Abstract
Cellulose derivatives functionalized with multidentate N‑donor atoms containing two, three and four amino functional groups (as marked N2–CL, N3–CL and N4–CL) have been prepared by grafting of linear aliphatic polyamines into the cellulose backbone through the Schiff base reaction. An increase in the adsorptive amounts of Cd2+ and Pb2+ with increasing N‑donor atoms of the grafted polyamines onto the cellulose backbone and the maximum capacity of N4–CL > N3–CL > N2–CL are found. The N4–CL with the highest N content (up to 5.2 mmol N g−1) exhibits the largest adsorptive capacities of 249.7 mg g−1 for Cd2+ and 401.2 mg g−1 for Pb2+. The adsorption of both the ions by the three cellulose derivatives is achieved within 30 min, is independent of pH in the range of 4.5–6 for Cd2+ and 4–6 for Pb2+, and can be satisfactorily fitted by Langmuir and pseudo-second-order equations. Thermodynamic parameters suggest an endothermic and endothermic nature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water contamination from heavy metals has become a growing concern worldwide due to the non-biodegradability, high toxicity, and probable carcinogen of heavy metals such as Pb2+ and Cd2+ for a serious threat to the human and eco-environmental health, even at low concentrations (Nordberg et al. 2007). Adsorption by solid materials is a suitable method for the removal of heavy metals from water at low concentrations (Liu et al. 2021). There has been an increasing need for chemists to develop the effective sorbents for the removal of heavy metals from the contaminated water.
Some sorbents derived from natural resources (such as microbial biomass, mineral, biochar, industrial wastes, and agricultural byproducts) have been developed to high-efficiently remove heavy metals (Joseph et al. 2019; Kaur et al. 2022). Cellulose, a non-toxic, regenerative, abundant, environmental-friendly, easily available, and biodegradable superior material, has been attracted a growing interest as ideal candidate for the removal of heavy metal ions such as Pb2+ and Cd2+ in water (Dhali et al. 2021; Abouzeid et al. 2019). The raw cellulose exhibits a low adsorption capacity due to the deficiency of binding groups with heavy metal ions (Lian et al. 2020). Some treatments (such as oxidation, sulfonation, acetylation, silylation, and adsorption of surfactants) were carried out for increasing the chemical activity of the cellulose (Dhali, et al. 2021). Modification is a good way to solve this problem through introducing some functional groups (such as hydroxyl (Kundu et al. 2019), carboxyl (Kundu et al. 2019), amino (Zhang et al. 2017), sulphonic acid (Gülü et al. 2003), acrylamide (Kumara and Kr. Sharma, 2019), thiourea (Zhou et al. 2014), amidoxime (Movaghgharnezhad et al. 2020), and sulfhydryl (Alipour et al. 2020)) in the structure of cellulose by advanced oxidation or grafting methods (Thakur et al. 2020). Grafting method as an effective route is used to functionalize the cellulosic materials for the improvement of the adsorption performances (Roy er al. 2009). More functional groups are found in cellulose after grating, leading to the significant enhancement of the reactive binding sites for the pollutants in the grafted cellulose derivatives (Abdel-Halim et al.2012; O’Connell er al. 2008). Obvious improvement in the adsorption capacities of heavy metal ions by the reactive grafted cellulose derivatives can be observed through the strong coordination of these functional groups with heavy metal ions compared with native cellulose (Alipour et al. 2020; Li et al. 2019). What’s more, the adsorption capacities of heavy metal ions by the reactive cellulose derivatives are related to the grafted density of the functional groups (Wu et al. 2020). For instance, polyethylenimine functionalized cellulose with the amino groups content of 2.61 mmol/g indicated the high adsorption capacities for Pb2+ (357.1 mg g−1) and Cd2+ (217.3 mg g−1) ions (Zhang et al. 2017). A cellulose modified with triethylenetetramine with an amino density of 2.3 mmol/g showed a maximum adsorption capacity of Pb2+ (192.3 mg g−1) and Cd2+ (87.0 mg g−1) ions (Gurgel and Gil 2009). A tetraethylenepentamine-functionalized cellulose with primary amine, secondary amine and tertiary amine exhibited the adsorption capacity of 75.1 mg g−1 for AsO43− ions (Yu et al. 2013). As indicated above, multi-amino-functionalized cellulose derivatives have high affinity for heavy metal ions due to the presence of multi-donor atoms as the electrostatic interaction sites and complexation sites, while, multi-amine groups as a chelating agent always exerted stronger affinity towards heavy metal ions than most mono-amine groups (Bois et al. 2003; Aguado et al. 2009). Reactive cellulose derivatives can be obtained using some modification technique (such as etherification, oxidation, esterification, silynation, alkaline treatment, and halogenation) (Hokkanen et al. 2016). Among them, halogenation is usually used to introduce the functional groups into the cellulose through the substitution reaction between 6-chloro-6-deoxycellulose and the compounds containing the amino groups (O’Connell et al. 2008). Zhao et al. (2014) reported a cellulose modified with ethylenediamine through the reaction of chlorinated cellulose and ethylenediamine as a solid phase extractant for the detection of Cu2+ ions. The same modification was proposed to prepare the cellulose modified with ethylenediamine by Torres et al. (2006) and da Silva Filho et al. (2006) for the removal of heavy metals. However, the halogenation is difficult even due to the relatively low reactivity of cellulose with chloride or bromine, and relies more on organic solvent which could harm the environment and human to some extent (Hokkanen et al. 2016; O’Connell et al. 2008). Another surface modification through the Schiff base reaction between dialdehyde cellulose and the compounds containing the primary amine groups with simple step, high efficiency and free organic solvent is also used to attach the functional groups onto the cellulose (Guo et al. 2014; Kobayashi et al. 2014). Adsorption capacities are attributed to the number of functional groups in reactive cellulose derivatives. Enhancement of the number of the functional groups is likely to be a key issue in the improvement of adsorption capacities.
