Abstract
An innovative way was found for the synthesis of efficient photocatalyst in the form of ZnS–ZnO nanocomposite from Cucumis melo pulp and chicken feather powder as a source of fuel and oxygen and sulfur, respectively, by combustion followed by hydrothermal method and is reported in this work. Different analytical techniques are employed, such as XRD, SEM, TEM, PL EDS, FT-IR, DRS, and EIS techniques for structural, morphological, optical, and electrochemical properties. The SEM analysis confirmed ZnS–ZnO nanocomposite formation. The XRD data of ZnS–ZnO nanocomposite resembled pure crystalline ZnO and ZnS nanoparticles. DRS confirmed the band gap of the synthesized material, which was calculated using a K-M plot and found to be 3.29 eV, 3.38 eV, and 3.12 eV for ZnO, ZnS nanoparticles, and ZnS-ZnO nanocomposite, respectively. The synthesized ZnS–ZnO nanocomposite showed enhanced photocatalytic activity towards MB [92%], RhB [98.4%], and CR [90.9%] dyes in water under UV light source. Radicals formation was confirmed by the ESR technique. TOC analysis was done to verify the degradation of dyes. ZnS–ZnO nanocomposite also exhibited potent or robust antibacterial activity.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Toxic and persistent organic pollutants in the form of dyes such as MB (methylene blue), CR (Congo red), and RhB (rhodamine B) negatively affect the quality of surface and underground water [1]. Photocatalytic mitigation of such pollutants is a widely explored method due to its inexpensiveness and ability to mineralize the organic compounds [2,3,4,5] eventually. During the photocatalytic decomposition of the dyes at a semiconducting material, light absorption leads to electron–hole pair formation, which performs as an oxidizing/reducing agent and aids in the breakdown of organic molecules [6, 7]. Several semiconductor materials have been investigated as photocatalysts, including metal oxides (MO), metal sulfides (MS), nitrides, oxynitrides, and their composites. Metal oxides nanoparticles such as TiO2, ZnO, V2O5, MgO, Bi2O, WO2, CuO, and Fe2O3 and their composites are more frequently explored [8, 9]. ZnO, an inexpensive, photostable, and non-toxic material, finds use for photocatalysis in several industries [10, 11]. However, it is sensitive to photo corrosion and challenging to use in aqueous solutions under UV light irradiation resulting in the significant reduction in their photocatalytic activity [12]. Nevertheless, some studies have shown that nano ZnO works with better photocatalytic activity than nano Fe2O3 and nano TiO2, even in aqueous solution [13,14,15,16,17]. Further, nanosizing and compositing improve properties of ZnO, such as surface area and surface defects. This provides a simple approach to efficient charge distribution and quantization of discrete energy levels.
On the other hand, nanostructured metal sulfides are cost-effective and efficient photocatalysts [18] across a broad solar spectrum range due to their narrow energy band gaps compared to metal oxides. This is because sulfides are available in various crystalline forms and structures and in various chemical compositions [19]. Also, due to their unique physical and chemical properties, they act as suitable sensors, reductants, and chemical/electrocatalysts. Among the most widely used semiconductor catalysts, group II B sulfides, especially CdS and ZnS, find a special place. The band gap of ZnS is wide, about 3.6 eV, which can easily absorb UV radiation. However, researchers have developed nano ZnS that, upon doping, can harness visible light by widening its conduction band [20].
Nanocomposites find applications in solar cells, linear optical devices, windows, and optoelectronic devices, and are widely used as semiconductors. In the family of oxide-sulfide composites, ZnO–ZnS nanocomposite finds technological application due to its high electrochemical stability, high electron mobility, and non-toxic nature [21]. ZnO–ZnS nanocomposite retains the good features of ZnO and ZnS and acts synergistically in photodegradation process [22]. It can be found that ZnS and ZnO can both be excited by UV light to generate electrons in the conduction band and holes in the valence band, respectively. Among them, the electrons in the conduction band of ZnS can migrate to that of ZnO, while the holes in the valence band of ZnO can quickly migrate to the valence band of ZnS. In the above-mentioned electron and hole migration process, efficient separation of photogenerated carriers can be achieved, and effective time of photogenerated carriers can be improved [22,23,24].
In many parts of the world, water-related diseases are leading to death, and the problem is growing at an alarming rate. Due to the widespread use of antibiotics, bacteria have developed resistance, further complicating the problem [25] and, hence, the need for effective antibacterial systems. Antibacterial agents find potential applications in the packaging, cosmetic, medical, and healthcare industries. Antimicrobial agents are often divided into organic and inorganic substances. However, organic antibacterial agents are reported to be more valuable than inorganic antibacterial agents due to their higher stability and increased safety [25]. One of the most explored uses of nanomaterials is as an antimicrobial agent against bacteria, viruses, and other pathogens. Among the various nanomaterials, metal oxides are widely studied due to their non-toxicity, stability, and biologically effective properties. Various nanomaterials such as ZnO, ZnS, Fe2O3, TiO2, Ag2O, CaO, MgO, and CuO [25, 26] have been used as effective bactericidal agents. Nanostructured ZnO with a band gap of 3.28 eV has a very high excitation energy of 60 m eV [27], while nano ZnS has a band gap of 3.6 eV. As a result, free electrons and holes have enough lifetimes to cause photogeneration of reactive oxygen species (ROS) from oxygen and water molecules adsorbed on the surface of nano ZnO. These ROS include highly reactive species such as superoxide free radicals, hydroxyl free radicals, and H2O2. These ROS damage cellular components such as nucleic acids, proteins, enzymes, and cell membranes and cause various levels of oxidative stress [9]. It has been reported that the size, morphology, and unique surface properties of ZnO and ZnS nanoparticles and bacteria significantly affect their antibacterial properties. Consequently, the surface area of ZnO and ZnS nanoparticles plays an active role in antibacterial activity; increasing the surface area increases the antibacterial properties, and decreasing the surface area decreases it [28].
As the demand for nanoparticles or nanocomposites has increased, so has the choice of methods and tools. Various methods have been reported for preparing ZnO–ZnS nanocomposite, including sol–gel, hydrothermal, solution combustion, thermoset water solution, and thermal treatment. Among the synthetic methods discussed in the literature, hydrothermal and flow processes are simple and efficient [1]. Maintaining high temperatures and pressures is necessary to construct expensive test specimens in the reported methods. To overcome this deficiency, scientists have adopted a straightforward green strategy, using available precursors, using environmentally friendly control experiments, and the obtained products can be used for various purposes [29]. Recently, it has been reported that ZnO–ZnS nanocomposites are prepared by a simple combustion method and used in the photocatalytic degradation of dyes such as methyl orange, rhodamine B, and tetracycline [22].
