Abstract
Objective
We herein evaluated the hemodynamics of a gastric tube in esophagectomy using a new noninvasive blood flow evaluation device utilizing near-infrared spectroscopy.
Methods
Thirty-two cases of subtotal esophagectomy and gastric tube reconstruction for esophageal cancer were studied. The new device measures the regional tissue saturation of oxygen (rSO2: 0–99%) and total hemoglobin index (T-HbI: 0–1.0) with a small sensor. We measured these values at the antrum (point A), final branch of the right gastroepiploic artery (point B) and planned anastomotic point (point C) before and after gastric tube formation. The values at the three points were compared, and the gradients at the three points from before to after gastric tube formation were compared.
Results
The mean values of rSO2 at point A, B, and C before gastric tube formation were 57.2%, 57.8% and 56.0%, and those after formation were 54.6%, 58.0% and 55.8%, respectively. There was no significant difference in the comparison of the rSO2 gradient before and after formation (p = 0.167). The mean values of T-HbI at point A, B, and C before formation were 0.126, 0.178 and 0.211, and those after formation were 0.167, 0.247 and 0.292, respectively. There was no significant difference in the gradient of the increase before and after formation (p = 0.461).
Conclusion
A new device has shown that the gastric tube used in our facility is one that maintains tissue saturation of oxygen and does not cause excessive congestion at anastomosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Reconstruction of the gastric tube during esophagectomy significantly changes the hemodynamics of the stomach wall. Inappropriate blood flow in the anastomotic site is one of the causes of anastomotic leakage. Thus far, the blood flow has been evaluated by the measurement of arterial blood flow in the gastric tube using laser Doppler and visualization by indocyanine green (ICG) fluorescence during operation.
The reconstructed gastric tube is mainly nourished by the right gastroepiploic artery and vein. Stem flaps and free flaps in plastic surgery are also fed by one artery and vein set. Free flaps are frequently discussed in the plastic surgery field, and their survival requires a good venous return as well as arterial blood flow. However, there are few reports focusing on venous return and congestion in the hemodynamic assessment of a reconstructed gastric tube.
In this study, we evaluated the hemodynamics before and after the formation of a reconstructed gastric tube in our facility using a new noninvasive blood flow evaluation device with near-infrared spectroscopy (NIRS) and assessed the characteristics of the gastric tube in esophagectomy.
Materials and methods
Materials
This study was approved by the Clinical Research Center of Tokyo Medical and Dental University Hospital as number M2016-129.
The subjects consisted of 32 consecutive patients who underwent subtotal esophagectomy and gastric tube reconstruction for esophageal cancer in our department from April 2017 to May 2018.
Gastric tube formation method
Our department usually performs subtotal esophagectomy, three-field lymph node dissection and retrosternal gastric tube reconstruction for thoracic esophageal cancer.
With regard to the abdominal operation, the dissection of the abdominal lymph nodes and gastric tube formation techniques is common to both open cases and laparoscopic cases. An incision at the greater omentum is made about 3 cm outside of the right gastroepiploic artery, and the left gastroepiploic arteriovenous is dissected. If the arcade of the left and right gastroepiploic arteries is present, it is preserved. Gastric tube formation is performed outside the body. The right gastric arteriovenous is dissected in the antrum, and the lesser curvature of the antrum is deeply cut using a curved stapler, as the right gastroepiploic artery-dominant region has a small diameter to ensure the extensibility of the gastric tube. The rest of the gastric wall is then cut near the lesser curvature using linear staplers to preserve the intramural vascular pathway for good blood flow to the tip of the gastric tube. We call this form of the gastric tube the “flexible” gastric tube (Fig. 1) [1]. The esophagogastric anastomosis is a cervical anastomosis with a circular stapler.