In order to enhance the adsorption capacities of heavy metals, in this work, three functionalized cellulose derivatives with two, three or four chelating N‑donor atoms in the pendant chains are prepared by introducing linear aliphatic polyamines (such as ethylenediamine, diethylenetriamine or triethylenetetramine) into the dialdehyde cellulose through the Schiff base reaction. These linear aliphatic polyamines can provide not just a variety of multiple coordination sites but also the flexible –CH2 − linkers which can allow N‑donor atoms to bend and rotate freely and better comply with the coordination of metal ions (Hu et al. 2012). The ability of the three functionalized cellulose derivatives containing chelating N‑donor atoms on their pendant chains in the removal of the Pb2+ and Cd2+ ions from aqueous solution is tested and compared.
Experimental
Chemicals
All the chemicals are of analytical grade and were obtained from Sinopharm Chemical Reagent Co., Shanghai, China. Solutions of Cd2+ or Pb2+ with the desired concentrations are prepared by dissolving the appropriate amount in deionized water. Dialysis bag (12, 000 MWCO, < 5 nm pore size) with 76 mm in diameter is purchased from Shanghai Yuanjv biological Technology Co., Ltd., Shanghai, China. All the chemicals are listed in Table S1. The instruments for the characterizations are described in Supporting Information.
Preparation of reactive cellulose derivatives
Microcrystalline cellulose is chemically oxidized to the dialdehyde cellulose by sodium metaperiodate, as described by Shen et al. (2015). The dialdehyde cellulose obtained has been purified by dialysis method to remove the excessive sodium metaperiodate, and then freeze-dried for use in next step. The aldehyde content of aldehyde cellulose was determined by the hydroxylamine hydrochloride method (Kim et al. 2004). The preparation process of dialdehyde cellulose and the determination of aldehyde groups are described in detail in Supporting Information.
The reactive cellulose derivatives are obtained by dispersing the dialdehyde cellulose into the solution polyamines (ethylenediamine, diethylenetriamine or triethylenetetramine) at pH 3 with the various molar ratio of aldehyde groups to primary amine groups (1:0.55, 1:1.1 and 1:2.2) using hydrothermal method under 90 °C for 2 h. The target product is separated by dialysis with changing the water once every 12 h for 5 days, and freeze-dried to get the powder of the reactive cellulose derivatives. The contents of the amino groups in the reactive cellulose derivatives are determinated by Kjeldahl method.
Adsorption studies
Batch adsorptive experiments were performed by adding a specific amount of sorbent into the metal solutions with known concentration in duplicate. 20 mg of reactive cellulose derivatives is added to 20 mL of Cd2+ or Pb2+ solution with a known concentration from 100 to 1000 mg L−1 with an interval of 100 mg L−1, at the pH in the range of 3–6 with an interval of 0.5 and at the temperature of 15, 30 or 45 °C during the pre-specified contact time from 10 to 60 min with an interval of 10 min. The initial solution pH is adjusted using a 0.005 mol L−1 of HCl solution. After adsorption, the suspension is filtered, and the residual concentration of both metal ions in the filtrate was determined by flame atomic absorption spectrometry (A6300c, Shimadzu Corporation, Japan). The amounts adsorbed are calculated as the Eq.S1 described in Supporting Information. The average values of metal concentrations are obtained by repeating the measurement for three times.
Desorption studies
100 mg of the loaded N4–CL was regenerated using 50 mL of 0.2 mol L–1 HCl solution as the eluent with stirring for 3 h (Ding et al. 2016). The regenerated N4–CL was filtrated, washed, and dried for the next cycle. The cycle was repeated four times.