Current research focuses on developing a green synthesis method for ZnS–ZnO nanocomposite using the simple combustion method. Cucumis melo extract is used as a fuel for ZnO nano synthesis. Chicken features are considered a soil pollutant with a natural biodegradation period of 7 years. This work is chosen as the source of sulfur for ZnS nano synthesis. After obtaining the ZnO and ZnS separately, the composite is made by treating the two solvothermal. Characterization of the as-prepared ZnO, ZnS nanoparticles, and ZnS–ZnO nanocomposite is carried out by X-ray diffraction (XRD), SEM, EDX, DRS, and FT-IR to determine phase formation, elemental mapping, energy band gap and vibrational stretching of functional group frequency. The synthesized ZnO, ZnS nanoparticles, and ZnS–ZnO nanocomposite were subjected to a comparative study of photocatalytic activity towards MB, RhB, and CR dyes in aqueous solution, and the antibacterial properties are presented.
Experimental
Materials and Methods
Zinc nitrate [Zn (NO3)2·6H2O] (99%, Sigma Aldrich)], chicken feathers (collected from the chicken stall near Bangalore University), and musk melon (Cucumis melo var) were purchased from a nearby market of Bangalore University. DI water, mannitol salt agar, blood agar, and MacConkey agar are used in the present study.
Synthesis of ZnO Nanoparticles
1 M of Zn (NO3)2·6H2O (100 mL) was dissolved in the Cucumis melo pulp (50 mL, which acts as a reducing agent and surfactant) and stirred for 1 h to form a uniform solution at 40 °C. The reaction mixture was transferred into the crucible to carry out the combustion reaction [30] in the muffle furnace at 500 ºC, and the combustion reaction took place within 5 min. Heating was continued for an additional 20 min. To ensure the completion of the reaction. The obtained product was calcinated at 600 ºC for 5 h to remove any organic materials in the development. Finally, ZnO nanoparticles were obtained.
Preparation of ZnS Nanoparticles
Waste chicken feathers were thoroughly washed with tap water followed by the DI water, water content was removed using a hot oven at 35 ºC for 3 h, and dried chicken feathers were powdered with the mixie jar. The obtained powder {(chicken feathers contain approximately 91% protein, and amino acid cystine is a significant source of keratin (7–20%) [31, 32], which we used as a source of sulfur for the preparation of nano ZnS} was mixed with the 1 M of Zn (NO3)2·6H2O in the presence of 10 mL of DI water. The mixture was ultrasonicated in a sonic bath at 35 kHz, followed by stirring on a magnetic stirrer to disperse the particles uniformly. The reaction mixture was transferred into the crucible to carry out the combustion reaction using a muffle furnace at 550 ℃ [33]. The combustion reaction took place on a flame for 10 min. The intermediate is allowed to heat for another 30 min at the same temperature to complete the reaction. The product is calcinated at 600 ℃ to remove any organic impurities. Finally, ZnS nanoparticles were obtained.
Preparation of ZnS–ZnO Nanocomposite
For the synthesis of ZnS–ZnO nanocomposite, we adopted the hydrothermal method [48]. The above ZnO and ZnS nanoparticles are taken in equal ratios (1:1) and dissolved in 30 mL DI water. The reaction mixtures were stirred for 2 h on a magnetic stirrer, then transferred into a 50 mL autoclave and kept heating at 150 ℃ for 10 h. ZnS–ZnO nanocomposite was, thus, formed and repeatedly filtered and washed with 60 mL water and 10 mL ethanol. The product was then dried at 80 ℃ for 2 h. ZnS–ZnO nanocomposite was prepared successfully in its purest form.
Preparation of Dye Solutions in an Aqueous Medium
100 ppm concentration of MB, RhB, and CR dyes was prepared by dissolving accurately weighed dyes in ultrapure water. The desired concentrated solution (20 ppm) was prepared from the stock solution by diluting the exact volume with water.
Photocatalytic Experiments
The photocatalytic degradation of ZnS–ZnO nanocomposite was assessed by measuring the degradation of MB (methylene blue), RhB [rhodamine B], and CR [Congo red] aqueous solutions by UV source. MB, RhB, and CR solutions are maintained at 26 ℃ using a jacketed reactor. In our experiment, 15 mg of the prepared catalyst was added to 100 ml of an aqueous solution of MB, RhB, and CR with an initial concentration of 20 ppm. An aqueous suspension containing MB, RhB, CR, and the corresponding catalyst mixture was mixed using a magnetic stirrer (400 rpm) in the photodegradation process. After different time intervals, MB, RhB, and CR were analyzed using a UV–VIS spectrophotometer (Perkin Elmer). All experiments were performed in a dark room. Finally, the photocatalytic efficiency was estimated [34, 35].
Antibacterial Activity
Antibacterial activity was screened by agar well diffusion method [9, 25, 25,26,27] against two bacterial strains, namely Gram-negative bacteria Pseudomonas aeruginosa and Klebsiella and Gram-positive bacteria Staphylococcus aureus and Streptococcus pneumonia. The suspension was prepared and grown on Mannitol salt agar. S. pneumoniae was grown on blood agar, and P. aeruginosa was grown on Mac Conkey agar; cultures were incubated for 24 h at 37 °C for bacteria. Cell density is adjusted to 1 × 108 cells/mL using 0.5 McFarland standard. The cell suspensions of all the cultures were adjusted to 1–2 × 105cells/mL.
The sample (20 µl), the standard [Ciprofloxacin (20 µL)], Staphylococcus aureus/Streptococcus pneumonia/ Pseudomonas aeruginosa were added to the 5 mm well on agar Petri dishes. The treated Petri dishes were incubated in an aerobic chamber at 37 °C for 24 h. The Petri dishes were observed for zone inhibition around the wells. After incubation, the zone of inhibition of each well was measured, and the values were noted. Triplicates were maintained in each compound, and the average values were calculated for the ultimate antibacterial activity.
Instruments and Measurements
The X-ray diffraction (XRD) patterns were analyzed using (PAN Analytica’s X-ray diffractometer) with Cu Ka radiation in step scan mode at a scan rate of 2º min−1 with two θ ranging from 10 to 90 θ. Morphology was observed using Ultra 55 FE-SEM (Karl Zeiss). FT-IR spectra were recorded using a Perkin Elmer IR-100300 spectrophotometer. The residual concentration of the dye in the reaction mixture at different time intervals was determined by a UV–visible spectrophotometer (Perkin Elmer). The mineralization rate of the solution was examined on the total organic carbon (TOC) analyzer (Multi N/C 3100).
