Abstract
A survey was conducted in the agricultural fields of mustard of Haridwar district, Uttarakhand, India, where a heavy population of Zeldia was recovered. The morphological, molecular, and phylogenetic characterization confirmed the nematode as Zeldia punctata. The phylogenetic analysis based on 18 and 28S rDNA placed the studied population of Z. punctata close to other populations of the same species. The principal component analysis (PCA) data revealed the high intraspecific and interspecific variations between the populations of Zeldia punctata from different geographical regions. The result indicated a variation between the Indian populations of Z. punctata. Additionally, the selected ecological factors indicated an impact of EC, temperature, and phosphorus on Z. punctata during the flowering and podding time of mustard crops in India. However, the PCA revealed no effect of ecological factors on the nematode diversity indices. In conclusion, Z. punctata, with its high individual numbers on mustard, may affect crop production due to activating the bacterial community of the soil and less space for plant-parasitic nematodes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mustard (Brassica rapa) is one of the important cash crops grown in India, belonging to the family Brassicaceae. The primary production of mustard occurred in the states of northern India. The mustard grown in the areas of Uttarakhand requires 120–155 days to get a mature harvest (Shekhawat et al. 2012; Dwivedi and Mishra 2020). The extensive application of fertilizers favors the growth of bacterivores in agricultural fields (Wasilewska 1979; Yeates 1984).The first description of the Zeldia punctata was given by Thorne (1925), as the species was classified as Acrobeles punctata under the genus Acrobeles von Linstow, 1877. Later, a new genus Zeldia Thorne, 1937 was erected in the family Cephalobidae, and the species was transferred under this new genus as a type species. The species was again described as Acrobeles glaphyrus Steiner, 1935; Z. serrata Heyns, 1962; Z. paucipunctata Andrássy, 1967 which was synonymized under Z. punctata by Allen and Noffsinger (1972). The distinctive morphological characteristics, including the presence of teeth-like structures on the cheilorhabdions of the genus Zeldia were observed by Steiner (1938) with a description of Z. odontocephala. Yeates (1967) described Zeldia punua from the sand under the Ammophilia arenaria (L.) link under this genus. Furthermore, seven new species were described under the genus Zeldia, namely Z. feria, Z. minor, Z. tridentata, Z. spinula, Z. acuta, Z. neoacuta, and Z. solata by Allen and Noffsinger (1972). However, due to overlapping morphological characteristics, Z. minor was synonymized under Z. punctata by Rashid et al. (1985). Zeldia spannata Waceke et al., 2005 recovered from the Mojave deserts, were described under this genus; however, the species transferred to genus Chiloplacus as C. spannatus (Abolafia and Peña-Santiago 2018). So far, the genus Zeldia comprises nine valid species and two species as species inquirenda, namely Z. punctulata Thorne, 1925 and Z. setosa Cobb, 1914 (Allen and Noffsinger 1972; Rashid et al. 1985).
Zeldia punctata has been reported in the USA (Thorne 1925; Allen and Noffsinger 1972), Mexico (Steiner 1935), South Africa (Heyns 1962 ), Brazil (Rashid et al. 1985), Namibia (Rashid and Heyns 1990), Senegal (De Ley et al. 1990), Greece (Boström 1992), Botswana (De Bruin and Heyns 1993), India (Tahseen et al. 1999), Pakistan (Tabassum and Shahina 2002), Spain (Abolafia and Peña-Santiago 2003), Hungary (Andrássy 2005) and Iran (Mehdizadeh and Shokoohi 2013).The description of the Z. punctata was found to be based on morphological studies with fewer studies on molecular characterization. Although molecular description of the species was provided by De Ley et al. (1999), Nadler et al. (2006), Kiontke et al. (2007), Holovachov et al. (2015), Abolafia and Veechi (2021), and Shokoohi et al. (2022) in the construction of phylogenetic trees to resolve the Rhabditida group without providing detailed description of the species. Furthermore, bacterial communities associated with Z. punctata from South Africa have been studied (Shokoohi et al. 2022). Therefore, the study’s aims were (1) to study Z. punctata based on morphology and molecular analyses and (2) to study the dynamic populations of Z. punctata using principal component analysis (PCA).
