Abstract
Pratylenchus bolivianus (Nematoda, Pratylenchidae) an important parasitic lesion nematode of ornamental and crop plants was found in association with rooibos (Aspalathus linearis) tea in the Cederberg region of South Africa. The population distribution and frequency of occurrence of P. bolivianus on the rooibos orchards were determined, and nematode characterization was done using a combination of traditional morphological characteristics, scanning electron microscopy (SEM), morphometrics and molecular marker by amplifying the D2-D3 expansion segment of the 28S ribosomal RNA gene. P. bolivianus occurred at 84.6% frequency in the sampled fields, with a mean population density that ranged between 10 and 770 lesion nematodes per 250 ml. The morphological features are similar to previous reports, with a slight variation in stylet length and ratio of ‘a’ due to intraspecific geographical variations. The en face view of the SEM shows pattern of the oral disc and first labial annule that is characteristic of P. bolivianus a pattern that falls under Group 2 classification. The phylogenetic relationships as inferred from Maximum Likelihood and Maximum Parsimony revealed a close relationship between the South African isolate of P. bolivianus and those published from other geographical locations. The study confirmed a morphological and genetic similarity between the amphimictic population of P. bolivianus from South Africa and those reported from Costa Rica.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rooibos, Aspalathus linearis (Burm.f.) R. Dahlgren (Fabales: Fabaceae), is a unique African herbal tea plant that grows in the extreme edaphic and climatic conditions of the Cederberg mountainous region of the Western Cape and Eastern Cape provinces of South Africa (SARC 2020). The tea plant grows naturally in the wild, but is also cultivated in increasing monocultures, to meet the growing demands for domestic consumption and exportation. Rooibos is a tea of choice to many, being exported to about 30 countries with Germany, Netherlands, Japan, the UK and the USA as major importers (SARC 2018). Rooibos is endemic to the Cederberg region of the Western Cape where the temperature at times can range between low temperatures of 0 °C in winter months to an extremely high of more than 45 °C in summer (SARC 2018).
A major biotic challenge to the cultivation of rooibos in South Africa is the problem caused by insect pests (Hatting 2017). About 40 phytophagous insects have been reported to be directly associated with rooibos (Stals 1997; Hatting 2015) out of which a clear-wing moth, Felderiola candescens (Felder and Felder 1874) (Sesiidae), a leafhopper, Molopopterus theae Theron, 1978 (Cicadellidae), and looper, Isturgia exerraria (Prout, 1925) (Geometridae) have been listed as principal species (Hatting 2017). However, very little information is available on the association of plant-parasitic nematodes with cultivated rooibos in South Africa. An amphimictic population of the root-lesion nematode, Pratylenchus bolivianus Corbett 1983 (Tylenchida: Pratylenchidae), was reported for the first time on rooibos orchards from South Africa (Daramola et al. 2018), and the morphological and molecular data of the South African population of this nematode and their distribution on rooibos orchards will be required to provide useful information that is crucial for recommending control and management options.
Nematode pests constitute a major constraint to the cultivation of agricultural crops worldwide, including herbaceous perennials (LaMondia 1995; Walker and Melin 1998). Root-lesion nematodes of the genus Pratylenchus Filipjev 1936 are among the most important nematode pests, causing damage to many economic crops, including herbaceous and ornamental plants worldwide (Castilo and Vovlas 2007; Jones et al. 2013). The root-lesion nematodes are essentially migratory endoparasites, feeding on plant roots and causing severe root damage through their feeding activities. Symptoms of damage by the nematodes vary on different host plants and may include stunted growth, reduced yield and general symptoms of nutrient deficiency, due to the inability of roots to adequately absorb and supply nutrients that are required for optimum growth and development. Severely affected young plants may wilt, due to badly damaged root and eventually, loss of plant stands may become inevitable under heavily infested soils. Damage caused by root-lesion nematodes often results in necrotic lesions on plant roots. The damaged roots can also predispose plants to fungal pathogens such as Verticillium and Fusarium, thereby resulting in disease complexes and increasing the extent of damage (Bucki et al. 2020).