The noninvasive blood flow evaluation device
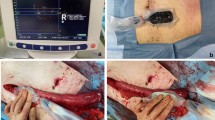
The new noninvasive blood flow evaluation device ‘toccare’ (Astem Co., Kanagawa, Japan) (Fig. 2a) was used to evaluate the hemodynamics of the gastric tube. This device has a small sensor probe with NIRS [2, 3]. The oxygenated and deoxygenated hemoglobin concentrations can be measured by Toccare according to the NIRS measurement principle by attaching the sensor to the examiner’s finger (Fig. 2b). The regional tissue saturation of oxygen (rSO2: 0% to 99%) and total hemoglobin index (T-HbI: 0 to 1.0) are then calculated and displayed. The rSO2 is the oxygen saturation of the peripheral blood in tissue; this differs from pulse oximeters, which measure arterial blood oxygen saturation. T-HbI is the absolute value that combines the oxygenated and deoxygenated hemoglobin concentrations and represents the total amount of hemoglobin per unit volume. Although it is an index, the unit is equivalent to mMol. Therefore, an increase in the T-HbI reflects an increase in the hemoglobin concentration in the measured tissue, i.e., congestion [4, 5]. The near-infrared light of this device can reach a depth of 4–5 mm from the surface of soft tissue, and this device measures the rSO2 and the T-HbI of the area, where the light can reach.
a New hemodynamic evaluation device, which measures the tissue oxygen saturation (rSO2: 0–99%) and total hemoglobin index (T-HbI: 0–1.0) of the area touched by the sensor probe. b rSO2 and T-HbI were measured before and after gastric tube formation using a small sensor attached to the examinee’s finger
Measurement of hemodynamics
In the abdominal portion of the surgery, after cutting the left gastric artery, left gastroepiploic artery and short gastric artery, we raised the stomach to the neck outside of the body and decided on the planned anastomotic point. We measured rSO2 and T-HbI before the formation of the gastric tube, with the stomach nourished by the right gastric artery and right gastroepiploic artery, at three locations: in the antrum (point A), the final branch of the right gastroepiploic artery (point B) and at the planned anastomotic point (point C) (Fig. 3a). In 19 of 32 cases, the values were also measured at the tip of the gastric tube (point D). We then performed gastric tube formation and measured these values again with the stomach nourished mainly by only the right gastroepiploic artery (Fig. 3b).
rSO2 and T-HbI were measured at three points: the antrum (point A), final branch of right gastroepiploic artery (point B) and planned anastomosis point (point C). a Before gastric tube formation, with the stomach nourished by the right gastric artery and right gastroepiploic artery. b After gastric tube formation, with the stomach nourished mainly by the right gastroepiploic artery
Statistical analyses
A repeated-measures analysis of variance (ANOVA) was used for comparisons of each of the three measurement points of the gastric tube before and after gastric tube formation. The Mann–Whitney U test was used for comparisons between two points. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [6], which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). Differences were considered to be significant at p < 0.05.
Results
The case characteristics are summarized in Table 1. The presence or absence of right and left gastroepiploic artery arcade (present: n = 11, absent: n = 19, unknown: n = 2), diabetes mellitus (present: n = 1, absent: n = 31), arteriosclerosis-related disease (including cerebral infarction, cardiac pectoris and arteriosclerosis obliterans; present: n = 7, absent: n = 25) and lymph node involvement around the lesser curvature (present: n = 8, absent: n = 24) were indicated. Anastomosis leakage was found in only 1 of the 32 cases.
The mean values of rSO2 before gastric tube formation in point A, B and C were 57.2%, 57.8% and 56.0%, and those after gastric tube formation were 54.6%, 58.0% and 55.8%, respectively (Fig. 4a). There was no significant difference among the rSO2 values at each point before gastric tube formation (p = 0.505). After tube formation, there was no significant difference in the rSO2 between points B and C (p = 0.078). In addition, there was no significant difference in the comparison of the oxygen saturation gradient before and after gastric tube formation (p = 0.167).