Materials and methods
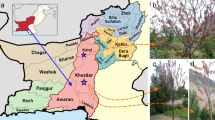
The present investigation was carried out in the fields of Haridwar district, Uttarakhand. The soils were collected from the mustard (Brassica rapa) fields of district Haridwar, Uttarakhand (29°54’29” N, 78°6’58” E; 265 m a.s.l.) viz., Bahadarabad, Khanpur, Laksar, Roorkee, Bhagwanpur and Narsan at a minimum depth of 5–30 cm (Southey 1974). Soil samples (250 g) were collected in transparent polyzipper bags labeled with the date, host plant, soil type, and locality. Further, the soil was brought to the Nematology Laboratory, Department of Zoology, Chaudhary Charan Singh University, Meerut, India, for further processing. The nematodes were extracted from 250 g of the soil, using modified Cobb’s (1918) sieving method and Baermann’s funnel techniques (Goodey 1957; Southey 1985).
Morphology and morphometry analysis
The adults were heat-killed in Ringer’s solution and then fixed in 50% TAF for 24 h. They were then fixed in 100% TAF for 5–7 days (Courtney et al. 1955); dehydrated in Seinhorst I and II (Seinhorst 1959). Further processing was done by following the Sharma and Chaubey (2023) description. The nematodes were permanently fixed on glass slides. The phase contrast microscope’s inbuilt software was used to perform observations and measurements in micrometers (µm). De Manian indices (de Man 1880) and other ratios were calculated. Images were captured with a Nikon Digital Sight camera and DIC optics (Y-TV55) on a Nikon microscope. Adobe®Photoshop®CS was used to edit the micrographs.
Scanning electron microscopy (SEM)
Some specimens preserved in glycerin were selected for their observation with a scanning electron microscope (SEM) according to the protocol described by Abolafia (2015). The nematodes were hydrated in distilled water, dehydrated in a graded ethanol-acetone series, critical point-dried, coated with gold, and observed with a Zeiss Evo scanning electron microscope (5 kV) (Model: EVO 18 Research, Country: India, Company: ZEISS).
Molecular characterization
The DNA was extracted from single virgin females (not having a plug at the vulva). The single virgin female was first washed with Ringer’s solution and then in Phosphate buffer saline (pH 7.2). Then, the nematode was transferred into a sterile Eppendorf tube (500 µl) with extraction buffer 20 µl (ddH20 17.7 µl, 10X Taq buffer with (NH4)2SO4 2 µl, 1% tween 2 µl, and proteinase K 0.1 µl). This buffer was frozen at -80°C for several days before being incubated at 65°C for 1 hour and subsequently at 95°C for 10 minutes. The lysates were chilled on ice, then centrifuged at 6000 × g for 2 minutes, and 3 µl of supernatant was used for PCR (Bharti et al. 2020). Partial sequence of 28S rDNA gene, D2–D3 domains were amplified using primers, D2A(5’-ACAAGTACCGTGAGGGAAAGTTG-3’) and D3B (5’-TCGGAAGGAACCAGCTACTA-3’) (De Ley et al. 1999) and partial sequence of 18S rDNA was amplified using primers NEM18SF: 5”-CGCGAATRGCTCATTACAACAGC-3” (forward) and NEM18SR: 5”-GGGCGGTATCTGATCGCC-3” (reverse) (Floyd et al. 2005). The 25 µl PCR product consisted of 7.5 µl nuclease-free distilled water, 1 µl each forward and reverse primer, 3 µl DNA extract, and 12.5 µl Dream Taq Green PCR master mix 2X (Thermo Scientific). The PCR amplifications were carried out using Verti 96 well fast thermocycler (AP Scientific) with heated lid pre-set at 95 ºC and subjected to the following cycling profile; as for the 18 S rDNA region, PCR conditions included 1 cycle of initial denaturation at 94 oC for 3 min; followed by 40 cycles of 94 oC (denaturation) for 30 s, 54 oC (annealing) for 30 s and 72 oC (primer extension) for 60 s, followed by a final extension at 72 oC for 10 min. For the D2-D3 rDNA region, PCR conditions included 1 cycle of initial denaturation at 95oC for 3 min, followed by 35 cycles of 95 oC (denaturation) for 45 s, 55 oC (annealing) for 45 s and 72 oC (primer extension) for 1 min, followed by a final extension at 72 oC for 10 min to confirm all of the PCR products are complete length. The amplified products were electrophoresed in 1% agarose (w/v) gel using 1x TAE buffer buffered agarose gel stained with ethidium bromide (45 min, 100 V) was used to assess amplification success. Bioserve Pvt. Ltd. Hyderabad (India) purified and sequenced the amplified PCR products. BioEdit was used to edit the newly discovered sequences (Hall 1999).