About 100 species of nematodes in the genus Pratylenchus have been recognized worldwide from a wide range of plant hosts (Geraert 2013; Bucki et al. 2020). In South Africa, approximately 21 nematode species in the genus have been reported in association with a wide range of field and horticultural crops such as soybean (Glycine max (L.) Merr.), banana (Musa acuminata (AAA) Cavendish subgroup), apple (Malus domestica Borkh.), maize (Zea mays L.), sunflower (Helianthus annuus L.) and various other economic crops. The species of the genus Pratylenchus nematodes that were reported include P. bolivianus Corbett 1983, P. brachyurus (Godfrey 1929) Filipjev and Schuurmans-Stekhoven 1941, P. coffeae (Zimmermann 1898) Filipjev and Schuurmans-Stekhoven 1941, P. crenatus Loof, 1960, P. delattrei Luc 1958, P. fallax Seinhorst 1968, P. flakkensis Seinhorst 1968, P. goodeyi Sher and Allen 1953, P. hexincisus Taylor and Jenkins 1957, P. hippeastri Inserra et al. 2007, P. loosi Loof 1960, P. pratensis (de Man 1880) Filipjev 1936, P. pseudopratensis Seinhorst 1968, P. neglectus (Rensch 1924) Filipjev and Schuurmans Stekhoven, 1941, P. penetrans (Cobb 1917) Filipjev and Schuurmans-Stekhoven 1941, P. scribneri Steiner 1943, Pratylenchus spp., P. tenuis Thorne and Malek 1968, P. teres Khan and Singh 1974, P. thornei Sher and Allen 1953, P. vulnus Allen and Jensen 1951 and P. zeae Graham 1951 (Van den Berg 1971; Smith 1982; Marias and Swart 1996; Kleynhans et al.1996; Fourie et al. 2001; Carta et al. 2002; Van den Berg et al. 2007; Daneel et al. 2015; Daramola et al. 2018; Knoetze et al. 2019). In some cases, severe damage such as stunted growth and wilting has been reported (Bolton et al. 1989) due to damage by the lesion nematodes.
The occurrence and damaging potential of P. bolivianus have been reported from South and Central American countries such as Bolivia, Chile, Colombia and Costa Rica (Corbett 1983; De Luca et al. 2011; Múnera Uribe 2015; Araya et al. 2016). Other records are from the UK, the Netherlands and the USA (Cotten et al. 1991; Amsing 1996; Waeyenberge et al. 2000; Troccoli et al. 2016). P. bolivianus has been reported as causing damage to plants such as Cape gooseberry, Physalis peruviana L., carnation, Dianthus caryophyllus L., oats Avena sativa L., potato, Solanum tuberosum L., tomato, Solanum lycopersicum L. and sword ferns, Nephrolepis exaltata (L.) Schott (Troccoli et al. 2016). The extent of damage caused by the nematode has raised some phytosanitary and quarantine concerns and the need to limit its spread in soil and plant debris. The occurrence of this nematode species on established monocultures in South Africa is a serious concern, especially when it is associated with an endangered plant species such as A. linearis. This is due to the associated risks of poor root development and depressed growth, along with foliage and yield reduction, which can have serious implications on rooibos production; thereby posing a potential threat to the budding tea industry.
Accurate identification of nematode species is important for taking phytosanitary and quarantine decisions and to limit the spread of nematode pests on agricultural crops. The use of traditional morphological and morphometrics for identifying species in the genus Pratylenchus is difficult and may be subjective, due to overlapping of characters and little morphological diversity. Many species in the genus show high levels of intraspecific variability; however, the use of molecular tools in combination with morphological identification provides a more reliable and accurate option for species delimitation.
In this study, a survey of the population distribution of P. bolivianus on rooibos monocultures in the Cederberg region of South Africa was conducted, and the South African population of the root-lesion nematode was characterized using morphological and molecular tools. The morphology and DNA sequences of this South African population were compared with other populations of P. bolivianus that have been previously reported from other geographical areas, therefore providing supporting data of the nematode identification.
Materials and methods
Field sampling and nematode extraction
A survey was conducted on rooibos monocultures in the Cederberg region of the Western Cape province of South Africa. Thirteen rooibos monocultures were sampled during the survey, which was based on road accessibility to farmland and the consent of the farmers to allow sampling. The sampling sites are located at Piketberg, Piekenierskloof, Alexanders Hoek, Citrusdal, Rietvlei and Skurfkop (Fig. 1).
Soil samples were obtained from the root zone of the rooibos plants. Each soil sample was a composite of 20–25 soil cores randomly taken from the same field at a depth of 25–30 cm with a hand trowel. The samples were placed in labelled plastic bags, sealed and transported to the laboratory for nematode extraction. Nematodes were extracted from a 250 ml sub-sample of soil by a decanting and sieving method (Cobb 1918), followed by the sugar flotation technique (Jenkins 1964). The nematode suspension was then concentrated to 20 ml, from which an aliquot of 5 ml was taken for observation and counting using a stereoscopic microscope. Nematodes with the characteristic features of Pratylenchus were picked and placed in distilled H20 inside cavity glass blocks for further morphological and molecular studies.
Morphological identification of nematodes and scanning electron microscopy (SEM)
Morphological identification of the nematodes was done under a Zeiss research compound microscope, equipped with a camera (Leica DFC 295), and LAS. 4.0 live measuring software. Specimen for light microscopy was killed with gentle heat and mounted on glass slides for microscopic observation. Permanent mounts were prepared using Seinhorst’s rapid technique (Seinhorst 1959). The diagnostic features, morphological characters and morphometrics of the lesion nematodes were taken using the compound microscope. Photomicrographs of the male and female specimens, including juveniles, were obtained, and all measurements were expressed in micrometres (µm).