Results and Discussion
X-Ray Diffraction (XRD): Structural Studies
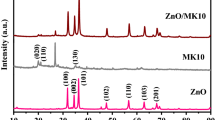
The structural phase and purity of the synthesized ZnS–ZnO nanocomposite were investigated by XRD analysis. The XRD peaks of the synthesized materials are shown in Fig. 1. Some of the peaks in the XRD pattern are very sharp and intense, indicating that the samples are good crystalline. The XRD pattern of ZnO showed diffraction peaks at 2θ values 23.80°, 34.51°, 36.21°, 47.52°, 56.61°, 62.90°, 67.91°, and 69.92°, with the corresponding Miller indices (hkl) of (100), (002), (101), (102), (110), (103), (112), and (201), respectively, indicating the formation of hexagonal structure and single phase formation of nano ZnO, which is in agreement with the Joint Committee on Powder Diffraction Standards (JCPDS) card no. 36-1451 [36,37,38], in addition to green-synthesized ZnS peaks position at 2θ values at 28.64°, 47.68°, 42.13°, 56.70° which corresponds to (hkl) values (111), (220), (101), (311) corresponding to the cubic structure with a = 5.36 Å is in agreement with the JCPDS card no. 00-001-07292 reflection planes, respectively [39], and both sets of peaks correspond to the formation of ZnS–ZnO nanocomposite. The observed peaks are in good agreement with bio-mediated synthesized ZnS–ZnO nanocomposite (JCPDS Card no. 89-1397 and 05-0566) [40], which confirms that ZnO crystallizes in the hexagonal wurtzite form with a = 3.2 Å, c = 5.2 Å and ZnS in cubic structure with a = 5.368 Å. Some additional peaks are observed in ZnS, which may be due to the oxidation of ZnS at higher temperatures. The average crystalline size of green-synthesized ZnO, ZnS nanoparticles, and ZnS–ZnO nanocomposite was found to be 30 nm, 24 nm, and 71.19 nm, respectively.
The crystallite size of green-synthesized ZnO, ZnS nanoparticles, and ZnS–ZnO nanocomposite was calculated using the Debye–Scherrer formula D = kλ/βcosθ. In this equation, D is the crystallite size, λ is the wavelength of the X-ray source, which is 1.5418 Å and θ is the Bragg’s diffraction angle in degree, k is constant equal to 0.9 and β is the full width at half maximum (FWHM) value in radians. The crystallite sizes were calculated from all three broad peaks, and the final crystallite size was obtained by averaging them. The average crystalline size for green-synthesized ZnS–ZnO nanocomposite and the average crystalline size calculated for the most intense green-synthesized nano ZnO peak plane is (101). It has been observed that the crystalline size has decreased; the lattice parameters which correspond to hexagonal ZnO structure increased from 3.2 to 8.6 Å; and c = 5.6 Å fell to 4.7 Å.
Scanning Electron Microscopy (SEM) and EDAX
The surface morphology analysis of green-synthesized ZnO, ZnS nanoparticles, and ZnS–ZnO nanocomposite revealed the formation of irregular nanospheres, octahedral, cubic, and disc shapes for ZnO nanoparticles, nano-pellets shapes were observed in the ZnS; while in ZnS–ZnO nanocomposite formed by the collection of Nano discs and nanoflowers with a good agglomeration of nanoparticles which may be due to the existence of interfacial surface tension phenomena [41, 42]. EDAX spectrum confirmed the purity of green-synthesized ZnO, ZnS nanoparticles, and ZnS–ZnO nanocomposite and the presence of Zn, O, and S only. The atomic % is mentioned in Fig. 2.
TEM and HRTEM analysis studied the morphology of ZnS–ZnO nanocomposite. As shown in Fig. 3a, ZnO nanoflakes with sparsely incorporated puff-like ZnS were found in ZnS–ZnO nanocomposite. The high-resolution (HR) TEM images (Fig. 3b) show a crystalline structure of heterojunction composites, typically two sets of 0.18 and 0.14 nm, corresponding to the (111) plane of ZnO and the (101) plane of ZnS, respectively. The selected area electron diffraction (SAED) pattern of ZnS–ZnO nanocomposite is shown in Fig. 3c; the diffraction rings centered around the transition point are observed, demonstrating the polycrystalline nature of the sample. These observations indicate that the heterojunction between ZnO, and ZnS is generated directly, suggesting that the combination mode facilitates the separation and transfer of photogenic charge carriers and enhances the photocatalytic activity. To sum up, these results from TEM confirmed the successful construction of ZnS–ZnO nanocomposite with the nanosphere and puff-like structures.
UV–Visible Diffused Reflection Spectroscopy (DRS) Analysis
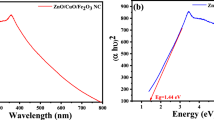
The optical properties of the bio-mediated synthesized ZnO, ZnS nanoparticles, and ZnS–ZnO nanocomposite were analyzed using UV–visible diffuse reflectance spectroscopy, as shown in Fig. 4a. The reflectance peak at 378 nm is due to the higher potential energies of O 2p orbitals when bonded with Zn and the reflectance peak at 352 nm is due to lower potential energies S orbitals. The optical bandgap energy (Eg) was calculated by Kubelka–Munk (K–M) plot using reflectance data [43, 44]. The Kubelka–Munk function E(hv) is generally applied to convert the diffused reflectance, and the optical bandgap was calculated by plotting [F(r)hv]2 against the hv (Photon energy), where F(r) is Kubelka–Munk function. The band gap energy of green-synthesized ZnO, ZnS nanoparticles, and ZnS–ZnO nanocomposite was found to be 3.29 eV, 3.38 eV, and 3.12 eV, respectively, as shown in Fig. 4b. Further results indicate the decreased band gap values for the composition suitable for photocatalysis.
FT-IR Spectral Analysis
FT-IR spectra of the synthesized ZnO, ZnS nanoparticles, and ZnS–ZnO nanocomposite were recorded in the 400 cm−1–1500 cm−1, as shown in Fig. 5, which further confirms the crystalline hexagonal wurtzite and cubic structures. The characteristic absorption peaks at 425 cm−1 and 665 cm−1 are attributed to ZnS–ZnO nanocomposite [36]. The 423 cm−1 and 670 cm−1 bands are associated with stretching vibrations of nano ZnS [22, 22, 23, 45]. The bands at 423 cm−1 and 840 cm−1 are due to stretching vibrations of nano ZnO [46].