Phylogenetic analyses
The 18S rDNA and D2-D3 sequences were BLAST (Basic Local Alignment Search Tool) for % similarity matches with the previously submitted sequences in the NCBI (National Centre for Biotechnology Information) (Altschul et al. 1990). For each amplified rDNA region (18S rDNA and 28S rDNA), an alignment of our data with sequences from other closely related species was generated using default ClustalW parameters in MEGA 7.0 (Kumar et al. 2016) and manually optimized in BioEdit (Hall 1999). Bayesian inference (BI) was used to infer the phylogenetic trees from the datasets. Macrolaimus sp. (DQ145639) and Diastolaimus grossus (DQ145636) for 28 S and Psilenchus hilarulus (MK639403), and Aphelenchus avenae (JQ348399) for 18S were utilized as outgroup taxa and, with all characteristics being equally weighted and gaps being treated as missing data (Nadler et al. 2006; Mehdizadeh and Shokoohi 2013; Holovachov et al. 2015; Abolafia and Vecchi 2021). MrBayes 3.2.7 was used to produce Bayesian phylogenetic reconstructions (Ronquist et al. 2012). The best-fit nucleotide substitution models used for the phylogenetic analysis were selected using jModel-Test 2.1.10 (Darriba et al. 2012). The General Time Reversible substitution model with gamma-distributed rate variation across sites and a proportion of invariable sites (GTR + G + I) was used as the optimal nucleotide substitution model for the analyses. One tree was preserved per 1000 generations after running Metropolis-coupled Markov chain Monte Carlo generations (MCMC) for 1 × 107cycles. The partial sequences of 18S rDNA (ON739024), and 28S rDNA (OM280054) were deposited in NCBI GenBank.
Population dynamics
The population densities of Z. punctata in the samples were calculated by applying the formulae proposed by Norton (1978) to determine the ecological index. The samples were collected during the podding (September-October) and flowering period (December-February) from the rhizosphere of mustard fields. The calculations are as follows:
1- Absolute frequency = (No. of samples containing a species)/ (No. of samples collected) × 100.
2- Relative frequency = (Frequency of a species)/ (Sum of frequencies of all spp.) × 100.
3- Relative density = (No. of individuals of a species in a sample)/ (Total of all individuals in a sample) × 100.
4- Absolute density = (No. of individuals of a species in a sample)/ (Volume or mass or units of the sample) × 100.
5- Prominence value = (Absolute density × √ (Absolute frequency))/10.
Soil physiochemical properties
The pH of collected soil samples was measured by pH meter (Systronics pH system 362). For measuring the pH of each sample, 20 g of soil sample was dissolved in 100 ml of distilled water. The pH meter was calibrated before taking the readings. For proper dissolution, the solution was stirred with a magnetic stirrer (Spinot), and the reading was measured with a pH meter. For the estimation of electrical conductivity (EC), the conductivity cell was first calibrated with 0.01 M KCl. After calibrations, reading was taken from the digital TDS/ Conductivity meter (model: MK-509). For the available phosphorus (AP), 0.5 M NaHCO3 used as extractant (Olsen et al. 1954) and reading was obtained from the Spectrophotometer.
Statistical analyses
Principal component analysis (PCA) was done to find out the morphological variations between the isolated nematodes in the present investigation and closely related species of the nematodes using XLSTAT (Addinsoft 2007). The measurements of the Zeldia species were collected from their original descriptions. The 2D plot was formed by using the PC1 and PC2 values of each isolate based on eigenvalues given by the software. The relationship of the population indices, including AF, RF, AD, RD, and PV, with the environmental factors (temperature) and edaphic (pH, available phosphorus, and EC) were determined through canonical correspondence analysis (CCA) using XLSTAT.
Results
Zeldia punctata (Thorne, 1925 ) Thorne, 1937
Measurements - see Table 1 for 20 individuals of females, males, and juveniles in good state of preservation.