For SEM, nematode specimens were fixed and then dehydrated in an increasing series of ethanol (70%, 80%, 90% and twice 100%). The specimens were critical point dried with CO2, mounted on SEM stubs and sputter-coated with Au/Pd at a thickness of about 200 Å (Eisenback 1986). The nematode specimens were viewed with a scanning electron microscope at 10 kV.
Molecular identification
DNA extraction, polymerase chain reaction and sequencing
Specimens for molecular identification were isolated from nematode samples (aliquots that were used for the morphological identification) and washed twice in distilled water inside a glass cavity block. DNA was extracted from individual single adults that were aseptically cut into bits of 2–3 pieces in a 10 µl lysis buffer (500 mM MgCl, 10 mM DTT, 4.5% Tween20, 0.1% gelatine and 3 μl proteinase K at 600 µg ml−1) and placed on the side of 0.5 mL Eppendorf tubes. The samples were placed in − 80 °C for 15 min, then incubated at 65 °C for 60 min and 95 °C for 15 min and kept at − 20 °C until future use or processed immediately for PCR assays.
Polymerase chain reaction for the amplification of the 28S rRNA was conducted using a modified method of Nguyen (2007) with KAPA2G™ Robust Hotstart ReadyMix (KAPA Biosystems) and the primers combination of D2A (ACAAGTACCGTGAGGGAAAGTTG) and D3B (TCGGAAGGAACCAGCTACTA) (Subbotin et al. 2006). The PCR conditions were as described by Tanha Maafi et al. (2003). The amplicons were separated on 1.5% agarose gel stained with ethidium bromide and visualized under UV light with a trans-illuminator imaging system.
PCR products were purified using the Nucleo-Fast Purification System (Macherey Nagel, Waltham, Massachusetts, USA). Sequencing of the purified DNA was done at the DNA Sequencing Unit of Stellenbosch University (Central Analytical Facilities, Stellenbosch University) and was performed in both directions using the Big Dye Terminator V1.3 sequencing kit, followed by the use of electrophoresis on the 3730 X 1DNA Analyser. The newly obtained sequences were submitted to the GenBank under the accession numbers MG871467, MW900157 and MW900158.
Sequence management and phylogenetic analyses
Sequence editing was done with Qiagen CLC Main Workbench (ver. 8.0) and the newly obtained sequence of the D2D3 expansion segment of the rRNA gene was compared with other closely related sequences on National Centre for Biotechnology Information (NCBI) using BLASTn (Altschul et al. 1997). The newly obtained sequence was further aligned against published gene sequences species within the genus Pratylenchus using the multiple alignment program for amino acid or nucleotide sequences MAFFT ver. 7 475 (Katoh and Standley 2013). Phylogenetic relationship within the closely related sequences was inferred using Maximum Parsimony (MP) and Maximum Likelihood (ML) methods and conducted using the software Molecular Evolution Genetics Analysis (MEGA) X ver. 10 (Kumar et al. 2018).
Results
Nematode population distribution
Nine genera of plant-parasitic nematodes were found in association with A. linearis from the sampled locations (Fig. 1). They include Aphelenchoides Fischer, 1894, Criconema Hofmanner and Menzel, 1914, Ditylenchus Filip'ev 1936, Hemicycliophora de Man, 1921, Longidorus Micoletzky, 1922, Neodolichorynchus estherae (Kleynhans 1992) Siddiqi, 2000, Pratylenchus, Scutellonema (Steiner 1937) Andrássy 1958 and Tylenchus Bastian, 1865. Thirteen fields were sampled and P. bolivianus was identified from 11 of the samples, thus occurring at a frequency of 84.6%. Higher population density of the P. bolivianus was recorded from Rietvlei and Citrusdal at 770/250 ml and 730/250 ml soil, respectively, while a lower nematode population of 10/250 ml was recorded from some fields in Alexanders Hoek and Piketberg (Fig. 2).
Morphological identification
Measurements of P. bolivianus (n = 10 females) L = 522.7 (481.4–587.3) µm; a = 22.3 (16.7–25.4); b = 5.3 (4.3–7.0); b' = 4.0 (3.5–5.0); c = 18.7 (16.8–20.5); V% = 80.8 (78.9–81.8) %; stylet = 17.5 (16.0–18.6) µm (Table 1). Males (n = 5): L = 478 (427.7–553.4); a = 27.1 (22.7–35.5); b = 5.3 (4.6–6.5); b' = 3.8 (3.6–4.0); c = 18.3 (16.9–19.9); stylet = 16.3 (15.6–17.5).