Photoluminescence Analysis
In theory, the rate of separation and migration of photogenerated electron–hole pairs significantly affects the photocatalytic activity of the catalyst. Using the PL technique, the migration and recombination of the charge carriers in ZnS–ZnO nanocomposite were studied, resulting in higher photodegradation efficiency. The emission spectra of synthesized ZnO, ZnS, and ZnS–ZnO nanocomposite are shown in Fig. 6 at 410 nm for excitation. ZnO and ZnS exhibit an intense emission peak at 430 and 440 nm, respectively. While ZnS–ZnO nanocomposite exhibits an intense emission peak at 450 nm, consistent with the previous findings [44]. As the emission peaks decreased drastically in ZnS–ZnO nanocomposite, this suggests that the recombination rate of photogenerated charge carriers is lower in ZnS–ZnO nanocomposite, resulting in faster charge transfer and separation rates. These results agree well with the enhanced photocatalytic degradation of dyes.
Photo Electrochemical Measurement
Photocurrent and electrochemical impedance were measured in a three-electrode system using the prepared samples as working electrodes, a platinum plate as a counter electrode, and a saturated Ag/AgCl electrode as a reference electrode. An aqueous 0.5 M Na2SO4 solution was used as the electrolyte. The working electrodes were prepared: 0.8 mg samples were ground, 2 mg carbon black, 20 µL Nafion, and 200 µL 2-propanol were added. Sonicated them in an Ultra-Sonicator for 60 min. The ink is dropped on a glassy carbon electrode (0.5 cm in diameter) and dried at room temperature. The loaded active material on the glassy carbon electrode is about 2 mg. Before spotting the ink on the glassy carbon electrode, it was polished with 0.05µ alumina powder and cleaned with acetone [47].
To assess the effectiveness of charge transfer and separation at the interface, photocurrent response and electrochemical impedance spectroscopy (EIS) were employed (Fig. 7). As shown in Fig. 7b, ZnS–ZnO nanocomposite is a higher photocurrent response as compared to the ZnS (1.8-fold), ZnO (2.4-fold) revealing the formation of the p–n heterojunction in hetero and binary composites contribute to the generation and transfer of the photogenic charge carriers. In addition, an electrochemical impedance test was conducted, and the results are depicted in Fig. 7b. In general, the magnitude of the arc dictates the rate at which the catalyst can transport electrons. The rate increases as the arc radius decreases [25, 48]. The ESI Nyquist plots (Fig. 7a) demonstrate that the ZnS–ZnO nanocomposite can improve the separation efficiency and free electrons transfer rate because the ZnS–ZnO nanocomposite sample has the smallest arc radius compared to the ZnS and ZnO samples. The charge transfer and recombination process can be depicted by the equivalent circuits RS and CPE, which represent the resistance of the solution and the constant phase element, which can be viewed as a double-layered capacitor (Fig. 7a inset). Rct, which means the electron transfer resistance, can be calculated using the arc radius in the EIS Nyquist diagram. The values of these electrical parameters are summarized in Table 1. Compared to pure ZnO, ZnS, the Rct values were reduced from 21.2 ohms to 3.9 ohms, which is responsible for the improved photocatalytic performance of the ZnS–ZnO nanocomposite.
Analysis of Photocatalytic Activity
The photocatalytic performance of the bio mediated synthesized ZnO, ZnS nanoparticles, and ZnS–ZnO nanocomposite was evaluated using photodegradation of 50 ppm aqueous solutions of Methylene blue [MB], Rhodamine B [RhB], and Congo red [CR] dye under UV light. Experimental set-up is shown in S-1. Visually, the blue, pink, and red colors of the MB, RhB, and CR solutions also fade with increasing irradiation time. MB, RhB, and CR were initially degraded, and no degradation was observed. The maximum absorption peaks of MB, RhB, and CR appeared at 580 nm, 546 nm, and 497 nm, respectively. Drop of intensities with the irradiation time for ZnO, ZnS nanoparticles, and ZnS–ZnO nanocomposite are shown in Fig. 8. Further, the photocatalytic activity of green-synthesized ZnO, ZnS nanoparticles, and ZnS encapsulated ZnO nanocomposite samples were analyzed with an increasing irradiation time of 70, 60, 60 min for MB, RhB and CR dyes. The degradation efficiency (C/C0) values were found to be 1–0.34—nano ZnO, 0.25—nano ZnS, and 0.027—(ZnS–ZnO nanocomposite) nanocomposite for [MB]; 1–0.24—nano ZnO, 0.19—nano ZnS, 0.015—(ZnS–ZnO nanocomposite) nanocomposite for [RhB], and 1–0.186—nano ZnO, 0.23—nano ZnS, 0.098—(ZnS–ZnO nanocomposite) nanocomposite for [CR], and for blank with the respective catalyst under visible light for 70, 60, 60 min, respectively, and these results are presented in Fig. 8a–c.
The photocatalytic efficiencies of green-synthesized ZnO, ZnS nanoparticles, and ZnS–ZnO nanocomposite were calculated by measuring the degradation of the MB, RhB, and CR dyes using the following equation [47].
where η is the photocatalytic efficiency, Ci is the initial absorbance intensity of MB, RhB, and CR, and Cf is the absorbance intensity of MB, RhB, and CR at the time t. The degradation percentage was calculated at 65.2% (ZnO), 75% (ZnS), and 92% (ZnS–ZnO nanocomposite) for [MB]; 70.3% (ZnO), 80.4% (ZnS), and 98.4% (ZnS–ZnO nanocomposite) for [RhB] and 70.36% (ZnO), 81.36% (ZnS),90.9% (ZnS–ZnO nanocomposite) for [CR] as shown in Fig. 10a2–c2. Kinetic values are mentioned in Table 2. The photodegradation of MB, RhB, and CR with ZnS–ZnO nanocomposite was found to be higher in photocatalytic efficiency when compared with ZnO and ZnS nanoparticles. Photocatalytic activity using various catalyst, from previous studies shows ZnS–ZnO nanocomposite is promising photocatalyst, and the comparison is illustrated in Table 1(S-2).
Kinetics Study
Photocatalysis of dye follows the pseudo-1st-order reaction. The kinetic values are estimated with the help of the following equation [22, 22, 46,47,48].
where C0 is the concentration of MB, RhB, and CR before irradiation, Ct is the concentration of the MB, RhB, and CR dyes after irradiation at t (min), and k is the rate constant. The rate constant for MB, RhB, and CR dyes in the presence of green-synthesized ZnO, ZnS nanoparticles, and ZnS–ZnO nanocomposite was calculated by plotting the ln(C/C0) against the time as shown in Fig. 9a1–c1. The rate constant for each sample was found to be 0.023, 0.020, 0.037 for [MB]; 0.016, 0.019, 0.031for [RhB] and 0.0212, 0.026, 0.065 for [CR] for ZnO, ZnS nanoparticles and ZnS–ZnO nanocomposite respectively; these results are summarized in Table 3. Green-synthesized ZnS–ZnO nanocomposite is a better photocatalyst than green-synthesized ZnO, ZnS nanoparticles and for MB, RhB, and CR dyes (50 ppm) in terms of degradation efficiency and rate constant due to the smaller crystalline size, lower charge transfer, lower bandgap, absorption of light in the visible region and lower recombination of charge carriers.
