Abstract
Light-mediated healthcare, including monitoring and therapy techniques, has evolved significantly over the past decades, owing to their advantages in minimal invasiveness and relatively low side effects. However, most of the clinically available light sources have suffered from their bulky size and rigidity, making them impractical for continuous on-body-type health monitoring and treatment applications. Recently, wearable and implantable healthcare systems using deformable light emitting diodes (LEDs) have been developed, which can be seamlessly integrated to any part of the body and exhibit the potential to improve efficacy of personalized and mobile photo-medicine. In this review, we discuss recent advances in wearable and implantable LED technologies for various biomedical applications. First, we present an overview of the state-of-the-art high-performance wearable and implantable LEDs, especially focusing on their light-emitting materials and unique device design approaches. We also discuss strategies for integrated light-based healthcare systems. Then, we discuss prominent examples of light-medicated sensing and therapeutic applications, whose accuracy and efficacy can be enhanced by wearable and implantable LEDs, ranging from non-invasive oxygen level sensing to various phototherapies. Finally, we conclude this review with a brief outlook on the future technologies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past decades, the light-based biomedicine has brought tremendous impact on modern healthcare due to its precision in biosensing and treatment, minimally invasive administration, and relatively low side-effects [1, 2]. Light sources with a narrow full-width half maximum (FWHM), such as light-emitting diode (LEDs) or laser, have been widely utilized for their high accuracy and selectivity of the treatment [3]. In particular, LED-based photomedicine offers distinct advantages, including the ability to irradiate large areas and enhanced safety due to reduced tissue damage, primarily attributed to the lower light intensity compared to laser-based treatments [4, 5]. The examples of the LED-based healthcare encompass various types of biosensing and therapeutic approaches (Fig. 1a).
a Representative applications of LED-based biosignal sensing and therapeutic applications, including PPG/pulse oximetry, photodynamic therapy, photobiomodulation, and optogenetics. The range of wavelength and required power intensity for each application is provided. b Schematic illustration of wearable and implantable LEDs for light-mediated healthcare system
For biosensing applications, biometric information about the targeted tissue can be collected by detecting reflected, scattered, or transmitted photons using photodetectors. Pulse oximetry is a representative example of light-based biosensing, which measures the blood oxygen saturation level using different light absorption characteristics between oxygenated and deoxygenated haemoglobins [6]. Pulse oximetry data can be further integrated with other biosensor data, including electrophysiological signals, temperature, and blood pressure, for comprehensive health monitoring [7].
For phototherapies, light can be used to trigger chemical/thermal reactions and/or ionic changes in the targeted tissues. LED-based phototherapies can be largely categorized into three types: photodynamic therapy (PDT) [8, 9], photobiomodulation (PBM) [10, 11], and optogenetic modulations [12, 13]. In PDT, light activates photosensitizers to generate reactive oxygen species (ROS) to destroy the unwanted tissues nearby the targeted region through oxidation of cell membranes [14]. PBM therapy uses light to stimulate mitochondrial chromophores [15], producing biochemical molecules (e.g., adenosine triphosphate (ATP), nitric oxide (NO), and ROS) and potentially used for pain reduction [16], facilitated wound healing [17], and tissue regeneration [18]. Optogenetic modulations are based on the light-mediated activation of photosensitive ion channels, pumps, or enzymes to control (stimulate and suppress) the activities of neurons or other tissues [19].
One of key considerations in designing such light-based healthcare devices is the possibility that a substantial amount of irradiated photons can be lost before reaching the targeted region. The loss of photons mainly occurs due to reflection, absorption, and scattering by the skin and surrounding tissues, which are predominantly composed of water and various organic compounds. The degree of light attenuation highly depends on the wavelength of incident light (light beams in Fig. 1b) [20, 21]. Ultraviolet (UV) light with a wavelength around 400 nm has a shallow penetration depth less than 0.5 mm, as majority of photons are scattered by the tissues. Thus, UV light cannot penetrate deep beyond the epidermis [22]. However, near-infrared (NIR) light with a wavelength around 700 nm can penetrate more than ~ 3 mm, reaching tissues beyond the dermis, although the NIR photons has lower energy (i.e., more difficult to activate target molecules) than UV photons [23]. When the light wavelength exceeds 1000 nm, the light penetration depth diminishes again as water absorbs a significant portion of photons in this range [24]. Hence, the selection of light source with appropriate wavelength considering the depth of the targeted tissues is a major device design factor.
Another key design consideration is the device form factor. Conventional LED devices consist of bulky and rigid LED arrays combined with optical guiding/focusing systems. The considerable size, weight, and rigidity of the conventional devices heighten physical burden and discomfort on the patients, especially under daily mobile environments [25]. Besides, phototherapies that rely on the irradiation from external light sources may not be practical for addressing deeply located lesions within the body due to the aforementioned light attenuation issues. To overcome these limitations, research efforts have been put on the development of unconventional LEDs, such as deformable LEDs that can be utilized for wearable and implantable healthcare systems and thus be seamlessly integrated to any organs on/in the body (Fig. 1b) [26,27,28]. These devices are expected to bring unparalleled advances in photo-medicine, offering enhanced light delivery efficiency to target tissues over a broad range of wavelengths and facilitating point-of-care-type long-term treatment potential.
Recent advancements in material engineering technologies have enabled the development of high-performance LEDs with deformable form factors (e.g., flexible [29, 30], rollable [31], foldable [32], and stretchable light-emitting devices [33]), strongly driven by the demands for deformable displays [34, 35]. Thanks to these technological advances, their biomedical applications have been also promoted. However, to utilize them as reliable light sources for healthcare applications, several issues need to be addressed [36]. For example, better performance, regarding narrower emission spectra and higher light intensity, is needed. To avoid unintended thermal damage to the tissues nearby the installed LEDs, it is important to minimize the amount of heat radiated from the device. The sustainable luminous performance without severe performance degradation even under repetitive mechanical deformations is also required to meet high standard for the medical device reliability. The development of fully biocompatible LEDs is also necessary to minimize potential biological side effects. Integration of auxiliary components (e.g., optical waveguides) is also needed to deliver sufficient amounts of light to deep target tissues [37]. Upconversion nanoparticles (UCNPs) can be considered to convert penetrative NIR light into high-energy visible or UV lights [38]. Wireless power transfer can be used to deliver power to deeply implanted light-emitting devices [39].
In this article, we review the latest research progress for developing deformable, wearable, and implantable LEDs, considering the material selection, device designs, and system integration toward highly efficient light-mediated healthcare systems. Three competitive LED technologies, including inorganic micro-LEDs (μ-LEDs), organic LEDs (OLEDs), and heavy-metal-free quantum dot LEDs (QLEDs), are compared in terms of their device structures, luminous performance, as well as their strengths and current challenges. Furthermore, we present a number of design approaches to achieve high mechanical stability of devices, enabling consistent illumination even when continuously deformed and conformally adhered to human skin or organs. System integration with auxiliary components, such as optical waveguides or UCNPs for efficient light delivery, as well as antennas for wireless power transmission, are then presented. We also review the representative applications for light-mediated biosensing and various phototherapies, whose performance can be facilitated by wearable and implantable LEDs. Finally, we conclude this review by presenting a brief summary and a future outlook.
Materials and Device Designs for Wearable and Implantable LEDs
Competing LED Technologies
Compared to conventional LEDs for indoor/outdoor lighting or information displays [40], wearable and implantable LEDs for biomedical applications require not only the higher level of luminous performance and stability, but also involve further challenges with regards to long-term compatibility with soft human tissues. For instance, although significant research progress has recently been achieved in the field of perovskite LEDs [41,42,43], the presence of lead (Pb) in the perovskite emitter yet remains a significant hurdle to the biomedical application of perovskite LEDs. Considering these issues, only the selected types of LEDs, including μ-LEDs, OLEDs, and QLEDs based on heavy-metal-free quantum dots (QDs), are considered as potential light-emitting devices for biomedical applications. At the current stage where multiple types of LEDs are being simultaneously developed, it would be meaningful to review the recent progress of each technology in this section. In Table 1, we summarize three competing LED technologies, with regard to their device structures, light emitters and current performance for each emission wavelength (i.e., NIR to UV light), as well as their inherent advantages and challenges.
-
(i)
Inorganic micro-LEDs
Since their initial invention by N. Holonyak in 1962, inorganic LEDs have been the most commonly employed lighting devices due to their exceptional luminous performance and durability. In the early stages of development, inorganic LEDs had their dimensions of 1 mm or more. Driven by the dramatic advancement in fabrication technology, chip sizes have continuously shrunk to microscale with their dimensions of 100 µm or even less. The typical device structure of μ-LED is based on the p–n junctions of epitaxial semiconductor, utilizing the recombination of injected charge carriers at the junction interface. GaN is the most widely used III–V compound semiconductors for μ-LEDs, based on its high epitaxial growth quality, high electron mobility (~ 103 cm2 V−1 s−1), and direct bandgap (~ 3.4 eV) corresponding to UV/blue light emission. In this regard, GaN can be utilized as the emissive material for blue-emitting μ-LEDs, and also as a layer to facilitate efficient carrier transport in μ-LEDs of various colors [44, 45]. Emissive materials for other color emission are also based on III–V compounds, including AlGaAs (NIR) [46], AlGaInP (red) [47], InGaN (green) [48], and AlGaN (UV) [49]. Using these materials, μ-LEDs generally employ multiple quantum well structures between p-doped GaN and n-doped GaN for their active layers, to improve the device efficiency by facilitating the radiative recombination of confined excitons [50].
All inner components of μ-LEDs are comprised of inorganic materials, resulting in remarkable operational stability (device lifetime > 100,000 h) and robustness against oxygen and humidity. Given that wearable and implantable LEDs are consistently exposed to humid environment, the exceptional stability of μ-LEDs establishes themselves as one of the most promising options for biomedical light sources [51]. Moreover, high brightness (~ 1,000,000 nits) and efficiency (external quantum efficiency (EQE) > 20% for NIR, red, green, and blue light) of μ-LEDs render them practical for a wide range of phototherapeutic approaches, which mostly require high irradiation intensity to activate light-sensitive materials and/or tissues.
Despite these advantages, challenges still persist for μ-LEDs, primarily arising from their small dimension. For example, a single μ-LED device, which is a point light source with a narrow emission angle, faces challenges in delivering light on wide area of tissues. In this regard, there is a strong demand for an array-type device in which the multiple μ-LED pixels are densely packed together, to deliver light in large area [52, 53]. However, the expensive chip transfer process remains as a major bottleneck for the fabrication of μ-LED arrays, resulting in a significant escalation in fabrication costs as the number of chips within an array increases [54, 55]. In addition, the reduction of device efficiency as chip size decreases presents a critical issue in fabricating high-resolution μ-LED arrays [56]. Scaling down of chip size leads to a greater involvement of carriers in non-radiative recombination dominated by surface defects, causing a significant decrease in EQE [57]. To deal with the issue, approaches based on the sidewall passivation of active materials have been reported by the number of previous studies [58].
-
(ii)
Organic LEDs
Since the first practical OLED device was developed by researchers in Kodak in 1987, OLEDs have emerged as major light-emitting devices especially used for information display applications [59]. OLED has a layered structure of organic functional materials between its anode and cathode, including a hole injection layer (HIL), a hole transport layer (HTL), a light-emitting layer (EML), and an electron transport layer (ETL). Holes are transported from an anode to EML via HIL and HTL, which are both the p-type organic semiconductors, while electrons are transported from a cathode to EML via ETL, which is the n-type organic semiconductor. Holes and electrons injected to EML recombine and generate excitons. In this process, four eigenstates are formed due to the spin statistics, resulting in 25% probability of singlet excitons and 75% probability of triplet excitons. OLEDs can emit wide range of wavelength from NIR to UV lights [60,61,62,63,64,65,66].
The first generation of organic emitters are fluorescence emitters, which utilize the light emission only from singlet excitons, while remaining 75% of triplet excitons do not participate in radiative recombination [67]. Such inefficient use of excitons in fluorescence emitters results in poor internal quantum efficiencies (IQEs) of less than 25%. From early 1990s, highly efficient phosphorescence emitters based on iridium (Ir)- or platinum (Pt)-based organometallic complexes have been developed as the second generation organic emitters [68, 69]. The phosphorescence emitters can utilize the radiative decay of triplet excitons as well as that of singlet excitons, thus exhibiting much higher IQEs. Recently, thermally activated delayed fluorescence (TADF) emitters, and hyperfluorescence emitters have been developed as the third and the fourth generation organic emitters [70, 71], respectively. These emitters have exhibited higher luminous efficiency than conventional emitters, but the stability of these materials are insufficient for being commercialized.