Result and discussion
Optimization of preparation conditions
The aldehyde content of the dialdehyde cellulose is found to be 1.85 mmol g–1 using hydroxylamine hydrochloride method. The N content in the cellulose derivatives is determined by Kjeldahl method and element analysis. Diethylenetriamine is used as an example to illustrate the effect of the raw material ratios on the N content of the cellulose derivatives. The molar ratios of aldehyde groups in the dialdehyde cellulose to primary amino groups in the diethylenetriamine are set at 1:0.5, 1:1 and 1:2. The N contents in the cellulose derivative are illustrated in Table S2. At the molar ratios of 1:0.5, the N contents of the cellulose derivative by Kjeldahl method and element analysis are 2.76 and 2.57 mmol g–1, respectively. At the molar ratios of 1:1, the N contents of the cellulose derivative by Kjeldahl method and element analysis are 4.44 and 4.37 mmol g–1, respectively. At the molar ratios of 1:2, the N contents of the cellulose derivative by the two methods are 4.31 and 4.39 mmol g–1, respectively. These results indicate that the optimal raw material ratio is 1:1. The low density of N atoms at the molar ratios of 1:0.5 is due to the Schiff base reaction between aldehyde groups and two primary amino groups of diethylenetriamine. The high density of N atoms at the molar ratios of 1:1 and 1:2 is attributed to the adequate suppl of primary amine, leading to that only one primary amino group in diethylenetriamine reacts with aldehyde group in the dialdehyde cellulose as illustrated in Scheme S1. These N‑donor atoms can play a leading role in the adsorptive removal of Pb2+ and Cd2+ ions.
Characterization
The fibrous nature of all the cellulose derivatives is remained in Fig. 1. It can be observed that the surface morphology of the dialdehyde cellulose by scanning electron microscope (SEM) looks smooth (Fig. 1a), whereas the three reactive cellulose derivatives exhibit rougher surface with wrinkles due to the grafting of polyamines (Fig. 1b–d). Compared with the dialdehyde cellulose, a slight increase in surface rugosity can be observed, while, the similar trend in the change of surface area in Table 1. The oxidation of sodium metaperiodate leads to a decrease in the surface area (Yu et al. 2021), whereas the introduction of polyamines onto the dialdehyde cellulose increases slightly the surface area in favor of the adsorption of heavy metal ions. EDS mapping images further confirm the presence of N element on three reactive cellulose derivatives after grating of polyamines through Schiff base reaction (Figure S1). In Table 1, the contents of N element in the three cellulose derivatives using the Kjeldahl method are determined to be 3.82 mmol g–1 for N2–CL, 4.43 mmol g–1 for N3–CL and 5.19 mmol g–1 for N4–CL, respectively. While, the contents of N element (3.78 mmol g–1 for N2–CL, 4.37 mmol g–1 for N3–CL and 5.21 mmol g–1 for N4–CL) are also measured by element analysis. There is no significant difference of the contents of N atoms in the three cellulose derivatives using the two measurement methods. The contents of N element in the three cellulose derivatives are directly related with the number of amine groups in the polyamines, indicating that the N4–CL can give the more N‑donor atoms for binding the metals.
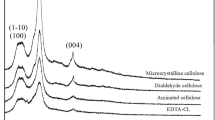
The X-ray diffraction (XRD) patterns of the microcrystalline cellulose and the three reactive cellulose derivatives are illustrated in Fig. 2. The diffraction peaks at 15.6°, 22.6° and 34.8° are attributed to the (1–10)/(101), (200) and (004) crystal faces of cellulose, respectively (French 2014). Peak intensity of the three reactive cellulose derivatives decreases due to the modification compared with microcrystalline cellulose and no change in the crystalline form for all the cellulose derivatives is observed.
Fourier transmission infrared (FT–IR) spectra of all the cellulose derivatives exhibit typical absorption bands of the cellulose backbone as indicated in Fig. 2b (Zhou et al. 2014). The broad band at 3450 cm−1 is ascribed to the presence of the O–H stretching vibration of –OH groups. The band at 2920 cm−1 is attributed to the C–H stretching vibration of –CH2 groups. The bands in the region 1158 and 1112 cm−1 is due to the C–O stretching vibration of C–OH and C–O–C groups. The broad band observed at 1031 cm−1 is attributed to the C–O–C stretching vibration from ether linkage, while the peak at 898 cm−1 is due to β–glycosidic linkages of glucose ring of cellulose. The reactive cellulose derivatives own the same characteristic absorption bands in the region 3100–3200 due to the N–H stretching vibrations from the presence of amino groups. The absorption band at 1422 cm−1 is due to the N–H bending vibrations of secondary amine groups, while, the absorption band at 1386 cm−1 is ascribed to the N–H bending vibrations of primary amine groups (Movaghgharnezhad et al. 2020). The absorption band at 1065 cm−1 is due to the C–N stretching vibration (Movaghgharnezhad et al. 2020). These illustrate that the polyamines have been grafted successfully onto the dialdehyde cellulose through Schiff base reaction.
In Fig. 3, the characteristic peaks of six carbon atoms of the glucose unit in cellulose for C1 at 104 ppm, C4 at 88 ppm, C2, C3, and C5 at 74 ppm, and C6 at 65 ppm can be observed by 13C nuclear magnetic resonance (CNMR) spectra in the solid state for all the cellulose derivatives (Kono et al. 2002). The signal at 175 ppm is assignable to carbonyl carbons of dialdehyde groups in the dialdehyde cellulose in Figure S2 (Varma et al. 1997), and then this peak disappears in Fig. 3 after the polyamines are grafted onto dialdehyde cellulose through the Schiff base reaction. A new signal is observed at 61.6 ppm which is due to the methylene carbon in polyamines (da Silva Filho et al. 2006). These results from the CNMR spectra are consistent with the FT–IR results.