Stability of the Green-Synthesized ZnS–ZnO Nanocomposite
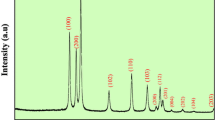
The reusability of ZnS–ZnO nanocomposite as a catalyst was analyzed using MB, RhB, and CR dyes under visible light. The used catalyst was collected by centrifuging and washed multiple times with ethanol and DI water. Further, the cleaned catalyst was dried at 75 ℃ for 5 h. The photocatalytic activity is studied for four cycles, and only slight changes occurred during every process of reuse after exposure to visible light for 70, 60, and 60 min for MB, RhB, and CR dyes, respectively. Degradation efficiency and kinetic parameters are shown in Fig. 10a1–c1. After the photocatalytic test, the catalyst ZnS–ZnO nanocomposite was collected and examined using XRD, as shown in Fig. 11.
The XRD pattern showed the same structure with small decreased peak intensities. The reusability test proves that green-synthesized ZnS–ZnO nanocomposite is a promising photocatalyst. The photocatalytic efficiency of MB, RhB, and CR dyes with four cycles is tabulated in Table 3.
TOC Analysis
To review the degree of CR, MB, RhB mineralization, TOC analysis was performed for the samples catalyzed by ZnO, ZnS, and ZnS–ZnO nanocomposite under optimized conditions [47]. The degree of RhB mineralization is defined based on TOC measurements from Eq. (3).
where TOCi and TOCf are initial and residual total organic carbon concentrations, respectively. Figure 12a–c compares the mineralization of CR, MB, and RhB in the photocatalytic process as a function of reaction time. The difference between the photodegradation and mineralization efficiencies is ascribed to the decomposition of RhB dye molecules into intermediates, which might have partially remained in the solution. It can be wholly mineralized by continuing the reaction for a little longer. The PFO reaction rate (R2) constant of CR, MB, and RhB mineralization is tabulated in Table 4. From Table 4, we conclude that, due to higher decomposition of dye, with significant rate constant values, ZnS–ZnO nanocomposite is efficient photocatalyst.
Photocatalysis Mechanism
The mechanism of MB, RhB, and CR degradation by the bio-mediated synthesized ZnS–ZnO nanocomposite is depicted in Fig. 13. The UV photons excite the electrons from the valence band, while the visible light photons excite the electrons from the defect energy levels of nano ZnO to the conduction band. The photogenerated electrons subsequently react with O2 to produce superoxide radical anions (O2−) and hydrogen peroxide (H2O2), and the holes from the nano ZnO valence bands react with water (H2O) to produce hydroxyl radicals (•OH) [2, 49, 50] Subsequently, hydrogen peroxide reacts with the electrons to form hydroxyl radicals, which in turn react with the MB, RhB, and CR, respectively, to produce harmless compounds such as CO2. NH3 and H2O. Both UV and visible light photons excite the electrons of nano ZnO and nano ZnS to the conduction band. Using the KM Plot, the band gap values for ZnO and ZnS nanoparticles are 3.29 and 3.38 eV, respectively. Due to the different work functions of the nano ZnO (3.29 eV) and ZnS (3.38 eV), after reaching the same Fermi level, the conduction band (CB) of ZnS–ZnO nanocomposite (3.12 eV) nanocomposite was situated above the CB of nano ZnO and ZnS. The valence band (VB) of ZnS–ZnO nanocomposite was positioned below the VB of nano ZnO and ZnS. The photoexcited electrons of nano ZnO were then transferred to nano ZnS, while the holes from the nano ZnO valence band were transferred to the valence band of nano ZnS. The major reason nano ZnO becomes less effective may be the hindered transfer of holes from reacting with water to produce hydroxyl radicals (•OH).
In addition, another possible tentative mechanism has been proposed. Because the Fermi level of ZnO is distinctly higher than that of ZnS, an internal electric field oriented from ZnO to ZnS between their interfaces and band bending is produced. Once the two materials are both excited by proper incident light, the excited electrons on the CB of ZnS will spontaneously slide into the VB of ZnO forced by the electric field and band bending and recombine with the holes in ZnO. Thus, the CB electron on ZnO with a more vital reduction ability acts as the main active species for proton generation, and the holes on the VB of ZnS act as the oxidation species. The transfer route of the charge carriers follows a slide path complying with the proposed S‐scheme heterojunction, which can effectively suppress charge recombination while retaining the strong redox abilities of the heterojunction [2, 2, 50,51,52,53,54]. This mechanism is further supported by PL spectrometry of •OH (Fig. 14). The green-synthesized ZnS–ZnO nanocomposite shows higher photocatalytic efficacy as compared to the individual green-synthesized ZnO and ZnS nanoparticles due to the agglomerated morphology, decreased band gap and size, contuses cross transfer of electrons from O to S atoms in the composite, to support the above-proposed mechanism, we also performed PL spectroscopy to confirm the formation of •OH radicals (Fig. 14). It was examined using coumarin as a probe molecule [48]. Coumarin is an insignificant fluorescent molecule, the reaction of •OH with coumarin gives rise to 7-hydroxyl coumarin (7HC) in aqueous solutions, which is fluorescence in the visible region. Dissolved oxygen plays a vital role in forming 7HC, hydroxylation of coumarin is not possible via one—e− oxidation, but it requires the intervention of •OH. In this process, 15 mg of ZnS–ZnO nanocomposite was dispersed in 100 mL of 0.5 mM aqueous coumarin solution. The solution was bubbled for 30 min before irradiation. The reaction was carried out under the visible light lamp. For 10 min interval, 2 mL solution was taken out, and PL intensity was measured using a spectrophotometer shown at 450 and 500 nm, which shows the formation of 7HC.
In addition, quantum yield was calculated using Eq. 4. In typical case, RhB in water used as a standard. The value was found to be 18.65% for ZnS–ZnO nanocomposite. Relevant graph is shown in S-4.
where Φx Quantum yield of the unknown sample (Unknown sample), Φstd Quantum yield of the Rhodamine-B (Internal standard), Gradx gradient obtained for the plot of the unknown sample, Gradstd gradient obtained for the plot of Rhodamine-B (1.09E8), η2 x refractive index of ethanol (1.3614), η2 std refractive index of the water (1.331).