When it comes to biomedical light sources, OLEDs can function as area light sources capable of distributing light over a wide region of human tissues. The high luminous efficiency of OLEDs leads to low power consumption for device operation, minimizing the risk of tissue damage caused by heat radiation. Moreover, the slim device design of OLED enables the fabrication of flexible light sources, allowing conformal integration on human tissues even under dynamic movement. However, as OLEDs generally require a high level of barrier properties (water vapour transmission rate (WVTR) < 10–5–7 g/m2/day) for their stable operation [72], operational reliability issues are raised for flexible OLEDs especially for their chronic use in vivo. Considering that flexible OLEDs for biomedical light sources should rely on soft barriers with biocompatible materials [33], instead of rigid encapsulation glasses, it is further challenging to secure sufficient barrier properties. Furthermore, the broad FWHM of conventional organic emitters (except for hyperfluorescence emitters) may lead to the decreased accuracy and selectivity of the treatment as well as the inadequate activation of light-sensitive materials.
-
(iii)
Quantum dot LEDs based on heavy-metal-free QDs
QLED, which utilizes the electroluminescence (EL) of colloidal QDs via carrier injection and recombination process, was first reported in 1994 by Alivisatos group [73]. QDs are colloidal semiconducting nanocrystals with their sizes around 10 nm. Based on the quantum confinement effect, the emission wavelength can be easily tuned by adjusting the size of nanoparticles. QLED has a similar device structure to OLED, comprised of a closely packed QD films for its EML, as well as hole/electron transport layers for efficient charge carrier injection to QDs [74,75,76]. While most high-performance OLEDs are composed of all-organic materials, QLEDs generally contain inorganic n-type materials for their ETLs, such as ZnO or ZnMgO nanoparticle films [77, 78], owing to their facile solution-processed synthesis, decent electron mobility (~ 10–3 cm2 V−1 s−1), and wide bandgap (~ 3.4 eV) capable of suppressing hole leakage.
The initial development of QLEDs was mainly based on CdSe QDs, but the contents of heavy metal (Cd) in QDs had brought environmental issues based on its toxicity, significantly limiting their potential applications. Since early 2010s, QLEDs based on heavy-metal-free QDs have been intensively studied as environmentally friendly alternatives [79, 80]. Based on the elaborate QD synthesis and profound understanding of charge/energy transport mechanism during device operation, high device efficiency of QLED with heavy-metal-free QDs was reported recently, exhibiting high EQEs for red- (InP QDs, 21.4%), green- (InP QDs, 17.6%), and blue- (ZnTeSe QDs, 20.2%) emitting QLEDs [81,82,83]. NIR- and UV-emitting QLEDs based on heavy-metal-free QDs were also reported, but exhibiting poor EQEs less than 10% [84, 85].
QLED shares most of the structural advantages of OLEDs for biomedical applications, such as efficient light delivery on wide area and mechanical flexibility, as it has a OLED-like device structure. Besides, owing to other inherent characteristics of QLEDs, including the ease of color tuning, narrow emission spectra (FWHM < 25 nm), and high brightness (> 300,000 nits), QLEDs are considered as promising technology that can greatly enhance the efficacy of versatile light-mediated healthcare applications [86, 87]. For example, Triana et al. reported the development of flexible, large-area QLEDs with high luminance performance and mechanical flexibility, highlighting their potential for applications in photomedicine [88]. The CdSe-based flexible QLEDs, weighing merely 1.4 g, featured an emissive pixel as large as 8 mm2. The device demonstrated a luminance of 42,214 cd/m2 at 5.8 V, which is equivalent to 19.6 mW/cm2. Given that most LED-based phototherapy requires irradiance levels between 10 and 30 mW/cm2, the luminous performance of the reported device substantiates its suitability as an ideal light source for photomedicine.
Nevertheless, the limited stability of flexible QLEDs, particularly in heavy-metal-free devices, remains a significant challenge. Still, the lifetime record for red-emitting InP-based QLEDs indicates a T90 lifetime of only 21 h, beginning with an initial brightness of 10,050 cd/m2 (roughly equivalent to 5 mW/cm2) [81]. It's noteworthy that to achieve a higher irradiance more than 5 mW/cm2, the device lifetime would be further compromised by accelerated degradation of device. Moreover, considering the limited protection capabilities of flexible encapsulants and the fatigue induced in functional layers by strain, the stability of flexible QLEDs is likely to be diminished in comparison to rigid QLEDs. For these reasons, only a few studies have reported the utilization of wearable and implantable heavy-metal-free QLEDs in phototherapy. To serve as a practical light source, it is crucial to implement further technological advancements in material processing and device fabrication.
Design Strategies for Wearable and Implantable LEDs
To achieve high-performance LEDs with longevity, materials with exceptional electronic and optoelectronic properties are indispensable for their internal components, including electrodes and active layers. Such materials are generally deposited onto rigid glasses or wafers via vacuum evaporation process, to achieve a highly uniform and crystalline structure facilitating carrier transport. However, most vacuum-deposited electronic materials have inherent rigidity and brittleness, often characterized by high Young's modulus (> 10 GPa). For example, indium tin oxide (ITO) has been the most widely used transparent electrode for conventional LEDs due to its high conductivity (~ 104 S/cm) as well as high transparency (> 80% for visible wavelength) [89]. However, the mechanical characteristics of ITO thin film is highly rigid and brittle, featuring high Young's modulus (~ 100 GPa), and low crack-on-set strain (< 1%) [90]. Hence, additional strategies are required to address the mechanical mismatch between the rigid electronic materials and soft human tissues [91,92,93], to utilize high-performance electronic materials for implementing high-performance wearable and implantable LEDs [94, 95]. In this section, we review the representative design strategies for wearable and implantable LEDs, especially focusing on the strain management methods for each design.
-
(i)
Stretchable μ-LED arrays with island-bridge structure
Large-area μ-LED array with high pixel resolution is expected to be one of the most promising biomedical light sources, as it enables not only uniform light delivery to wide regions of human tissues, but also provides the precise targeting of specific regions down to the micro-scale. Based on the development of a scalable and facile chip transfer process, high-resolution μ-LED arrays of more than 104 ppi of pixel density have been recently reported [96]. However, conventional high-resolution μ-LED arrays may not be applicable for wearable and implantable LEDs, as μ-LED chips are transfer printed on rigid substrates. Rigid light sources cannot adhere seamlessly to the curvilinear surface of skin or tissues, resulting in unexpected positional fluctuations and uneven illuminance with reduced light-delivery efficiency. Moreover, in the case of implantable devices for in vivo treatment, the rigidity of the device may result in side effects such as tissue damage or inflammation.
To deal with the issue, there have been extensive research efforts to fabricate μ-LED arrays which utilize island-bridge structures with stretchable interconnections. The exploitation of serpentine-shaped interconnection has been the most fundamental design approach for imparting stretchability on μ-LED arrays [97, 98]. As demonstrated in the left image of Fig. 2a, the fabrication of stretchable μ-LED array composed of 6 × 6 μ-LEDs was reported by Kim et al. The μ-LEDs based on AlGaInP exhibited deep red emission (~ 670 nm), and had a dimension of 100 × 100 × 2.5 μm. Each pixel was connected with serpentine-shaped, micro-ribbons of metal thin films, which serve as both electrical interconnections and structural bridges. The device was then transfer printed onto a pre-strained soft substrate to induce the 3D buckling of serpentine-shaped interconnections after the release of pre-strain. The 3D buckled structure of serpentine-shaped interconnections can effectively reduce the strain applied on the pixels during the stretching of the array. In this regard, the devices were able to endure 48% of uniaxial stretching without failure. After being sealed with soft poly(dimethylsiloxane) (PDMS) substrates (~ 400 μm), the μ-LED array was readily applied as subdermal light sources for illumination of tissues in vivo (Fig. 2a, right).
Fig. 2 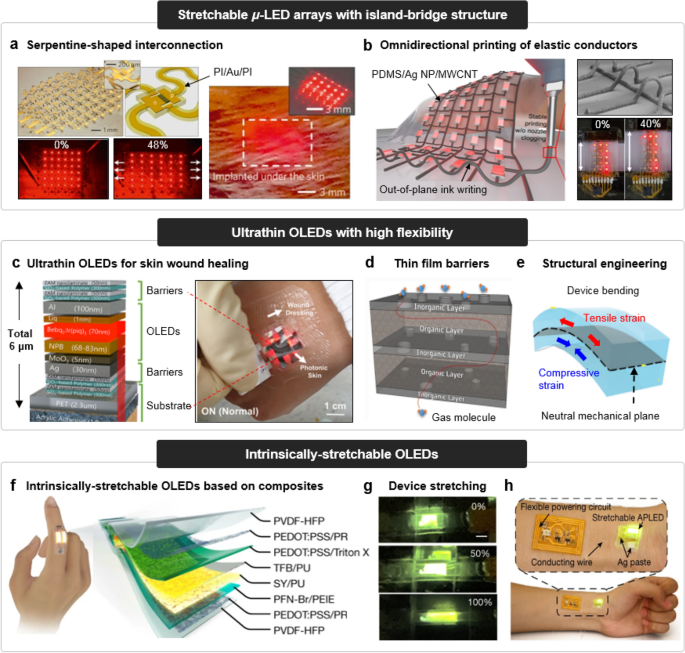
Reproduced with permission from Ref. [97], Copyright 2010, Springer Nature. b Stretchable μ-LED array with a omnidirectionally printed conductive ink for its interconnection (left), SEM image of out-of-plane-printed conductive ink (upper right), and stretching of device (lower right). Reproduced with permission from Ref. [106], Copyright 2023, Springer Nature. (ii) Ultrathin OLEDs with high flexibility. c Device structure of ultrathin OLED (left), and its application to wearable patch for skin wound healing. Reproduced with permission from Ref. [108], Copyright 2020, Wiley–VCH GmbH. d Inorganic–organic hybrid thin film barrier. Reproduced with permission from Ref. [xx], Copyright 2018, Springer Nature. e Structural engineering for efficient strain management, based on neutral mechanical plane. Reproduced with permission from Ref. [111], Copyright 2023, Wiley–VCH GmbH. (iii) Intrinsically stretchable OLEDs. f Device structure of intrinsically stretchable OLED. g Optical images of device stretching. h Application to wearable light sources for real-time heartbeat display. Reproduced with permission from Ref. [119], Copyright 2022, Springer Nature
Device design approaches for wearable and implantable LEDs with high deformability. (i) Stretchable μ-LED arrays with island-bridge structure. a Stretchable μ-LED array with a serpentine-shaped interconnection (left), and its application to sub-dermal light source (right).
The use of geometrically designed interconnection, however, resulted in relatively lower pixel density, compared to conventional μ-LED arrays with straight interconnections. To overcome these issues, research has focused on inherently stretchable interconnections with linear designs. Various conducting materials, including liquid metals (e.g., eutectic gallium indium (E-GaIn) [99]), conductive nanocomposites [100], and micro-cracked metal films [101, 102], have been employed in this regard. Chung et al. reported the stretchable μ-LED array, based on the omnidirectional printing of conductive ink for its stretchable interconnection (Fig. 2b) [103]. The conductive ink consists of an emulsified elastic nanocomposite with immiscible, non-volatile solvents (chloroform and diethylene glycol) and conductive fillers [Ag nanoparticles (Ag NPs) and multi-walled carbon nanotubes (MWCNTs)] embedded in PDMS. While the conventional system of printed elastic conductors only allows the layer-wise deposition of inks, the unique rheological properties of the conductive ink enabled the three-dimensional omnidirectional printing. Based on this technique, the stretchable μ-LED arrays with 40% strain endurance were fabricated.