a Mean and standard deviation of rSO2 before and after gastric tube formation. After tube formation, there was no significant difference in the rSO2 between points B and C (p = 0.078). The values of rSO2 in one case of anastomotic leakage were also indicated. b Mean and standard deviation of T-HbI before and after gastric tube formation. There was no significant difference in the gradient of the increase before and after gastric tube formation (p = 0.461). The values of T-HbI in one case of anastomotic leakage were also indicated
The mean T-HbI values before gastric tube formation in point A, B and C were 0.126, 0.178 and 0.211, and those after gastric tube formation were 0.167, 0.247 and 0.292, respectively (Fig. 4b). The T-HbI at each point before gastric tube formation significantly increased from point A to point B (p = 0.002) and from point B to point C (p = 0.025). There was a significant increase from point A to point B after gastric tube formation (p = 0.003). There was no significant difference in the gradient of the increase before and after gastric tube formation (p = 0.461).
Considering the 19 cases in which measurement was performed up to the tip of the gastric tube (point D), The mean rSO2 values before gastric tube formation at points A, B, C and D were 57.1%, 57.2%, 55.3% and 53.8%, and those after gastric tube formation were 55.1%, 57.4%, 55.0 and 53.6%, respectively (Fig. 5a). Before tube formation, there was no significant difference in the rSO2 values between points C and D (p = 0.539). After tube formation, there was no significant difference in the rSO2 values between points C and D (p = 0.759). The oxygen saturation gradient before and after gastric tube formation did not differ to a statistically significant extent (p = 0.800).
a Mean and standard deviation of rSO2 before and after gastric tube formation at Point A, B, C and D. After tube formation, there was no significant difference in the rSO2 values between points C and D (p = 0.759). The values of rSO2 in one case of anastomotic leakage were also indicated. b Mean and standard deviation of T-HbI before and after gastric tube formation at Point A, B, C and D. There was no significant difference in the gradient of the increase from before to after gastric tube formation (p = 0.618). The values of T-HbI in one case of anastomotic leakage were also indicated
The mean T-HbI values before gastric tube formation at points A, B, C and D were 0.132, 0.189, 0.217 and 0.327, and those after gastric tube formation were 0.177, 0.249, 0.277 and 0.429, respectively (Fig. 5b). Before tube formation, there was a significant increase from point C to point D (p = 0.003). After tube formation, there was a significant increase from point C to point D (p = 0.039). There was no significant difference in the gradient of the increase from before to after gastric tube formation (p = 0.618).
In one case of anastomotic leakage, for Points A, B, C and D, the respective values of rSO2 were 54%, 57%, 51% and 48% before gastric tube formation and 63%, 57%, 52% and 54% after gastric tube formation. For Points A, B, C and D, the respective values of T-HbI were 0.22, 0.27, 0.28 and 0.37 before gastric tube formation and 0.33, 0.43, 0.30 and 0.45 after gastric tube formation.
We also evaluated the differences in blood flow with and without diabetes, lymph node involvement around the lesser curvature and other items, as shown in Table 1. Our comparison of the values and gradients of rSO2 and T-HbI after gastric tube formation showed no significant differences in any of the items (data not shown).
Discussion
Reconstruction after esophagectomy is one of the most important steps of this procedure. The stomach is often used for reconstruction. Postoperative anastomotic leakage is a serious and sometimes fatal complication of esophageal cancer surgery. Gastric tube blood flow at the anastomotic site is one of the most important factors for ensuring a good outcome [7,8,9]. Therefore, various studies have reported on methods of gastric tube formation and ensuring a good blood flow at the anastomotic site [10,11,12,13].
To evaluate the gastric tube blood flow, the measurement of the arterial blood flow in the wall using a Doppler blood flow meter has been attempted in some studies [14,15,16,17]. In recent years, the ICG method has been used to evaluate the micro blood flow for free flaps in the field of plastic surgery and for the blood flow evaluation in liver resection [18,19,20,21]. ICG has also been proven effective during esophagectomy and gastric tube reconstruction [22,23,24]. However, this method cannot evaluate the venous return or tissue blood washout [24].