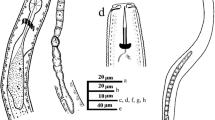
The South African population of the amphimictic species of P. bolivianus being reported in this study from the rhizosphere of A. linearis is characterized by a small-sized body that is almost straight. The lip region is almost continuous with the body having three distinct lip annuli (Figs. 3 and 4). The en face view by SEM (Fig. 4a–c) shows a lip region with an oval oral aperture positioned on the labial disc with two amphidial openings, one on each side of the oral aperture. The oral disc and the dorsal and ventral submedian lip sectors appear fused together with divisions between the submedian and lateral segments and form almost a rectangular-shaped configuration (Fig. 4a–c), according to the lip pattern arrangement proposed for Group 2 of Pratylenchus species described by Corbett and Clark (1983).
The stylet is strong, robust and conus, with round prominent stylet knobs (Fig. 3a). The dorsal orifice gland opening (DOGO) is posterior to the stylet base. Pharyngeal procorpus is cylindrical and narrows anteriorly to a well-developed median bulb, which is ovate and has a conspicuous central valve (Fig. 3a). The pharyngeal lobe overlaps the intestine ventrally and laterally (Fig. 3d). The excretory pore is located anterior to the level of cardia with nerve ring encircling the isthmus. Vulva is posteriorly located at about 81% of the body length (Fig. 3b). The reproductive system is monodelphic with a single anteriorly positioned ovary and a post-vulva uterine sac that is 25 µm long (Fig. 3d). Spermatheca is rounded, to spherical, but not very conspicuous.
The tail is conical and tapers asymmetrically with a characteristic thickened cuticle and coarse annulation towards the tail end (Figs. 3b and 4g). Tail tip is smooth and rounded with 16–19 tail annuli at the terminus. Lateral fields have four lines, with two outer bands that are narrower than the centre band the in mid-body. Phasmids are pore-like and centred in lateral fields and located near the mid-tail (Fig. 4g–i).
Males are similar to females except for their smaller size and sexual dimorphism (Fig. 3c). The anterior region is also slightly more slender and the lip region higher when compared to the female (Fig. 5k–l). Testis are short and outstretched, spicules are paired (14.4–17.8 µm), and the gubernaculum is 5.6 µm (4.3–6.8 µm) long and slightly curved. The tail is conical and enveloped by a bursa.
The morphology and morphometric descriptions of the South African population agree with the original description of P. bolivianus by Corbett 1983. It, however, differs from the original (pm) population and those described from Colombia (Múnera Uribe 2015) by presence of both male and female nematodes. The descriptions of the South African population (including SEM studies) are also similar to the amphimictic population given by Araya et al. (2016) and those described from Florida (Troccoli et al. 2016) except for slightly smaller body length (481–587 µm vs 560–744 µm) which may be linked to geographical intraspecific variability.
Molecular identification and phylogenetic analyses
Newly obtained nucleotide sequences of the South African isolate of P. bolivianus which were derived from the amplification of the 28S expansion segment of the ribonucleic RNA gene (MG871467, MW900157 and MW900158), produced sequences of approximately 760 base pairs and were close in similarity (with one nucleotide difference) to the Costa Rica isolate. The DNA sequences of the amphimictic population from South African were aligned with published sequences of P. bolivianus and closely related species from other geographical locations using Boleodorus sp. from China as the outgroup (Araya et al. 2016). A total of 23 nucleotide sequences were obtained.
The result of the molecular identification showed that the South African isolates are genetically similar to other published P. bolivianus isolates, with strong bootstrap support. Estimates of the evolutionary divergence between the sequences revealed that the pairwise distance of the South African nucleotide isolate, compared with isolates of P. bolivianus from other geographical locations, ranged between 1 and 4 nucleotide base pair differences.
Analysis of the phylogenetic relationship within the closely related Pratylenchus species as inferred from the ML and MP (Fig. 5) shows that there is a congruence in the position of the South African population, which merged and clustered together with other sequences of P. bolivianus, thus clearly separating it from other Pratylenchus species within the group. The South African isolate is also a closer taxon to the amphimictic population from Costa Rica within the P. bolivianus clade as shown in the phylogenetic analysis.
Discussion
The root-lesion nematodes, Pratylenchus, have been recognized as one of the major constraints to crop production worldwide (Castillo and Vovlas 2007). Nematodes belonging to the genus have been implicated, as important nematode pests of agricultural crops, due to their wide host ranges and their ability to cause a significant reduction to the quality and quantity of agricultural outputs, including ornamental and horticultural crops. Although economic damage by P. bolivianus has been reported from South America, Europe, China and America on some cultivated and ornamental crops (Corbett 1983; De Luca et al. 2011; Múnera Uribe 2015; Araya et al. 2016; Troccoli et al. 2016); there is a more recent report on the association of this nematode species rooibos in South Africa (Daramola et al. 2018). The current study confirms a widespread distribution of P. bolivianus on rooibos orchards in the Western Cape of South Africa. The potential for this root-lesion nematode to cause damage on rooibos orchards can be of economic importance, given the percentage frequency of its occurrence and the population densities of the nematodes that were recorded in some fields. Further investigation on yield reduction assessment will, however, confirm the damage potential of the nematode on rooibos tea plants.