-
(ii)
Ultra-flexible OLEDs
High-performance OLED can function as a practical area light source for wide range of light-mediated healthcare applications, enabling uniform illumination across the broad region of human tissues. In addition, OLEDs can be fabricated on various flexible polymeric substrates, such as polyethylene terephthalate (PET) or polyethylene naphthalate (PEN) thin films [104]. In this regards, flexible OLEDs have been intensively studied for various biomedical applications [105]. Jeon et al. reported the wearable photonic skin based on ultrathin flexible OLEDs (Fig. 2c) [106]. The photonic skin consists of a red-emitting OLED, fabricated on a 2.3-μm-thick flexible PET substrate. The total thickness of the photonic skin, including the PET substrate, active layers of OLED, and top/bottom encapsulation barriers, was 6 µm, exhibiting outstanding mechanical deformability. The flexible OLED recorded comparable luminous performance with its rigid counterpart, as well as had a long device lifetime of more than 100 h until its initial irradiance (5 mW/cm2) decreased to 90% under ambient conditions. Such advantageous features of flexible OLEDs for biomedical approaches demonstrated their potentials through 70% enhancement in epidermal regeneration after skin wound healing.
Although the number of recent studies have shown the proof-of-concept of flexible OLEDs in light-mediated healthcare, the insufficient long-term reliability of flexible OLEDs and QLEDs under humid environments needs to be further addressed. Compared to the conventional encapsulation materials, such as a glass sheet with its extremely low WVTR (< 10–7 g/m2/day), it is challenging to attain such low permeability with soft and flexible barriers [107]. Reported so far, the most prevalent strategy to achieve high-performance thin film barrier is known as the layer-by-layer deposition of organic–inorganic hybrid materials (Fig. 2d) [108, 109]. For organic layers, a few-hundred-nanometer-thick polyimide (PI) or parylene thin films have been widely used for biocompatible encapsulation, but these polymeric thin films often exhibit low barrier properties (WVTR > 10–2 g/m2/day). To complement the high permeability of organic layers, a few-nanometer-thick inorganic layers, such as the nanolaminate of aluminum oxides, are inserted between organic barriers [110]. The WVTR of thin film barriers can be further reduced by depositing the multiple dyads of organic–inorganic hybrids, as a thicker barrier generally offers better protection performance. However, the total thickness of the thin film barriers should be carefully optimized, considering the strain distribution of the flexible device. As exhibited in Fig. 2e, the active layers of OLED (especially for brittle ITO) should be positioned on a neutral mechanical plane where tensile and compressive strain are balanced, to ensure mechanical stability during the bending deformation [111, 112].
-
(iii)
Intrinsically stretchable OLEDs
Intrinsically stretchable OLED is considered as the ultimate form of future wearable and implantable LEDs. The rubber-like mechanical properties of intrinsically stretchable OLEDs make them highly compatible with soft human skin or tissues. Without the need for specially designed device structures such as serpentine-shaped interconnections or 3D buckling, this type of LED demonstrates exceptional endurance to mechanical deformations. The fabrication of an intrinsically stretchable LED necessitates that all the layers within the device should be inherently soft and stretchable. Consequently, conventional non-stretchable materials like ITO, vacuum-deposited metal electrodes, and other crystalline electronic materials are unsuitable. Instead, intrinsically stretchable conductors and semiconducting materials must be employed as the electrodes and active inner layers of the devices to maintain their functionality in a deformed state [113].
Since the first intrinsically stretchable OLED was demonstrated by Pei’s group in 2011 [114], several studies have been reported [115, 116]. The major strategies for the fabrication of intrinsically stretchable OLED lies in the composite formation of heterogeneous materials, to impart softness on various electronic/optoelectronic materials. For instance, a percolated network of Ag nanowires (Ag NWs) composite or surfactant-modified poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS) have been widely used for stretchable electrodes, as these composite materials can maintain their conductivities even under the applied strain [117, 118]. For intrinsically stretchable EMLs, organic light-emitting composites have been studied. These composites are generally composed of organic light-emitters (e.g., SuperYellow, the derivative of poly(1,4-phenylenevinylene)), and organic additives, such as elastomers or surfactants. The role of additives in the composite is to enhance the mechanical stretchability of the composite. However, the composite formation approach may result in the low EL performance of the device, as the insulating polymeric additives suppress the efficient carrier transport and recombination inside the device.
Recently, intrinsically stretchable OLEDs with high device performance was reported by Zhang et al., based on the sophisticated material engineering of stretchable electrodes and EML (Fig. 2f) [119]. For stretchable anodes and cathodes, the composite of PEDOT:PSS and polyrotaxane (PR) was used. With 5 wt% of PR contents, the stretchable composite electrodes exhibited high conductivity (700 S/cm), as well as the minimal resistance change under applied strain (R/R0 < 3 under the 100% of uniaxial stretching). For stretchable EML, the composite of SuperYellow and polyurethane (PU) was utilized. The spontaneous phase separation occurred during the spin coating of EML, resulted in the nanoconfined structure of SuperYellow nanofibers being homogenously distributed in the elastomeric PU matrix. Consequently, the intrinsically stretchable OLEDs exhibited high maximum brightness of 7450 nits at 15 V, as well as a maximum stretchability of 100% (Fig. 2g). The device was readily applied to wearable LEDs, achieving real-time displaying of pulse signals of heartbeat (Fig. 2h).
Intrinsically stretchable OLEDs are the promising solution for wearable and implantable LEDs, due to their mechanical alignment with soft human tissues. Nevertheless, several challenges must be addressed. For example, the reported EL performance as well as the operational stability of intrinsically stretchable OLEDs are still insufficient. The repeated deformation of stretchable electrodes may induce a gradual increase in their resistance, resulting in the degradation of luminous performance. Further research on the material engineering of stretchable electrodes, EML, and charge transport layers is thus required to dramatically enhance the EL performance and mechanical stability
System Integration of Wearable and Implantable LEDs
Auxiliary Components for Efficient Light Delivery
Efficient light delivery to deeply located tissue is often impeded by light attenuation, due to the scattering and absorption of photons within tissues. To address this challenge, several strategies have been developed for incorporating auxiliary components into implantable light-emitting systems. These strategies include methods for establishing a direct optical connection between a light source and internal targeted tissues with fiber waveguides or an in situ conversion of low-energy photons to high-energy photons in deep tissues with functional nanoparticles.
Optical waveguides, which confine the spread of light to one dimension, are effective measures to deliver photons into deep target sites (Fig. 3a) [1, 121]. Waveguides can also serve as a physical barrier, preventing photons from directly interacting with biological tissues, thus minimizing the loss of photons during their delivery. Conventional optical fibers typically consist of a combination of high-refractive-index core layer with low-refractive-index cladding layer [122]. This type of structure enables total reflection of the light, capable of confining majority of photons within the fiber. Silica or plastic were widely utilized for conventional optical fibers (Fig. 3b), but their intrinsic rigidity may lead to adverse effects due to mechanical incompatibility with human tissues. Consequently, research efforts have focused on developing optical fibers with features of flexibility and biocompatibility (Fig. 3c). Natural polymers with refractive index higher than those of biological tissues, such as cellulose and silk, have been explored for the fabrication of biocompatible and biodegradable optical fibers (Fig. 3d) [123, 124].

Reproduced with permission from Ref. [123], Copyright 2009, Wiley–VCH GmbH. d Optical images of H-shaped waveguides composed of biodegradable polymers dissolved after soaking into phosphate-buffered saline solution. Reproduced with permission from Ref. [162], Copyright 2016, Springer Nature. e Optical image (left) and schematic illustration (right) of the hydrogel fiber including cells for optogenetics. Reproduced with permission from Ref. [125], Copyright 2013, Springer Nature. f Optical image of multifunctional fiber. g Schematic illustration of multifunctional fiber implanted into tumor tissues for immunotherapeutics delivery and tumor impedance measurement. Reproduced with permission from Ref. [127], Copyright 2021, Springer Nature. (ii) Upconversion nanoparticles. h Schematic illustration of the operating mechanisms of upconversion nanoparticle. Reproduced with permission from Ref. [128], Copyright 2009, Wiley–VCH GmbH. (i) Various excitation/emission wavelengths depending on the type of upconversion nanoparticles. j Microscopic images of green light (left) and near infrared light (right) propagation in tissue with upconversion nanoparticles. Reproduced with permission from Ref. [129], Copyright 2017, American Chemical Society
Auxiliary components for efficient light delivery. (i) Optical fiber waveguides. a Microscopic images of light propagation in tissue without (left) and with waveguide (right), Reproduced with permission from Ref. [158], Copyright 2016, Springer Nature. b Optical image of silica fiber. c Optical image of silk fiber.
Moreover, many researches have explored for the fabrication of optical fibers with multi-functionality. For example, optical sensing and therapeutic functions can be performed by integrating fiber with polymeric hydrogels containing genetically modified cells (Fig. 3e) [125, 126]. The composition of hydrogel was engineered to exhibit sufficient transparency and deformability to be utilized as an implantable light-guiding matrix. Furthermore, the intrinsic softness of the hydrogel eliminates the risk of tissue damage from mechanical inconsistency with fibers and tissues. Optical communication of the implanted hydrogels with target tissues could be processed through fiber, which was demonstrated by the detection of cellular toxicity and optogenetic therapy. Another study developed multifunctional optical fiber integrated with a sensing electrode and hollow drug-injectable channel (Fig. 3f) for immunotherapeutic approaches [127]. The immune checkpoint blockade antibodies can be locally delivered via optical fiber, which allows long-term treatment while avoiding systemic toxicity through direct administration into the target tissues. In addition, the photosensitizer was coated on the fiber to elicit the PDT for enhancing antitumor efficacy. The treatment outcomes can be analyzed in real-time by sensing electrodes embedded in the fiber, which measures tumor impedance to determine the therapeutic response (Fig. 3g).
Another solution for the administration of high-energy photons into deep tissues involves the use of UCNPs, typically composed of lanthanide-doped transition metals (Fig. 3h) [128]. Although such bulk materials exhibit similar upconverting properties, important features, such as total efficiency and other ensemble effects could be only achieved in nanoparticle cases. The upconversion nanoparticles are capable of absorbing multiple incident photons with low energy to emit one photon with high energy based on anti-stokes emission. Hence, considering the higher-energy photons, such as UV light, have limited penetration depths compared to visible or NIR light, UCNPs can be a practical solution for the phototreatment of deep target sites with high-energy photons. The excitation and emission wavelengths of the light from/to the UCNPs can be tailored depending on the material compositions and the doping concentration (Fig. 3i) [129,130,131]. For instance, UCNP whose composition is averaged to 78:20:2 of Y:Yb:Er could absorb near-infrared light with a wavelength of 900 nm in tissues and emit green light at a wavelength of 550 nm. As a result, the use of UCNP allows deeper propagation of green light within deep tissues, compared to using a bare green laser as an external light source (Fig. 3j).
Auxiliary Components for Power Transmission
Light-mediated healthcare system also demands a continuous power supply for device operation, prompting advancements in system integration to achieve a stable power supply system. Consequently, the mount of the battery within the light-emitting system, connected to implanted light sources, has been initially suggested (Fig. 4a) [132,133,134]. The rigid battery, integrated with other bulky device components (e.g., optofluidic devices and power transfer module) are located outside of the skull (Fig. 4b), and a soft flexible wire connects them to the implanted light source (Fig. 4c), thereby minimizing the risk of side effects by avoiding mechanical mismatch. While such battery-powered devices offer high-performance and stable current delivery to light sources, they necessitate periodic replacement of battery and the head-mounted configuration to shield device components against external stress for long-term operation of the system. The scalable size of the battery and its configuration on the head (Fig. 4d) limits the miniaturization of the system, potentially impinging on the free movement of patients in their daily lives.

Reproduced with permission from Ref. [132], Copyright 2019, Springer Nature. b Optical image of the device components in head-mounted configuration. Reproduced with permission from Ref. [133], Copyright 2017, Springer Nature. c Schematic illustration of soft and flexible fiber connected to wearable battery. Reproduced with permission from Ref. [134], Copyright 2017, Elsevier. d Optical image of the scalable head-mounted configuration including battery. (ii) Battery-free system. Reproduced with permission from Ref. [133], Copyright 2017, Springer Nature. e Schematic illustration of the energy harvesting system implanted into the mouse brain. f Optical image of the flexible and wireless energy harvester. g Optical image of the wireless energy harvester implanted into the rat brain. Reproduced with permission from Ref. [160], Copyright 2015, Springer Nature. h Schematic illustrations of the midfield powering coupled enhances power transmission depth and intensity. Reproduced with permission from Ref. [141], Copyright 2014, National Academy of Science. (iii) Wirelessly rechargeable battery-powered system. i Schematic illustration of the wirelessly rechargeable battery-powered system implanted into the brain. j Schematic exploded view of the wirelessly rechargeable battery-powered system. k Schematic illustration of the stable wireless power transmission scenario. Reproduced with permission from Ref. [142], Copyright 2021, Springer Nature
Auxiliary components for power transfer. (i) Battery-powered system. a Schematic illustration of the head-mounted configuration including battery.