From the above discussion, we conclude that ZnS–ZnO nanocomposite derived from bio mediated is a promising catalyst for photodegradation of dyes.
Antibacterial Properties of ZnO, ZnS Nanoparticles, and ZnS–ZnO Nanocomposite
The antibacterial property of green-synthesized ZnO, ZnS nanoparticles, and ZnS–ZnO nanocomposite was studied using Gram-negative bacteria, Pseudomonas aeruginosa, Klebsiella and Gram-positive bacteria, Staphylococcus aureus, and Streptococcus pneumonia. ZnS–ZnO nanocomposite has shown good antibacterial activity against these pathogenic strains. ZnS–ZnO nanocomposite showed profound activity towards Staphylococcus aureus, Streptococcus pneumonia, and Pseudomonas aeruginosa. But significantly less activity towards Klebsiella as compared to the individual nanoparticles; nanocomposite shows good activity, as shown in Fig. 15.
During the incubation time (24 h), the free radicals produced by the nanoparticles can interact with the bacterial cells causing death or inhibition of growth. ZnS–ZnO nanocomposite exhibited higher activity than ZnO and ZnS nanoparticles. As the concentration of nanoparticles increases, the antibacterial activity increases due to an increase in the number of free radicals produced. These free radicals will oxidize the cellular components of the microorganism, causing its death. Maximum antibacterial activity was observed for 600 μg/ concentration of ZnO, ZnS nanoparticles, and ZnS–ZnO nanocomposite for both types of strains; the results are tabulated in Table-2 S-3. Antibacterial activity of ZnO and ZnS was found to be less than ZnO, ZnS nanoparticles, and ZnS–ZnO nanocomposite against both types of bacterial strains under identical conditions. This is the first report for antibacterial studies using ZnS–ZnO nanocomposite.
Conclusion
In conclusion, ZnS–ZnO nanocomposite has been synthesized using musk Cucumis melo and waste pollutant chicken feathers. A green, simple, eco-friendly, feasible, and use of convenient combustion technique followed by a hydrothermal method has been developed. The size, structure, and properties of ZnS–ZnO nanocomposite were enhanced when compared with ZnO and ZnS nanoparticles prepared by the hydrothermal method. XRD, TEM, and FT-IR results explained that the ZnS–ZnO nanocomposite has formed under the present conditions. The PXRD pattern depicts a confirmed single-phase formation and crystallite size in the nano-range. Photocatalytic and antibacterial studies were conducted, and the results proved highly reliable. Elemental composition was confirmed by EDAX studies and had a deleterious effect on the properties of ZnS–ZnO nanocomposite. Further, ZnS–ZnO nanocomposite was found to be an efficient photocatalyst for the degradation of MB, RhB, and CR, dyes listed as class II health hazard dyes because of their toxicity to humans, animals, and the environment. ZnS–ZnO nanocomposite showed significant antibacterial activity toward gram-positive bacteria compared to gram-negative bacteria.
Data Availability
The data supporting these studies finding are available from the corresponding author upon reasonable request.
References
F. Davar, A. Majedi, A. Mirzaei, Green synthesis of ZnO nanoparticles and its application in the degradation of some dyes. J. Am. Ceram. Soc. 98, 1739–1746 (2015)
V.V. Gawade, N.L. Gavade, H.M. Shinde, S.B. Babar, A.N. Kadam, K.M. Garadkar, Green synthesis of ZnO nanoparticles by using Calotropis procera leaves for the photodegradation of methyl orange. J. Mater. Sci. Mater. Electron. 28, 14033–14039 (2017)
S.S.M. Hassan, W.I.M.E. Azab, H.R. Ali, M.S.M. Mansour, Green synthesis and characterization of ZnO nanoparticles for photocatalytic degradation of anthracene. Adv. Natl. Sci: Nanosci Nanotechnol 6, 045012 (2015)
H. Sadiq, F. Sher, S. Sehar, E.C. Lima, S. Zhang, H.M.N. Iqbal, F. Zafar, M. Nuhanović, Green synthesis of ZnO nanoparticles from Syzygium Cumini leaves extract with robust photocatalysis applications. J. Mol. Liq. 335, 116567 (2021)
M. Abdullah, P. John, Z. Ahmad, M.N. Ashiq, S. Manzoor, M.I. Ghori, M.U. Nisa, A.G. Abid, K.Y. Butt, S. Ahmed, Visible-light-driven ZnO/ZnS/MnO2 ternary nanocomposite catalyst: synthesis, characterization and photocatalytic degradation of methylene blue. Appl. Nanosci. (Switzerland). 11, 2361–2370 (2021)
D. Suresh, R.M. Shobharani, P.C. Nethravathi, M.A. Pavan Kumar, H. Nagabhushana, S.C. Sharma, Artocarpus gomezianus aided green synthesis of ZnO nanoparticles: luminescence, photocatalytic and antioxidant properties. Spectrochim. Acta A Mol. Biomol. Spectrosc. 141, 128–134 (2015)
C. Liu, Y. Wang, D. Meng, X. Yu, Y. Wang, J. Liu, C. Lu, K. Xu, Enhanced visible light photocatalytic performance of ZnO/ZnS/CuS ternary nanocomposites. Mater. Lett. 122, 197–200 (2014)
Y. Li, W. Zhang, J. Niu, Y. Chen, Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 6, 5164–5173 (2012)
T.S. Aldeen, H.E. Ahmed-Mohamed, M. Maaza, ZnO nanoparticles prepared via a green synthesis approach: physical properties, photocatalytic and antibacterial activity. J. Phys. Chem. Solids 160, 110313 (2022)
M.Y. Guo, A.M.C. Ng, F. Liu, A.B. Djuriŝić, W.K. Chan, H. Su, K.S. Wong, Effect of native defects on photocatalytic properties of ZnO. J. Phys. Chem. C 115, 11095–11101 (2011)
A. El-Golli, M. Fendrich, N. Bazzanella, C. Dridi, A. Miotello, M. Orlandi, Wastewater remediation with ZnO photocatalysts: green synthesis and solar concentration as an economically and environmentally viable route to application. J. Environ. Manag. 286, 112226 (2021)