Influence of pH
The influence of solution pH on the amounts adsorbed of the both metal ions by the three reactive cellulose derivatives is indicated in Fig. 4a. the amounts adsorbed of the both metal ions by the three reactive cellulose derivatives are affected strongly by the initial pH of solution. When the solution pH goes up from 3 to 4, a 1- to 2.5-fold increase in the amounts adsorbed of both metal ions are found, respectively, which is attributed to the electrostatic repulsion between metal cations and the protonated surface of the three reactive cellulose derivatives (Liu et al. 2019). Whereas, the amounts adsorbed of both metal ions by the three reactive cellulose derivatives are independent of pH in the range of 4.5–6 for Cd2+ ions and 4–6 for Pb2+ ions because weak acidity conditions make the coordination of N-donor atoms with both metal ions easily. These phenomena indicate that the acid effect of H+ ions play a significant role in the holding of both metal ions from the aqueous solution and can effectively weaken the coordination alibility of N-donor atoms with both metal ions. The current results agree well with the previous reports (Wang et al. 2019). In addition, the amounts adsorbed of the both metal ions follow the order N4–CL > N3–CL > N2–CL. The precipitation of both metal ions will be formed at pH > 6. Therefore, all the experiments are carried out at pH 5.
a Influences of pH on the amounts adsorbed of both the ions by the three cellulose derivatives: Concentration of metals = 500 mg L−1, time = 30 min, volume of solution = 20.0 mL, dosage of sorbent = 1 g L−1, temperature = 30 °C; b Influences of contact time on the amounts adsorbed of both the ions by the three cellulose derivatives: Concentration of metals = 500 mg L−1, pH = 5, volume of solution = 20.0 mL, dosage of sorbent = 1 g L−1, temperature = 30 °C; and the adsorptive amounts of (c) Cd2+ and (d) Pb2+ ions by the three cellulose derivatives and native cellulose: Time = 30 min, pH = 5, volume of solution = 20.0 mL, dosage of sorbent = 1 g L−1, temperature = 30 °C
Influence of contact time
To assess the influences of contact time on the amounts adsorbed of both metal ions, the experiments with various contact time were carried out. In Fig. 4b, the amounts adsorbed of both metal ions by the three reactive cellulose derivatives rise abruptly in the inception period, which is attributed to the adequate available N-donor atoms in the three reactive cellulose derivatives. After 30 min, the amounts adsorbed of both metal ions by the three reactive cellulose derivatives retain steady state due to the exhaustion of the available N-donor atoms. Therefore, operational condition of 40 min is enough for all the trials.
Influence of initial concentrations
The dependence of the amounts adsorbed of the both metal ions on their different initial concentrations with a constant dose of 1 g L−1 is illustrated in Fig. 4c and d. A gradual increase in the amounts adsorbed of Cd2+ ions by the three reactive cellulose derivatives occurs when the initial concentrations of Cd2+ go up to 300 mg L−1, demonstrating the unsaturation of the adsorbed amounts (Fig. 4c). The adsorption of Pb2+ ions by the three reactive cellulose derivatives is in line with changes in the similar trend with the adsorption of Cd2+ ions (Fig. 4d). There is an insignificant change in the amounts adsorbed of Cd2+ ions by the three reactive cellulose derivatives with increasing the concentrations of Cd2+ ions from 300 to 600 mg L−1, indicating that the three reactive cellulose derivatives for the adsorption of Cd2+ ions have been saturated with the amounts adsorbed of 209.5 mg g−1 for N2–CL, 234.6 mg g−1 for N3–CL and 249.7 mg g−1 for N4–CL maintaining the order of N4–CL > N3–CL > N2–CL. The saturation stage of the Pb2+ adsorption is also entirely independent of the initial concentrations of Pb2+ ions from 400 to 600 mg L−1. The amounts adsorbed of the N2–CL, N3–CL, and N4–CL for Pb2+ ions are found to be 344.8 mg g−1, 377.2 mg g−1 and 401.2 mg g−1, respectively, following the same order with the adsorption of Cd2+ ions. For a comparison, the microcrystalline cellulose unmodified for the adsorption of the both metal ions are also given in Fig. 4c and d. The amounts adsorbed of Cd2+ and Pb2+ ions by the microcrystalline cellulose unmodified are 3.1 and 4.8 mg g−1, respectively. Grafting the various polyamines on microcrystalline cellulose can evidently enhance the removal capability of both metal ions in varying degrees, and then results in an 80-fold increase in the amounts adsorbed of Cd2+ and Pb2+ ions, inferring that the binding sites of the three reactive cellulose derivatives for metal ions are originated from the grafted polyamines on the microcrystalline cellulose. Hence, the removal of microcrystalline cellulose for both metal ions is promoted by grafting the polyamines. Among them, the N4–CL exhibits the highest amounts adsorbed for Cd2+ and Pb2+ ions, which is comparable to that of amino-functionalization cellulose, and much more than the amounts adsorbed of other cellulose-based sorbents reported previously (Table 2) due to the high density of grating for the N-donor atoms. These results indicate that the N4–CL with more excellent chelating groups can serve as a good substitute for the removal of metal ions.
