethyl cellulose fiber as a fast binding and biodegradable adsorbent of heavy metals. J. Taiwan Inst. Chem. Eng. 57, 104–110 (2015)
J. Qu, Q. Meng, X. Lin, W. Han, Q. Jiang, L. Wang, Q. Hu, L. Zhang, Y. Zhang, Microwave-assisted synthesis of β-cyclodextrin functionalized celluloses for enhanced removal of Pb(II) from water: Adsorptive performance and mechanism exploration. Sci. Total. Environ. 752, 141854 (2021)
T.M. Albayati, A.M. Doyle, Shape-selective adsorption of substituted aniline pollutants from wastewater. Adsorpt. Sci. Technol. 31, 459–468 (2013)
E. Abu-Danso, S. Peräniemi, T. Leiviskä, A. Bhatnagar, Synthesis of S-ligand tethered cellulose nanofibers for efficient removal of Pb(II) and Cd(II) ions from synthetic and industrial wastewater. Environ. Pollut. 242, 1988–1997 (2018)
B. Fu, F. Xie, Facile in situ synthesis of cellulose microcrystalline-manganese dioxide nanocomposite for effective removal of Pb(II) and Cd(II) from water. Environ. Sci. Pollut. Res. 27, 5108–5121 (2020)
L. Gurgel, L.F. Gil, Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by succinylated mercerized cellulose modified with triethylenetetramine. Carbohyd. Polym. 77, 142–149 (2009)
O.K. Júnior, L. Gurgel, R. Freitas, L.F. Gil, Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by mercerized cellulose and mercerized sugarcane bagasse chemically modified with EDTA dianhydride (EDTAD). Carbohyd. Polym. 77, 643–650 (2009)
I.M. Kenawy, M. Hafez, M.A. Ismail, M.A. Hashem, Adsorption of Cu(II), Cd(II), Hg(II), Pb(II) and Zn(II) from aqueous single metal solutions by guanyl-modified cellulose. International Journal of Biological Macromolecules. Int. J. Biol. Macromol. 107, 1538–1549 (2018)
J. Liu, T.-H. Xie, C. Deng, K.-F. Du, N. Zhang, J.-J. Yu, Y.-L. Zou, Y.-K. Zhang, Welan gum-modified cellulose bead as an effective adsorbent of heavy metal ions (Pb2+, Cu2+, and Cd2+) in aqueous solution. Sep. Sci. Technol. 49, 1096–1103 (2014)
S.S. Pillai, B. Deepa, E. Abraham, N. Girija, P. Geetha, L. Jacob, M. Koshy, Biosorption of Cd(II) from aqueous solution using xanthated nano banana cellulose: equilibrium and kinetic studies. Ecotoxicol. Environ. Saf. 98, 352–360 (2013)
K. Taksitta, P. Sujarit, N. Ratanawimarnwong, S. Donpudsa, K. Songsrirote, Development of tannin-immobilized cellulose fiber extracted from coconut husk and the application as a biosorbent to remove heavy metal ions. Environ Nanotechnol Monit Manag 14, 100389 (2020)
A. Vázquez-Guerrero, R. Cortés-Martínez, R. Alfaro-Cuevas-Villanueva, E.M. Rivera-Muñoz, R. Huirache-Acuña, Cd(II) and Pb(II) adsorption using a composite obtained from moringa oleifera lam. Cellulose nanofibrils impregnated with iron nanoparticles. Water 13, 89 (2021)
T. Xiang, Z.L. Zhang, H.Q. Liu, Z.Z. Yin, L. Li, X.M. Liu, Characterization of cellulose-based electrospun nanofiber membrane and its adsorptive behaviours using Cu(II), Cd(II), Pb(II) as models. Sci. China Chem. 56, 567–575 (2013)
H.M.F. Freundlich, Über die adsorption in lösungen. Zeitschrift Fur Physikalische Chemie 57, 385–470 (1906)
I. Langmuir, The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 40, 1361–1403 (1918)
M.J. Tempkin, V. Pyzhev, Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physicochim. URSS 12, 217–222 (1940)
M.M. Dubinin, L.V. Radushkevich, Equation of the characteristic curve of activated charcoal. Chem. Zentralbl. 1, 875 (1947)
I. Tan, A.L. Ahmad, B.H. Hameed, Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 154, 337–346 (2008)
A.E. Mahdi, N.S. Ali, HSh. Majdi, T.M. Albayatia, M.A. Abdulrahman, D.J. Jasim, K.R. Kalash, I.K. Salih, Effective adsorption of 2-nitroaniline from wastewater applying mesoporous material MCM-48: equilibrium, isotherm, and mechanism investigation. Desalin. Water Treat. 300, 120–129 (2023)
A.E. Mahdi, N.S. Ali, K.R. Kalash, I.K. Salih, M.A. Abdulrahman, T.M. Albayati, Investigation of equilibrium, isotherm, and mechanism for the efficient removal of 3-nitroaniline dye from wastewater using mesoporous material MCM-48, progress in color colorants. Coating 16, 387–398 (2023)
S. Lagergren, About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetenskapsakademiens Handlingar 24, 1–39 (1898)
Y.S. Ho, G. McKay, Pseudo-second-order model for sorption processes. Process Biochem. 34, 451–465 (1999)
M.J.D. Low, Kinetics of chemisorption of gases on solids. Chem. Rev. 60, 267–312 (1960)
W.J. Weber, J.C. Morris, Kinetics of adsorption of carbon from solutions. J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 89, 31–63 (1963)
N.S. Ali, E.H. Khader, R.H. Khudhur, M.A. Abdulrahman, I.K. Salih, T.M. Albayati, Removal of anionic azo dye from wastewater using Fe3O4 magnetic nanoparticles adsorbents in a batch system. Desalin. Water Treat. 317, 100033 (2024)
V. Vadivelan, K.V. Kumar, Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 286, 90–100 (2005)
Y. Liu, Is the free energy change of adsorption correctly calculated? J. Chem. Eng. Data 54, 1981–1985 (2009)
M. Talib, Al-Bayati, Removal of aniline and nitro-substituted aniline from wastewater by particulate nanoporous MCM-48. Part Sci Technol An Int. J. 32, 616–623 (2014)
Funding
This work is supported by Financial support by Basic Scientific Research Foundation of the Education Department of Liaoning Province of China (No. LJKZZ20220053).
Author information
Authors and Affiliations
Contributions
Jingxin Zheng: Investigation, formal analysis, data curation, writing—original draft preparation. Lu Yang: Methodology, formal analysis, writing—original draft preparation. Baohong Ding: Formal analysis, validation, data curation. Nan You: Conceptualization, validation, project administration. Hongtao Fan: Methodology, project administration.
Corresponding authors
Ethics declarations
Conflict of Interest
No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, J., Yang, L., You, N. et al. Chelating Cellulose for Removal of Heavy Metals. Korean J. Chem. Eng. 41, 2729–2739 (2024). https://doi.org/10.1007/s11814-024-00230-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-024-00230-1