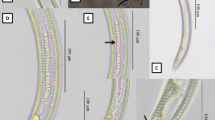
Female Lateral field with three incisures (Fig. 2d, f). Lip region with six lips amalgamated in pairs; labial probolae bifurcate, 2–5 μm long; primary axils U-shaped, secondary axils V-shaped (Figs. 1b, c, 2a, b). Pharyngeal corpus cylindrical (4–8 times isthmus length). Excretory pore at level of isthmus (Fig. 1a). Ovary reflexed at oviduct, posteriorly reflexed to vulva usually straight without flexures (Fig. 1g). Vagina short, straight and distally thick walled 5.15–7.91 μm long, occupying 14–19% of body diameter (Figs. 1e, 2c). Postvulval uterine sac distinct with 19.08–28.14 μm long. Post uterine sac 0.5–0.6 times the corresponding body diameter in length. Female tail conical (Fig. 2e), with concave ventral side (Fig. 1f, i).
Male Body 0.70–0.81 mm long, ventrally curved, appearing J- shaped (Fig. 1j). Reproductive system monarchic, with testis ventrally reflexed anteriorly located on the left side of the intestine (Fig. 1h). Spicules curved ventrally, long, broad, 2.0-2.5 times gubernaculum’s length, somewhat rounded and reduced manubrium, conical calamus, lamina slightly curved with a hump with a ventral velum, and a thin cylindrical tip (Fig. 1d). Gubernaculum well developed with manubrium straight, crura with a tip (Fig. 1d). Genital papilla observed, eight pairs consisting of three pairs of preanal papillae, four pairs of caudal papillae, and one pair of subdorsal papillae and phasmid. The preanal papillae present sub-ventrally, the caudal papillae where two pairs observed near tail terminus and two pairs arranged near the phasmid. Phasmids were observed anterior to the caudal papillae at 20–25% of tail length. Tail conical in shape, short with a pointed terminus.
Third stage juveniles (J3) Body 0.43–0.65 mm long becomes straight after fixation. Cuticle showed similarity with the adults. Lip region 7.37–10.62 μm wide with a narrow stoma. Distinct cephaloboid pharynx differentiated into three sections viz., corpus, isthmus and basal bulb. Nerve ring visible at the corpus level opposite to excretory pore. Deirids obscure. Rectum distinct, straight 1.0-1.2 of anal body diameter. Prominent anus. Tail conical in shape with a pointed terminus. General morphology showed similarity with adult female, except for the absence of reproductive system.
Remarks and morphometric comparison
Zeldia punctata is a widespread species that has been reported in different countries worldwide (Shokoohi and Abolafia 2019). The morphological and morphometrical analysis revealed the present isolate belongs to Z. punctata. The Indian population, compared with the American population (Thorne 1925), differs in tail length (43–56 vs. 33 μm). The Indian population of Z. punctata is similar to the specimens studied from India by Tahseen et al. (1999). Compared with Pakistani population of the same species (Tabassum and Shahina 2002), they differ in tail length (43–56 vs. 32–40 μm). Compared with the Spanish material examined by Abolafia and Peña-Santiago (2003), they differ in body length (0.74–0.86 vs. 0.62–0.82 mm), and shorter neck (184–223 vs. 202–216 μm) and spermatheca (11–16 vs. 37 μm). In comparison to the material examined from Hungary by Andrássy (2005), Indian specimens have shorter body length (0.74–0.86 vs. 0.64–0.94 mm) and shorter neck (184–223 vs. 210–230 μm). The comparative morphometrics for Z. punctata is given in Tables 2 and 3, respectively.
Molecular characterization
Pairwise distances (Kimura 2-parameter) based on the D2-D3 region revealed the genetic distances between the populations of Z. punctata described from other geographical regions. The Indian population showed the lowest genetic distance (0.0081) from the USA population (DQ145662). The highest genetic distance (0.7717) was observed between the USA (EU195988) and Sweden (KU180685) populations. The average overall genetic distance between the different populations of Z. punctata was 0.0579, ranging from 0.001to 0.7717. The lowest genetic distance (0.001) was observed between the USA (DQ145662) and Sweden (KU180685) population (Table 4). The Indian population showed the lowest distance (0.0081) with a population from the USA (DQ145662) and the highest distance (0.0185) with a population from Sweden (KU180685).