Isotherm
Adsorption isotherms of the both metal ions by the three reactive cellulose derivatives were correlated with the Langmuir (Langmuir 1918), Freundlich (Freundlich 1906) or Dubinin–Radushkevich (D–R) (Dubinin & Radushkevich 1947) model. The linear equations of Langmuir and Freundlich models are described in Supporting Information. Take the N4–CL for example (Table 3), the results do not fit well with Fruendlich model (r2 > 0.718), while, the adsorptive data of the N4–CL for both metal ions are found to be well represented by Langmuir model (r2 > 0.998). The n values from Fruendlich mode are higher than 1.0, indicating the favorable adsorption of Cd2+ and Pb2+ ions by the N4–CL (Sun et al. 2011). The theoretical amounts adsorbed of Cd2+ and Pb2+ ions by the N4–CL at 30 °C from Langmuir model are calculated to be 269.8 and 429.2 mg g−1, which are consistent with their experimental values (249.7 mg g−1 for Cd2+ and 401.2 mg g−1 for Pb2+). The similar fitting results have been obtained by the N2–CL (Table S3) and N3–CL (Table S4). The relatively low R2 values in the range of 0.778–0.979 from D–R isotherm are obtained. A large gap in the adsorptive capacities between the values from D–R isotherm and the values from experiment is observed. The E values ranged from 12.0 to 13.1 kJ mol−1 reveal the characteristics of chemisorption for both the ions (Saleh 2018). These illustrate that the Langmuir isotherm more suited to describe the adsorption of Cd2+ and Pb2+ ions by the three reactive cellulose derivatives. Furthermore, the results show that the values of b and qmax for the three reactive cellulose derivatives are in following order: N4–CL > N3–CL > N2–CL, indicating that the N4–CL in relation to the N2–CL and N3–CL maintained the higher amounts adsorbed and stability constant for the adsorption of Cd2+ and Pb2+ ions.
Kinetic
The experimental data are fitted using pseudo-first-order kinetic model (PDOKM) (Lagergren 1898) and pseudo-second-order kinetic model (PSOKM) (Ho and McKay 1999). The linear equations of two models are described in Supporting Information. In Table 4, it is clear to see that the kinetic adsorption data of Cd2+ and Pb2+ ions by the three reactive cellulose derivatives are appreciable closer to the PSOKM with the high values of r2 (> 0.990) than PDOKM with relatively low values of r2 in the range of 0.873–0.908, suggesting that the adsorption of both metal ions by the three reactive cellulose derivatives not follow the PDOKM. The computed values of the amounts adsorbed of the three reactive cellulose derivatives for the both metal ions from the PSOKM are very close to their experimental values. These reflect that the adsorption of both metal ions by the three reactive cellulose derivatives belongs to the PSOKM. The similar trends in the order of qcal values for the three reactive cellulose derivatives (N4–CL > N3–CL > N2–CL) are also observed. These values of N4–CL are significantly higher than those of N3–CL and N2–CL under the same conditions, illustrating that the N4–CL exhibits significantly better capability of metal removal.
Thermodynamics
The calculated equations of Gibbs free energy change (ΔG0), enthalpy change (ΔH0) and entropy change (ΔS0) are calculated as described by Liu (Liu 2009) and listed in Supporting Information. In Table 5, for both metal ions, the negative values of ΔG° gradually decreased with the temperature on the rise, demonstrating that the adsorption of both metal ions by the three reactive cellulose derivatives is more spontaneous at higher temperature. This is can be inferred from the variation of lnb value with increasing temperature. Linear plots of lnb vs. 1/T are fitted to calculated the values of ΔH0 and ΔS0 (Figure S3). The positive values of ΔH0 for both metal ions illustrate that adsorption of both metal ions by the three reactive cellulose derivatives is endothermic and favorable at higher temperature. The positive values of ΔS0 for both metal ions demonstrate an increase in randomness during the adsorption.
Regeneration of the N 4 –CL
Four consecutive cycles of adsorption–desorption are exhibited in Fig. 5. The adsorptive amounts of the N4–CL drop down to 200.5 mg g−1 for Cd2+ and 347.9 mg g−1 for Pb2+ in the first cycle. The adsorptive amounts of both the ions decrease with increasing cycle numbers. The adsorptive amounts of Cd2+ (180.6 mg g−1) and Pb2+ (325.5 mg g−1) are found after 4 cycles, illustrating that the N4–CL has good reusability. There are no notable changes in the SEM images after metal adsorption (Figure S4), whereas a drop in the N contents of the regenerated N4–CL with increasing cycle numbers is found (Table S5), illustrating the decrease in adsorptive amounts due to part loss of N–donor for chelating metals.