To overcome such limitations, a battery-free implantable lighting system based on a radiofrequency (RF) energy-harvesting circuit has been proposed [135,136,137]. The antenna coil in the circuit serves as an energy-harvester connected to multiple device components and light sources (Fig. 4e). Because the miniaturized system did not contain any large components such as a battery (Fig. 4f), it enables full implantation near the target region inside the body without the need for configuration (Fig. 4g). However, the wireless power transmission is highly influenced by the angular orientations between the implanted device and external power sources [138,139,140]. Factors such as the location and the posture of individuals significantly impact the charging behavior of the implanted battery. The introduction of the parity-time-symmetry concept can achieve robust wireless power transfer regardless of the motion and position of the objects. Furthermore, midfield powering can increase the power transmission depth and allow the miniaturization of power-receiving components, broadening the applicability of the implantable light-emitting systems (Fig. 4h) [141].
However, the wireless power transfer system cannot provide high current density for light sources in case of desired applications. Therefore, in response to the shortcomings of both battery-powered and battery-free power transfer systems, recent research has reported a hybrid approach by combining a rechargeable battery and wireless power-receiving modules within the implantable light-emitting system (Fig. 4i) [142]. This system ensures a continuous power supply to the implantable battery through wireless charging while also providing a high level of current delivery to the LEDs simultaneously. Nevertheless, stable power transmission during the free movement of the object is still not guaranteed. As a solution, an implantable light-emitting system adopting a wirelessly controllable Bluetooth module as a control unit has been proposed (Fig. 4j). The individual object can be monitored by real-time communication with a smartphone, enabling various controls for each object without the need for specially equipped facilities targeting moving objects. For example, it will be possible to order for object the rest in a fixed place for facile charging, if the implanted battery is discharged (Fig. 4k).
Biomedical Applications of Wearable and Implantable LEDs
Light-Emitting System for Biosignal Sensing
Light-mediated signal sensing has been extensively used in modern healthcare, as it has several advantages, including non-invasiveness and convenience for continuous monitoring. The most representative examples of light-mediated biosignal sensing are photoplethysmography (PPG) and pulse oximetry. PPG relies on the principle that changes in blood flow lead to variations in light absorption. This method involves positioning a single light source and a photodetector on a slender part of the body, such as a fingertip, to record alterations in the absorption of transmitted or reflected light with each heartbeat as blood moves to the periphery [143]. On the other hand, pulse oximetry is utilized to monitor oxygen saturation level in blood flows. In contrast to PPG, pulse oximetry gauges light absorption at two distinct wavelengths, as blood oxygen saturation is determined by the ratio of absorbance at these two wavelengths. Conventional systems for pulse oximetry have used a combination of red- and NIR-emitting LEDs, as this range of light (700–1000 nm) can penetrate deep into the peripheral blood vessels in the dermis. However, several studies have recently shown that the combination of shorter wavelengths of LEDs, such as red- and green-emitting LEDs, can also be effective for this purpose [144].
The integration of wearable and implantable optoelectronic system into light-mediated signal sensing can greatly enhance their accuracy by implementing more conformal fit to the tissue at the measurement site, regardless of the natural movement of the human body. In this respect, Yokota et al. presented a wearable pulse oximetry device in their study, employing highly flexible polymer light-emitting diodes (PLEDs) that emitted red and green light along with an organic photodiode (Fig. 5a) [145]. The organic photodiode collects the reflected photons. This ultrathin PLED had a total thickness of around 3 µm and demonstrated remarkable flexibility and bendability to be easily attached to the curved surface of the skin without any operational issues. Consequently, the decent stability of the device under ambient conditions was proved by measuring PPG signals using a single light source of red-emitting light (Fig. 5b), and oxygen saturation level of blood flows using the combination of red- and green-emitting LEDs (Fig. 5c). Owing to the conformal contact between the device and the skin, these signals were notably free from noise and exhibited consistent repeatability. Recently, there have been consistent efforts to enhance the performance of wearable systems for pulse oximetry, including the effective form factor of the device, improved light out-coupling modes, and the development of self-powered systems [146].

Reproduced with permission from Ref. [145], Copyright 2016, American Association for the Advancement of Science (AAAS). (ii) Implantable optoelectronic system for local tissue oximetry. d Implantable miniaturized optoelectronic system for local tissue oximetry. e Schematic illustration of the device implanted on femoral artery and veins of mouse (left), and oxygenation level of the local tissue (right). f Schematic illustration of the device implanted on deep brain region of mouse (left), and oxygenation level of the brain region (right). Reproduced with permission from Ref. [147], Copyright 2019, American Association for the Advancement of Science (AAAS)
Wearable and implantable light-emitting systems for biosignal sensing. (i) Wearable optoelectronic system for pulse oximetry. a Ultra-flexible organic optoelectronic system, comprised of red- and green-emitting PLEDs and an organic photodetector. b PPG sensing data. c Measurement of oxygen saturation level of blood flows using pulse oximetry.
While the wearable light-emitting system measures the oxygen saturation level of blood flows from the peripheral blood vessels, the monitoring for the oxygenation level of deep local tissue necessitates an implantable light-emitting system. Zhang et al. reported the wireless, battery-free optoelectronic systems as sub-dermal implants for local tissue oximetry (Fig. 5d) [147]. The system comprises high-performance optoelectronic components including μ-LEDs and a microscale inorganic photodetector, and the electronic module for wireless power collection, circuit control, and data transmission to external receivers. Wireless power harvesting is achieved through magnetic resonant coupling, while data are transmitted via infrared (IR) communication. The compact size, lightweight construction (~ 80 mg), flexible design, and biocompatible encapsulation materials of this system make it suitable for implantation, minimizing tissue disruption and ensuring robust/long-term functionality. Implanted to the femoral artery and vein region (Fig. 5e) and deep brain region of mice (Fig. 5f), changes in oxygenation levels in deep local tissues can be measured. Recently, the technique has been further developed to a wireless, implantable catheter-type oximeter, which is capable of measuring cardiac oxygen saturation [148].
Light-Emitting System for Therapeutic Approaches
The phototherapeutic approaches based on light-emitting system can be classified depending on the light-mediating mechanism, exhibiting different characteristics and/or requirements of light sources such as light intensity, wavelength, and auxiliary materials for processing. In this section, we explain the detailed mechanism of various phototherapies and introduce the application examples of light-emitting system based on wearable and implantable LEDs.
-
(i)
Photodynamic therapy
In general, PDT involves three components: a photosensitizer, a light source, and ROS (Fig. 6a) [149, 150]. The protocol of PDT consists of multi-staged reactions to destroy the unwanted cells or tissues, such as tumors. First, a photosensitizer is administered into the target site in the absence of light. Subsequently, the target site is exposed to suitable light with a proper wavelength capable of exciting the injected photosensitizer. The photosensitizer absorbs illuminated light and generates ROS from tissue oxygen in situ, which could kill the target cells. Laser has been widely used as a light source for the conventional PDT, but the fluctuation of illuminated light intensity to the target site due to the dynamic motion of organs reduces the reliability and safety. On the contrary, deformable LED could offer reliable illumination in dynamic biological tissues, but its weak light intensity may not be sufficient to deliver enough photons to the target deep tissue.
Fig. 6 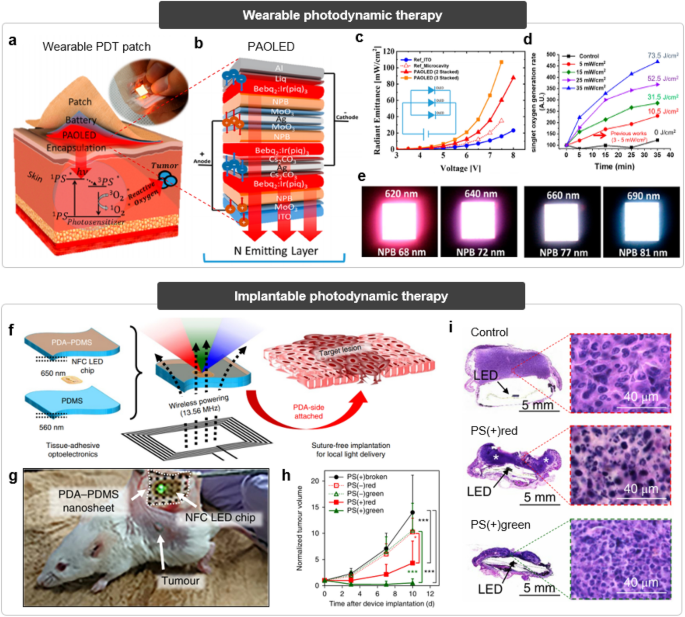
Reproduced with permission from Ref. [151], Copyright 2020, American Chemical Society. e Optical images of the emitted light with various wavelengths from stacked OLEDs according to the combination of OLEDs. (ii) Implantable photodynamic therapy. f Schematic illustration of the implantable optoelectronic system for wireless photodynamic therapy. g Optical image of the implanted light-emitting system on the tumor tissue. h Relative tumor volume change of experimental groups after photodynamic therapy. i Histopathological images of the tumor tissues after treatment in experimental groups. Reproduced with permission from Ref. [153], Copyright 2019, Springer Nature
Wearable and implantable light-emitting systems for photodynamic therapy. (i) Wearable photodynamic therapy. a Schematic illustration of the photodynamic therapy mechanism using wearable LED. Inset shows wearable LED patch. b Schematic exploded view of the parallel-stacked OLEDs. c Relative radiant emittance depending on the stacked OLED layer numbers. d Singlet oxygen generation rate versus time depending on the power input intensity.
To address this limitation by boosting-up the light output intensity, Jeon et al. proposed a parallel-stacked flexible OLEDs for wearable PDTs (Fig. 6b) [151]. The multi-layer stacking of OLEDs allows high power at low voltage through electrically stacked circuit design, compensating for the low power output of a single OLED (Fig. 6c). This design of the proposed device leads to a higher singlet oxygen generation ratio compared to previous approaches, underscoring its potential for wearable PDTs (Fig. 6d). Furthermore, the stacked design enables the adjustment of the color for output light through the combination of stacked OLED [152]. For example, a white OLED can be realized by combining a cyan OLED and a red OLED. The color and intensity of the output light can be fine-tuned by controlling the NPB thickness of the OLEDs (Fig. 6e). This adaptable approach can be used with various types of photosensitizers for PDT.
When it comes to the deep target tissues, however, an external light source based on wearable LEDs may not be a practical option for PDT due to the light attenuation within the tissues. The combination of wearable light sources and optical fibers has been explored, but the inflammation risk and degradation in light transmission efficiency through fiber still exist. Therefore, the wirelessly powered light-emitting system that can be implanted into tissues for mediating PDT has been proposed (Fig. 6f) [153]. To minimize the fluctuation of the light intensity, adhesive elastomeric hydrogel was utilized as a substrate of the light-emitting system to conformally integrate with the target tissues for stable fixation. After the implantation of such light-emitting system to target site, power was wirelessly transmitted to the implanted system from power-supplying cages, leading to in situ LED radiation with desired wavelength for activating photosensitizers (Fig. 6g). The antitumor efficacy of the suggested PDT platform was confirmed by suppressed tumor volume and histological images, superior compared to those of the control groups (Fig. 6h, i).
-
(ii)
Photobiomodulation
PBM generally employs red or NIR light for improving tissue function and regeneration by regulating the generation of specific chemical species (e.g., ATP, NO, and ROS) (Fig. 7a) [15, 154, 155]. PBM includes all therapies that could enhance biological activity, such as wound healing, stimulating neurogenesis, increasing mitochondrial function, protecting tissue aging, and improving blood flow. In terms of cellular level, illuminated light with a desired wavelength and intensity can actuate some light-absorbing components in cells (e.g., cytochrome c oxidase or light-sensitive transient receptor potential channels), leading to upregulation of healthy biomolecules for therapeutic effects.