Phylogenetic relationships
The phylogenetic analysis based on 18S and 28S rDNA confirmed the species as Z. punctata. Based on the phylogenetic analysis inferred from 18S rRNA sequences; the Indian population formed a clade with the Netherlands population with a 1.00 posterior probability (Fig. 3).The phylogenetic tree based on the 28S rDNA sequences showed that the present Indian population placed close to the Swedish population of Z. punctata (MZ254892) with a 1.00 posterior probability. The Indian population was also placed close to other molecularly identified Z. punctata from the USA (AF147070; DQ145662; EU195988) and Sweden (KU180685) (Fig. 4).
Principal component analysis (PCA)
The principal component analysis based on the morphometric data of Z. punctata reported from different geographical locations, including India (Tahseen et al. 1999), USA (Allen and Noffsinger 1972), Brazil (Rashid et al. 1985), Namibia (Rashid and Heyns 1990), Senegal (De Ley et al. 1990), Greece (Bostrom 1992), Botswana (De Bruin and Heyns 1993), Pakistan (Tabassum and Shahina 2002), Spain (Abolafia and Peña-Santiago 2003), Iran (Mehdizadeh and Shokoohi 2013) and the present study revealed the morphometric variations (Table 5).
The Indian populations showed variations in the morphometry from the populations reported from other countries; however, the result indicated that the present population from India was close to the Iranian and Mexican populations of Z. punctata. The PCA based on the morphometry data of the female revealed an accumulated variability of 46.71% (Fig. 5; Table 5).The contribution of variation among the characters displayed in PC1 and PC2 was found to be 25.71% and 21.0%, respectively (Fig. 5). Three parameters, a (r = 0.606), and V% (r = 0.782) showed a positive correlation across populations of Z. punctata in PC1. Whereas neck length (r = -0.757), and tail length (r = -0.754) showed a negative correlation across populations of Z. punctata in PC1 (Fig. 5). In addition, L (r = 0.725), b (r = 0.728), and c’ (r = 0.738) showed positive correlation across populations of Z. punctata in PC2. The result also showed that some populations of Z. punctata were placed close together (Fig. 5; Table 6). The result grouped different populations of Z. punctata into four groups, including (1) formerly studied population from India (Tahseen et al. 1999), USA (Allen and Noffsinger 1972), and South Africa, (2) Pakistan, Brazil, Botswana, Greece, and USA (Thorne 1925), (3) Namibia, Spain, and Senegal, and (4) Mexico, Iran, and present population from India (Fig. 5). The result indicated that two populations from Indian placed separately based on the female morphometrics. Additionally, the result revealed the high interspecific and intraspecific variations in the populations of Z. punctata studied.
Population dynamics
The highest prominence value was observed in the Narsan and Laksar (88.2; 85.4), whereas the lowest was in the Bahadarabad (64.6) during the podding stages in 2021. The 2022 sampling revealed the highest prominence value in the Narsan and Bhagwanpur (91.6; 83.1) and the lowest in the Roorkee (48.9) (Table 7). The PCA analysis revealed that 77.36% of the variation, PC1 contributed 58.37%, and PC2 contributed 18.99%, where environmental factors like temperature and edaphic factors like pH, available phosphorus (AP) and electrical conductivity (EC) played a role in the population dynamics of Z. punctata (Fig. 6). The available phosphorus showed a positive correlation with the population dynamics of Z. punctata in Bhagwanpur, Bahadrabad, and Khanpur regions during flowering. The edaphic factor EC positively correlated with Roorkee and Khanpur in 2021 during the podding of mustard. The result showed no correlation between the present study’s diversity indices and ecological factors.