Accurate and precise diagnosis of nematode species is an important factor to be considered in order to effectively mitigate the problem of yield reduction and losses that is linked to nematode pests of economic crops. Identification of nematode species has been traditionally hinged on morphological and morphometric features, which has played a very significant role in characterizing important nematode species. However, despite its importance, morphological diagnosis is greatly hampered by phenotypic plasticity, interspecific similarities and a reducing number of skilled taxonomy specialists Bucki et al. (2020). Moreover, delimitation of some cryptic species within the genus Pratylenchus can be cumbersome and subjective, thus leading to erroneous identification. In the current investigation, the population of P. bolivianus associated with A. linearis in South Africa was successfully characterized and the morphological and morphometric data are provided in combination with a molecular identification by the amplification of the 28S ribosomal RNA gene. The result of this study is congruent with previous studies on morphological and molecular characterization of P. bolivianus from other geographical locations (Araya et al. 2016; Troccoli et al. 2016). The study also confirms the presence of the amphimictic population of P. bolivianus in South Africa, which is similar, morphologically and genetically to the amphimictic population from Costa Rica, but differs slightly in terms of smaller body size, which can be linked to intraspecific variation as suggested by (Araya et al. 2016). The presence of amphimictic and parthenogenetic populations in some species of Pratylenchus has been reported (Luc 1987). A comprehensive report on the possibility of variations in the morphological features of P. bolivianus from different geographical locations despite their genetic similarities has been discussed (Troccoli et al. 2016). The current study provides additional data on morphological variation observed in the amphimictic South Africa population which is a variant from the original type description of the parthenogenetic population from Bolivia Corbett (1983) and other descriptions Valenzuela and Raski 1985; Araya et al. 2016 and Troccoli et al. 2016). The presence of this amphimictic population in South Africa may also be influenced by the Mediterranean climate that is characterized by extremely hot summer temperatures, which favours the presence of males as suggested by Troccoli et al. (2016). Damage to herbaceous perennials such as A. linearis by nematode pests could be fatal and result in heavy economic losses, thereby posing a significant threat to manufacturing companies that rely heavily on this unique plant species that grow exclusively in the fynbos biome of South Africa. There is also a need to promote the conservation of this important herbaceous tea plant, especially when they are confirmed to be highly susceptible (Hatting 2017) to a significant number of insect pests including plant-parasitic nematodes.
Conclusion
The South African population of the root-lesion nematode, P. bolivianus, reported in the current investigation is widespread and abundant in the sampled rooibos orchards from the Cederberg region with extreme climatic conditions, located in the Western Cape province of South Africa. Photomicrographs and SEM images of the nematode species are provided with morphometric data. The isolates are genetically similar to the amphimictic population reported from Costa Rica with very slight intraspecific morphological variations. The combined identification method used in this study therefore offers a useful tool for characterizing this important root-lesion nematode.
References
Allen MW, Jensen HJ (1951) Pratylenchus vulnus, new species (Nematada: Prarylenchinae), a parasite of trees and vines in California. Proc Helminthol Soc Wash 18:4750
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res 25(17):3389–3402. https://doi.org/10.1093/nar/25.17.3389