Fig. 7 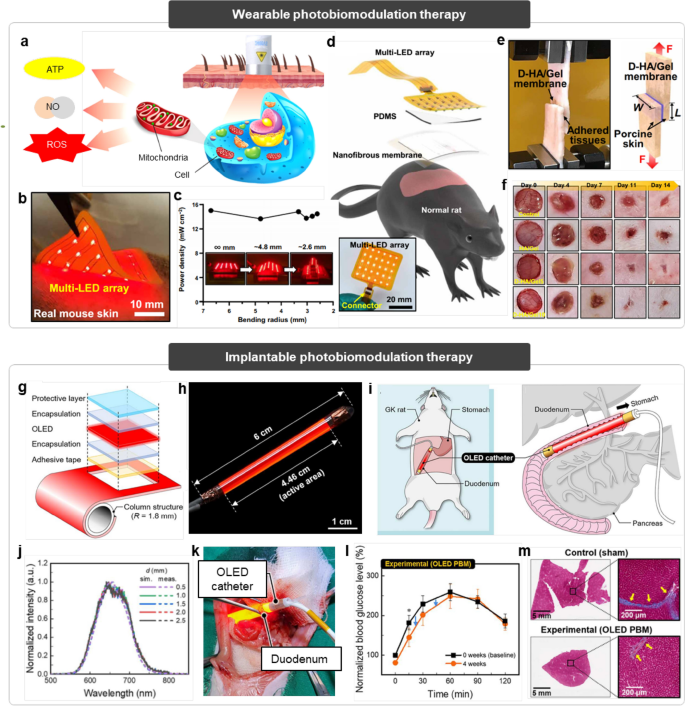
Reproduced with permission from Ref. [156], Copyright 2022, American Association for the Advancement of Science (AAAS). (ii) Implantable photobiomodulation therapy. g Schematic exploded view of the OLED catheter. h Optical image of the OLED catheter. i Schematic illustrations of the OLED catheter implanted in duodenum of the rat. j Measured and simulated spectral intensity of the emitted light from OLED catheter. k Optical image of the OLED catheter implanted in duodenum of the rat. l Relative change in blood glucose level of rats after photobiomodulation using OLED catheter. m Histopathological images of the livers showing collagen deposition in control group (top) and experimental group (bottom). Reproduced with permission from Ref. [157], Copyright 2023, American Association for the Advancement of Science (AAAS)
Wearable and implantable light-emitting systems for photobiomodulation therapy. (i) Wearable photobiomodulation therapy. a Schematic illustration of the photobiomodulation. b Optical image of the multi-LED array applied on the mouse skin for biomodulation. c Power density of the LED array depending on the bending radius. Inset shows the deformed LED array. d Schematic illustration of the multi-LED array integrated with nanofibrous membrane for photobiomodulation. e Optical image (left) and schematic illustration (right) of the nanofibrous membrane adhered with porcine skin. f Optical images of the wound healing efficacy depending on the membrane composition.
Conventional PBM therapies have utilized a laser as a high-intensity light source for direct irradiation to the target area, to maximize the efficacy of the treatment. However, LEDs have been recently considered as an alternative light source for PBM due to their enhanced safety, easy operation method, and a wide area of irradiation [11]. In particular, flexible wearable patches integrated with multi-LED array was proposed for PBM to a large area of tissue at one time (Fig. 7b) [156]. The soft and flexible encapsulation layers allow reliable irradiation of the LED arrays even under deformation (Fig. 7c), ensuring stable operation while applied onto the curved tissues. The substrate of the patch consists of an adhesive nanofibrous membrane to mimic the extracellular matrix, facilitating conformal adhesion between the patch and the tissue (Fig. 7d, e). This adhesive integration improves the light delivery efficiency to the tissue from the LED as well as aids in joining the separated surface of the wound, leading to the enhancement of the wound healing efficacy dramatically (Fig. 7f).
However, such attachable patch-type LEDs have limitations in efficiently delivering light to the inner parts of organs. Hence, an effective photobiomodulation platform for versatile inner-body phototherapeutics has been proposed (Fig. 7g) [157]. This platform comprises an ultrathin flexible OLED integrated onto a cylindrical catheter (Fig. 7h), enabling minimally invasive administration into small-sized organs such as duodenum and intestine (Fig. 7i). The symmetrical structure of this platform, along with the intrinsic characteristics of the OLED, allows axial uniform illumination distribution in all directions regardless of the wavelength (Fig. 7j). This not only minimizes the risk of low-temperature burns by preventing the local hotspot around the target sites, but also delivers the desired intensity of light to the target area with high accuracy. The airtight structure of this platform, in which OLED is tightly encapsulated within biocompatible materials, guarantees stable illumination even in the dynamic aqueous environment in the organs (Fig. 7k). The advantages of the platform are synergistically effective for implantable biomodulation in organs, which was successfully demonstrated in diabetic rats, exhibiting the improved glucose levels in the duodenum (Fig. 7l) and the reduced fibrosis in the liver (Fig. 7m).
-
(iii)
Optogenetics
Optogenetics is a cutting-edge method for precisely modulating cellular activity through the use of genetically engineered target cells made responsive to light [13, 19, 134]. This technique enables exceptional spatial control of target cells by selective genetic modification, allowing researchers to manipulate cell behavior with unprecedented precision (Fig. 8a). Furthermore, ultrahigh temporal resolution could be achieved due to the fast response of channel rhodopsin, differentiating from other therapeutic methods. However, high cost and difficulty in operation, mainly stemming from deep penetration of light and introduction of genetics, limits the clinical translation of optogenetics. To solve the light delivery issues, LED presents a preferred option for a light source due to its safety and rapid response time compared to lasers. Nevertheless, conventional methods for delivering light to deep brain regions, such as implanting bulk LEDs or employing optical fibers, have encountered challenges related to biocompatibility and spatial resolution [158].
Fig. 8 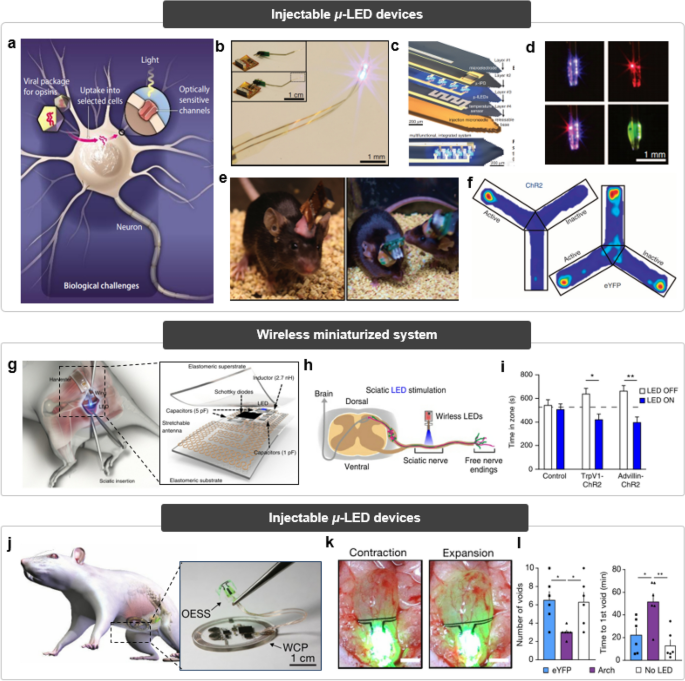
Reproduced with permission from Ref. [163], Copyright 2013, American Association for the Advancement of Science (AAAS). b Optical images of the injectable micro-LED device. Inset shows the connection with wireless power system. c Schematic exploded view of the injectable micro-LED device. d Optical images of the device emitting lights with wavelengths of 675 and 450 nm (top left and top right) and concurrently (bottom left). This device could also emit light with wavelength of 530 nm by coating of fluorescein. e Optical images of the lightweight micro-LED system (left) and conventional rigid light-emitting system (right) implanted into the mouse brain. f Heat maps of mouse activity reflecting the positional preference. Reproduced with permission from Ref. [159], Copyright 2023, American Association for the Advancement of Science (AAAS). (ii) Wireless miniaturized system for optogenetics. g Schematic illustration of the implantable, miniaturized optoelectronic system implanted onto the sciatic nerve of the rat. h Schematic illustration of the wireless optical stimulation on sciatic nerve using the system. i Place aversion of the mice after optical stimulation. Reproduced with permission from Ref. [160], Copyright 2015, Springer Nature. (iii) Wireless closed-loop system for optogenetics. j Schematic illustration of the wireless, closed-loop optoelectronic system implanted on the bladder. Inset shows the optical image of the system. k Optical images of the system seamlessly applied on the surface of the bladder with contracted state (left) and expanded state (right). l Voiding patterns from rats according to genetic modification. Reproduced with permission from Ref. [161], Copyright 2019, Springer Nature
Implantable light-emitting systems for optogenetic therapies. (i) Injectable micro-LED system for optogenetics. a Schematic illustration of the mechanisms of optogenetics.
To address these issues, injectable and cellular-scale optoelectronic system was developed (Fig. 8b) [159]. The system integrates multilayers of device components to perform various functions inside the brain (Fig. 8c), such as electrical/thermal/optical monitoring and stimulation. In addition, multi-colored LEDs that can be activated concurrently or independently as desired are also incorporated within the system, capable of irradiating light of the required wavelength for the possibility of integration with various photomedicine (Fig. 8d). The lightweight and wireless optoelectronic system does not disturb the free movement of the mouse, compared to the conventional rigid configuration of powering systems (Fig. 8e). Such wireless and optical stimulation implemented by the system was successfully demonstrated by neurobiological behavior test (Fig. 8f). The place preference of the mouse was clearly observed because they have learned that dopamine can be secreted when they move to a specific location, as pre-designed by optogenetics.
Despite many advantages of such system to optogenetics, its clinical potential is still far limited because a part of the system that is exposed outside the head could interfere with patients’ daily life, in terms of inflammation risks and convenience. Further, such an external mount system is unsuitable for highly mobile areas including peripheral nerves. Consequently, a fully implantable miniaturized optoelectronic system has been developed for interfacing with neural tissues (Fig. 8g) [160]. This system, encapsulated by soft and stretchable layers, includes wireless energy harvesting components connected to the implanted LED, enabling wireless optoelectronic functionality in dynamically challenging neural tissues. Despite the miniaturized dimension of the device, capacitors included in the electric circuit allow reliable illumination inside the body without the issue of power shortage. Such rigid components are fully encapsulated with elastomeric substrates to sustain mechanical consistency with the soft neuronal tissues. The operation of the suggested device was demonstrated through sciatic nerve stimulation (Fig. 8h), exhibiting pain behavior induced by wireless optical stimulation that leads to place aversion in mice (Fig. 8i). This result also indicates that this miniaturized implantable device does not impair the motor-related nerve.
Continuous neuromodulation, when not tailored to the patient's specific condition, can result in adverse effects such as patient discomfort and an increased risk of injury. To address this concern, Mickle et al., have integrated a biophysical sensory component with an optical stimulation interface to establish a closed-loop system for on-demand optogenetic neuromodulation (Fig. 8j, left) [161]. Soft biophysical sensor continuously measures organ function and transmits biosignal data to control module, and the control module actuates microscale inorganic light-emitting diodes in response to pathological behaviors to activate the opsin in real time. Additionally, the system incorporates a wireless control and power module (Fig. 8j, right) for both power supply and data transmission, which allows continuous communicating with an external processing unit and power supplier. The wide area of the energy harvester, for efficient wireless power transfer, impedes direct implantation of the system around the target tissue, so the system structures, in which the energy harvester is placed in the subcutaneous region and the light source is placed at the target site, and the catheter connected to each other, was implemented. Using a strain gauge sensor to detect bladder volume changes (Fig. 8k), the system can provide feedback optical stimulation to the bladder, controlling voiding patterns in genetically modified mice (Fig. 8l). This closed-loop optoelectronic approach demonstrates the potential for acute treatment responses by dynamically countering pathological abnormalities in real time.