Discussion
The description of Z. punctata, recovered from sewage, based on six females from India was first provided by Tahseen et al. (1999); however, the description lacks male morphology and molecular data of the species. Furthermore, this species was described by Tabassum and Shahina (2002), Abolafia and Peña-Satiago (2003), and Mehdizadeh and Shokoohi (2013) describing females. Allen and Noffsinger (1972), Rashid et al. (1985), and Andrássy (2005) provided a description of males with limited morphology and morphometry. Likewise, the morphology of the juveniles was given by De Bruin and Heyns (1993). The present study is the first report on the molecular data of 18 and 28 S rDNA of Z. punctata from the Indian subcontinent with a detailed description of male. The morphological and morphometric data were found to be consistent with the type species (Thorne 1925) and redescribed by Thorne (1937), and Allen and Noffsinger (1972). Zeldia feria Allen and Noffsinger, 1972 can be distinguished from Z. punctata in terms of conspicuous punctuation, body length (0.77–0.98 vs. 0.74–0.86 mm), excretory pore (obscure vs. present), and tail (rounded vs. pointed). Zeldia punctata can be differentiated from Zeldia punua Yeates, 1967 by having shorter margins at labial probolae, phasmid (middle of tail vs. at the level of anus), and a longer tail. The description of Zeldia punua Yeates, 1967 is based on only three specimens, and the redescription given by Allen and Noffsinger (1972) is based on one specimen. Zeldia punctata can be distinguished from Z. odontocephala Steiner, 1938; Z. tridentata Allen and Noffsinger, 1972; Z. spinula Allen and Noffsinger, 1972; Z. acuta Allen and Noffsinger, 1972; Z. neoacuta Allen and Noffsinger, 1972 and Z. solata Allen and Noffsinger, 1972 as all the species possess accessory tooth associated with cheilorhabdions which is absent in the studied population. The species has been reported from sugarcane fields, sewage, beach sand, marginal soil, grapes,citrus tree, grasses, volcanic sandy soil, bulrush, date palm, sweet orange, tuberose, and maize fields. The present study also revealed mustard fields as a new host to Z. punctata.
The presence of available phosphorus was found to increase the population of nematodes. The use of fertilizers in agricultural fields has led to an increase in available phosphorus and nitrogen (Wasilewska 1979; Yeates 1984). During the flowering season, the Z. punctata population in the regions of Bahadarabad, Bhagwanpur, and Khanpur was positively correlated with the available phosphorus. The PV (81.8/84.2) of Bhagwanpur increased as the availability of phosphorus (158.5 ± 1.1; 175.1 ± 1.07) increased in this area. Similarly, the highest available phosphorus (189.5 ± 1.0; 192.3 ± 1.1) was observed in the Bahadarabad region, which stimulated bacterial populations and recorded PV (74.6/75.8). During the podding season in 2021, the population of Khanpur with a recorded PV (73.44) was found to be positively correlated with EC (0.174 ± 0.1). Likewise, the EC (0.224 ± 0.2) of Roorkee also showed a positive correlation with the Z. punctata population (PV = 78.6) during podding seasons. In 2021, the EC of Roorkee (0.224 ± 0.2) and Bhagwanpur (0.256 ± 0.2) was observed to be the highest during the podding season, which stimulated the Z. punctata population with a recorded PV of 78.6 and 81.8, respectively.
The phylogenetic analyses were found consistent with the results of Abolafia and Veechi (2021), and Kiontke et al. (2007). However, due to the few sequences available for Zeldia, molecular comparison with other species is not possible. In conclusion, Z. punctata is a free-living bacterivores nematode distributed worldwide. Because of their feeding behavior, they may impact crop production through microbial changes in the soil. In addition, they may also affect the plant-parasitic dynamics in the soil. The present result showed that EC, temperature, and phosphorus in mustard during the growing season affect Z. punctata population dynamics. Therefore, the species’ ecological role is suggested to be more studied using various soil physicochemical properties and concerning plant-parasitic nematodes, aiming for crop yield to increase.
Data availability
The data and material of this manuscript are available from the corresponding author on reasonable request.