R.D.C. Soltani, G. Shams Khoramabadi, H. Godini, Z. Noorimotlagh, The application of ZnO/SiO2 nanocomposite for the photocatalytic degradation of a textile dye in aqueous solutions in comparison with pure ZnO nanoparticles. Desalin. Water Treat. 56(9), 2551–2558 (2015)
S. Reais, R. Mohammadi, Z. Khammar, G. Paimard, S. Abdalbeygi, Z. Sarlak, M. Rouhi, Photocatalytic detoxification of aflatoxin B1 in an aqueous solution and soymilk using nano metal oxides under UV light: KINETIC and isotherm models. Lwt 154, 112638 (2022)
P. Pascariu, I.V. Tudose, M. Suchea, E. Koudoumas, N. Fifere, A. Airinei, Preparation and characterization of Ni, Co doped ZnO nanoparticles for photocatalytic applications. Appl. Surf. Sci. 448, 481–488 (2018)
M. Anpo, K. Chiba, M. Tomonari, S. Coluccia, M. Che, M.A. Fox, Photocatalysis on native and platinum-loaded TiO2 and ZnO catalysts—origin of different reactivities on wet and dry metal oxides. Bull. Chem. Soc. Jpn 64(2), 543–551 (1991)
J. Villaseñor, P. Reyes, G. Pecchi, Photodegradation of pentachlorophenol on ZnO. J. Chem. Technol. Biotechnol. 72(2), 105–110 (1998)
P.V. Kamat, R. Huehn, R. Nicolaescu, A “sense and shoot” approach for photocatalytic degradation of organic contaminants in water. J. Phys. Chem. B 106(4), 788–794 (2002)
P.V. Raleaooa, A. Roodt, G.G. Mhlongo, D.E. Motaung, R.E. Kroon, O.M. Ntwaeaborwa, Luminescent, magnetic and optical properties of ZnO–ZnS nanocomposites. Phys. B Condense Matter. 507, 13–20 (2017)
G.K. Weldegebrieal, Synthesis method, the antibacterial and photocatalytic activity of ZnO nanoparticles for azo dyes in wastewater treatment: a review. Inorg. Chem. Commun. 120, 108140 (2020)
N. Pauzi, N.M. Zain, N.A.A. Yusof, Gum arabic as a natural stabilizing agent in green synthesis of ZnO nanofluids for antibacterial application. J. Environ. Chem. Eng. 8, 103331 (2020)
M. Shabaani, S. Rahaiee, M. Zare, S.M. Jafari, Green synthesis of ZnO nanoparticles using loquat seed extract; biological functions and photocatalytic degradation properties. LWT. 134, 110133 (2020)
X. Jin, J. Chen, F. Chen, H. Duan, Z. Wang, J. Li, Solid-state synthesis of ZnO/ZnS photocatalyst with efficient organic pollutant degradation performance. Catalysts 12(9), 981 (2022)
W. Liu, T. He, Y. Wang, Ge. Ning, Xu. Zhenggang, X. Chen, Hu. Xinjiang, Wu. Yaohui, Y. Zhao, Synergistic adsorption-photocatalytic degradation effect and norfloxacin mechanism of ZnO/ZnS@ BC under UV-light irradiation. Sci. Rep. 10(1), 11903 (2020)
W. Xitao, Lv. Rong, W. Kang, Synthesis of ZnO@ ZnS–Bi2 S3 core–shell nanorod grown on reduced graphene oxide sheets and its enhanced photocatalytic performance. J. Mater. Chem. A 2(22), 8304–8313 (2014)
H. Chemingui, T. Missaoui, J.C. Mzali, T. Yildiz, M. Konyar, M. Smiri, N. Saidi, A. Hafiane, H.C. Yatmaz, Facile green synthesis of zinc oxide nanoparticles (ZnO NPs): antibacterial and photocatalytic activities. Mater. Res. Express. (2019). https://doi.org/10.1088/2053-1591/ab3cd6
T.U. Doan-Thi, T.T. Nguyen, Y.D. Thi, K.H. Ta-Thi, B.T. Phan, K.N. Pham, Green synthesis of ZnO nanoparticles using orange fruit peel extract for antibacterial activities. RSC Adv. 10, 23899–23907 (2020). https://doi.org/10.1039/d0ra04926c
S. Saif, A. Tahir, T. Asim, Y. Chen, M. Khan, S.F. Adil, Green synthesis of ZnO hierarchical microstructures by Cordia myxa and their antibacterial activity. Saudi J. Biol. Sci. 26, 1364–1371 (2019). https://doi.org/10.1016/j.sjbs.2019.01.004
M. Ramesh, M. Anbuvannan, G. Viruthagiri, Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 136, 864–870 (2015). https://doi.org/10.1016/j.saa.2014.09.105
F.T. Thema, E. Manikandan, M.S. Dhlamini, M. Maaza, Green synthesis of ZnO nanoparticles via Agathosma betulina natural extract. Mater. Lett. 161, 124–127 (2015)
Z. Ahsani-Namin, R. Norouzbeigi, H. Shayesteh, Green mediated combustion synthesis of copper zinc oxide using Eryngium planum leaf extract as a natural green fuel: excellent adsorption capacity towards Congo red dye. Ceram. Int. 48(14), 20961–20973 (2022)
S. Isarankura-Na-Ayutthaya, S. Tanpichai, J. Wootthikanokkhan, Keratin extracted from chicken feather waste: extraction preparation, and structural characterization of the keratin and keratin/biopolymer films and electrospuns. J. Polym. Environ. 23, 506–516 (2015). https://doi.org/10.1007/s10924-015-0725-8
V. Muthukumaraswamy-Rangaraj, A. Achazhiyath-Edathil, P. Kadirvelayutham, F. Banat, Chicken feathers as an intrinsic source to develop ZnS/carbon composite for Li-ion battery anode material. Mater. Chem. Phys. 248, 122953 (2020)
C. Mallikarjunaswamy, V. Lakshmi-Ranganatha, R. Ramu, Udayabhanu, G. Nagaraju, Facile microwave-assisted green synthesis of ZnO nanoparticles: application to photodegradation, antibacterial and antioxidant. J. Mater. Sci. Mater. Electron. 31, 1004–1021 (2020). https://doi.org/10.1007/s10854-019-02612-2
D. Jiang, J. Xue, L. Wu, W. Zhou, Y. Zhang, X. Li, Photocatalytic performance enhancement of CuO/Cu2O heterostructures for photodegradation of organic dyes: Effects of CuO morphology. Appl. Catal. B 211, 199–204 (2017). https://doi.org/10.1016/j.apcatb.2017.04.034
J.H. Li, R.Y. Hong, M.Y. Li, H.Z. Li, Y. Zheng, J. Ding, Effects of ZnO nanoparticles on the mechanical and antibacterial properties of polyurethane coatings. Prog. Org. Coat. 64, 504–509 (2009). https://doi.org/10.1016/j.porgcoat.2008.08.013
M. Sundararajan, P. Sakthivel, A.C. Fernandez, Structural, optical and electrical properties of ZnO-ZnS nanocomposites prepared by simple hydrothermal method. J. Alloys Compd. 768, 553–562 (2018)