Amsing JJ (1996) Population dynamics and damage potential of the root-lesion nematode, Pratylenchus bolivianus, on Alstroemeria. Nematologica 42:71–79. https://doi.org/10.1163/187529296X00076
Andrássy I (1958) Hoplolaimus tylenchifonnis Daday, 1905 (syn. H. coronatus Cobb, 1923) und die Gattungen der Unterfamilie Hoplolaiminae Filipjev, 1936. Nematologica 3:44–56. https://doi.org/10.1163/187529258X00337
Araya TZ, Padilla WP, Archidona-Yuste A, Cantalapiedra-Navarrete C, Liébanas G, Palomares-Rius JE, Castillo P (2016) Root-lesion nematodes of the genus Pratylenchus (Nematoda: Pratylenchidae) from Costa Rica with molecular identification of P. gutierrezi and P. panamaensis topotypes. Eur J Plant Pathol 145(4):973–998. https://doi.org/10.1007/s10658-016-0884-z
Bastian CH (1865) Monograph on the anguillulidae, or free nematoids, marine, land and freshwater; with descriptions of 100 new species. Trans Linn Soc Lond 25:73–184
Bolton C, De Waele D, Loots GC (1989) Plant-parasitic nematodes on field crops in South Africa III Sunflower. Rev De Nématol 12(1):69–75
Bucki P, Qing X, Castillo P, Gamliel A, Dobrinin S, Alon T, Braun MS (2020) The genus Pratylenchus (Nematoda: Pratylenchidae) in Israel: From taxonomy to control practices. Plants 9:1475. https://doi.org/10.3390/plants9111475
Carta LK, Handoo ZA, Skantar AM, Van Biljon J, Botha M (2002) Redescription of Pratylenchus teres Khan & Singh, 1974 (Nemata: Pratylenchidae), with the description of a new subspecies from South Africa, and a phylogenetic analysis of related species. African Plant Protection 8:13–24
Castillo P, Vovlas N (2007) Pratylenchus (Nematoda: Pratylenchidae): diagnosis, biology, pathogenicity, and management. In: Hunt DJ, Perry RN (eds) Nematology monographs and perspectives, vol 6. Brill, Leiden, The Netherlands, pp 529
Cobb NA (1917) A new parasitic nema found infesting cotton and potatoes. J Agric Res 11:27–33
Cobb NA (1918) Estimating the nematode population of the soil. Agricultural Technology Circular I. Bureau of Plant Industry, Department of Agriculture, United States, pp 1–48
Corbett DCM (1983) Three new species of Pratylenchus with a redescription of P. andinus Lordello, Zamith & Boock, 1961 (Nematoda: Pratylenchidae). Nematologica 29:390–403. https://doi.org/10.1163/187529283X00276
Corbett DCM, Clark SA (1983) Surface feature in the taxonomy of Pratylenchus species. Rev De Nématol 6:85–98
Cotten J, Barlett PW, Webb RM (1991) A first record of the root-lesion nematode, Pratylenchus bolivianus Corbett in England and Wales. Plant Pathol 40:311–312. https://doi.org/10.1111/j.1365-3059.1991.tb02383.x
Daneel M, De Jager K, Van den Bergh I, De Smet M, De Waele D (2015) Occurrence and pathogenicity of plant-parasitic nematodes on commonly grown banana cultivars in South Africa. Nematropica 45(1):118–127
Daramola F, Knoetze R, Malan AP (2018) First report of the root lesion nematode, Pratylenchus bolivianus, on Aspalathus linearis in South Africa. Plant Dis 102(9):1860. https://doi.org/10.1094/PDIS-02-18-0215-PDN
De Luca F, Reyes A, Troccoli A, Castillo P (2011) Molecular variability and phylogenetic relationships among different species and populations of Pratylenchus (Nematoda: Pratylenchidae) as inferred from the analysis of the ITS rDNA. Eur J Plant Pathol 130:415–426. https://doi.org/10.1007/s10658-011-9763-9
de Man JG (1880) Die Einheimischen, frei in der reinen Erde und im siissen Wasser lebende Nematoden. Vorlaüfiger Bericht und descriptiv-systematischer Theil. Tijdschr Nederl Dierk Vereen 5:1–104
de Man JG (1921) Nouvelles recherches sur les nématodes libres terricoles de la Hollande. Capita Zoologica 1:3–62
Eisenback JD (1986) Comparison of techniques useful for preparing nematodes for scanning electron microscopy. J Nematol 18(4):479–487
Felder C, Felder R (1874) Lepidoptera von Dr. Cajetan Felder, Rudolf Felder und Alois F. Rogenhofer. Atlas. [Sesiidae]. Reise der österreichischen Fregatte Novara um die Erde in den Jahren 1857, 1858, 1859 unter den Befehlen des Commodore B. von Wüllerstorf-Urbair. Zoologischer Theil. Zweiter Band. Zweite Abtheilung (fasc. 4): 2–9