The reconstructed gastric tube is mainly nourished by the right gastroepiploic artery and vein. The blood supply of the cranial 20% of the greater curvature narrow gastric tube is received through a microscopic network of capillaries and arterioles [25]. It is thought that the hemodynamics are similar to those of stem flaps or free flaps in that they are fed by one artery and vein set. This means that its survival requires good venous return as well as good arterial blood flow. The blood flow evaluation of free flaps has been discussed frequently in the field of plastic surgery. A recent report described NIRS as noninvasive, able to detect changes in the blood flow in real time, and having both excellent sensitivity and specificity [26].
This new device was originally developed to measure the tissue oxygen saturation of fetal brain tissue through an internal examination of a pregnant woman [2, 3]. In addition, its use has been attempted in the field of plastic surgery, because the blood flow state of the reconstructed flap can be easily evaluated. Tsuge et al. reported that, in cases of transverse rectus abdominis flap reconstruction in plastic surgery, the rSO2 decreased as the distance increased, gross congestion was observed at points, where the average rSO2 was below 45%, and ICG did not stain at points with a value of 41.3%. When sites with an average rSO2 below 45% were resected, flap necrosis was not seen [27].
In the present study, we used the same device to evaluate the venous return as well as the arterial blood flow in the reconstructed gastric tube. We could measure the rSO2 and T-HbI values of the reconstructed gastric tube during surgery easily and noninvasively. Since the near-infrared light of this device reaches up to 4–5 mm deep in soft tissue, it should be able to determine the condition of almost all layers, from the serosa to the mucous membrane, of the gastric wall.
As mentioned in the Methods section, we believe the “flexible” gastric tube in our facility achieves a good blood flow to the oral side of the gastric tube by preserving the intramural vascular pathway and contributes to a low anastomotic leakage rate. Evaluating the blood flow by the ICG method showed that the proximal region of the right gastroepiploic artery was rapidly enhanced though the intramural vascular pathway. In addition, the rate of anastomotic leakage was only 1.8% (11 cases) among the 615 patients who had undergone the same operation as those in this study since 2000, with only 1 patient developing gastric tube necrosis [1]. We, therefore, evaluated the hemodynamics of our gastric tube using this device, making this one of the few reports to quantitatively evaluate the oxygenation and congestion of the gastric tube.
There was no significant difference in the rSO2 between the three points before gastric tube formation. This means that the planned anastomotic point is well-oxygenated by the right gastric artery and right gastroepiploic artery. In addition, there was no significant difference between points B and C after gastric tube formation. This means that the planned anastomotic point, which is located beyond the periphery of the right gastroepiploic artery, remains well-oxygenated, even after gastric tube formation.
The T-HbI at each point before gastric tube formation increased toward the tip, indicating that the venous return decreases and the blood tends to stagnate as it travels to the tip. In addition, the T-HbI at each point tended to increase after gastric tube formation as well. This seems to be due to the overall tendency toward congestion over time, since the main blood vessels, such as the left gastric artery and vein, were cut off. However, there was no significant difference in the gradient of the three points on comparing values before and after gastric tube formation. If venous return in the intramural vascular pathway is not maintained on the oral side of the last branch of the right gastroepiploic artery and vein, it is expected that the congestion on the oral side will worsen and the gradient will increase after gastric tube formation. In this study, there was no change in the gradient from before to after gastric tube formation, and it is considered that after formation, the venous return on the oral side is maintained at the same level as before gastric tube formation.
Furthermore, in 19 cases in which 4 points of the gastric tube were measured, the rSO2 value tended to be slightly decreased (although not to a statistically significant extent) at the tip. The T-HbI value increased significantly from the planned anastomotic point to the tip from before to after gastric tube formation. There was no significant difference in the gradient from the antrum to the tip of T-HbI from before to after gastric tube formation. From these results, although venous return is maintained at the same level up to the tip of the gastric tube even after the formation of the gastric tube, the T-HbI value gradually increases toward the oral side, and rSO2 may decrease with time. In this study, the cut-off value of rSO2 for gastric tube necrosis and a high risk of anastomotic leakage in gastric tube reconstruction could not be determined. If anastomosis is performed at the tip of the tube, some cases might develop anastomotic leakage due to an impaired blood flow. Therefore, it is thought that anastomosis should be performed on the anal side as much as possible.