Conclusion
In this review, we provide an overview of the recent advancements in the development of wearable and implantable LEDs for light-mediated healthcare, focusing on material selection, device designs, and system integration. We compared three competitive LED technologies: μ-LEDs, OLEDs, and heavy-metal-free QLEDs, examining their device structures, luminous performance, as well as their advantages and current challenges. Additionally, we discussed various design approaches aimed at ensuring the high mechanical stability of LEDs, allowing for consistent illumination even when they are conformally attached to human skin or organs. We also covered system integration with auxiliary components like optical waveguides or UCNPs for effective light delivery, as well as antennas for wireless powering. Finally, we reviewed the representative applications of light-mediated signal sensing (e.g., PPG/oximetry), and various phototherapies (e.g., PDT, PBM therapies, and optogenetics), all facilitated by wearable and implantable LEDs.
Technological advancements would further improve the treatment efficacy of light-mediated healthcare using wearable and implantable LEDs. In Fig. 9, we proposed technical pathways for next-generation wearable and implantable LEDs, with regards to material engineering, device designs, and system integration. For all three competing LEDs, researches on sophisticated material engineering and device fabrication strategies should be further studied to bring out the dramatic enhancement of device performance and stability. If LEDs utilizing inorganic nanorods or heavy-metal-free perovskites as their light-emitters follow the performance and longevity of conventional LEDs, they might become a mainstream for wearable and implantable LED applications, owing to their inherent advantages. Moreover, the barrier performance of biocompatible soft encapsulants would be further improved to effectively protect the organic active layers vulnerable to humidity, as well as to minimize the side effects caused by direct exposure of rigid, abiotic components to human tissues.
In view of device design strategies, the development of large-area LED arrays with high pixel resolution should be preceded to facilitate the precise illumination of targeted tissues, either focusing on specific localized regions or provide uniform irradiation across wide areas. In addition, the form factors of LED devices would be further evolved to achieve higher mechanical compliance, such as rollable or crumpable devices. While the device performance and stability of intrinsically stretchable LEDs reported previously are yet inferior compared to those of conventional LEDs, there is room for improvement. This improvement can be achieved through the synthesis of soft functional materials that possess both excellent mechanical stability and electronic/optoelectronic properties. Also, the development of injectable devices coupled with the 4D printing technique would be expected for minimal invasiveness for the implant of devices in the future.
To implement the complete level of light-emitting system for biomedical applications, it is essential to develop and harmonize various components to create a seamlessly integrated system. The ideal system should include state-of-the-art powering components and data communication elements for functioning as a human–machine interface. The current wirelessly rechargeable battery system, which addresses the limitations of both batteries and RF harvesters, is likely to progress into a self-powered system that is free from power supplying issues. With regard to data communication between bio-interfaces and processing units, unidirectional data acquisition over wired circuits has been developed into the form of bidirectional wireless communication and closed-loop systems, so feedback stimulation based on acquired bio-information from patient can now be manually performed. The potential for integration with data science, leveraging artificial intelligence technology, holds the promise of enabling active data analysis and automated feedback stimulation tailored to specific conditions of individual patients. The ideal form of potential biomedical light-emitting system could offer breakthroughs for a variety of unmet clinical challenges.
Data availability
As this is a review paper, we think that the data availability statement is not particularly required for this manuscript.
References
G.-H. Lee, H. Moon, H. Kim, G.H. Lee, W. Kwon, S. Yoo, D. Myung, S.H. Yun, Z. Bao, S.K. Hahn, Nat. Rev. Mater. 5, 149 (2020)
X. Ai, J. Mu, B. Xing, Theranostics 6, 2439 (2016)
K.C. Smith, Photomed. Laser Surg. 23, 78 (2005)
R.A. Weiss, D.H. McDaniel, R.G. Geronemus, A.W. Margaret, L.B. Karen, G.M. Munavalli, S.G. Bellew, Dermatol. Surg. 31, 1199 (2005)
M.A. Hadis, P.R. Cooper, M.R. Milward, P.C. Gorecki, E. Tarte, J. Churm, W.M. Palin, J. Biophoton. 10, 1514 (2017)
A. Jubran, Crit. Care 3, R11 (1999)
S. Marathe, D. Zeeshan, T. Thomas, and S. Vidhya, in 2019 International Conference on Vision Towards Emerging Trends in Communication and Networking (ViTECoN), vol. 1 (2019)
M.M. Kim, A. Darafsheh, Photochem. Photobiol. 96, 280 (2020)
D. Kessel, J. Clin. Med. 8, 1581 (2019)
K.D. Desmet, D.A. Paz, J.J. Corry, J.T. Eells, M.T.T. Wong-Riley, M.M. Henry, E.V. Buchmann, M.P. Connelly, J.V. Dovi, H.L. Liang, D.S. Henshel, R.L. Yeager, D.S. Millsap, J. Lim, L.J. Gould, R. Das, M. Jett, B.D. Hodgson, D. Margolis, H.T. Whelan, Photomed. Laser Surg. 24, 121 (2006)
V. Heiskanen, M.R. Hamblin, Photochem. Photobiol. Sci. 17, 1003 (2018)
E. Ronzitti, C. Ventalon, M. Canepari, B.C. Forget, E. Papagiakoumou, V. Emiliani, J. Opt. 19, 113001 (2017)
A. Bansal, S. Shikha, Y. Zhang, Nat. Biomed. Eng. 7, 349 (2023)
C.A. Robertson, D.H. Evans, H. Abrahamse, J. Photochem. Photobiol. B. 96, 1 (2009)
M.R. Hamblin, Photochem. Photobiol. 94, 199 (2018)
R.T. Chow, P.J. Armati, Photomed. Laser Surg. 34, 599 (2016)
I. Khan, S.U. Rahman, E. Tang, K. Engel, B. Hall, A.B. Kulkarni, P.R. Arany, Sci. Rep. 11, 13371 (2021)
N.H.C. Souza, R.A. Mesquita-Ferrari, M.F.S.D. Rodrigues, D.F.T. da Silva, B.G. Ribeiro, A.N. Alves, M.P. Garcia, F.D. Nunes, E.M. da Silva Junior, C.M. França, S.K. Bussadori, K.P.S. Fernandes, J. Cell. Mol. Med. 22, 4922 (2018)
B.R. Rost, J. Wietek, O. Yizhar, D. Schmitz, Nat. Neurosci. 25, 984 (2022)
V.V. Barun, A.P. Ivanov, A.V. Volotovskaya, V.S. Ulashchik, J. Appl. Spectrosc. 74, 430 (2007)
L. Finlayson, I.R.M. Barnard, L. McMillan, S.H. Ibbotson, C.T.A. Brown, E. Eadie, K. Wood, Photochem. Photobiol. 98, 974 (2022)
M. Meinhardt, R. Krebs, A. Anders, U. Heinrich, H. Tronnier, J. Biomed. Opt. 13, 044030 (2008)
T.A. Henderson, L.D. Morries, Neuropsychiatr. Dis. Treat. 11, 2191 (2015)
H. Arimoto, M. Egawa, Skin Res. Technol. 21, 94 (2015)
G. Romano, G. Insero, S.N. Marrugat, F. Fusi, Biomol. Concepts 13, 256 (2022)
D.T. Phan, S. Mondal, L.H. Tran, V. Thi Mai Thien, H. Van Nguyen, C.H. Nguyen, S. Park, J. Choi, J. Oh, Flex. Print. Electron. 6, 045002 (2021)
S. Choi, Y. Jeon, J.H. Kwon, C. Ihm, S.Y. Kim, K.C. Choi, Adv. Sci. 9, 2204622 (2022)
Y. Lee, J. Kim, J.H. Koo, T.-H. Kim, D.-H. Kim, Korean J. Chem. Eng. 35, 1 (2018)
P. Jia, M. Lu, S. Sun, Y. Gao, R. Wang, X. Zhao, G. Sun, V.L. Colvin, W.W. Yu, Adv. Mater. Interfaces 8, 2100441 (2021)
J. Park, H. Seung, D.C. Kim, M.S. Kim, D.-H. Kim, Adv. Funct. Mater. 31, 2009281 (2021)
E.G. Jeong, J.H. Kwon, K.S. Kang, S.Y. Jeong, K.C. Choi, J. Inf. Display 21, 19 (2020)
D.C. Kim, H. Yun, J. Kim, H. Seung, W.S. Yu, J.H. Koo, J. Yang, J.H. Kim, T. Hyeon, D.-H. Kim, Nat. Electron. 4, 671 (2021)
T. Sekitani, H. Nakajima, H. Maeda, T. Fukushima, T. Aida, K. Hata, T. Someya, Nat. Mater. 8, 494 (2009)
J.H. Koo, D.C. Kim, H.J. Shim, T.-H. Kim, D.-H. Kim, Adv. Funct. Mater. 28, 1801834 (2018)
D. Zhang, T. Huang, L. Duan, Adv. Mater. 32, 1902391 (2020)
J. Seung Lee, J. Kim, Y. Ye, T. Kim, Adv. Drug Deliv. Rev. 186, 114339 (2022)