Filipjev IN (1936) On the classification of the Tylenchinae. Proc Helminthol Soc Wash 3:80–82
Filipjev IN, Schuurmans Stekhoven JH (1941) A manual of agricultural Helminthology. Brill, Leiden, pp 878
Fischer M (1894) Lher eine Clematis-Iirankheit. Rer, physiol. Lab. Lanciwirtsch. Inst. Univ. Idalle, vol 3, pp 1–11
Fourie H, Mc Donald AH, Loots GC (2001) Plant-parasitic nematodes in field crops in South Africa. 6. Soybean Nematol 3:447–454. https://doi.org/10.1163/156854101753250773
Geraert E (2013) The Pratylenchidae of the World: Identification of the Family Pratylenchidae (Nematoda: Tylenchida). Academia Press, Ghent, Belgium
Godfrey GH (1929) A destructive root disease of pineapple and other plants due to Tylenchus brachyurus. Phytopathol 19:611–629
Graham TW (1951) Nematode root rot of tobacco and other plants. South Carolina Agricultural Experiment Station Bulletin, 390
Hatting JL (2017) Major insect pests and their natural enemies associated with cultivation of Rooibos, Aspalathus linearis (Burm. f.) R. Dahlgren, in South Africa: A review. S Afr J Botany 110:118–123. https://doi.org/10.1016/j.sajb.2016.07.016
Hatting JL (2015) Rooibos. In: Prinsloo G, Uys V (eds) Insects of Cultivated Plants and Natural Pastures in Southern Africa. Entomological Society of Southern Africa, Pretoria, pp 298–309
Hofmanner B, Menzel R (1914) Neue arten freilebender Nematoden aus der Schweiz. Zool Anz 44:80–91
Inserra RN, Troccoli A, Gozel U, Bernard EC, Dunn D, Duncan L (2007) Pratylenchus hippeastri n. sp. (Nematoda: Pratylenchidae) from amaryllis in Florida with notes on P. scribneri and P. hexincisus. Nematology 9:25–42
Jenkins WRB (1964) A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Dis Rep 48:693
Jones JT, Haegeman A, Danchin EG, Gaur HS, Helder J, Jones MG, Kikuchi T, Manzanilla-López R, Palomares-Rius JE, Wesemael WM, Perry RN (2013) Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol 14(9):946–961. https://doi.org/10.1111/mpp.12057
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30(4):772–780. https://doi.org/10.1093/molbev/mst010
Khan E, Singh DB (1974) Five new species of Pratylenchus (Nematoda: Pratylenchidae) from India. Indian J Nematol 4:199–211
Kleynhans KPN (1992) New species of Tylenchorhynchus Cobb, 1913, Paratrophurus Arias, 1970 and Histotylenchus Siddiqi, 1971 from South Africa and Namibia (Nemata: Belonolaimidae). Phytophylactica 24:235–251
Kleynhans KPN, Van Den Berg E, Swart A, Marais M, Buckley NH (1996) Plant nematodes in South Africa. Plant Protection Research Institute Handbook No. 8. Pretoria, Business Print, pp 165
Knoetze R, Van den Berg E, Girgan C, Van der Walt L (2019) First report of the root-lesion nematode, Pratylenchus hippeastri, on apple in South Africa. J Plant Dis Prot 126:607–609. https://doi.org/10.1007/s41348-019-00259-z
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
LaMondia JA (1995) Response of perennial herbaceous ornamentals to Meloidogyne hapla. Suppl J of Nematol 27:645–648
Loof PAA (1960) Taxonomic studies on the genus Pratylenchus (Nematoda). Tijdschrift Voor Plantenziekten 66:29–90
Luc M (1958) Les nématodes et le fl étrissement des cotonniers dans le SudOuest de Madagascar. Coton et Fibres Tropicales 13:1–18
Luc MA (1987) A reappraisal of Tylenchina (Nemata). 7. The family Pratylenchidae Thorne, 1949. Rev De Nématol 10:203–218
Marais M, Swart A (1996) Plant-parasitic nematodes of the Lower Orange River irrigation area South Africa. Afr Plant Prot 2(1):25–30
McDonald A, Fourie H, Loots G (2001) Plant-parasitic nematodes in field crops in South Africa 6 Soybean. Nematology 3(5):447–454. https://doi.org/10.1163/156854101753250773
Micoletzky H (1922) Die FreilebendenErd-Nematoden Archiv Für Naturgeschichte. Berlin A87:1–650
Múnera Uribe GD (2015) Nematodes associated with (Physalis peruviana L.) plants in 24 Colombian municipalities. In: Abstracts, 47th Annual Meeting of the Organization of Nematologists of Tropical America, Varadero, Cuba, pp 53 [Abstr.]