References
Abolafia J, Peña-Santiago R (2003) Nematodes of the Order Rhabditida from Andalucía Oriental, Spain. The Genera Nothacrobeles Allen & Noffsinger, 1971 and Zeldia Thorne, 1937. J Nematol 35(3):233–243
Abolafia J, Peña-Santiago R (2018) On the identity of Chiloplacus subtenuis Rashid & Heyns, 1990 and C. tenuis Rashid & Heyns, 1990 (Rhabditida: Cephalobidae), with description of C. mysteriosus sp. n. Nematology 20(1):49–66. https://doi.org/10.1163/15685411-00003124
Abolafia J, Shokoohi E (2019) Soil and freshwater rhabditid nematodes (Nematoda, Rhabditida) from Iran: a compendium. Universidad de Jaén, Spain, pp 1–223
Abolafia J, Vecchi M (2021) Redescription and phylogenetic analysis of the type species of the genus Panagrellus Thorne, 1938 (Rhabditida, Panagrolaimidae), P. pycnus Thorne, 1938, including the first SEM study. J Nematol 53(1):1–20. https://doi.org/10.21307/jofnem-2021-080
Addinsoft (2007) XLSTAT, analyse de données et statistique avec MS Excel. Addinsoft, New York
Allen MW, Noffsinger EM (1972) A review of the genus Zeldia Thorne, 1937 (Nematoda: Cephalobidae) with descriptions of seven new species. Proc Helminthol Soc Wash 39:206–223
SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Andrássy I (1967) Ergebnisse der zoologischen Forschungen von Dr. Z. Kaszab in der Mongolei. 92. Weitere Bodennematoden aus den Jahren 1964 und 1965. Opusc Zool Budapest 7:203–233
Andrássy I (2005) Free-living nematodes of Hungary (Nematoda Errantia). Pedozoologica Hungarica 3 1:518
Bharti L, Bhat AH, Chaubey AK, Abolafia J (2020) Morphological and molecular characterisation of Merlinius brevidens (Allen, 1955) Siddiqi, 1970 (Nematoda: Rhabditida: Merlinidae) from India. J Nat Hist 54(23–24):1477–1498. https://doi.org/10.1080/00222933.2020.1810352
Boström S (1992) Some Cephalobidae (Nematoda: Rhabditida) from Crete, Greece. Fundam Appl Nematol 15:289–295
Cobb NA (1918) Estimating the nematode population of soil. United States Department Agri Tech Circu 1:1–48
Courtney WD, Polley D, Miller VL (1955) TAF, an improved fixative in nematode technique. Plant Dis Rep 39:570–571
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772
De Bruin S, Heyns J (1993) Cephalobidae (Nematoda: Rhabditida) from Botswana. Phytophylactica 25:1–10
De Ley P, Geraert E, Coomans A (1990) Seven cephalobids from Senegal (Nematoda: Rhabditida). J Afr Zool 104(4):287–304
De Ley P, Félix MA, Frisse LM, Nadler SA, Sternberg PW, Thomas WK (1999) Molecular and morphological characterisation of two reproductively isolated species with mirror-image anatomy (Nematoda: Cephalobidae). Nematology 1:591–612
De Man JG (1880) Die einheimischen, frei in der reinen Erde und im süssen Wasser lebenden Nematoden. Tijdschriftder Nederlandsche Dierkundige Vereeniging 5:1–104
Dwivedi S, Mishra R (2020) Effect of integrated plant nutrient management practices on quality characteristics of mustard seed. J Pharmacogn Phytochem 9(6S):175–179
Floyd RM, Rogers AD, Lambshead PJD, Smith CR (2005) Nematode-specific PCR primers for the 18S small subunit rRNA gene. Mol Ecol Notes 5(3):611–612. https://doi.org/10.1111/j.1471-8286.2005.01009.x
Goodey JB (1957) Laboratory methods for work with plant and soil nematodes.Technical Bulletin No. 2 Ministry of Agriculture, Fisheries and Food, London, 47 pp
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment and analysis program for Windows 95/98.NT. Nucleic Acids Symp Ser 41:95–98
Heyns J (1962) Osstellahamata n. gen., n. sp., Zeldia serrata n. sp. and Acrobeles thornei n. sp., three new nematodes from South Africa (Rhabditida: Cephalobidae). Nematologica 8(4):301–306
Holovachov O, Camp L, Nadler SA (2015) Sensitivity of ribosomal RNA character sampling in the phylogeny of Rhabditida. J Nematol 47(4):337
Kiontke K, Barrière A, Kolotuev I, Podbilewicz B, Sommer R, Fitch DH, Félix MA (2007) Trends, stasis, and drift in the evolution of nematode vulva development. Curr Biol 17(22):1925–1937
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Mehdizadeh S, Shokoohi E (2013) The genera Nothacrobeles Allen & Noffsinger, 1971 and Zeldia Thorne, 1937 (Nematoda: Rhabditida: Cephalobidae) from southern Iran, with description of N. abolafiai sp. n. Zootaxa 3637(3):325–340. https://doi.org/10.11646/zootaxa.3637.3.5