H. Zhang, H. Zhao, X. Zhao, C. Xu, D. Franklin, A. Vázquez-Guardado, W. Bai, J. Zhao, K. Li, G. Monti, W. Lu, A. Kobeissi, L. Tian, X. Ning, X. Yu, S. Mehta, D. Chanda, Y. Huang, S. Xu, B.E. Perez White, J.A. Rogers, Adv. Funct. Mater. 31, 2100576 (2021)
X. Zhu, J. Zhang, J. Liu, Y. Zhang, Adv. Sci. 6, 1901358 (2019)
J. Kim, J. Seo, D. Jung, T. Lee, H. Ju, J. Han, N. Kim, J. Jeong, S. Cho, J.H. Seol, J. Lee, Proc. Natl. Acad. Sci. 117, 16856 (2020)
Y. Huang, E.-L. Hsiang, M.-Y. Deng, S.-T. Wu, Light Sci. Appl. 9, 105 (2020)
J.S. Kim, J.-M. Heo, G.-S. Park, S.-J. Woo, C. Cho, H.J. Yun, D.-H. Kim, J. Park, S.-C. Lee, S.-H. Park, E. Yoon, N.C. Greenham, T.-W. Lee, Nature 611, 688 (2022)
Y.-H. Kim, J. Park, S. Kim, J.S. Kim, H. Xu, S.-H. Jeong, B. Hu, T.-W. Lee, Nat. Nanotechnol. 17, 590 (2022)
S.Y. Lee, S. Jeon, J. Ahn, J. Bang, H.K. Woo, K. Lee, B.K. Jung, T. Park, D. Son, J.-P. Ahn, S.J. Oh, Appl. Surf. Sci. 563, 150229 (2021)
S. Nakamura, T. Mukai, M. Senoh, Appl. Phys. Lett. 64, 1687 (1994)
H.S. Wasisto, J.D. Prades, J. Gülink, A. Waag, Appl. Phys. Rev. 6, 041315 (2019)
I. Schnitzer, E. Yablonovitch, C. Caneau, T.J. Gmitter, Appl. Phys. Lett. 62, 131 (1993)
B.O. Jung, W. Lee, J. Kim, M. Choi, H.-Y. Shin, M. Joo, S. Jung, Y.-H. Choi, M.J. Kim, Sci. Rep. 11, 4535 (2021)
B. Moran, M.J. Holmes, J. Flemish, R. Armitage, Z. Ren, H. Lotfi, T. Chung, H.J. Kim, H. Masui, R. Pathak, J.C. Tan, B.H. Tan, Y.M. Tio, S. Lim, K.B. Lim, O. Shchekin, SID Symp. Digest Tech. Pap. 54, 414 (2023)
M.A. Khan, N. Maeda, J. Yun, M. Jo, Y. Yamada, H. Hirayama, Sci. Rep. 12, 2591 (2022)
M.S. Wong, S. Nakamura, S.P. DenBaars, 2020 ECS. J. Solid State Sci. Technol. 9, 015012 (2019)
H.E. Lee, J.H. Shin, J.H. Park, S.K. Hong, S.H. Park, S.H. Lee, J.H. Lee, I.-S. Kang, K.J. Lee, Adv. Funct. Mater. 29, 1808075 (2019)
M. Asad, Q. Li, M. Sachdev, W.S. Wong, Nano Energy 73, 104724 (2020)
V. Poher, N. Grossman, G.T. Kennedy, K. Nikolic, H.X. Zhang, Z. Gong, E.M. Drakakis, E. Gu, M.D. Dawson, P.M.W. French, P. Degenaar, M.A.A. Neil, J. Phys. D Appl. Phys. 41, 094014 (2008)
T. Wu, C.W. Sher, Y. Lin, C.F. Lee, S. Liang, Y. Lu, S.W.H. Chen, W. Guo, H.C. Kuo, Z. Chen, Appl. Sci. 8, 9 (2018)
J.-E. Ryu, S. Park, Y. Park, S.-W. Ryu, K. Hwang, H.W. Jang, Adv. Mater. 35, 2204947 (2023)
F. Olivier, S. Tirano, L. Dupré, B. Aventurier, C. Largeron, F. Templier, J. Lumin. 191, 112 (2017)
S. Hang, C.-M. Chuang, Y. Zhang, C. Chu, K. Tian, Q. Zheng, T. Wu, Z. Liu, Z.-H. Zhang, Q. Li, H.-C. Kuo, J. Phys. D Appl. Phys. 54, 153002 (2021)
M.S. Wong, D. Hwang, A.I. Alhassan, C. Lee, R. Ley, S. Nakamura, S.P. DenBaars, Opt. Express 26, 21324 (2018)
B. Geffroy, P. le Roy, C. Prat, Polym. Int. 55, 572 (2006)
K. Tuong Ly, R.-W. Chen-Cheng, H.-W. Lin, Y.-J. Shiau, S.-H. Liu, P.-T. Chou, C.-S. Tsao, Y.-C. Huang, Y. Chi, Nat. Photon. 11, 63 (2017)
Z. Li, D. Yang, C. Han, B. Zhao, H. Wang, Y. Man, P. Ma, P. Chang, D. Ma, H. Xu, Angew. Chem. Int. Ed. 60, 14846 (2021)
Y. Chen, D. Zhang, Y. Zhang, X. Zeng, T. Huang, Z. Liu, G. Li, L. Duan, Adv. Mater. 33, 2103293 (2021)
H. Lee, J. Hyuk Kwon, SID Symp. Digest Tech. Pap. 52, 321 (2021)
Y. Luo, S. Li, Y. Zhao, C. Li, Z. Pang, Y. Huang, M. Yang, L. Zhou, X. Zheng, X. Pu, Z. Lu, Adv. Mater. 32, 2001248 (2020)
W. Jang, M. Lee, H. Kweon, H.W. Park, J. Yang, S. Kim, H. Jo, C. Lee, J.H. Cho, K. Kwak, D.H. Kim, B. Kim, M.S. Kang, ACS Photon. 8, 2519 (2021)
H. Park, C. Na, H. Lee, S.M. Cho, Korean J. Chem. Eng. 40, 667 (2023)
G. Hong, X. Gan, C. Leonhardt, Z. Zhang, J. Seibert, J.M. Busch, S. Bräse, Adv. Mater. 33, 2005630 (2021)
H. Sasabe, J. Kido, Eur. J. Org. Chem. 2013, 7653 (2013)
B. Minaev, G. Baryshnikov, H. Agren, Phys. Chem. Chem. Phys. 16, 1719 (2014)
F.B. Dias, K.N. Bourdakos, V. Jankus, K.C. Moss, K.T. Kamtekar, V. Bhalla, J. Santos, M.R. Bryce, A.P. Monkman, Adv. Mater. 25, 3707 (2013)
C.-Y. Chan, M. Tanaka, Y.-T. Lee, Y.-W. Wong, H. Nakanotani, T. Hatakeyama, C. Adachi, Nat. Photon. 15, 203 (2021)
J. Lewis, Mater. Today 9, 38 (2006)
V.L. Colvin, M.C. Schlamp, A.P. Alivisatos, Nature 370, 354 (1994)
X. Dai, Y. Deng, X. Peng, Y. Jin, Adv. Mater. 29, 1607022 (2017)
W.K. Bae, J. Lim, Korean J. Chem. Eng. 36, 173 (2019)
T. Lee, B.J. Kim, H. Lee, D. Hahm, W.K. Bae, J. Lim, J. Kwak, Adv. Mater. 34, 2106276 (2022)
J.-H. Kim, C.-Y. Han, K.-H. Lee, K.-S. An, W. Song, J. Kim, M.S. Oh, Y.R. Do, H. Yang, Chem. Mater. 27, 197 (2015)
B.S. Mashford, M. Stevenson, Z. Popovic, C. Hamilton, Z. Zhou, C. Breen, J. Steckel, V. Bulovic, M. Bawendi, S. Coe-Sullivan, P.T. Kazlas, Nat. Photon. 7, 407 (2013)
J. Lim, W.K. Bae, D. Lee, M.K. Nam, J. Jung, C. Lee, K. Char, S. Lee, Chem. Mater. 23, 4459 (2011)
J. Lim, M. Park, W.K. Bae, D. Lee, S. Lee, C. Lee, K. Char, ACS Nano 7, 9019 (2013)
Y.-H. Won, O. Cho, T. Kim, D.-Y. Chung, T. Kim, H. Chung, H. Jang, J. Lee, D. Kim, E. Jang, Nature 575, 634 (2019)
W.-C. Chao, T.-H. Chiang, Y.-C. Liu, Z.-X. Huang, C.-C. Liao, C.-H. Chu, C.-H. Wang, H.-W. Tseng, W.-Y. Hung, P.-T. Chou, Commun. Mater. 2, 96 (2021)
T. Kim, K.-H. Kim, S. Kim, S.-M. Choi, H. Jang, H.-K. Seo, H. Lee, D.-Y. Chung, E. Jang, Nature 586, 385 (2020)
S. Chen, M. Tang, Q. Jiang, J. Wu, V.G. Dorogan, M. Benamara, Y.I. Mazur, G.J. Salamo, P. Smowton, A. Seeds, H. Liu, ACS Photon. 1, 638 (2014)
A. Wang, H. Shen, S. Zang, Q. Lin, H. Wang, L. Qian, J. Niu, L. Song Li, Nanoscale 7, 2951 (2015)
M.A. Triana, E.-L. Hsiang, C. Zhang, Y. Dong, S.-T. Wu, ACS Energy Lett. 7, 1001 (2022)
Q. Lin, Y. Zhu, Y. Wang, D. Li, Y. Zhao, Y. Liu, F. Li, W. Huang, Adv. Mater. 35, 2210385 (2023)
M.A. Triana, S.-T. Wu, Y. Dong, SID Symp. Digest Tech. Pap. 51, 1748 (2020)
M.K. Choi, J. Yang, D.C. Kim, Z. Dai, J. Kim, H. Seung, V.S. Kale, S.J. Sung, C.R. Park, N. Lu, T. Hyeon, D.-H. Kim, Adv. Mater. 30, 1703279 (2018)
K. Alzoubi, M.M. Hamasha, S. Lu, B. Sammakia, J. Display Technol. 7, 593 (2011)
M.K. Choi, J. Yang, T. Hyeon, D.-H. Kim, Npj Flex. 2, 10 (2018)
J.-K. Song, M.S. Kim, S. Yoo, J.H. Koo, D.-H. Kim, Nano Res. 14, 2919 (2021)
J.H. Koo, S. Jeong, H.J. Shim, D. Son, J. Kim, D.C. Kim, S. Choi, J.-I. Hong, D.-H. Kim, ACS Nano 11, 10032 (2017)
J.I. Kwon, G. Park, G.H. Lee, J.H. Jang, N.J. Sung, S.Y. Kim, J. Yoo, K. Lee, H. Ma, M. Karl, T.J. Shin, M.H. Song, J. Yang, M.K. Choi, Sci. Adv. 8, eadd0697 (2022)
J. Yoo, S. Li, D.-H. Kim, J. Yang, M.K. Choi, Nanoscale Horiz. 7, 801 (2022)
V.W. Lee, N. Twu, I. Kymissis, Inf. Display 32, 16 (2016)
R.-H. Kim, D.-H. Kim, J. Xiao, B.H. Kim, S.-I. Park, B. Panilaitis, R. Ghaffari, J. Yao, M. Li, Z. Liu, V. Malyarchuk, D.G. Kim, A.-P. Le, R.G. Nuzzo, D.L. Kaplan, F.G. Omenetto, Y. Huang, Z. Kang, J.A. Rogers, Nat. Mater. 9, 929 (2010)
N. Kim, J. Kim, J. Seo, C. Hong, J. Lee, A.C.S. Appl, Mater. Interfaces 14, 4344 (2022)
G.-H. Lee, Y.R. Lee, H. Kim, D.A. Kwon, H. Kim, C. Yang, S.Q. Choi, S. Park, J.-W. Jeong, S. Park, Nat. Commun. 13, 2643 (2022)
K. Tybrandt, J. Vörös, Small 12, 180 (2016)
Y. Lee, J.W. Chung, G.H. Lee, H. Kang, J.-Y. Kim, C. Bae, H. Yoo, S. Jeong, H. Cho, S.-G. Kang, J.Y. Jung, D.-W. Lee, S. Gam, S.G. Hahm, Y. Kuzumoto, S.J. Kim, Z. Bao, Y. Hong, Y. Yun, S. Kim, Sci. Adv. 7, eabg9180 (2023)
S.H. Kim, G.W. Baek, J. Yoon, S. Seo, J. Park, D. Hahm, J.H. Chang, D. Seong, H. Seo, S. Oh, K. Kim, H. Jung, Y. Oh, H.W. Baac, B. Alimkhanuly, W.K. Bae, S. Lee, M. Lee, J. Kwak, J.-H. Park, D. Son, Adv. Mater. 33, 2104690 (2021)
B. Lee, H. Cho, S. Moon, Y. Ko, Y.-S. Ryu, H. Kim, J. Jeong, S. Chung, Nat. Electron. 6, 307 (2023)
K.-H. Choi, H.-J. Nam, J.-A. Jeong, S.-W. Cho, H.-K. Kim, J.-W. Kang, D.-G. Kim, W.-J. Cho, Appl. Phys. Lett. 92, 223302 (2008)
Y. Jeon, H.-R. Choi, J.H. Kwon, S. Choi, K.M. Nam, K.-C. Park, K.C. Choi, Light Sci. Appl. 8, 114 (2019)
Y. Jeon, H.-R. Choi, K.-C. Park, K.C. Choi, J. Soc. Inf. Disp. 28, 324 (2020)
Y. Jeon, H. Lee, H. Kim, J.H. Kwon, Micromachines (Basel) 13, 1478 (2022)
S. Lee, J.-H. Han, S.-H. Lee, G.-H. Baek, J.-S. Park, JOM 71, 197 (2019)
B.-H. Kwon, C.W. Joo, H. Cho, C. Kang, J.-H. Yang, J.-W. Shin, G.H. Kim, S. Choi, S. Nam, K. Kim, C.-W. Byun, N.S. Cho, S. Kim, A.C.S. Appl, Mater. Interfaces 13, 55391 (2021)
J.H. Kwon, Y. Jeon, S. Choi, H. Kim, K.C. Choi, J. Inf. Display 19, 135 (2018)
J.H. Jang, S. Li, D.-H. Kim, J. Yang, M.K. Choi, Adv. Electron. Mater. 9, 2201271 (2023)