Nguyen KB (2007) Methodology, morphology and identification. In: Nguyen K, Hunt DJ (eds) Entomopathogenic nematodes: systematics, phylo-geny and bacterial symbionts. Brill, Leiden, The Netherlands, pp 59–119
Prout LB (1925) New species of Geometridae (Lepidoptera) in the collections of the South African Museum. Ann South Afr Museum 19(4):579–600
Rensch D (1924) Aphelenchus neglectus sp. n. eine neue parasitäre Nematodenart. Sonderabdruck aus dem Zoologischen Anzeiger 59:277–280
Seinhorst JW (1959) A rapid method for the transfer of nematodes from fixative to anhydrous glycerine. Nematologica 4:67–69
Seinhorst JW (1968) Three new Pratylenchus species with a discussion of the structure of the cephalic framework and of the spermatheca in this genus. Nematologica 14:497–510
Sher SA, Allen MW (1953) Revision of the genus Pratylenchus (Nematoda: Tylenchidae). Univ Calif: Publs ZOOS 57:441–470
Siddiqi MR (2000) Tylenchida parasites of plants and insects, 2nd edn. UK, CABI Publishing, Wallingford
Smith PC (1982) Nematode pests of grapevines. In: Keetch DP, Heyns J (eds) Nematology in Southern Africa. Department of Agriculture and Fisheries, South Africa, Science Bulletin, No. 400, pp 88–95
South African Rooibos Council (2018) Rooibos Industry Fact Sheet. Available at https://sarooibos.co.za/wp/wp-content/uploads/2018/08/SARC-2018-Fact-Sheet-1.pdf
South African Rooibos Council (2020) Rooibos Industry Fact Sheet. Available at https://sarooibos.co.za/wp/wp-content/uploads/2020/12/SARC-2020-Information-sheet.pdf
Stals R (1997) Plantvretende insekte van rooibos [Plant eating insects of rooibos]. Unpublished report to Rooibos Production and Technical Services. South African National Collection of Insects. ARC-Plant Protection Research Institute, Pretoria
Steiner G (1937) Opuscula miscellanea nematologica. V Proc. helnzinth. Soc Wash 4:33–38
Steiner G (1943) Description of Pratylenchus scribneri. In: Sherbakoff CD, Stanley WW (eds) The more important diseases and insect pests of crops in Tennessee. Tenn. Agr. Sta. Bulletin, 186, ppThorne G, Malek RB (1968) Nematodes 142
Subbotin SA, Sturhan SA, Chizov VN, Vovlas N, Baldwin JG (2006) Phylogenetic analysis of Tylenchida Thome, 1949 as inferred from D2 and D3 expansion fragments of the 28S rRNA sequences. Nematology 8:455–474. https://doi.org/10.1163/156854106778493420
Tanha Maafi Z, Subbotin SA, Moens M (2003) Molecular identification of cyst forming nematodes (Heteroderidae) from Iran and a phylogeny based on the ITS sequences of rDNA. Nematology 5:99–111. https://doi.org/10.1163/156854102765216731
Taylor DP, Jenkins WR (1957) Variation within the nematode genus Pratylenchus with the descriptions of P. hexincisus n. sp. and P. subpenetrans n. sp. Nematologica 2:159–174
Theron JG (1978) Southern African species of the genus Molopopterus Jacobi (Hemiptera: Cicadellidae), with a note on a species attacking rooibos tea. J Entomol Soc Southern Africa 41:31–44
Thorne G, Malek RB (1968) Nematodes of the Northern Great Plains. Part I. Tylenchida (Nemata: Secernentea). Technical Bulletin No. 31. South Dakota Agricultural Experiment Station, South Dakota State University, Brookings, South Dakota
Troccoli A, Subbotin SA, Chitambar JJ, Janssen T, Waeyenberge L, Stanley JD, Duncan LW, Agudelo P, Uribe GEM, Franco J, Inserra RN (2016) Characterisation of amphimictic and parthenogenetic populations of Pratylenchus bolivianus Corbett, 1983 (Nematoda: Pratylenchidae) and their phylogenetic relationships with closely related species. Nematology 18(6):651–678. https://doi.org/10.1163/15685411-00002981
Valenzuela A, Raski DJ (1985) Pratylenchus australis n. sp. and Eutylenchus fueguensis n. sp. (Nematoda: Tylenchina) from Southern Chile. J Nematol 17:330–336
Van den berg E (1971) The root-lesion nematodes of South Africa (genus Pratylenchus, family Hoplolaimidae). Techn. Comm. Dep. Agric. Techn. Seru. Repub. S. Afi., No. 99, pp 13
Van den Berg E, Marias M, Tiedt LR (2007) Plant nematodes in South Africa 9 Check-list of plant nematodes from the Goegap and Witsand Nature Reserves, Northern Cape Province, with a description of a new Rotylenchus species (Hoplolaimidae: Nematoda). Afri Plant Prot 13:28–35
Waeyenberge L, Ryss A, Moens M, Pinochet J, Vrain TC (2000) Molecular characterization of 18 Pratylenchus species using rDNA restriction fragment length polymorphism. Nematology 2:135–142. https://doi.org/10.1163/156854100509024
Walker JT, Melin JB (1998) Host status of herbaceous perennials to Meloidogyne incognita and M arenaria. J Nematol 30(4S):607–610
Zimmermann A (1898) De Nematoden der Koffiewortels. Meded. Plantentuin 27:1–64
Acknowledgements
The authors are grateful to DG Malan for the transportation and logistics during the field survey and to LR Tiedt for assisting with scanning electron microscopy. The project was supported financial assistance of the National Research Foundation (NRF) (Grant no: 99679) towards this research is hereby acknowledged. Opinions expressed and conclusions arrived at are those of the authors and are not necessarily to be attributed to the NRF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Daramola, F.Y., Lewu, F.B. & Malan, A.P. Distribution and characterization of Pratylenchus bolivianus (Nematoda, Pratylenchidae) on rooibos (Aspalathus linearis) tea from South Africa. J Plant Dis Prot 128, 1291–1301 (2021). https://doi.org/10.1007/s41348-021-00471-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-021-00471-w