In one case of anastomotic leakage, the rSO2 at Points C and D was maintained after gastric tube formation, and there was no excessive increase in the T-HbI. Anastomotic leakage may have occurred for reasons other than an impaired blood flow.
Given the above, the new device with NIRS showed that our gastric tube maintains the tissue saturation of oxygen to the anastomosis and does not cause excessive congestion, a result that supports our gastric tube concept.
The present study was associated with two limitations. First, this study was limited by being performed in a single facility, so we were unable to compare our flexible gastric tube with a narrow gastric tube or subtotal gastric tube. Second, as there was only one case of anastomotic leakage, it was not possible to sufficiently compare cases with and without anastomotic leakage.
In the future, I would like to conduct collaborative research with other institutions and compare the hemodynamics with other gastric tube shapes. We also hope to examine whether or not the rSO2 and T-HbI values at the anastomotic point can predict anastomotic leakage by increasing the number of cases.
Conclusion
A new device with NIRS showed that the gastric tube in our facility is one that maintains tissue saturation of oxygen to the anastomosis and does not cause excessive congestion.
References
Nakajima Y, Kawada K, Tokairin Y, Hoshino A, Okada T. Flexible gastric tube: a novel gastric tube formation method to prevent anastomotic leakage. Ann Thorac Surg. 2020. https://doi.org/10.1016/j.athoracsur.2019.12.084.
Kanayama N, Niwayama M. Examiner's finger-mounted fetal tissue oximetry. J Biomed Opt. 2014;19:067008.
Uchida T, Kanayama N, Mukai M, Furuta N, Itoh H, Suzuki H, et al. Examiner's finger-mounted fetal tissue oximetry: a preliminary report on 30 cases. J Perinat Med. 2016;44:745–9.
Ebihara A, Tanaka Y, Konno T, Kawasaki S, Fujiwara M, Watanabe E. Detection of cerebral ischemia using the power spectrum of the pulse wave measured by near-infrared spectroscopy (in English). J Biomed Opt. 2013;18:106001.
Uchida T, Kanayama N, Kawai K, Niwayama M. Craniofacial tissue oxygen saturation is associated with blood pH using an examiner's finger-mounted tissue oximetry in mice. J Biomed Opt. 2016;21:40502.
Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Allaix ME, Herbella FA, Patti MG. Hybrid trans-thoracic esophagectomy with side-to-side stapled intra-thoracic esophagogastric anastomosis for esophageal cancer. J Gastrointest Surg. 2013;17:1972–9.
Alanezi K, Urschel JD. Mortality secondary to esophageal anastomotic leak. Ann Thorac Cardiovasc Surg. 2004;10:71–5.
Aminian A, Panahi N, Mirsharifi R, Karimian F, Meysamie A, Khorgami Z, et al. Predictors and outcome of cervical anastomotic leakage after esophageal cancer surgery. J Cancer Res Ther. 2011;7:448–53.
Miyawaki Y, Sato H, Fujiwara N, Sugita H, Sakuramoto S, Okamoto K, et al. Evaluation of the associations between gastric tube preparation methods and the incidence of cervical anastomotic leakage after esophagectomy for thoracic esophageal cancer. Dig Surg. 2019;37:154–62.
Akiyama H, Miyazono H, Tsurumaru M, Hashimoto C, Kawamura T. Use of the stomach as an esophageal substitute. Ann Surg. 1978;188:606–10.
Shu YS, Sun C, Shi WP, Shi HC, Lu SC, Wang K. Tubular stomach or whole stomach for esophagectomy through cervico-thoraco-abdominal approach: a comparative clinical study on anastomotic leakage. Ir J Med Sci. 2013;182:477–80.