M.K. Choi, J. Yang, K. Kang, D.C. Kim, C. Choi, C. Park, S.J. Kim, S.I. Chae, T.-H. Kim, J.H. Kim, T. Hyeon, D.-H. Kim, Nat. Commun. 6, 7149 (2015)
D.C. Kim, H.J. Shim, W. Lee, J.H. Koo, D.-H. Kim, Adv. Mater. 32, 1902743 (2020)
J. Liang, L. Li, X. Niu, Z. Yu, Q. Pei, Nat. Photon. 7, 817 (2013)
J.-H. Kim, J.-W. Park, Sci. Adv. 7, eabd9715 (2023)
H. Yin, Y. Zhu, K. Youssef, Z. Yu, Q. Pei, Adv. Mater. 34, 2106184 (2022)
J. Liang, L. Li, K. Tong, Z. Ren, W. Hu, X. Niu, Y. Chen, Q. Pei, ACS Nano 8, 1590 (2014)
X. Fan, W. Nie, H. Tsai, N. Wang, H. Huang, Y. Cheng, R. Wen, L. Ma, F. Yan, Y. Xia, Adv. Sci. 6, 1900813 (2019)
Z. Zhang, W. Wang, Y. Jiang, Y.-X. Wang, Y. Wu, J.-C. Lai, S. Niu, C. Xu, C.-C. Shih, C. Wang, H. Yan, L. Galuska, N. Prine, H.-C. Wu, D. Zhong, G. Chen, N. Matsuhisa, Y. Zheng, Z. Yu, Y. Wang, R. Dauskardt, X. Gu, J.B.-H. Tok, Z. Bao, Nature 603, 624 (2022)
W. Liu, C. Zhang, R. Alessandri, B.T. Diroll, Y. Li, H. Liang, X. Fan, K. Wang, H. Cho, Y. Liu, Y. Dai, Q. Su, N. Li, S. Li, S. Wai, Q. Li, S. Shao, L. Wang, J. Xu, X. Zhang, D.V. Talapin, J.J. de Pablo, S. Wang, Nat. Mater. 22, 737 (2023)
M. Humar, S.J.J. Kwok, M. Choi, A.K. Yetisen, S. Cho, S.-H. Yun, J. Nanophoton. 6, 414 (2017)
D.R. Sparta, A.M. Stamatakis, J.L. Phillips, N. Hovelsø, R. van Zessen, G.D. Stuber, Nat. Protoc. 7, 12 (2012)
S.T. Parker, P. Domachuk, J. Amsden, J. Bressner, J.A. Lewis, D.L. Kaplan, F.C. Omenetto, Adv. Mater. 21, 2411 (2009)
D. Shan, C. Zhang, S. Kalaba, N. Mehta, G.B. Kim, Z. Liu, J. Yang, Biomaterials 143, 142 (2017)
M. Choi, J.W. Choi, S. Kim, S. Nizamoglu, S.K. Hahn, S.H. Yun, Nat. Photon. 7, 987 (2013)
J. Guo, M. Zhou, C. Yang, Sci. Rep. 7, 7902 (2017)
A.L. Chin, S. Jiang, E. Jang, L. Niu, L. Li, X. Jia, R. Tong, Nat. Commun. 12, 5138 (2021)
J. Lee, B. Yoo, H. Lee, G.D. Cha, H.-S. Lee, Y. Cho, S.Y. Kim, H. Seo, W. Lee, D. Son, M. Kang, H.M. Kim, Y. Il Park, T. Hyeon, D.-H. Kim, Adv. Mater. 29, 3169 (2017)
S. Han, B.W. Hwang, E.Y. Jeon, D. Jung, G.H. Lee, D.H. Keum, K.S. Kim, S.H. Yun, H.J. Cha, S.K. Hahn, ACS Nano 11, 9979 (2017)
G. Liang, H. Wang, H. Shi, H. Wang, M. Zhu, A. Jing, J. Li, G. Li, J. Nanobiotechnol. 18, 154 (2020)
L. Zhang, D. Jin, M.H. Stenzel, Biomacromol 22, 3168 (2021)
R. Qazi, A.M. Gomez, D.C. Castro, Z. Zou, J.Y. Sim, Y. Xiong, J. Abdo, C.Y. Kim, A. Anderson, F. Lohner, S.H. Byun, B. Chul Lee, K.I. Jang, J. Xiao, M.R. Bruchas, J.W. Jeong, Nat. Biomed. Eng 3, 655 (2019)
J.G. McCall, R. Qazi, G. Shin, S. Li, M.H. Ikram, K.I. Jang, Y. Liu, R. Al-Hasani, M.R. Bruchas, J.W. Jeong, J.A. Rogers, Nat. Protoc. 12, 219 (2017)
J.W. Jeong, J.G. McCall, G. Shin, Y. Zhang, R. Al-Hasani, M. Kim, S. Li, J.Y. Sim, K.I. Jang, Y. Shi, D.Y. Hong, Y. Liu, G.P. Schmitz, L. Xia, Z. He, P. Gamble, W.Z. Ray, Y. Huang, M.R. Bruchas, J.A. Rogers, Cell 162, 662 (2015)
K.L. Montgomery, A.J. Yeh, J.S. Ho, V. Tsao, S.M. Iyer, L. Grosenick, E.A. Ferenczi, Y. Tanabe, K. Deisseroth, S.L. Delp, A.S.Y. Poon, Nat. Methods 12, 969 (2015)
Y. Zhang, D.C. Castro, Y. Han, Y. Wu, H. Guo, Z. Weng, Y. Xue, J. Ausra, X. Wang, R. Li, G. Wu, A. Vázquez-Guardado, Y. Xie, Z. Xie, D. Ostojich, D. Peng, R. Sun, B. Wang, Y. Yu, J.P. Leshock, S. Qu, C.J. Su, W. Shen, T. Hang, A. Banks, Y. Huang, J. Radulovic, P. Gutruf, M.R. Bruchas, J.A. Rogers, Proc. Natl. Acad. Sci. U.S.A. 116, 21427 (2019)
S. Il Park, G. Shin, J.G. McCall, R. Al-Hasani, A. Norrisf, L. Xia, D.S. Brenner, K.N. Noh, S.Y. Bang, D.L. Bhatti, K.I. Jang, S.K. Kang, A.D. Mickle, G. Dussor, T.J. Price, R.W.G. Iv, M.R. Bruchas, J.A. Rogers, Proc. Natl. Acad. Sci. U.S.A. 113, 8169 (2016)
H. Kim, S. Yoo, H. Joo, J. Lee, D. An, S. Nam, H. Han, D.-H. Kim, S. Kim, Sci. Adv. 8, abo4610 (2022)
S. Yoo, J. Lee, H. Joo, S.H. Sunwoo, S. Kim, D.H. Kim, Adv. Healthc. Mater. (2021). https://doi.org/10.1002/adhm.202100614
H. Joo, Y. Lee, J. Kim, J.-S. Yoo, S. Yoo, S. Kim, A.K. Arya, S. Kim, S.H. Choi, N. Lu, H.S. Lee, S. Kim, S.-T. Lee, D.H. Kim, Sci. Adv. 7, eabd4639 (2022)
J.S. Ho, A.J. Yeh, E. Neofytou, S. Kim, Y. Tanabe, B. Patlolla, R.E. Beygui, A.S.Y. Poon, Proc. Natl. Acad. Sci. U.S.A. 111, 7974 (2014)
C.Y. Kim, M.J. Ku, R. Qazi, H.J. Nam, J.W. Park, K.S. Nam, S. Oh, I. Kang, J.H. Jang, W.Y. Kim, J.H. Kim, J.W. Jeong, Nat. Commun. 12, 535 (2021)
T.-H. Kim, C.-S. Lee, S. Kim, J. Hur, S. Lee, K.W. Shin, Y.-Z. Yoon, M.K. Choi, J. Yang, D.-H. Kim, T. Hyeon, S. Park, S. Hwang, ACS Nano 11, 5992 (2017)
C.M. Lochner, Y. Khan, A. Pierre, A.C. Arias, Nat. Commun. 5, 5745 (2014)
T. Yokota, P. Zalar, M. Kaltenbrunner, H. Jinno, N. Matsuhisa, H. Kitanosako, Y. Tachibana, W. Yukita, M. Koizumi, T. Someya, Sci. Adv. 2, e1501856 (2023)
H. Jinno, T. Yokota, M. Koizumi, W. Yukita, M. Saito, I. Osaka, K. Fukuda, T. Someya, Nat. Commun. 12, 2234 (2021)
H. Zhang, P. Gutruf, K. Meacham, M.C. Montana, X. Zhao, A.M. Chiarelli, A. Vázquez-Guardado, A. Norris, L. Lu, Q. Guo, C. Xu, Y. Wu, H. Zhao, X. Ning, W. Bai, I. Kandela, C.R. Haney, D. Chanda, R.W. Gereau, J.A. Rogers, Sci. Adv. 5, eaaw0873 (2023)
W. Lu, W. Bai, H. Zhang, C. Xu, A.M. Chiarelli, A. Vázquez-Guardado, Z. Xie, H. Shen, K. Nandoliya, H. Zhao, K. Lee, Y. Wu, D. Franklin, R. Avila, S. Xu, A. Rwei, M. Han, K. Kwon, Y. Deng, X. Yu, E.B. Thorp, X. Feng, Y. Huang, J. Forbess, Z.-D. Ge, J.A. Rogers, Sci. Adv. 7, eabe0579 (2023)
D.E.J.G.J. Dolmans, D. Fukumura, R.K. Jain, Nat. Rev. Cancer 3, 380 (2003)
A. Bansal, F. Yang, T. Xi, Y. Zhang, J.S. Ho, Proc. Natl. Acad. Sci. 115, 1469 (2018)
Y. Jeon, I. Noh, Y.C. Seo, J.H. Han, Y. Park, E.H. Cho, K.C. Choi, ACS Nano 14, 15688 (2020)
S.S. Lee, S.M. Cho, Korean J. Chem. Eng. 19, 463 (2002)
K. Yamagishi, I. Kirino, I. Takahashi, H. Amano, S. Takeoka, Y. Morimoto, T. Fujie, Nat. Biomed. Eng. 3, 27 (2019)
F. Salehpour, J. Mahmoudi, F. Kamari, S. Sadigh-Eteghad, S.H. Rasta, M.R. Hamblin, Mol. Neurobiol. 55, 6601 (2018)
M.R. Hamblin, J. Neurosci. Res. 96, 731 (2018)
S.Y. Lee, S. Jeon, Y.W. Kwon, M. Kwon, M.S. Kang, K.-Y. Seong, T.-E. Park, S.Y. Yang, D.-W. Han, S.W. Hong, K.S. Kim, Sci. Adv. 8, 1646 (2022)
J.H. Sim, J. Kwon, H. Chae, S.-B. Kim, H. Cho, W. Lee, S.H. Kim, C.-W. Byun, S. Hahn, D.H. Park, S. Yoo, Sci. Adv. (2023). https://doi.org/10.1126/sciadv.adh8619
D. Kim, T. Yokota, T. Suzuki, S. Lee, T. Woo, W. Yukita, M. Koizumi, Y. Tachibana, H. Yawo, H. Onodera, M. Sekino, T. Someya, Proc. Natl. Acad. Sci. 117, 21138 (2020)
T. Kim, J.G. McCall, Y.H. Jung, X. Huang, E.R. Siuda, Y. Li, J. Song, Y.M. Song, H.A. Pao, R.-H. Kim, C. Lu, S.D. Lee, I.-S. Song, G. Shin, R. Al-Hasani, S. Kim, M.P. Tan, Y. Huang, F.G. Omenetto, J.A. Rogers, M.R. Bruchas, Science 340, 211 (2013)
S. Il Park, D.S. Brenner, G. Shin, C.D. Morgan, B.A. Copits, H.U. Chung, M.Y. Pullen, K.N. Noh, S. Davidson, S.J. Oh, J. Yoon, K.I. Jang, V.K. Samineni, M. Norman, J.G. Grajales-Reyes, S.K. Vogt, S.S. Sundaram, K.M. Wilson, J.S. Ha, R. Xu, T. Pan, T. Il Kim, Y. Huang, M.C. Montana, J.P. Golden, M.R. Bruchas, R.W. Gereau, J.A. Rogers, Nat. Biotechnol. 33, 1280 (2015)
A.D. Mickle, S.M. Won, K.N. Noh, J. Yoon, K.W. Meacham, Y. Xue, L.A. McIlvried, B.A. Copits, V.K. Samineni, K.E. Crawford, D.H. Kim, P. Srivastava, B.H. Kim, S. Min, Y. Shiuan, Y. Yun, M.A. Payne, J. Zhang, H. Jang, Y. Li, H.H. Lai, Y. Huang, S. Il Park, R.W. Gereau, J.A. Rogers, Nature 565, 361 (2019)
S. Nizamoglu, M.C. Gather, M. Humar, M. Choi, S. Kim, K.S. Kim, S.K. Hahn, G. Scarcelli, M. Randolph, R.W. Redmond, S.H. Yun, Nat. Commun. (2016). https://doi.org/10.1038/ncomms10374
J.C. Williams, T. Denison, Sci. Transl. Med. (2013). https://doi.org/10.1126/scitranslmed.3003100
Acknowledgements
This work was supported by the Gachon University research fund of 2023 (GCU-202300980001). This research was further supported by Institute for Basic Science (IBS-R006-A1).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cha, G.D., Kim, DH. & Kim, D.C. Wearable and Implantable Light-Emitting Diodes and Their Biomedical Applications. Korean J. Chem. Eng. 41, 1–24 (2024). https://doi.org/10.1007/s11814-023-00006-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-023-00006-z