R.K. Tukhtaev et al., Production of powder electroluminophors based on zinc sulfide under combustion conditions, in Doklady physical chemistry, vol. 395, (Kluwer Academic Publishers-Plenum Publishers, 2004)
G. Murugadoss, V. Ramasamy, Structural and optical study of mixed structure of ZnO(hexagonal)/ ZnS(cubic) nanocomposites. Spectrochim. Acta A Mol. Biomol. Spectrosc. 93, 290–294 (2012)
S. Munyai, L.M. Mahlaule-Glory, N.C. Hintsho-Mbita, Green synthesis of Zinc sulphide (ZnS) nanostructures using S. frutescences plant extract for photocatalytic degradation of dyes and antibiotics. Mater. Res. Express. 9, 015001 (2022)
M. Schmitt, C. Dietlin, J. Lalevée, Towards visible LED illumination: ZnO–ZnS nanocomposite particles. ChemistrySelect 5, 985–987 (2020). https://doi.org/10.1002/slct.201904699
A.R. Harikrishnan, P. Dhar, P.K. Agnihotri, S. Gedupudi, S.K. Das, Effects of interplay of nanoparticles, surfactants and base fluid on the surface tension of nanocolloids. Eur. Phys. J. E 40, 1–14 (2017)
N.T.K. Thanh, N. Maclean, S. Mahiddine, Mechanisms of nucleation and growth of nanoparticles in solution. Chem. Rev. 114(15), 7610–7630 (2014)
A.B. Murphy, Band-gap determination from diffuse reflectance measurements of semiconductor films, and application to photoelectrochemical water-splitting. Sol. Energy Mater. Sol. Cells 91, 1326–1337 (2007). https://doi.org/10.1016/j.solmat.2007.05.005
S.S. Kumar, P. Venkateswarlu, V.R. Rao, G.N. Rao, Synthesis, characterization and optical properties of zinc oxide nanoparticles. Int. Nano Lett. 3, 1–6 (2013)
P.V. Raleaooa, A. Roodt, G.G. Mhlongo, D.E. Motaung, R.E. Kroon, O.M. Ntwaeaborwa, Luminescent, magnetic and optical properties of ZnO-ZnS nanocomposites. Physica B 507, 13–20 (2017)
A. Sadollahkhani, I. Kazeminezhad, J. Lu, O. Nur, L. Hultman, M. Willander, Synthesis, structural characterization and photocatalytic application of ZnO@ ZnS core shell nanoparticles. RSC Adv. 4, 36940 (2014)
P.S. Nandisha, A. Sowbhagya, Bio-mediated synthesis of CuO nano bundles from Gomutra (cow urine): synthesis, characterization, photodegradation of the malachite green dye and SBH mediated reduction of 4-nitrophenol. Mater. Sci. Eng. B 295, 116607 (2023)
P.S. Nandisha, Sowbhagya, S. Yallappa, Synthesis and characterization of ternary NiO@Bi2MoO6–MoS heterojunction with enhanced photodegradation efficiency towards indigo carmine dye. Solid State Sci. 139, 107157 (2023)
S. Ali, S. Saleem, M. Salman, M. Khan, Synthesis, structural and optical properties of ZnS–ZnO nanocomposites. Mater. Chem. Phys. (2020). https://doi.org/10.1016/j.matchemphys.2020.122900
A.M. Mohammed, S.S. Mohtar, F. Aziz, M. Aziz, A. Ul-Hamid, Cu2O/ZnO-PANI ternary nanocomposite as an efficient photocatalyst for the photodegradation of Congo Red dye. J. Environ. Chem. Eng. (2021). https://doi.org/10.1016/j.jece.2021.105065
W. Shi, N. Chopra, Controlled fabrication of photoactive copper oxide-cobalt oxide nanowire heterostructures for efficient phenol photodegradation. ACS Appl. Mater. Interfaces 4, 5590–5607 (2012). https://doi.org/10.1021/am301488c
D. Ayodhya, G. Veerabhadram, A review on recent advances in photodegradation of dyes using doped and heterojunction-based semiconductor metal sulfide nanostructures for environmental protection. Mater Today Energy 9, 83–113 (2018). https://doi.org/10.1016/j.mtener.2018.05.007
S. Chakraborty, J.J. Farida, R. Simon, S. Kasthuri, N.L. Mary, Averrhoe carrambola fruit extract assisted green synthesis of ZnO nanoparticles for the photodegradation of congo red dye. Surfaces Interfaces. (2020). https://doi.org/10.1016/j.surfin.2020.100488
A.Q. Malik, T. Ul, G. Mir, O. Amin, M. Sathish, D. Kumar, Synthesis, characterization, photocatalytic effect of CuS–ZnO nanocomposite on photodegradation of Congo red and phenol pollutant. Inorg. Chem. Commun. 143, 109797 (2022). https://doi.org/10.1016/J.INOCHE.2022.109797
M. Abdullah, P. John, Z. Ahmad, M.N. Ashiq, S. Manzoor, M.I. Ghori, M.U. Nisa, A.G. Abid, K.Y. Butt, S. Ahmed, Visible-light-driven ZnO/ZnS/MnO2 ternary nanocomposite catalyst: synthesis, characterization and photocatalytic degradation of methylene blue. Appl. Nanosci. 11, 2361–2370 (2021)
S. Munyai, L.M. Mahlaule-Glory, N.C. Hintsho-Mbita, Green synthesis of Zinc sulphide (ZnS) nanostructures using S. frutescences plant extract for photocatalytic degradation of dyes and antibiotics. Mater. Res. Express 9, 015001 (2022)
Acknowledgements
The authors are thankful to G. Shanker, professor, Gajendra babu, Vinay. G, Eti Chetan, research scholars, Department of Chemistry, Bangalore University, Bengaluru, INDIA, for continuous support to our research work.
Author information
Authors and Affiliations
Contributions
PSN work design, original draft writing, experimental work, and formal analysis. S work on design, editing, and revision of the draft. BU spectral analysis, editing, and revision of the draft. MH formal analysis and spectral analysis, and revision of the draft. KLN formal analysis, editing, revision of the draft.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest.
Ethical Approval
This article does not contain any studies involving animals performed by any authors. Also, this article has no studies involving human participants functioned by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nandisha, P.S., Sowbhagya, Pasha, M.A. et al. Bio-mediated Synthesis of ZnS–ZnO Nanocomposite from Cucumis melo Pulp and Chicken Feathers: Photodegradation of Dyes and Antibacterial Activities. Korean J. Chem. Eng. 41, 515–531 (2024). https://doi.org/10.1007/s11814-024-00058-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-024-00058-9