Conclusion
Results exhibit that the immobilization of linear aliphatic polyamines onto the cellulose backbone is successful in forming the high-efficient sorbents with high adsorptive amounts for the enhanced removal of Cd2+ and Pb2+ ions from water. It can be inferred that the binding sites of the N2–CL, N3–CL and N4–CL are provided by the multidentate N‑donor atoms from the linear aliphatic polyamines which have ability to adsorb Cd2+ and Pb2+ ions from water, and hence the adsorptive amounts of both the ions by the N2–CL, N3–CL and N4–CL are significantly enhanced by the introduction of the linear aliphatic polyamines. Among the three cellulose derivatives, the N4–CL contains more N‑donor atoms and exhibits higher adsorptive amounts for both the ions. The adsorption of both the ions by the N2–CL, N3–CL and N4–CL is thermodynamically feasible and spontaneous. These suggest that cellulose derivatives functionalized with multidentate N‑donor atoms can be used as potential sorbents for the enhanced removal of metals from water.
Data availability
The Supporting Information is available free of charge on the Springer Publications website at DOI:
References
Abdel-Halim ES, Al-Deyab SS (2012) Chemically modified cellulosic adsorbent for divalent cations removal from aqueous solutions. Carbohydr Polym 87:1863–1868
Abouzeid RE, Khiari R, El-Wakil N, Dufresne A (2019) Current state and new trends in the use of cellulose nanomaterials for wastewater treatment. Biomacromol 20:573–597
Abu-Danso E, Peräniemi S, Leiviskä T, Bhatnagar A (2018) Synthesis of S-ligand tethered cellulose nanofibers for efficient removal of Pb(II) and Cd(II) ions from synthetic and industrial wastewater. Environ Pollut 242:1988–1997
Aguado J, Arsuaga JM, Arencibia A, Lindo M, Gascón V (2009) Aqueous heavy metals removal by adsorption on amine-functionalized mesoporous silica. J Hazard Mater 163:213–221
Alipour A, Zarinabadi S, Azimi A, Mirzaei M (2020) Adsorptive removal of Pb(II) ions from aqueous solutions by thiourea-functionalized magnetic ZnO/nanocellulose composite: optimization by response surface methodology (RSM). Int J Biol Macromol 151:124–135
Bois L, Bonhommé A, Ribes A, Pais B, Raffin G, Tessier F (2003) Functionalized silica for heavy metal ions adsorption. Colloids Surfaces A 221:221–230
da Silva Filho EC, de Melo JCP, Airoldi C (2006) Preparation of ethylenediamine-anchored cellulose and determination of thermochemical data for the interaction between cations and basic centers at the solid/liquid interface. Carbohyd Res 341:2842–2850
Dhali K, Ghasemlou M, Daver F, Cass P, Adhikari B (2021) A review of nanocellulose as a new material towards environmental sustainability. Sci Total Environ 775:145871
Ding Z, Xin H, Wan Y, Wang S, Gao B (2016) Removal of lead, copper, cadmium, zinc, and nickel from aqueous solutions by alkali-modified biochar: batch and column tests. J Ind Eng Chem 33:239–245
Dubinin MM, Radushkevich LV (1947) Equation of the characteristic curve of activated charcoal. Chem Zentralbl 1:875
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21:885–896
Freundlich HMF (1906) Über die adsorption in lösungen. Zeitschrift Fur Physikalische Chemie 57:385–470
Fu B, Xie F (2019) Facile in situ synthesis of cellulose microcrystalline-manganese dioxide nanocomposite for effective removal of Pb(II) and Cd(II) from water. Environ Sci Pollut Res 27:5108–5121
Ge H, Huang H, Xu M, Chen Q (2016) Cellulose/poly(ethylene imine) composites as efficient and reusable adsorbents for heavy metal ions. Cellulose 23:2527–2537
Gülü G, Gürda G, Özgümü S (2003) Competitive removal of heavy metal ions by cellulose graft copolymers. J Appl Polym Sci 90:2034–2039
Guo M, Wang H, Sun L, Li Y (2014) Synthesis, characterization and properties of cellulose-grafted glycine derivatives. J Appl Polym Sci 131:40929
Gurgel LVA, Gil LF (2009) Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by succinylated mercerized cellulose modified with triethylenetetramine. Carbohyd Polym 77:142–149
Gurgel LVA, Júnior OK, de Freitas Gil RP, Gil LF (2008) Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by cellulose and mercerized cellulose chemically modified with succinic anhydride. Biores Technol 99:3077–3083
Ho YS, McKay G (1999) Pseudo-second-order model for sorption processes. Process Biochem 34:451–465