Ndoye JM, Dia A, Ndiaye A, Fall B, Diop M, Ndiaye A, et al. Arteriography of three models of gastric oesophagoplasty: the whole stomach, a wide gastric tube and a narrow gastric tube. Surg Radiol Anat. 2006;28:429–37.
Miyazaki T, Kuwano H, Kato H, Yoshikawa M, Ojima H, Tsukada K. Predictive value of blood flow in the gastric tube in anastomotic insufficiency after thoracic esophagectomy. World J Surg. 2002;26:1319–23.
Schilling MK, Mettler D, Redaelli C, Buchler MW. Circulatory and anatomic differences among experimental gastric tubes as esophageal replacement. World J Surg. 1997;21:992–7.
Tsekov C, Belyaev O, Tcholakov O, Tcherveniakov A. Intraoperative Doppler assessment of gastric tube perfusion in esophagogastroplasty. J Surg Res. 2006;132:98–103.
Ikeda Y, Niimi M, Kan S, Shatari T, Takami H, Kodaira S. Clinical significance of tissue blood flow during esophagectomy by laser Doppler flowmetry. J Thorac Cardiovasc Surg. 2001;122:1101–6.
Yamamoto M, Sasaguri S, Sato T. Assessing intraoperative blood flow in cardiovascular surgery. Surg Today. 2011;41:1467–74.
Holm C, Mayr M, Hofter E, Becker A, Pfeiffer UJ, Muhlbauer W. Intraoperative evaluation of skin-flap viability using laser-induced fluorescence of indocyanine green. Br J Plast Surg. 2002;55:635–44.
Saito T, Yano M, Motoori M, Kishi K, Fujiwara Y, Shingai T, et al. Subtotal gastrectomy for gastric tube cancer after esophagectomy: a safe procedure preserving the proximal part of gastric tube based on intraoperative ICG blood flow evaluation. J Surg Oncol. 2012;106:107–10.
Kang Y, Lee J, Kwon K, Choi C. Dynamic fluorescence imaging of indocyanine green for reliable and sensitive diagnosis of peripheral vascular insufficiency. Microvasc Res. 2010;80:552–5.
Shimada Y, Okumura T, Nagata T, Sawada S, Matsui K, Hori R, et al. Usefulness of blood supply visualization by indocyanine green fluorescence for reconstruction during esophagectomy. Esophagus. 2011;8:259–66.
Ishiguro T, Kumagai Y, Ono T, Imaizumi H, Honjo H, Suzuki O, et al. Usefulness of indocyanine green angiography for evaluation of blood supply in a reconstructed gastric tube during esophagectomy. Int Surg. 2012;97:340–4.
Kumagai Y, Ishiguro T, Haga N, Kuwabara K, Kawano T, Ishida H. Hemodynamics of the reconstructed gastric tube during esophagectomy: assessment of outcomes with indocyanine green fluorescence (in english). World J Surg. 2014;38:138–43.
Liebermann-Meffert DM, Meier R, Siewert JR. Vascular anatomy of the gastric tube used for esophageal reconstruction. Ann Thorac Surg. 1992;54:1110–5.
Steele MH. Three-year experience using near infrared spectroscopy tissue oximetry monitoring of free tissue transfers. Ann Plast Surg. 2011;66:540–5.
Tsuge I, Enoshiri T, Saito S, Suzuki S. A quick evaluation of TRAM flap viability using fingerstall-type tissue oximetry. Plast Reconstr Surg Glob Open. 2017;5:e1494.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yamaguchi, K., Nakajima, Y., Matsui, T. et al. The evaluation of the hemodynamics of a gastric tube in esophagectomy using a new noninvasive blood flow evaluation device utilizing near-infrared spectroscopy. Gen Thorac Cardiovasc Surg 68, 841–847 (2020). https://doi.org/10.1007/s11748-020-01350-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-020-01350-1