Nadler SA, Bolotin E, Stock SP (2006) Phylogenetic relationships of Steinernema Travassos, 1927 (Nematoda: Cephalobina: Steinernematidae) based on nuclear, mitochondrial and morphological data. Syst Parasitol 63(3):159–179. https://doi.org/10.1007/s11230-005-9009-3
Norton DC (1978) Ecology of plant parasitic nematodes. John Wiley and Sons, New York, p 268
Rashid F, Heyns J (1990) Description of Namibinema scaphovulva n. gen., n. sp. and Zeldia punctata (Thome, 1925) from Namibia (Nematoda: Cephalobidae). Phytophylactica 22(4):397–403
Rashid F, Geraert E, Sharma RD (1985) Morphology, taxonomy and morphometry of some Cephalobidae (Nematoda: Rhabditida) from Brazil with descriptions of two new genera and four new species. Nematologica 30 (1984): 251–299
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. https://doi.org/10.1093/sysbio/sys029
Seinhorst JW (1959) A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica 4:67–69
Sharma H, Chaubey AK (2023) Molecular and phenotypic characterization of Hemicriconemoides rosae (Rathour 2003) from mustard rhizosphere in India. JOBAZ 84(1):1–15. https://doi.org/10.1186/s41936-023-00338-6
Shekhawat K, Rathore SS, Premi OP, Kandpal BK, Chauhan JS (2012) Advances in agronomic management of Indian mustard (Brassica juncea (L.) Czernj. Cosson): an overview. Int J Agron. 2012:1–14. https://doi.org/10.1155/2012/408284
Shokoohi E, Mashela PW, Machado RA (2022) Bacterial communities associated with Zeldia punctata, a bacterivorous soil-borne nematode. Int Microbiol 1–10. https://doi.org/10.1007/s10123-021-00207-8
Southey JF (1974) New or unusual host-plant records for plant-parasitic nematodes, 1971–73. J Plant Pathol 23(1):45–46
Southey JF (1985) Laboratory methods for work with plant and soil nematodes.Ministry of Agriculture, Fisheries and Food, Reference Book 402, London
Steiner G (1935) Opuscula Miscellanea Nematologica, I. Proc Helm Soc Wash 2(1):41–45
Steiner (1938) Opuscula Miscellanea Nematological VII. (1). Observations on nematodes associated with Irish potatoes grown in South Carolina. Proc Helm Soc Wash 5:35–40
Tabassum KA, Shahina F (2002) Re-description of four known cephalobids (Nemata: Cephalobidae) from Pakistan. Pak J Nematol 20:25–37
Tahseen Q, Ahmad I, Jairajpuri MS (1999) Observations on three species of the subfamily Acrobelinae (Nematoda: Cephalobidae) from India. Nematology 1:527–537
Thorne G (1925) The genus Acrobeles Von Linstow, 1887. Trans Am Micros Soc 44:171–210
Thorne G (1937) A revision of the nematode family Cephalobidae Chitwood and Chitwood 1934. Proc Helm Soc Wash 4:1–16
Von Linstow O (1877) Helminthologische Beobachtungen. Arch Naturgesch Jahrg 42 Bd 1:1876
Waceke JW, Bumbarger DJ, Mundo-Ocampo M, Subbotin SA, Baldwin JG (2005) Zeldia spannata sp. n. (Nematoda: Cephalobidae) from the Mojave Desert. Calif JNMS 8(1):57–67
Wasilewska L (1979) The structure and function of soil nematode communities in natural ecosystem agrocenoses. Pol J Environ Stud 5:97–145
Yeates GW (1967) Studies on nematodes from dune sands. 5 Acrobelinae. NZ J Sci 10:527–547
Yeates GW (1984) Variation in soil nematode diversity under pasture with soil and year. Soil Biol Biochem 16:95–102
Acknowledgements
The authors thank the Department of Zoology, Chaudhary Charan Singh University, Meerut, for providing the necessary laboratory facilities for conducting the experiments. Head SAIF AIIMS, New Delhi, is also being acknowledged for providing scanning electron microscopic facilities.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
The study was designed by HS and AKC. Interpretation of the data was done by HS and ES. Manuscript writing was done by HS. Tables and reference settings were done by LB and HS. Errors and grammatical mistakes in the manuscript were removed and edited by AKC and ES. Then, the final manuscript was read and approved by all authors.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, H., Bharti, L., Chaubey, A.K. et al. Comparative morphometrics, molecular characterization, and dynamic population of Zeldia punctata associated with mustard crops from India. Biologia 79, 1761–1775 (2024). https://doi.org/10.1007/s11756-024-01658-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-024-01658-w