Hokkanen S, Bhatnagar A, Sillanpaä M (2016) A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res 91:156173
Hu Z, Schneider CM, Price CN, Pye WM, Dawe LN, Kerton FM (2012) Coordination chemistry of α-ω-bis(pyridylimine) ligands containing flexible linkers with copper(I). Eur J Inorg Chem 11:1773–1782
Joseph L, Jun BM, Flora J, Park CM, Yoon Y (2019) Removal of heavy metals from water sources in the developing world using low-cost materials: a review. Chemosphere 229:142–159
Júnior OK, Gurgel L, Freitas R, Gil LF (2009) Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by mercerized cellulose and mercerized sugarcane bagasse chemically modified with EDTA dianhydride (edtad). Carbohyd Polym 77:643–650
Kaur J, Sengupta P, Mukhopadhyay S (2022) Critical review of bioadsorption on modified cellulose and removal of divalent heavy metals (Cd, Pb, and Cu). Ind Eng Chem Res 61:1921–1954
Kenawy IM, Hafez M, Ismail MA, Hashem MA (2018) Adsorption of Cu(II), Cd(II), Hg(II), Pb(II) and Zn(II) from aqueous single metal solutions by guanyl-modified cellulose. Int J Biol Macromol 107:1538–1549
Kim UJ, Wada M, Kuga S (2004) Solubilization of dialdehydecellulose by hot water. Carbohyd Polym 56:7–10
Kobayashi M, Suzawa I, Ichishima E (2014) Highly reactive dialdehydes of cellulose andα-cyclodextrin. Agri Biol Chem 54:1705–1709
Kono H, Yunoki S, Shikano T, Fujiwara M, Takai M (2002) CP/MAS 13CNMR study of cellulose and cellulose derivatives. 1. Complete assignment of the CP/MAS spectrum of the native cellulose. J Am Chem Soc 124:7506–7511
KumaraSharma, R. RKr (2019) Synthesis and characterization of cellulose based adsorbents for removal of Ni(II), Cu(II) and Pb(II) ions from aqueous solutions. React Funct Polym 140:82–92
Kundu D, Mondal SK, Banerjee T (2019) Development of β-cyclodextrin-cellulose/hemicellulose-based hydrogels for the removal of Cd(II) and Ni(II): synthesis, kinetics, and adsorption aspects. J Chem Eng Data 64:2601–2617
Lagergren S (1898) About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetenskapsakademiens Handlingar 24:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Li Y, Guo C, Shi R, Zhang H, Gong L, Dai L (2019) Chitosan/nanofibrillated cellulose aerogel with highly oriented microchannel structure for rapid removal of Pb(II) ions from aqueous solution. Carbohyd Polym 223:115048
Lian Z, Li Y, Xian H, Ouyang X, Lu Y, Peng X, Hu D (2020) EDTA-functionalized magnetic chitosan oligosaccharide and carboxymethyl cellulose nanocomposite: synthesis, characterization, and Pb(II) adsorption performance. Int J Biol Macromol 165:591–600
Liu Y (2009) Is the free energy change of adsorption correctly calculated? J Chem Eng Data 54:1981–1985
Liu J, Xie T-H, Deng C, Du K-F, Zhang N, Yu J-J, Zou Y-L, Zhang Y-K (2014) Welan gum-modified cellulose bead as an effective adsorbent of heavy metal ions (Pb2+, Cu2+, and Cd2+) in aqueous solution. Sep Sci Technol 49:1096–1103
Liu Y, Liu R, Li M, Yu F, He C (2019) Removal of pharmaceuticals by novel magnetic genipin-crosslinked chitosan/graphene oxide-SO3H composite. Carbohyd Polym 220:141–148
Liu Y, Nie P, Yu F (2021) Enhanced adsorption of sulfonamides by a novel carboxymethyl cellulose and chitosan-based composite with sulfonated graphene oxide. Bioresource Technol 320:124373
Movaghgharnezhad S, Mirabi A, Toosi MR, Rad AS (2020) Synthesis of cellulose nanofibers functionalized by dithiooxamide for preconcentration and determination of trace amounts of Cd(II) ions in water samples. Cellulose 27:8885–8898
Nongbe MC, Bretel G, Ekou T, Ekou L, Yao BK, Grognec EL, Felpin F-X (2018) Cellulose paper grafted with polyamines as powerful adsorbent for heavy metals. Cellulose 25:4043–4055
Nordberg GF, Fowler BA, Nordberg M, Friberg L (2007) Handbook on the toxicology of metals, 3rd edn. Academic Press, Imprint
O’Connell DW, Birkinshaw C, O’Dwyer TF (2008) Heavy metal adsorbents prepared from the modification of cellulose: a review. Bioresource Technol 99:6709–6724
Pillai SS, Deepa B, Abraham E, Girija N, Geetha P, Jacob L, Koshy M (2013) Biosorption of Cd(II) from aqueous solution using xanthated nano banana cellulose: equilibrium and kinetic studies. Ecotoxicol Environ Saf 98:352–360
Qu J, Tian X, Jiang Z, Cao B, Akindolie MS, Hu Q, Feng C, Feng Y, Meng X, Zhang Y (2020) Multi-component adsorption of Pb(II), Cd(II) and Ni(II) onto microwave-functionalized cellulose: kinetics, isotherms, thermodynamics, mechanisms and application for electroplating wastewater purification. Journal of hazardous materials. J Hazard Mater 387:121718
Roy D, Semsarilar M, Guthrie JT, Perrier S (2009) Cellulose modification by polymer grafting: a review. Chem Soc Rev 38:2046–2064
Saleh TA (2018) Simultaneous adsorptive desulfurization of diesel fuel over bimetallic nanoparticles loaded on activated carbon. J Clean Prod 172:2123–2132
Shen G, Zhang X, Shen Y, Zhang S, Fang L (2015) One-step immobilization of antibodies for α-1-fetoprotein immunosensor based on dialdehyde cellulose/ionic liquid composite. Anal Biochem 471:38–43
Sun XF, Wang SG, Cheng W, Fan M, Tian BH, Gao BY, Li X-M (2011) Enhancement of acidic dye biosorption capacity on poly(ethylenimine) grafted anaerobic granular sludge. J Hazard Mater 189:27–33
Taksitta K, Sujarit P, Ratanawimarnwong N, Donpudsa S, Songsrirote K (2020) Development of tannin-immobilized cellulose fiber extracted from coconut husk and the application as a biosorbent to remove heavy metal ions. Environ Nanotechnol Monit Manage 14:100389
Thakur V, Sharma E, Guleria A, Sangar S, Singh K (2020) Modification and management of lignocellulosic waste as an ecofriendly biosorbent for the application of heavy metal ions sorption. Mater Today 32:608–619
Torres JD, Faria EA, Prado AGS (2006) Thermodynamic studies of the interaction at the solid/liquid interface between metal ions and cellulose modified with ethylenediamine. J Hazard Mater B 129:239–243
Varma AJ, Chavan VB, Rajmohanan PR, Ganapathy S (1997) Some observations on the high-resolution solid-state CP-MAS carbon-13 NMR spectra of periodate-oxidized cellulose. Polym Degrad Stabil 58:257–260
Wang F, Zhu Y, Xu H, Wang A (2019) Preparation of carboxymethyl cellulose-based macroporous adsorbent by eco-friendly pickering-MIPEs template for fast removal of Pb2+ and Cd2+. Front Chem 7:603
Wei W, Kim S, Song MH, Bediako JK, Yun YS (2015) Carboxymethyl cellulose fiber as a fast binding and biodegradable adsorbent of heavy metals. J Taiwan Inst Chem Eng 57:104–110
Wu Q, He H, Zhou H, Xue F, Zhu H, Zhou S, Wang L, Wang S (2020) Multiple active sites cellulose-based adsorbent for the removal of low-level Cu(II), Pb(II) and Cr(VI) via multiple cooperative mechanisms. Carbohyd Polym 233:115860
Xiang T, Zhang ZL, Liu HQ, Yin ZZ, Li L, Liu XM (2013) Characterization of cellulose-based electrospun nanofiber membrane and its adsorptive behaviours using Cu(II), Cd(II), Pb(II) as models. Sci China Chem 56:567–575
Yu X, Tong S, Ge M, Wu L, Zuo J, Cao C, Song W (2013) Synthesis and characterization of multi-amino-functionalized cellulose for arsenic adsorption. Carbohyd Polym 92:380–387
Yu H, Zheng L, Zhang T, Ren J, Cheng W, Zhang L, Meng P (2021) Adsorption behavior of Cd (II) on TEMPO-oxidized cellulose in inorganic/organic complex systems. Environ Res 195:110848
Zhang C, Su J, Zhu H, Xiong J, Liu X, Li D, Chen Y, Li Y (2017) The removal of heavy metal ions from aqueous solutions by amine functionalized cellulose pretreated with microwave-H2O2. RSC Adv 7:34182–34191
Zhao L, Yan H, Yang C, Pan J (2014) Fabrication and application of a copper(II)-selective extraction disc prepared from chlorinated polyvinyl chloride and ethylenediamine-functionalized cellulose. Anal Methods 6:2930–2935
Zhou Y, Wang X, Zhang M, Jin Q, Gao B, Ma T (2014) Removal of Pb(II) and malachite green from aqueous solution by modified cellulose. Cellulose 21:2797–2809
Acknowledgments
Not applicable.
Funding
This work is supported by Scientific Foundation of Liaoning Education Department (LJKZZ20220053).
Author information
Authors and Affiliations
Contributions
BD: Methodology, Formal analysis, Writing—Original draft preparation. LY: Investigation, Formal analysis, Data curation, Writing—Original draft preparation. HF: Conceptualization, Project administration. NY: Conceptualization, Project administration Validation, Resources, Data curation. HW: Formal analysis, Data curation, Writing—Reviewing and Editing.
Corresponding authors
Ethics declarations
Conflict of interest
No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ding, B., Yang, L., Fan, H. et al. Cellulose derivatives functionalized with multidentate N‑donor atoms: comparative adsorption of cadmium (II) and lead (II) ions from water. Cellulose 30, 6387–6400 (2023). https://doi.org/10.1007/s10570-023-05273-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05273-x